Abstract
NK cell responses to HIV/SIV infection have been well studied in acute and chronic infected patients/monkeys, but little is known about NK cells during viral transmission, particularly in mucosal tissues. Here we report a systematic study of NK cell responses to high-dose vaginal exposure to SIVmac251 in the rhesus macaque female reproductive tract (FRT). Small numbers of NK cells were recruited into the FRT mucosa following vaginal inoculation. The influx of mucosal NK cells preceded local virus replication and peaked at one week, and was thus in an appropriate time frame to control an expanding population of infected cells at the portal of entry. However, NK cells were greatly outnumbered by recruited target cells that fuel local virus expansion, and were spatially dissociated from SIV RNA+ cells at the major site of expansion of infected founder populations in the transition zone and adjoining endocervix. The number of NK cells in the FRT mucosa rapidly decreased in the second week, in an inverse relationship to the peak of local SIV RNA+ cells. Mucosal NK cells produced IFN-γ and MIP-1α/CCL3, but lacked several markers of activation and cytotoxicity, and this was correlated with inoculum-induced upregulation of the inhibitory ligand HLA-E and downregulation of the activating receptor CD122/IL2Rβ. Examination of SIVΔnef-vaccinated monkeys suggested that recruitment of NK cells to the genital mucosa was not involved in vaccine-induced protection from vaginal challenge. In summary, our results suggest that NK cells play at most a limited role in defenses in the FRT against vaginal challenge.
Keywords: Natural Killer Cells, Female Genital Mucosa, Vagina, Cervix, Rhesus Macaque, SIV, CCL3, IFN-γ, HLA-E, CD122, granzyme H, IFN-γ, IP-10, SIVΔnef Vaccine, ISH, FISH, IHC
Introduction
While antiretroviral therapies have converted HIV-1 infection to a chronic and largely manageable disease for many individuals, it is clear that ending the pandemic depends on more effective measures for prevention, particularly for the principal route of sexual mucosal transmission and in the population of young women in the pandemic’s epicenter at sub-Saharan Africa, who are the most at risk of heterosexual acquisition of HIV-1 (1). To that end, we have been seeking design principles for development of an effective vaccine to prevent HIV-1 transmission to women in the SIV-rhesus macaque non-human primate model. We report here the results of an investigation into the role of NK cells in preventing transmission by analyses of the NK cell responses to high dose vaginal challenge with pathogenic SIVmac251 in naïve animals, and animals vaccinated with the live attenuated vaccine, SIVΔnef.
We focused these studies on the immunological and virological events of transmission in the early stages of infection at the portal of entry for two reasons. First, it had previously been shown that the SIV-infected founder populations were initially quite small in the monkey FRT mucosa tissues, in spite of vaginal exposure to high doses of SIVmac251 (2, 3). Consequently, there should be an opportunity for pre-existing or rapidly induced innate or adaptive immune responses to operate at high effector to target ratios to prevent or restrict the establishment of the initial virus-replicating population. Second, since local expansion of the infected founder population usually precedes the dissemination of infection into the circulation and the establishment of a robust self-propagating systemic infection in mainly lymphoid tissues (2, 3), pre-existing and rapid immune responses could efficiently prevent or attenuate systemic infection by constraining this local expansion.
The innate immune system, particularly natural killer (NK) cells, could potentially play important roles in this early window of opportunity, as a rapid responder population that could kill the infected cells through either antibody-dependent cellular cytoxicity (ADCC) or antibody-independent (NK cytotoxicity) mechanisms (4). NK cells also express β-chemokines, including MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5, which could inhibit continued propagation of infection by blocking viral entry (4, 5). Furthermore, preservation of NK cell functions has been associated with improved disease outcome in patients and monkeys chronically infected with HIV-1 and SIV, respectively (6–8). Increased NK cell activity has been correlated with protection in HIV-1 highly exposed seronegative subjects (HESN) (9). Studies on the activating and inhibitory receptors on NK cells have revealed a protective correlation between certain KIR and HLA polymorphisms (10–13) as well as a role of NK cells in controlling viral infection in vivo based on HIV-1 adaptation to KIR receptors (14). However, little is thus far known about NK cell responses in mucosal tissues, particularly at the early stage of SIV/HIV mucosal transmission. We address in part that gap in our knowledge of the role of NK cells in protecting the FRT in early infection by analyzing and comparing NK cell responses in the lower FRT mucosa to high dose vaginal challenge with pathogenic SIVmac251, in naïve animals and in animals vaccinated with the live attenuated vaccine, SIVΔnef.
Material and Methods
Tissues from Δnef-vaccinated and/or SIV-infected animals
Archived genital tissues from previous studies of SIV high dose vaginal infection (2) and SIV Δnef vaccines (15, 16) were used in this study. Briefly, fresh tissues obtained at necropsy were fixed in 4% paraformaldehyde, Streck’s, or SAFE Tissue Fixative, and embedded in paraffin, as previously reported (2).
Immunohistochemistry (IHC)
Single and double immunohistochemical staining, fluorescent immunohistochemical staining, and quantitative image analysis on confocal microscopy were performed as described elsewhere (17, 18). The primary antibodies used in this study were summarized in Table I.
Table I.
Primary antibodies used in this study
| Antibodies | Clone/Catalog # | Tissue Fixation | Antigen Retrieval |
|---|---|---|---|
| NKG2A | Epitomics (T3308) | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| Granzyme H | Sigma HPA029200 | PFA | 1 mM EDTA Buffer pH8.0 98°C 20 min |
| Granzyme B | Spring Bioscience E2580 | PFA | 1 mM EDTA Buffer pH8.0 98°C 20 min |
| IFN-γ | Abcam ab25101 | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| IL-2Rβ (CD122) | Novus Biologicals NBP1-19140 | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| HLA-E | MEM-E/06 ab3984 | PFA | 1 mM EDTA Buffer pH8.0 98°C 20 min |
| CD3 | AbD Serotech MCA 1477 | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| CCL3/MIP-1α | Neomarkers RB-10489-P | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| CCL4/MIP-1β | R&D AF-271-NA | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| CCL5/RANTES | R&D AF-278-NA | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| Ki67 | Neomarkers RM9106 | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| HLA-DR/DQ/DP | DAKO M0073 | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| CD69 | Novacastra NCL-CD69 CH11 | SAFE | 1 mM EDTA Buffer pH8.0 98°C 20 min |
| CD38 | Vector VP-C348 SPC32 | SAFE | 1 mM EDTA Buffer pH8.0 98°C 20 min |
| IP10 | R&D AF-266-NA | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| CD107a | LifeSpan LS-B580 | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| CD68 | DAKO KP1 M0814 | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
| Vimentin | Neomarkers RB-9063 | PFA | 10 mM Citrate Buffer pH6.0 98°C 20 min |
In situ hybridization (ISH)
SIV RNA was detected in paraformaldehyde-fixed and paraffin-embedded tissues by in situ hybridization as previously described (2). Briefly, sections in depth of 5 μm were deparaffinized in xylene, rehydrated in PBS, and permeablized sequentially in HCl, digitonin, and proteinase K. The sections were then acetylated and hybridized to 35S-labeled SIV-specific riboprobes. After wash and digestion with ribonucleases, the sections were coated with nuclear-track emulsion before exposure and development. For fluorescent ISH (FISH), digoxigenin-labeled SIV-specific riboprobes were used and followed by sequential staining with Goat anti-digoxigenin Abs (Roche) and Donkey anti-Goat Abs conjugated with Alexa Fluor 555 (Invitrogen) (17, 18).
Statistical tests
The Wilcoxon rank-sum test was used to measure the variations of NK cell, macrophage, and SIV RNA+ cell densities over the course of infection, vaccination, and challenge. The Paired t-test was used to compare the number of NK cells between vaginal and cervical tissues from the same animal. The Spearman’s Rank Correlation test was used to analyze the relationship between mucosal NK cells and local SIV RNA+ cells. Statistical analysis in this study was carried out using PRISM 4.
Results
NK cell responses in naïve animals: location of NKG2A+CD3− NK cells
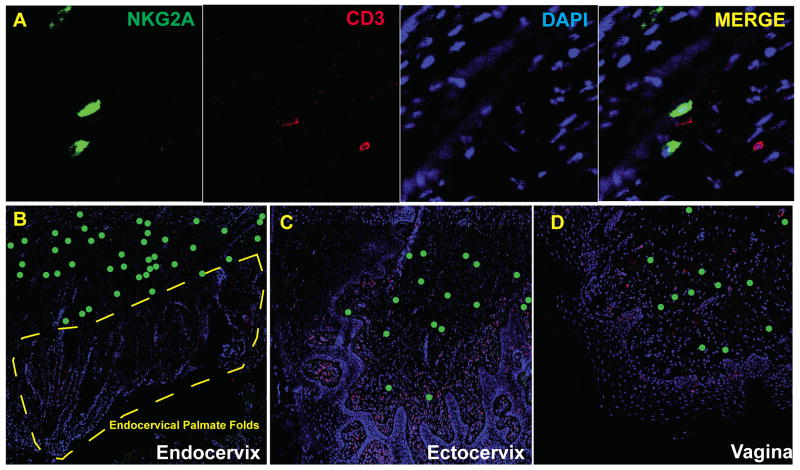
We first systematically examined early NK cell responses in the vaginal and cervical mucosa of naïve unvaccinated rhesus macaque female genital tract (FRT) at time points within four weeks of high-dose vaginal inoculation of SIVmac251 (1–2×105 TCID50). NK cells were defined as NKG2A+CD3− cells (Fig. 1A) as previously reported (19–23), detected in fixed and paraffin-embedded necropsy samples using immunohistochemistry. In general, NK cells were mainly found in vaginal and cervical submucosa (Fig. 1B–D). In contrast to gut mucosal tissues (24), we did not find intraepithelial NK cells in vagina or cervix, and we found very few NK cells at the site where infected founder populations have been consistently sighted (2, 3) in the palmate folds of endocervical tissues in any of the 32 cervical tissues examined (Fig. 1B).
FIGURE 1.
Spatial distribution of NK cells in the lower FRT mucosa of rhesus macaques. NK cells in rhesus macaques were defined as NKG2A+CD3− using immunohistochemistry (A). NK cells were mainly located in the submucosal area in endocervix (B), ectocervix (C), and vagina (D). There were remarkably few NK cells in the palmate folds of the endocervix (B). All images were taken from stained sections of tissues collected 7 days post infection. NKG2A (Green), CD3 (Red). In (B), (C), and (D), NK cells were labeled as green dots for better visibility at the magnification shown.
NK cell responses in naïve animals: rapid expansion and then contraction following vaginal challenge
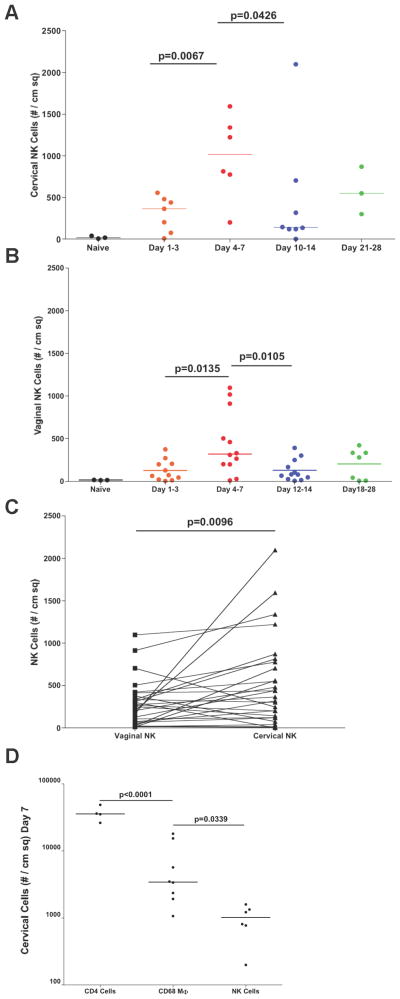
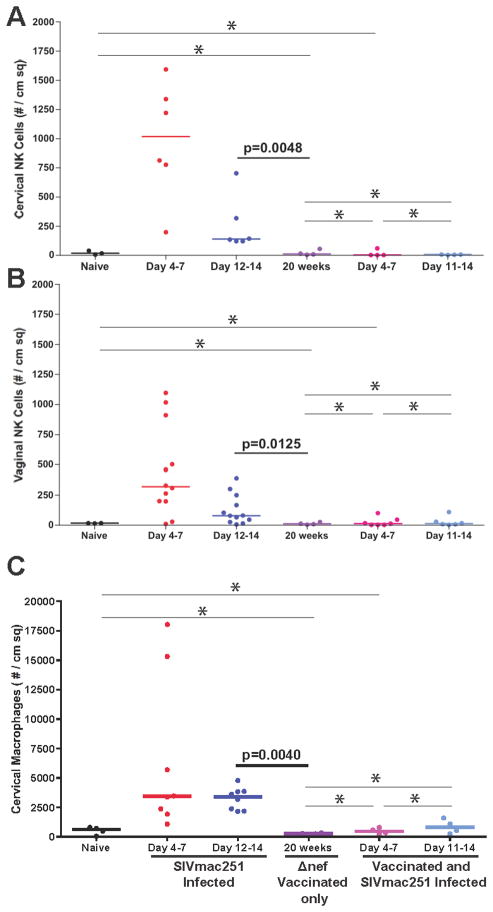
We quantified the number of NK cells in vaginal (48 animals) and cervical (32 animals) tissues in naïve animals prior to and following SIV vaginal challenge. As shown in Fig. 2A and 2B, NK cells were rapidly recruited into the cervical and vaginal mucosa after vaginal inoculation, with significantly higher numbers in cervix compared to vagina (cervical median = 1017, vaginal median = 319) at the end of the first week (Fig. 2C). However, even at the peak of expansion, NK cells were greatly outnumbered by expanded populations of the CD4+ T cells that fuel local virus expansion (2, 3), and by macrophages Fig. 2D).
FIGURE 2.
NK cells are recruited into the lower FRT mucosa after vaginal challenge. The density of NK cells in the genital mucosa was quantified in cervical (A) and vaginal (B) tissues. In the same individual animals, there were more mucosal NK cells in the cervix than vagina (C). However, even at the peak of recruitment, NK cells were significantly outnumbered by recruited CD4 T cells and macrophages (D). Data used in (D) were from tissues collected 7 days post infection. Each point or line represents an individual animal.
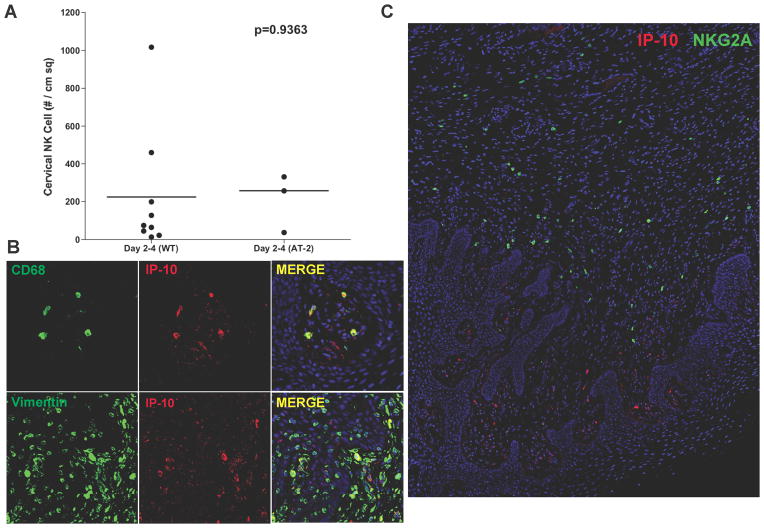
While small foci of SIV RNA+ cells have been detected in the cervix as early as 3–4 days (2, 25) post vaginal inoculation, it seemed unlikely that viral replication per se would be the driving force for the early NK cell influx into the genital mucosa, given the magnitude of local infection. The recruitment of NK cells to cervical tissues of animals vaginally inoculated with infectious SIV (WT-SIV) or AT-2 inactivated virus (AT-2-SIV) is consistent with this conclusion. As shown in Fig. 3A, the densities of NK cells were comparable between WT-SIV and AT-2-SIV groups through 4 days after vaginal inoculation. Moreover, the decrease in numbers of mucosal NK cells in both vagina and cervix during the second week (the peak of infection in the FRT), when there remained only 13.6% (cervix) and 24.8% (vagina) (percentage of median) NK cells of the peak values (Fig. 2A and 2B), also argues against viral replication-driven NK cell recruitment.
FIGURE 3.
(A). AT-2 inactivated SIV was as potent as WT in recruiting NK cells into the FRT. Each point represents an individual animal. (B). Macrophages (CD68+) and fibroblasts (Vimentin+) were the major CXCL10/IP-10-expressing cell population in the FRT mucosa. (C). These CXCL10+ cells were in close proximity to NKG2A+ NK cells in the submucosa. All images were from tissues collected 7 days post infection.
NK cells are most likely recruited by chemokine expression in the FRT. Since CXCL10/IP-10 is well known as a potent NK cell chemoattractant, we examined its expression profile in the FRT mucosa of infected animals. Macrophages (CD68+) and fibroblasts (vimentin+) were the major CXCL10-producing cell populations in the genital mucosa (Fig. 3B). These CXCL10+ cells resided close to the basal layer of epithelium and were often found in close proximity to majority of NKG2A+ NK cells in the submucosa (Fig. 3C). We favor local recruitment of NK cells by these CXCL10+ cells rather than recruitment by CXCL10 in the inoculum (26), which we would expect to elicit a general and immediate recruitment of NK cells to the mucosal border, rather than the observed focal and delayed recruitment three days after exposure.
NK cell responses in naïve animals: Relationships between NK cells and SIV RNA+ cells
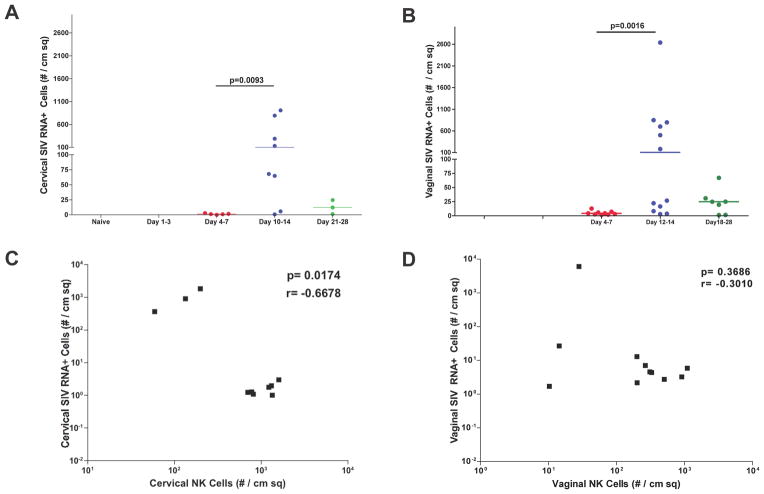
We next investigated the potential role of NK cells recruited in the first week of infection in containing local viral replication by examining the density and spatial relationships between the mucosal NK cells and SIV RNA+ cells. We enumerated SIV RNA+ cells detected by in situ hybridization (ISH), and show that SIV RNA+ cells were barely detectable in the first week, and then increased to peak in the second week (Fig. 4A and 4B). Since the mucosal NK cells peaked in the first week, when the local expansion of infected founder foci of infected cells had just begun to expand, there was an expected negative correlation between the densities of SIV RNA+ cells and NK cells, which was significant in cervix but not vagina (Fig. 4C and 4D). However, in montage images of the transformation zone (TZ) where SIV RNA+ cells are consistently concentrated in early infection (2, 3), there was complete spatial separation of NKG2A+CD3− NK and SIV RNA+ cell populations (Fig. 5). Indeed, in all animals examined, the SIV RNA+ cells were always located in the endocervix close to the TZ where there were few if any NK cells (Fig. 1b). Although these images are snapshots of interactions of cells in FRT tissues, the spatial dissociation between NK cells and SIV RNA+ cells (Fig. 5) does not support the hypothesis that recruited NK cells contain infection by contact-dependent mechanisms in the endocervix and TZ where expanding founder populations of infected cells have been consistently documented (2, 3). However, this spatial dissociation does not exclude a possible role for NK cells in eliminating infected cells at sites close to NK cells before the SIV RNA reaches a detectable level.
FIGURE 4.
Increase in the density of SIV RNA+ cells in cervix and vagina and negative correlation with the density of mucosal NK cells at the end of the first week following vaginal inoculation. (A and B). SIV RNA+ cells detected by ISH were enumerated in cervix and vagina. Each point represents an individual animal. (C and D). In cervical and vaginal tissues, the densities of NK cells were negatively correlated with the densities of SIV RNA+ cells at the end of the first week.
FIGURE 5.
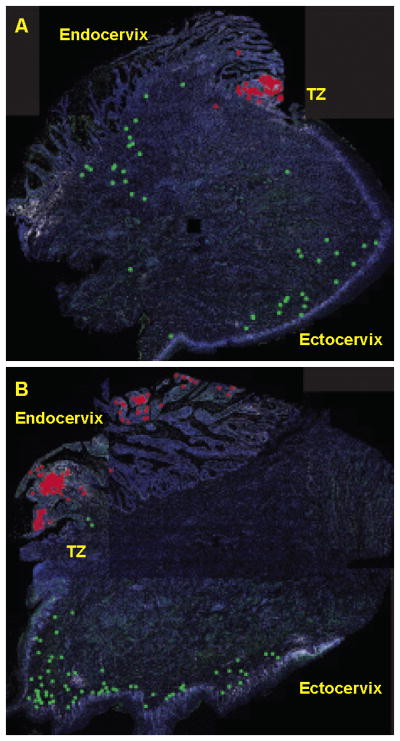
Spatial dissociation between cervical NK cells and SIV RNA+ cells. Montage images of the density and spatial distribution of mucosal NK cells and SIV RNA+ cells in entire cervical tissue sections. (A and B). NK cells and SIV RNA+ cells are too small to be visible in montage images of entire tissue section at the resolution shown. The NK cells and SIV RNA+ cells were therefore labeled as green and red dots, respectively. All images were from tissues collected 7 days post infection. TZ-transformation zone.
NK cell responses in naïve animals: functional and activation markers of NK cells in FRT tissues
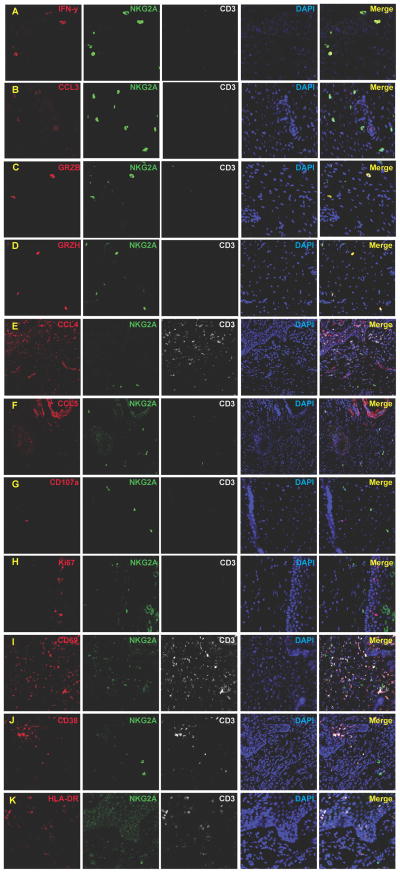
We examined the chemokine and cytokine expression profiles of cervical NK cells at 7 days post vaginal inoculation for evidence that might indirectly support the hypothesis that NK cells play a protective role in containing local infection following vaginal exposure to SIV. The majority of cervical NK cells produced IFN-γ (74.3–98.1%, median 80.3%, n=7) (Fig. 6A) as well as the HIV-1 inhibitory β-chemokine MIP-1α (CCL3) (Fig. 6B), but not MIP-1β (Fig. 6E) or RANTES (Fig. 6F) (not shown), and only ~ one in four cervical NK cells expressed granzyme B (10.3–29.2%, median 25.4%, n=7) (Fig. 6C) and less than half of the NK cells were positive for the more abundant NK cytotoxic effector (27), granzyme H (14.6–42.6%, median 38.5%, n=7) (Fig. 6D). Moreover, none of the NK cells were positive for the degranulation marker, CD107a (Fig. 6G), and were also not activated or replicating by the phenotypic markers, Ki67−, CD69−, CD38−, and HLA-DR− (Fig. 6H–K).
FIGURE 6.
Cervical NK cells express IFN-γ (A), MIP-1α (CCL3) (B), granzyme B (C) and granzyme H (D). However, the majority are CCL4− (E), CCL5− (F), CD107a− (G), Ki67− (H), CD69− (I), CD38− (J), and HLA-DR− (K).
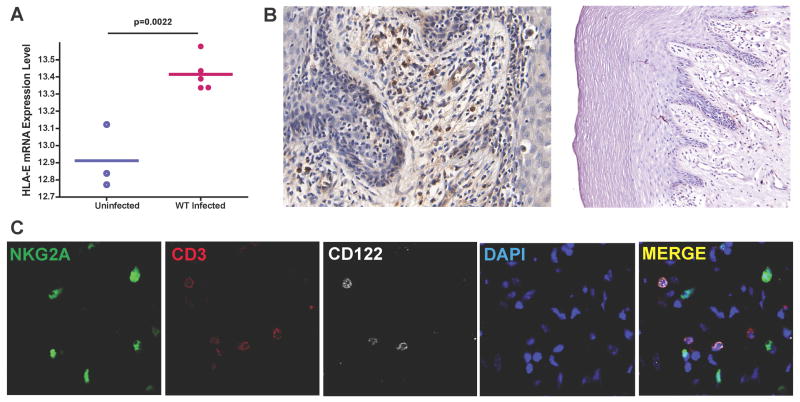
In seeking an explanation for the latter phenotype, we had noticed in unpublished microarray analyses in which we compared transcriptional profiles of cervical tissues from uninfected to vaginally infected animals, that expression of the HLA-E (Mamu-E) inhibitory ligand of NKG2A receptors was up-regulated in cervical tissues within 4 days after virus infection (Fig. 7A). By immunohistochemical staining, HLA-E+ cells were detectable as soon as 24 hours post challenge (Fig. 7B). These HLA-E+ cells included Langerhans cells in the genital epithelium and cells in the submucosa close to epithelium, where NK cells usually reside (Fig. 7B). Consistent with the inhibitory function of HLA-E, the resident NK cell population was CD122 (IL2Rβ) negative (Fig. 7C), and therefore would be unable to respond to IL-2 and IL-15. Collectively, the cervical NK cells recruited by SIV vaginal inoculation thus lacked many markers of activation and cytotoxic function that might be expected for an effector population.
FIGURE 7.
(A). Microarray analysis comparison of gene expression in uninfected versus infected cervical tissue reveals up-regulation of HLA-E expression in cervical tissues after vaginal challenge. (B). The HLA-E expressing cells in cervical-vaginal submucosa as early as 24 h post infection include Langerhans cells in epithelium, shown to the right. (C). NKG2A+CD3− NK cells in cervical mucosa were CD122 (IL2Rβ) negative.
FRT-NK cell density does not correlate with protection by SIVmac239Δnef-vaccination
SIVmac239Δnef vaccination has been shown to protect against high dose vaginal challenge (15, 16, 28) as e.g. vaccinated animals either had sterilizing immunity or lower peak and set point viral loads compared to unvaccinated animals at 20 weeks post-vaccination (R.K. Reeves, manuscript in preparation). We evaluated potential NK cell contributions to this protection by asking whether vaccinated animals had denser vaginal and cervical NK cell populations compared to naïve animals prior to and after vagina challenge. To the contrary, we found that the NK cell populations prior to challenge were not statistically different between naïve and vaccinated animals in cervix or vagina (Fig. 8A, B), and, surprisingly, high dose vaginal challenge of vaccinated animals with pathogenic SIVmac251 did not even induce the influx of NK cells or macrophages (Fig. 8C) into the genital mucosa that we had documented (Fig. 1) in unvaccinated animals.
FIGURE 8.
NK cell population densities in the FRT mucosa do not correlate with SIVmac239Δnef vaccine protection before or after high dose vaginal challenge. NK cells in the cervical (A) and vaginal (B) mucosa were quantified by IHC in tissues from naïve, SIVmac251 vaginally infected, SIVmac239Δnef-vaccinated, vaccinated plus vaginally challenged rhesus macaques. There was no correlation between NK cell density prior to and after vaginal challenge with protection, and NK cell densities did not increase following challenge in the vaccinated animals. (* = NOT significant-NS). Macrophage recruitment was also inhibited in the vaccinated animals (C).
Discussion
Here we report characterization of the NK cell response to SIV infection in the female genital mucosa of rhesus macaques after a high dose vaginal exposure. In this animal model, the first 3–4 days after infection define a time frame of vulnerability for the virus in establishing and expanding infected founder populations and thus a window of opportunity for the host immune system, to inhibit viral replication and prevent mucosal transmission. We did find that NK cells were recruited into the genital mucosa in this window of opportunity by mechanisms that can be at least partially attributed to cells in the FRT expressing CXCL10/IP-10, the most potent NK cell chemoattractant (29), in close proximity to NK cells. The peak values of recruited cervical NK cells at 7 days post-vaginal exposure were negatively correlated with the number of SIV RNA+ cells in cervix. Moreover, most of the NK cells in the FRT mucosa are IFN-γ+ and MIP-1α/CCL3+, a β-chemokine, which has been shown to block viral entry in culture (4, 5). Collectively, these results initially provided evidence to support the hypothesis that the NK cells recruited into the genital mucosa could contribute to containing SIV infection in the early window of opportunity at the portal of entry.
However, we document aspects of the quantity, location and quality of observed mucosal NK cell responses that are inconsistent with NK cells as effective gatekeepers to prevent viral transmission. First, only a very small number of NK cells were recruited into the genital mucosa relative to the orders of magnitude greater recruitment of CD4 T cells that fuel the local expansion of viruses and infected cells prior to systemic dissemination and infection (2, 25). The numbers of NK cells also declined at the peak of virus replication in the lower FRT tissues, similar to decreased numbers of NK cells in the gut mucosa of SIV-infected animals two to four weeks following SIV vaginal transmission (23, 30). Thus, the effective in vivo ratio of NK effector cells to infected targets was even more unfavorable for a protective role.
Second, the spatial dissociation between recruited NK cells and expanding populations of SIV RNA+ cells in the endocervix does not support a model in which mucosal NK cells kill their targets by cell-cell contact-mechanisms, such as ADCC and death receptor-mediated cytotoxicity (31). Third, the recruited NK cells lacked many phenotypic markers associated with a functioning effector population, such as evidence of degranulation (CD107a), activation (CD38, CD69, and HLA-DR), and other cytokines/chemokines previously associated with an activated effector population of NK cells, although CD69 and Ki67 expression on NK cells in blood and gut has been reported (23, 32). In the FRT, we show increased expression of the inhibitory HLA-E ligand and down-regulation of activating receptor CD122 following vaginal exposure to SIV, highlighting the rapidly altered balance between activating and inhibitory signals during mucosal transmission, as has been shown in other acute and chronic infections (33–35).
SIVmac239Δnef vaccination provides robust protection against subsequent WT-SIV challenge by parenteral and mucosal routes (16, 28). Although safety issues have precluded advancing this and other live attenuated viruses as candidates for an effective HIV-1 vaccine, understanding the correlates of protection could provide critical insights to guide HIV-1 vaccine development. We have been focusing particularly on identifying correlates associated with the Δnef vaccine-mediated maturation of protection, defined by little protection if animals are vaginally challenged at 5 weeks post vaccination, but substantial protection at 15–20 weeks or even 40 weeks post vaccination. In this study, we assessed the role of NK cells in the FRT mucosa in mediating the vaccine-induced protection in animals that have been vaccinated for 20 weeks (versus naïve animals). Surprisingly, NK cell numbers in the FRT mucosa of 20-week-vaccinated animals prior to challenge were comparable to the naïve animals. Even more remarkably, we observed no influx of either NK cells or macrophages into the FRT mucosa in the vaccinated animals following high dose vaginal challenge. We show elsewhere that the formation of immune complexes following vaginal challenge, and interaction of these immune complexes with Fc receptor for IgG, FcγRIIb, induce an inhibitory program that blocks CD4 T cell recruitment (Smith, A., et al., ms. in preparation). This inhibitory program may similarly be involved in blocking NK cell as well as macrophage recruitment. Thus, our studies collectively provide only limited evidence of correlations between NK cells in the FRT mucosa of naïve animals and containment of infection at the portal of entry, and no evidence of a role for NK cells in protection against vaginal challenge in SIVmac239Δnef-vaccinated animals.
Acknowledgments
We thank Dr. Ronald Desrosiers for providing SIVmac239Δnef; Jacqueline Gillis, Fay Eng Wong, and Elizabeth Curran for assistance with tissue processing; and Dr. Angela Carville and other members of the NEPRC Division of Veterinary Resources for their expert animal care.
Footnotes
This work was supported by the International AIDS Vaccine Initiative and NIH grants AI071306, AI090735, AI095985 and RR00168 (currently OD011103).
References
- 1.2012. UN Joint Programme on HIV/AIDS (UNAIDS), Global Report: UNAIDS Report on the Global AIDS Epidemic.
- 2.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 5.Oliva A, Kinter AL, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, Li Y, Romano JW, Fauci AS. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest. 1998;102:223–231. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, Kaplan J. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis. 1999;180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21:227–233. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 8.Banks ND, Kinsey N, Clements J, Hildreth JE. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses. 2002;18:1197–1205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 9.Tomescu C, Abdulhaqq S, Montaner LJ. Evidence for the innate immune response as a correlate of protection in human immunodeficiency virus (HIV)-1 highly exposed seronegative subjects (HESN) Clin Exp Immunol. 2011;164:158–169. doi: 10.1111/j.1365-2249.2011.04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265:29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrington M, Martin MP, van Bergen J. KIR-HLA intercourse in HIV disease. Trends Microbiol. 2008;16:620–627. doi: 10.1016/j.tim.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashirova AA, Thomas R, Carrington M. HLA/KIR restraint of HIV: surviving the fittest. Annu Rev Immunol. 2011;29:295–317. doi: 10.1146/annurev-immunol-031210-101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMichael AJ, Jones EY. Genetics. First-class control of HIV-1. Science. 2010;330:1488–1490. doi: 10.1126/science.1200035. [DOI] [PubMed] [Google Scholar]
- 14.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyand MS, Manson K, Montefiori DC, Lifson JD, Johnson RP, Desrosiers RC. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73:8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RP, Lifson JD, Czajak SC, Cole KS, Manson KH, Glickman R, Yang J, Montefiori DC, Montelaro R, Wyand MS, Desrosiers RC. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73:4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AJ, Li Q, Wietgrefe SW, Schacker TW, Reilly CS, Haase AT. Host genes associated with HIV-1 replication in lymphatic tissue. J Immunol. 2010;185:5417–5424. doi: 10.4049/jimmunol.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith AJ, Schacker TW, Reilly CS, Haase AT. A role for syndecan-1 and claudin-2 in microbial translocation during HIV-1 infection. J Acquir Immune Defic Syndr. 2010;55:306–315. doi: 10.1097/QAI.0b013e3181ecfeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter DL, Shieh TM, Blosser RL, Chadwick KR, Margolick JB, Hildreth JE, Clements JE, Zink MC. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry. 1999;37:41–50. [PubMed] [Google Scholar]
- 20.Webster RL, Johnson RP. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology. 2005;115:206–214. doi: 10.1111/j.1365-2567.2005.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavilio D, Benjamin J, Kim D, Lombardo G, Daucher M, Kinter A, Nies-Kraske E, Marcenaro E, Moretta A, Fauci AS. Identification of NKG2A and NKp80 as specific natural killer cell markers in rhesus and pigtailed monkeys. Blood. 2005;106:1718–1725. doi: 10.1182/blood-2004-12-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bostik P, Takahashi Y, Mayne AE, Ansari AA. Innate immune natural killer cells and their role in HIV and SIV infection. HIV Ther. 2010;4:483–504. doi: 10.2217/HIV.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16− natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sips M, Sciaranghella G, Diefenbach T, Dugast AS, Berger CT, Liu Q, Kwon D, Ghebremichael M, Estes JD, Carrington M, Martin JN, Deeks SG, Hunt PW, Alter G. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 2012;5:30–40. doi: 10.1038/mi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 26.Del Prete GQ, Scarlotta M, Newman L, Reid C, Parodi LM, Roser JD, Oswald K, Marx PA, Miller CJ, Desrosiers RC, Barouch DH, Pal R, Piatak M, Jr, Chertova E, Giavedoni LD, O’Connor DH, Lifson JD, Keele BF. Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J Virol. 2013;87:4584–4595. doi: 10.1128/JVI.03507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sedelies KA, Sayers TJ, Edwards KM, Chen W, Pellicci DG, Godfrey DI, Trapani JA. Discordant regulation of granzyme H and granzyme B expression in human lymphocytes. J Biol Chem. 2004;279:26581–26587. doi: 10.1074/jbc.M312481200. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RP. Live attenuated AIDS vaccines: hazards and hopes. Nat Med. 1999;5:154–155. doi: 10.1038/5515. [DOI] [PubMed] [Google Scholar]
- 29.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- 30.Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, Kuzmichev YV, Carville A, Johnson RP. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011;118:3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 32.Reeves RK, Evans TI, Gillis J, Johnson RP. Simian immunodeficiency virus infection induces expansion of alpha4beta7+ and cytotoxic CD56+ NK cells. J Virol. 2010;84:8959–8963. doi: 10.1128/JVI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. J Infect Dis. 2010;202(Suppl 2):S297–301. doi: 10.1086/655657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 35.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]