Abstract
Wilson’s disease (WD) is a human disorder of copper homeostasis caused by mutations in the copper-transporting ATPase ATP7B. WD is characterized by copper accumulation, predominantly in the liver and brain; hepatic pathology; and wide differences between patients in the age of onset and the spectrum of symptoms. Several factors contribute to the phenotypic variability of WD. The WD-causing mutations produce a wide range of changes in stability, activity, intracellular localization, and trafficking of ATP7B; the non-pathogenic genetic polymorphisms may contribute to the phenotype. In Atp7b−/− mice, a mouse model of WD, an abnormal intracellular distribution of copper in the liver triggers distinct changes in the transcriptome; these mRNA profiles might be used to more specifically define disease progression. The major effect of accumulating copper on lipid metabolism and especially cholesterol homeostasis in mice and humans suggests the importance of fat and cholesterol metabolism as modifying factors in WD.
Keywords: Wilson’s disease, ATP7B, copper, Atp7b−/− mice, cholesterol
Wilson disease is a monogenic disorder with a wide spectrum of phenotypic manifestations
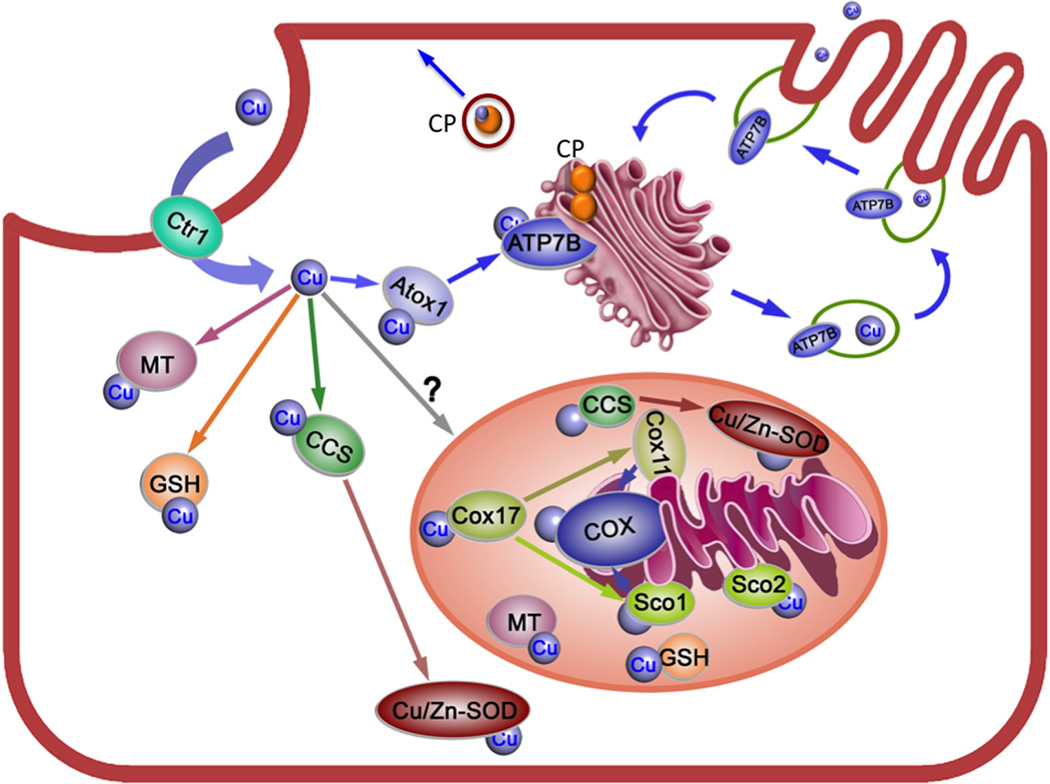
WD is an autosomal recessive disease caused by mutations in the gene ATP7B located on chromosome 13.1 The gene encodes a copper-transporting ATPase of the same name. ATP7B is a large trans-membrane protein expressed most highly in the liver, where it performs two important physiologic functions. Using the energy of ATP hydrolysis, ATP7B transfers copper from the cytosol into the lumen of the trans-Golgi network (TGN), where copper is incorporated into copper-dependent enzymes, such as ceruloplasmin (CP) (Fig. 1). When the cytosolic concentration in hepatocytes exceeds a certain threshold, ATP7B travels from the TGN to vesicles located in the vicinity of the apical membrane. There, ATP7B facilitates copper sequestration for further vesicle-mediated excretion into the bile (Fig. 1). In mammals, the liver is the main organ that regulates body copper homeostasis. In WD, mutations in ATP7B are associated with reduced copper efflux from the liver, massive copper accumulation, and a wide spectrum of pathologies, which range from mild inflammation to fulminant liver failure.1
Figure 1.
Copper distribution pathways in the hepatocytes. Copper (depicted by the blue balls) enters a hepatocyte via the high affinity copper transporter Ctr1 located at the basolateral membrane. In the cytosol, copper binds to glutathione (GSH), copper chaperones Atox1 and CCS, and to a yet-to-be-identified mitochondrial copper chaperone. A set of chaperones in the mitochondria (Cos11, Cox17, Sco1 and Sco2) work together to deliver copper to cytochrome C oxidase (COX). ATP7B is located in the trans-Golgi network (TGN), where it accepts copper from Atox1 and transfers copper into the TGN lumen. Copper is then incorporates into ceruloplasmin (CP), which is constitutively secreted across the basolateral membrane into the blood. When cellular copper is elevated, ATP7B traffics from the TGN to vesicles in the vicinity of the apical membrane; vesicle fusion with this membrane results in the release of copper into the bile. Inactivation of ATP7B in WD is associated with the loss of copper export from the liver, impaired copper incorporation into ceriloplasmin, and development of pathology. Excess copper binds to metallothionein (MT).
In tissues other than the liver, ATP7B is less abundant. The brain, kidneys, ovary, placenta, mammary gland, intestine, and lung express ATP7B along with another highly homologous transporter, ATP7A. The loss of ATP7B activity in these tissues has less dramatic consequences on tissue morphology and function, possibly due to compensatory activity of ATP7A. Functional compensation, however, is partial, and with time copper accumulates and triggers pathological changes. A large percentage of WD patients (up to 60% in some studies2) exhibit neurologic symptoms (tremors, dystonia, dysarthria, muscle weakness). Other, less frequent presentations include psychiatric symptoms such as depression, psychosis, and insomnia; renal dysfunction; hemolytic anemia; and relatively mild changes in cardiac function.3–9 Copper is thought to mediate its negative effects by participating in the Fenton-like reactions that increase production of reactive oxygen species and non-specific modifications of DNA, proteins, and lipids. Recent data from murine models of WD indicate that such broad non-specific damage may occur later in the disease, whereas the initial effects of accumulating copper are more selective.10,11 These effects involve distinct changes in the pattern of cellular mRNA,10 alterations in the expression and function of mitochondria enzymes,11 and changes in metabolic profiles inside cells and in the extracellular milieu.10 Animal models, such as the Atp7b−/− mice discussed in this review, have served as important experimental tools in identifying most of these effects and have provided important insights into the mechanisms of copper-induced pathology in WD.12
Direct genotype–phenotype correlations in WD have been difficult to identify.13 Consequently, precise definition of WD phenotypes may require in-depth characterization of the molecular and cellular consequences of copper misbalance. In WD, a significant interplay between the genetic, metabolic, and dietary factors is likely, because the disease is characterized by the great variability of symptoms observed in patients, even among monozygotic twins.14,15 Dietary factors were shown to influence disease progression in animal models of WD.16–18 In recent years, the mechanisms of phenotypic variability in WD have been the subject of significant interest and scrutiny.19–23 It has become apparent that the nature of phenotypic diversity is multifactorial. The disease is associated with numerous mutations that show varied consequences and can be further influenced by genetic polymorphisms in ATP7B as well as other genes.24,25 Metabolic factors, such as dietary copper levels and fat and cholesterol metabolism, appear to significantly contribute to disease manifestations.15 Altogether, the combination of factors produces a palette of phenotypes that evolve with time, posing challenges for diagnosis and treatment. This review briefly summaries our recent findings regarding molecular and cellular factors that contribute to variable disease manifestations and highlights future directions for uncovering the mechanisms of phenotypic variability in WD.
WD mutations have a broad spectrum of effects on ATP7B structure and function
WD is associated with over 500 mutations, the majority of which are missense mutations (http://www.wilsondisease.med.ualberta.ca/database.asp).26 Although some disease-causing amino acid substitutions are relatively common and may account for 10–40% of all ATP7B mutations found within a population (H1069Q in Caucasians and African Americans and R778L in Chinese and Japanese patients), the vast majority of mutations are rare. The large number, low frequency, and compound-heterozygous nature of WD mutations complicate genetic testing and make characterization of their consequences a daunting task. Nevertheless, studies from several laboratories, including work done by our group, have yielded information about the properties of various mutants. These studies have been aided by a recent high-resolution structure of the bacterial copper-ATPase LCopA that was used as a template for generating a molecular model for the core of ATP7B.27–29
At the molecular level, several primary consequences of WD mutations have been identified; these effects are not mutually exclusive. Many missense mutations, including H1069Q and R778L, decrease protein stability and lower the amount of ATP7B in cells.30–33 The majority of mutations are inactivating, i.e., they completely disrupt the copper-transport function of ATP7B.34 However, recent characterization of 24 WD-related mutations showed that a relatively large number of ATP7B variants tested in this study (25–30%) were able to bind and hydrolyze ATP and had at least partial copper-transport activity.34 This is an important finding, because partial activity of ATP7B may explain mild phenotypes in some cases of the disease. It addition, drugs that would stabilize ATP7B and increase its activity may represent a promising avenue for designing novel treatments for WD. Improved therapies are highly desirable because current treatments have side effects, do not alleviate increased predisposition to cancer, and take a long time to take an effect.35–37
The WD-causing mutations also alter ATP7B localization and/or trafficking in cells. Normally, the primary localization of ATP7B is in the TGN; ATP7B traffics from the TGN to vesicles in response to copper elevation or other stimuli (Fig. 1). The WD-causing mutations may prevent ATP7B targeting to the TGN by causing retention of ATP7B in the endoplasmic reticulum (ER) and increased protein degradation.31,34,38 This effect is usually due to significant structural changes caused by a mutation and an inability of the mutant to overcome the ER quality control. However, in some cases ATP7B retention in the ER can be counteracted by metabolic factors. We have recently characterized an interesting genetic polymorphism, which causes such reversible ER retention of ATP7B. The 2623A/G basepair change in ATP7B produces a Gly875→Arg substitution in the ATP7B protein. The ATP7B-Arg875 variant is less stable compared to a more common ATP7B-Gly875, and under basal conditions it is trapped in the ER, at least in a cell model system.39 However, a modest and physiologically relevant increase of copper levels in the cell growth medium (1–10 mM) stabilizes the ATP7B-Arg875 variant, allowing it to escape the ER quality control and reach the TGN. Once in its correct cellular location, ATP7B-Arg875 shows diminished (compared to a common ATP7B-Gly875 variant) but easily measurable copper transport activity (i.e., the ATP7B-Arg875 variant no longer behaves as an impaired disease-causing mutant).39 Based on these in vitro data, one may hypothesize that individuals with the 2623A/G genetic background would be more severely affected by the dietary copper deficiency. In WD patients, this genetic polymorphism in combination with otherwise mild WD mutations may yield more deleterious phenotypes compared to individuals with the ATP7B-Gly875 background.
Characterization of the ATP7B-Arg875 variant has raised another interesting issue related to the differences between various forms of copper accumulation and their respective contributions to WD phenotypes and response to treatment. Specifically, we found that the abnormal cellular behavior of the ATP7B-Arg875 variant is corrected only by exogenously added copper. This observation was puzzling, because the low copper-transport activity of ATP7B-Arg875 was expected to produce a gradual elevation of copper in the cytosol that, in turn, was expected to stabilize ATP7B-Arg875 and trigger its movement from the ER to the TGN. Yet, this did not occur in two examined cell lines (HEK293 cells and skin fibroblasts). Experiments in Menkes fibroblasts (patient cells that have lost copper-export function and accumulate copper) clearly illustrate different effects of exogenously added copper and endogenously accumulating copper on ATP7B-Arg875. When expressed in Menkes fibroblasts, the recombinant ATP7B-Arg875 is trapped in the ER, even though copper levels in these cells are higher that the copper content of normal fibroblasts after exposure to an additional 10 mM of copper(the conditions necessary for the ER exit, see above).39 Addition of copper to the growth medium of Menkes fibroblasts triggers ATP7B-Arg875 relocalization to the TGN, along with the restoration of copper transport to the TGN lumen.39
The likely explanation for the observed requirement for exogenous copper is that the endogenous copper in Menkes fibroblasts is not free but bound by metallothioneins,40 proteins with extremely high affinity for copper that act as intracellular copper chelators. The metal-binding sites in ATP7B have lower affinity for copper than metallothioneins,41 and consequently ATP7B may remain copper-free when metallothioneins are expressed. In a copper-free state, the ATP7B-Arg875 variant is destabilized;39 its degradation results in further loss of copper export, higher copper levels in the cytosol, greater induction of metallothioneins, and so on. Further experiments are necessary to directly test this scenario. Nevertheless, it seems important to take into account contributions of metallothionein expression when one considers the effects of accumulating copper in WD, especially because metallothionein levels may differ considerably between various cells and tissues, as well as between individuals. Strong induction and high expression of metallothioneins not only protect against copper overload (and therefore decrease disease manifestations), but also result in sequestration of most (if not all) exchangeable copper. Since exchangeable copper is necessary to accommodate mitochondria function and Cu, Zn-superoxide dismutase in the cytosol, high expression of metallothioneins may leave cells vulnerable to drug-mediated chelation during treatment.
Time-dependent changes in the intracellular localization of accumulating copper are coupled to distinct changes of hepatic transcriptome
Identification of the main site(s) of action for elevated copper in a cell is an important issue that so far has not been broadly discussed in the WD literature. The loss of ATP7B activity results in copper elevation in the cytosol; this copper is sequestered and “deactivated” by metallothioneins. It is not entirely clear which cellular compartment contains the exchangeable (and hence most reactive) copper, and where the effects of high copper are most damaging. This question has been partially addressed using Atp7b−/− mice. This animal strain lacks the functional ATP7B in all tissues and recapitulates several important features of human WD, including hepatic copper overload, loss of holoceruloplasmin, elevated copper in the urine, and markedly abnormal hepatic histology.12 In our laboratory, we have analyzed the age-dependent changes in the subcellular distribution of copper in Atp7b−/− hepatocytes in parallel with studies of liver pathology and mRNA profiles.42,43 These experiments have produced somewhat unexpected and informative results. We found that early in the disease (in 6- to 7-week-old mice), copper accumulates primarily in the cytosol (where it is bound by low molecular weight proteins, most likely upregulated metallothioneins) and in the nuclei.42 Mitochondrial and microsomal fractions show relatively little increase in copper content compared to cell cytosol and nuclei. At this stage of the disease, liver histology remains essentially normal, although elevated transaminases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) in the serum indicate hepatocyte stress. With time (12–20 weeks after birth), copper continues to accumulate in hepatocytes and begins to form highly concentrated deposits inside and outside of the cell.42 Coincidentally, copper concentration in the nuclei and cytosol begins to decrease, although it remains high throughout the cell; at this stage of the disease, the liver shows marked pathologic changes. Finally, concentration of copper in the cytosol and nuclei decreases significantly and most accumulated copper can be found in the membranous cellular compartment (possibly lysosomes); at this stage, one observes regeneration of liver parenchyma along with marked proliferation of bile ducts43,42 and tumor development.
Analysis of mRNA profiles using liver tissues from the control and Atp7b−/− mice reveals specific changes in the transcriptome that accompany these three major stages of the disease. The initial response to elevated copper includes changes in the mRNA for proteins associated with regulation of cell cycle, chromosome maintenance, mRNA splicing, and lipid metabolism.10 Subsequent proteomic and bioinformatic analyses confirmed that these changes are initiated in the nuclei. Downregulation of cholesterol biosynthesis is also a nuclear event, apparently caused by a diminished signaling by the LXR/RXR nuclear receptors.44,45 The functional outcome of copper overload (i.e., changes in the RNA handling machinery, cell cycle proteins, and nuclear receptor signaling) is consistent with the major accumulation of copper in the nucleus. Although copper concentrations in the Atp7b−/− cytosol and the nuclei are similar,42 the increase in the copper-chelating metallothioneins is much less apparent in the nuclei compared to the cytosol. This observation suggests that a more exchangeable copper could be present in the nucleus and initiate a cascade of reactions leading to pathology development.
Later in the disease, when elevated copper is distributed throughout the cell, the mRNA profile suggests a broader involvement of many cell compartments including the ER, mitochondria, and the endocytic pathway.42 Finally, at the stage of copper sequestration (in animals older than 30–40 weeks), when copper is seen in highly concentrated (and presumably inert) copper deposits, no nuclear involvement is observed, whereas the mRNA for proteins involved in the lysosomal and endosomal pathways are upregulated. It should be noted that the total content of copper in the liver during these complex events remains unchanged and even decreases over time. Thus, one has to conclude that specific intracellular localization rather than total copper content determines liver response at every given time and defines pathology progression. Therefore, the mRNA profiles could be instructive in better defining stages of the disease and inferring the form of accumulated copper (bound to metallothionein in the cytosol or sequestered in the deposits). Availability of such time-dependent markers of disease progression may also be helpful for fine-tuning treatments.
Our studies have not directly addressed the role of mitochondria in the liver pathology of Atp7b−/− mice, largely due to insufficient resolution of X-ray fluorescence imaging. Cellular fractionation indicates that mitochondria from Atp7b−/− livers accumulate copper, and recent studies in LPP rats (another murine model of WD) indicate that mitochondrial dysfunction might be the key event precipitating liver failure in animals (and possibly humans).46 Changes in mitochondrial enzymes, especially complex IV (which was downregulated) and thioredoxin 2 (which was upregulated) were also reported for the 3- to 4-month-old toxic milk mice (an inbred strain with a missense mutation in Atp7b11). It should be noted that the Atp7b−/− mice, unlike LPP rats, do not develop liver failure, even though both strains of animals have no functional Atp7b and accumulate large amounts of copper. Comparison of these species may yield valuable information about metabolic and genetic factors that contribute to this important variation in a disease manifestation.
Communication of the copper status between organs is mediated by a small copper carrier
It is well established that the onset of hepatic disease in WD patients, on average, precedes neurologic manifestations. This likely reflects a rapid and much greater accumulation of copper in the liver compared to the brain and other tissues. It has been speculated that copper accumulation in the brain is aided by necrotic changes in the liver and the release of copper from damaged hepatocytes. While such mechanism is likely, especially at the late stage of the disease, recent studies in Atp7b−/− mice point to an additional and more specific mechanism that contributes to a time-dependent copper redistribution between tissues. Live animal imaging of orally administered radioactive copper revealed age-dependent decrease in copper uptake by Atp7b−/− livers and redirection of copper flow to other tissues.47,48 The decrease in hepatic copper entry is associated with the downregulation of mRNA for Ctr1, a high-affinity copper uptake transporter, as well as a decreased amount of this protein at the plasma membrane.47
The decrease in copper uptake by the liver is also coupled to elevation of urinary copper, suggesting that kidneys represent a secondary route for excretion of excess copper from the body when the liver route is not operational. We found that the urinary copper is bound to a small (1.5–2 kDa apparent molecular weight) molecule, which we have termed small copper carrier (SCC).47 In mice, SCC is low in the urine at the first stage of the disease, when most copper is absorbed by the liver. During the second stage of the disease (when liver pathology is most pronounced, see above) the SCC–copper levels increase. Significantly, elevation of urinary copper is selective, i.e., no increase in iron, zinc, magnesium, or other metals is observed.47 This result speaks in favor of the specificity of the regulatory mechanisms that redirect copper flow (in the form of SCC–copper complex) from the liver to the kidneys. In other words, it seems unlikely that hepatocyte damage is a primary source of elevated copper in the urine, at this stage. With time, other metals, as well as protein content, increase in the urine.47 In mice, this nonspecific elevation is the urinary content of various metals is not associated with worsening of liver histology. Consequently, it seems likely that the observed metallo- and proteinurea could be caused by damage from the kidneys and may not originate entirely in the liver.
Dysregulation of lipid homeostasis is an early and major metabolic effect of copper overload
Studies of the Atp7b−/− liver transcriptome have demonstrated that dysregulation of lipid metabolism occurs before pathology onset in the liver and is, by far, the most significantly altered metabolic process43. The mRNA levels for HMG-CoA reductase, a rate-limiting enzyme in cholesterol biosynthesis, are markedly reduced in Atp7b−/− mouse liver; this is also true for human WD liver10. Measurements of the cholesterol and triglyceride levels in the liver and the serum are consistent with the results of mRNA profiling and indicate downregulation of cholesterol biosynthesis as well as changes in lipolysis.43 Recent follow-up studies in humans49,50 indicate that cholesterol metabolism is dysregulated in WD patients, although specific changes in the serum cholesterol and triglyceride levels differ for humans and mice.49 In addition, a recent report using a different mouse model of WD (tx-j mice) confirmed the effects of copper overload on lipid metabolism and showed that “maternal choline supplementation prevented the transcriptional deficits in fetal tx-j liver,”51 although this later impact was attributed to epigenetic effects of DNA methylation. Altogether, these findings suggest the important role that individual diet as well as individual fat and cholesterol metabolism may play in the onset and progression of WD. In this regard, a recent clinical report on markedly varied WD manifestations in monozygotic twins is especially interesting.15 The study describes two patients with a homozygous H1069Q mutation who were presented with a greatly dissimilar course of disease (acute liver failure versus mild inflammation).15 The main apparent differences between the two patients were their previous history of eating disorders and diet at the time of disease onset.15
Current data also suggest that a tight link between copper homeostasis and lipid metabolism exists in physiologic situations other than WD. For example, in mice and in fish,52 copper elevation downregulates cholesterol and fatty acid biosynthesis in tissues. Copper deficiency has an opposite effect: reduced hepatic copper concentrations or dietary copper restrictions are associated with a more pronounced hepatic steatosis in human and rats, respectively.53 It should be noted that Atp7b−/− mice do not appear to have steatosis. This is in a stark contrast to human WD livers, where steatosis is a frequent disease manifestation. Recent studies in cultured human cell lines (from a healthy individual) revealed that additions of very high copper concentrations to the cell growth medium increases expression of genes encoding enzymes related to cholesterol and fatty acid biosynthesis, including HMG-CoA reductase. Again, this is in contrast to the ATP7B−/− liver (in both humans and mice) in which the transcript for this protein is significantly downregulated and for which transcript levels can be restored (in mice) by the viral-mediated expression of active ATP7B in the liver.54
A potential explanation of the discrepancy between specific consequences of copper elevation on lipid metabolism in human and mouse liver may be related to known species variations in the regulation of lipid metabolism. In addition, there could be species-to-species differences in the amount of exchangeable/reactive copper. Our studies indicate that both mice and humans express metallothionein in response to copper overload, however the increase in metallothionein levels in mouse liver is much higher than that in human liver (D. Huster, personal communication). As was discussed above, high expression of metallothionein in mice would greatly diminish exchangeable copper, whereas medium levels of metallothionein in human liver may leave some copper unsequestered and active. Thus, even if the total levels of copper accumulation in mouse and human liver may be comparable, there could be a significant difference in the amount of active copper. Clearly, the species- and cell-specific responses to copper require further studies. Better understanding of physiologic connections between the lipid and copper metabolisms in several species would help to validate various experimental models of human WD and identify key metabolic pathways that may modify disease progression.
The mechanism by which elevated copper modulates lipid biosynthesis in Atp7b−/− liver is not entirely clear. Our data suggest that cholesterol sensing in the ER and SREBP1-mediated signaling are not significantly perturbed. The Ingenuity network analysis points to several upstream regulators, most notably the LXR/RXR pair, that may be affected by copper.45,55 It would be important to test this hypothesis directly and determine whether copper affects LXR/RXR signaling directly by binding and/or oxidizing each of the proteins, or less indirectly by modifying (oxidizing) LXR/RXR effector molecules and ligands. In either case, genetic and epigenetic changes that influence the LXR/RXR signaling may be important modifying factors in WD, and further studies of this pathway are likely to be informative.
In conclusion, the in vitro studies with ATP7B mutants and the experiments using Atp7b−/− mice have yielded important information about factors that may contribute to phenotypic variability in WD. Future studies should be focused on the characterization of disease-causing mutants in the physiologically relevant cells and tissue and a thorough mechanistic analysis of the metabolic effects of copper accumulation.
Acknowledgments
I thank Dr. Nan Yang for help with preparation of the figure. This work was supported by the grants PO1 GM067166 and RO1 DK071865 from the National Institute of Health to SL
References
- 1.Das SK, Ray K. Wilson's disease: an update. Nat Clin Pract Neurol. 2006;2:482–493. doi: 10.1038/ncpneuro0291. [DOI] [PubMed] [Google Scholar]
- 2.Brewer GJ, Yuzbasiyan-Gurkan V. Wilson disease. Medicine (Baltimore) 1992;71:139–164. doi: 10.1097/00005792-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Toppo A, Rath B, Harbhajanka A, Lalita Jyotsna P. Hemolytic Anemia as a Presenting Feature of Wilson's Disease: A Case Report. Indian J Hematol Blood Transfus. 2010;26:101–102. doi: 10.1007/s12288-010-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimbrean PC, Schilsky ML. Psychiatric aspects of Wilson disease: a review. Gen Hosp Psychiatry. 2014;36:53–62. doi: 10.1016/j.genhosppsych.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Rich AM, Lajoie TM. Wilson's disease--treatment of psychiatric manifestations in pregnancy. Psychosomatics. 2012;53:175–177. doi: 10.1016/j.psym.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Arat N, et al. P wave dispersion is prolonged in patients with Wilson's disease. World J Gastroenterol. 2008;14:1252–1256. doi: 10.3748/wjg.14.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soni D, Shukla G, Singh S, Goyal V, Behari M. Cardiovascular and sudomotor autonomic dysfunction in Wilson's disease--limited correlation with clinical severity. Auton Neurosci. 2009;151:154–158. doi: 10.1016/j.autneu.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Meenakshi-Sundaram S, et al. Cardiac involvement in Wilson's disease--an electrocardiographic observation. J Assoc Physicians India. 2004;52:294–296. [PubMed] [Google Scholar]
- 9.Ghosh L, Shah M, Pate S, Mannari J, Sharma K. Wilson's disease presenting with hypokalemia, hypoparathyroidism and renal failure. J Assoc Physicians India. 2012;60:57–59. [PubMed] [Google Scholar]
- 10.Huster D, et al. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem. 2007;282:8343–8355. doi: 10.1074/jbc.M607496200. [DOI] [PubMed] [Google Scholar]
- 11.Roberts EA, Robinson BH, Yang S. Mitochondrial structure and function in the untreated Jackson toxic milk (tx-j) mouse, a model for Wilson disease. Mol Genet Metab. 2008;93:54–65. doi: 10.1016/j.ymgme.2007.08.127. [DOI] [PubMed] [Google Scholar]
- 12.Lutsenko S. Atp7b−/− mice as a model for studies of Wilson's disease. Biochem Soc Trans. 2008;36:1233–1238. doi: 10.1042/BST0361233. [DOI] [PubMed] [Google Scholar]
- 13.Ferenci P. Phenotype-genotype correlations in patients with Wilson's disease. Ann N Y Acad Sci. 2014 doi: 10.1111/nyas.12340. [DOI] [PubMed] [Google Scholar]
- 14.Czlonkowska A, Gromadzka G, Chabik G. Monozygotic female twins discordant for phenotype of Wilson's disease. Mov Disord. 2009;24:1066–1069. doi: 10.1002/mds.22474. [DOI] [PubMed] [Google Scholar]
- 15.Kegley KM, et al. Fulminant Wilson's disease requiring liver transplantation in one monozygotic twin despite identical genetic mutation. Am J Transplant. 2010;10:1325–1329. doi: 10.1111/j.1600-6143.2010.03071.x. [DOI] [PubMed] [Google Scholar]
- 16.Saito A, Nakayama K, Hara H. Mild zinc deficiency and dietary phytic acid accelerates the development of fulminant hepatitis in LEC rats. J Gastroenterol Hepatol. 2007;22:150–157. doi: 10.1111/j.1440-1746.2006.04506.x. [DOI] [PubMed] [Google Scholar]
- 17.Yonezawa K, et al. Soy protein isolate enhances hepatic copper accumulation and cell damage in LEC rats. J Nutr. 2003;133:1250–1254. doi: 10.1093/jn/133.5.1250. [DOI] [PubMed] [Google Scholar]
- 18.Kato J, et al. Hepatic iron deprivation prevents spontaneous development of fulminant hepatitis and liver cancer in Long-Evans Cinnamon rats. J Clin Invest. 1996;98:923–929. doi: 10.1172/JCI118875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee S, et al. Genetic defects in Indian Wilson disease patients and genotype-phenotype correlation. Parkinsonism Relat Disord. 2014;20(1):75–81. doi: 10.1016/j.parkreldis.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal A, et al. Wilson Disease Mutation Pattern with Genotype-Phenotype Correlations from Western India: Confirmation of p.C271* as a Common Indian Mutation and Identification of 14 Novel Mutations. Ann Hum Genet. 2013;77:299–307. doi: 10.1111/ahg.12024. [DOI] [PubMed] [Google Scholar]
- 21.Mihaylova V, et al. Neurological symptoms, genotype-phenotype correlations and ethnic-specific differences in Bulgarian patients with Wilson disease. Neurologist. 2012;18:184–189. doi: 10.1097/NRL.0b013e31825cf3b7. [DOI] [PubMed] [Google Scholar]
- 22.Horslen S, Hahn SH. Genotype-phenotype correlation in Wilson disease. J Clin Gastroenterol. 2010;44:387–388. doi: 10.1097/MCG.0b013e3181d96ac4. [DOI] [PubMed] [Google Scholar]
- 23.Wright LM, et al. Hepatocyte GP73 expression in Wilson disease. J Hepatol. 2009;51:557–564. doi: 10.1016/j.jhep.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferenci P. Polymorphism of methylenetetrahydrofolate reductase as disease modifier - a deja-vu in Wilson disease? J Hepatol. 2011;55:753–755. doi: 10.1016/j.jhep.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Litwin T, Gromadzka G, Czlonkowska A. Apolipoprotein E gene (APOE) genotype in Wilson's disease: impact on clinical presentation. Parkinsonism Relat Disord. 2012;18:367–369. doi: 10.1016/j.parkreldis.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kenney SM, Cox DW. Sequence variation database for the Wilson disease copper transporter, ATP7B. Hum Mutat. 2007;28:1171–1177. doi: 10.1002/humu.20586. [DOI] [PubMed] [Google Scholar]
- 27.Gourdon P, et al. Crystal structure of a copper-transporting PIB-type ATPase. Nature. 2011;475:59–64. doi: 10.1038/nature10191. [DOI] [PubMed] [Google Scholar]
- 28.Gourdon P, Sitsel O, Lykkegaard Karlsen J, Birk Moller L, Nissen P. Structural models of the human copper P-type ATPases ATP7A and ATP7B. Biol Chem. 2012;393:205–216. doi: 10.1515/hsz-2011-0249. [DOI] [PubMed] [Google Scholar]
- 29.Schushan M, Bhattacharjee A, Ben-Tal N, Lutsenko S. A structural model of the copper ATPase ATP7B to facilitate analysis of Wilson disease-causing mutations and studies of the transport mechanism. Metallomics. 2012;4:669–678. doi: 10.1039/c2mt20025b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Granillo A, Sedlak E, Wittung-Stafshede P. Stability and ATP binding of the nucleotide-binding domain of the Wilson disease protein: effect of the common H1069Q mutation. J Mol Biol. 2008;383:1097–1111. doi: 10.1016/j.jmb.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 31.Dmitriev OY, Bhattacharjee A, Nokhrin S, Uhlemann EM, Lutsenko S. Difference in stability of the N-domain underlies distinct intracellular properties of the E1064A and H1069Q mutants of copper-transporting ATPase ATP7B. J Biol Chem. 2011;286:16355–16362. doi: 10.1074/jbc.M110.198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne AS, Kelly EJ, Gitlin JD. Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation. Proc Natl Acad Sci U S A. 1998;95:10854–10859. doi: 10.1073/pnas.95.18.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Berghe PV, et al. Reduced expression of ATP7B affected by Wilson disease-causing mutations is rescued by pharmacological folding chaperones 4-phenylbutyrate and curcumin. Hepatology. 2009;50:1783–1795. doi: 10.1002/hep.23209. [DOI] [PubMed] [Google Scholar]
- 34.Huster D, et al. Diverse functional properties of Wilson disease ATP7B variants. Gastroenterology. 2012;142:947–956. doi: 10.1053/j.gastro.2011.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez B, Burguera J, Berenguer M. Response to different therapeutic approaches in Wilson disease. A long-term follow up study. Ann Hepatol. 2012;11:907–914. [PubMed] [Google Scholar]
- 36.Shimizu N, et al. Effects of long-term zinc treatment in Japanese patients with Wilson disease: efficacy, stability, and copper metabolism. Transl Res. 2010;156:350–357. doi: 10.1016/j.trsl.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Weiss KH, et al. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013;11:1028–1035. e1021–e1022. doi: 10.1016/j.cgh.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Huster D, et al. Defective cellular localization of mutant ATP7B in Wilson's disease patients and hepatoma cell lines. Gastroenterology. 2003;124:335–345. doi: 10.1053/gast.2003.50066. [DOI] [PubMed] [Google Scholar]
- 39.Gupta A, et al. Cellular copper levels determine the phenotype of the Arg875 variant of ATP7B/Wilson disease protein. Proc Natl Acad Sci U S A. 2011;108:5390–5395. doi: 10.1073/pnas.1014959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leone A, Pavlakis GN, Hamer DH. Menkes' disease: abnormal metallothionein gene regulation in response to copper. Cell. 1985;40:301–309. doi: 10.1016/0092-8674(85)90144-8. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Z, et al. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J Biol Chem. 2011;286:11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ralle M, et al. Wilson disease at a single cell level: intracellular copper trafficking activates compartment-specific responses in hepatocytes. J Biol Chem. 2010;285:30875–30883. doi: 10.1074/jbc.M110.114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huster D, et al. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am J Pathol. 2006;168:423–434. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burkhead JL, Ralle M, Wilmarth P, David L, Lutsenko S. Elevated copper remodels hepatic RNA processing machinery in the mouse model of Wilson's disease. J Mol Biol. 2011;406:44–58. doi: 10.1016/j.jmb.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilmarth PA, et al. A systems approach implicates nuclear receptor targeting in the Atp7b(−/−) mouse model of Wilson's disease. Metallomics. 2012;4:660–668. doi: 10.1039/c2mt20017a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zischka H, et al. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J Clin Invest. 2011;121:1508–1518. doi: 10.1172/JCI45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray LW, et al. Urinary copper elevation in a mouse model of Wilson's disease is a regulated process to specifically decrease the hepatic copper load. PLoS One. 2012;7(6):e38327. doi: 10.1371/journal.pone.0038327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng F, Lutsenko S, Sun X, Muzik O. Imaging copper metabolism imbalance in Atp7b (−/−) knockout mouse model of Wilson's disease with PET-CT and orally administered 64CuCl2. Mol Imaging Biol. 2012;14:600–607. doi: 10.1007/s11307-011-0532-0. [DOI] [PubMed] [Google Scholar]
- 49.Seessle J, et al. Alterations of lipid metabolism in Wilson disease. Lipids Health Dis. 2011;10:83. doi: 10.1186/1476-511X-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cacciatore S, Tenori L. Brain cholesterol homeostasis in Wilson disease. Med Hypotheses. 2013;81:1127–1129. doi: 10.1016/j.mehy.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Medici V, et al. Maternal choline modifies fetal liver copper, gene expression, DNA methylation, and neonatal growth in the tx-j mouse model of Wilson disease. Epigenetics. 2013;9 doi: 10.4161/epi.27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos EM, et al. Identifying health impacts of exposure to copper using transcriptomics and metabolomics in a fish model. Environ Sci Technol. 2010;44:820–826. doi: 10.1021/es902558k. [DOI] [PubMed] [Google Scholar]
- 53.Aigner E, et al. A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010;105:1978–1985. doi: 10.1038/ajg.2010.170. [DOI] [PubMed] [Google Scholar]
- 54.Roybal JL, et al. Early gestational gene transfer with targeted ATP7B expression in the liver improves phenotype in a murine model of Wilson's disease. Gene Ther. 2012;19:1085–1094. doi: 10.1038/gt.2011.186. [DOI] [PubMed] [Google Scholar]
- 55.Lutsenko JLBaS. The Role of Copper as a Modifier of Lipid Metabolism. Lipid Metabolism. 2013;Chapter 3:39–61. [Google Scholar]