Abstract
RNA interference has broad therapeutic potential due to its high specificity and ability to potentially evade drug resistance. Three cationic α-cyclodextrin:poly(ethylene glycol) polyrotaxanes derived from polymer axle different sizes (MW 2000, 3400 and 10000) have been synthesized for delivering siRNA. These polyrotaxanes are able to condense siRNA into positively charged particles that are < 200 nm in diameter, enabling their facile internalization into mammalian cells. The cationic polyrotaxanes display cytotoxicity profiles that are >102-fold lower than the commercial standard bPEI and gene silencing efficiencies that are comparable to both Lipofectamine 2000 and bPEI. Our findings suggest that the cationic polyrotaxanes display a size-activity relationship, wherein, the higher molecular weight polyrotaxanes (PEG3400 and 10000) are able to condense and deliver siRNA better than the lower molecular weight material (PEG2000).
Keywords: gene delivery, siRNA, cyclodextrins
Introduction
There is a growing recognition of the vast therapeutic potential of RNAi in cancer, neurological disorders, and cardiovascular diseases. Advantages such as high specificity and the ability to act “upstream” from conventional chemotherapeutic agents gives them the ability to potentially target any protein and evade drug resistance. Safe delivery of siRNA to affected cells may offer target specific treatment of genetic diseases1-4, however, the clinical success of this approach is limited by the absence of efficient, low toxicity delivery vehicles. Viral and non-viral vectors have been studied for this purpose, but they both suffer from several key limitations. Although efficient and persistent, viral vectors are challenged by issues such as large-scale production, immunogenicity, and safety, whereas non-viral vectors are limited primarily by lack of efficiency. The scalability and modest host immunogenicity relative to viral vectors has garnered considerable attention for non-viral gene delivery approaches. Many different non-viral delivery vehicles have been explored for siRNA delivery, including nanoparticle complexes based on cyclodextrin-oligomers5-9, cationic lipids10-12, lipid/Ca2+ mixtures13, Au14 or PLGA15. All these vehicles are capable of forming nanoparticles that are smaller than 200 nm in diameter and can efficiently deliver siRNA into target cells. They can also protect the nucleic acid from enzymatic degradation and enhance the cell permeability of the siRNA cargo; however, most of these vehicles possess undesirable toxicity, immunogenicity and/or serum stability profiles. Use of clinically approved biodegradable and bio-compatible materials for constructing new non-viral delivery vehicles can potentially address these problems.
Cyclodextrins (CD) are naturally occurring oligosaccharides that are extensively used in the pharmaceutical industry to improve the bioavailability of hydrophobic drugs, prevent undesired side effects and improve permeability across biological membranes. CDs have been extensively used for gene delivery due to the aforementioned properties and their ability to stabilize the nucleic acids in biological media.16 A variety of CD-based systems such as CD polymers17, CD dendrimers18, and CD polyrotaxanes19 have been developed and have shown potential as materials for non-viral siRNA delivery. Recently, Davis and co-workers have demonstrated the in vivo efficacy of targeted CD-oligomer:siRNA particles as delivery vehicles against melanoma in humans.4
Polyrotaxanes (PR) are supramolecular structures comprised of cyclic molecules threaded onto an “axle” that is endcapped by bulky groups at the terminal positions of the “axle”. The construction of such supramolecular architectures from FDA approved materials such as α-cyclodextrin (α-CD) and poly(ethylene glycol) (PEG) makes them extremely attractive for biomedical applications19. Such CD-based PR have been used extensively for biomedical applications such as hydrogels for drug delivery20, biodegradable drug delivery constructs21, scaffolds for tissue engineering22 and gene delivery23-31.
Cationic CD PR have been studied for their DNA complexation and transfection ability in cells where cationic substituents have been introduced by post-modification reactions after the macrocycle has been threaded onto the polymer backbone23-25. Cationic α-CD PR have been synthesized where the endcaps are linked via disulfide bonds, rendering them degradable in the reducing environment of the cell26-28. PR:pDNA complexes also have been encapsulated within a lipid bilayer to improve their biological performance.29 PR have also been synthesized with endcaps that act as targeting moieties to enhance their uptake via receptor mediated endocytosis30. While these materials have previously been studied for their siRNA complexation ability, their efficiency as siRNA delivery vectors has not yet been reported to our knowledge31.
Herein, we report the first study employing cationic α-CD PR as delivery vectors for siRNA. A family of three linear α-CD:PEG-based cationic polyrotaxanes (PR+) were prepared for the purpose of siRNA complexation and cellular delivery. We anticipated that the mobility of the threaded cationic CDs on the PEG chain would promote enhanced complexation with siRNA to generate potent non-viral vectors at lower N/P ratios than other cyclodextrin polymer constructs. A lower N/P ratio on complexes usually translates into lower surface charges of the formulated complexes hence ensuring improved cell viability, circulation profiles, and reduced off-target effects.
Experimental Methods
Materials
All solvents were of reagent grade, purchased from commercial sources, and used without further purification, except DMF and toluene, which were dried over CaH2 under N2, filtered and distilled under reduced pressure. α-CD; poly(ethylene glycol) of molecular weights 2,000, 3,400, 10,000 and 35,000; 1,1-carbonyldiimidazole (CDI), and N,N’-dimethyl ethylenediamine were obtained from Sigma-Aldrich. 2,4,6-Trinitrobenzenesulfonic acid was obtained from Research Organics. NIH3T3-GFP cells were purchased from Cell Biolabs and CHO-GFP cells were kindly provided by Prof. Z.R. Lu, Case Western University. Plasmid purification kits, anti-GFP siRNA and Allstart negative control siRNA were purchased from Qiagen. Block-IT AlexaFluor 555-labeled siRNA was purchased from Life Technologies. Dialysis membranes of MWCO = 6 – 8,000 were purchased from Fisher Scientific. 1H NMR spectra were recorded on a 300 MHz VARIAN INOVA 300 NMR spectrometer at 30 °C. Chemical shifts were referenced to the residual protonated solvent peak.
Branched poly(ethylene imine) (bPEI) polymer and lipofectamine 2000 (L2k) cationic lipid were used as positive controls for all the studies performed. Both bPEI and L2k were used as used as transfection controls since they are known to be highly effective delivery agents for pDNA and siRNA.
Synthesis of Cationic Polyrotaxanes
Synthesis of 3.4k PR
H2N-PEG3400-NH2 (250 mg, 0.0746 mmol) was dissolved in 10 g α-CD in 100 mL H2O and stirred for 2 d. An aqueous (10%) solution of 2,4,6-trinitrobenzene sulfonic acid (TNBS) (5.00 mL, 1.70 mmol) was pre-activated by addition of NaHCO3 until the solution was pH 8. This solution was then added and stirred at 20 °C for an additional 2 d. The crude solution was then washed with 18 MΩ H2O and centrifuged at 9000 rpm to sediment the crude PR product. This operation was repeated until the aqueous solution was colorless; the crude PR solid was then dried under vacuum to give a yellow powder. Yield: 1.441 g. Loading: ~9.60 α-CD, 25.94% threading efficiency. MW: 13.27 kDa. 1H NMR (Supplement 1) (300 MHz, DMSO-d6, δ): 8.99-8.92 (s, TNBS), 5.81-5.60 (b, CD C2-OH), 5.61-5.34 (d, CD C3-OH), 4.90-4.67 (s, CD C1H), 4.57-4.34 (s, CD C6-OH), 3.85-3.17 (m, CD C2H, C3H, C4H, C5H, and C6-H2; PEG CH2).
Synthesis of 2k PR
This material was prepared using the same procedure as above, except that H2N-PEG2k-NH2 (250 mg, 0.125 mmol), 5 g α-CD in 50 mL H2O, and TNBS (8.38 mL, 2.85 mmol) were used. Yield: 0.850 g. Loading: ~7.8 α-CD, 34.4% threading efficiency. MW: 10.03 kDa. 1H NMR (Supplement 2) (400 MHz, DMSO-d6, δ): Same as above.
Synthesis of 10k PR
This material was produced using the same procedure as above, except that H2N-PEG10k-NH2 (2.2 g, 0.220 mmol), 10 g α-CD in 100 mL H2O, and TNBS (13.0 mL, 4.42 mmol) were used. Yield: 8.673 g. Loading: ~12.86 α-CD, 11.63% threading efficiency. MW: 22.5 kDa. 1H NMR (Supplement 3) (300 MHz, DMSO-d6, δ): 9.09-9.01 (s, TNBS), 5.76-5.63 (b, CD C2-OH), 5.63-5.35 (d, CD C3-OH), 4.90-4.70 (s, CD C1H), 4.60-4.40 (s, CD C6-OH), 3.86-3.17 (m, CD C2H, C3H, C4H, C5H, and C6H2; PEG CH2).
Synthesis of 3.4k DMEDA PR+
The 3.4k PR precursor (500 mg, 0.0377 mmol) was dissolved in minimal DMSO under Ar with CDI (1.76 g, 10.85 mmol) and stirred for 1 d at 20 °C. N,N-Dimethylethylenediamine (DMEDA) (4.74 mL, 43.41 mmol) was then added under Ar, and the reaction mixture was stirred for another2 d. The solution was then dialyzed (Fisher-brand, MWCO 6-8 kDa) three times against H2O and dried under vacuum to give a red solid. Yield: 121 mg. Loading: ~7.38 α-CD, 20%; ~6.11 DMEDA/CD, 45.05 DMEDA/mol. MW: 14.92 kDa. 1H NMR (Supplement 5) (300 MHz, D2O, δ): 5.37-4.91 (b, CD C2-OH, C3-OH), 4.55-4.29 (b, CD C1H), 4.30-3.96 (b, CD C6-OH), 3.96-3.47 (m, CD C2H, C3H, C4H, C5H, and C6H2; PEG CH2), 3.40-3.09 (b, DMEDA CONHCH2), 2.65-2.37 (b, DMEDA CH2-NMe2), 2.40-2.08 (d, DMEDA N-(CH3)2).
Synthesis of 2k DMEDA PR+
The same procedure as above was used, except that 2k PRTX (500 mg, 0.0499 mmol), CDI (1.89 g, 11.65 mmol), and DMEDA (5.08 mL, 46.6 mmol) were utilized. Yield: 156 mg. Loading: ~2.48 α-CD, 11%; ~2.08 DMEDA/CD, 5.16 DMEDA/mol. MW: 5.29 kDa. 1HNMR (Supplement 6) (300 MHz, D2O, δ): 5.37-4.91 (b, CD C2-OH, C3-OH), 4.55-4.29 (b, CD C1H), 4.29-4.00 (b, CD C6-OH), 4.00-3.47 (m, CD C2H, C3H, C4H, C5H, and C6H2; PEG CH2), 3.37-3.15 (b, DMEDA CONH-CH2), 2.64-2.43 (b, DMEDA CH2-NMe2), 2.37-2.16 (d, DMEDA N-(CH3)2).
Synthesis of 10k DMEDA PR+
The same procedure as above, except that 10k PRTX (3.0 g, 0.133 mmol), CDI (8.34 g, 51.43 mmol), and DMEDA (5.62 mL, 51.55 mmol) was used. Yield: 828 mg. Loading: ~10.00 α-CD, 9%; ~1.71 DMEDA/CD, 17.13 DMEDA/mol. MW: 21.66 kDa. 1H NMR (Supplement 7) (300 MHz, D2O, δ): 5.35-4.91 (b, CD C2-OH, C3-OH), 4.54-4.28 (b, CD C1H), 4.29-3.94 (b, CD C6-OH), 3.80-3.51 (m, CD C2H, C3H, C4H, C5H, and C6H2; PEG CH2), 3.38-3.08 (b, DMEDA CONH-CH2), 2.60-2.34 (b, DMEDA CH2-NMe2), 2.34-2.07 (d, DMEDA N-(CH3)2).
HILIC-ESI MS Analysis of PR+
The PR+ were analyzed for free α-CD and DMEDA-modified α-CD content using a commercial 2.1 × 30 mm Waters BEH amide column, 1.7 Om particle size, mounted on a Thermo Accela UPLC system (Thermo Fisher Scientific, Waltham, MA, USA), with a Thermo LTQ Velos mass spectrometer serving as the detector. The temperature of the column oven was maintained at 40 °C. The injection volume was 10 μL and a mobile phase gradient of 85%-50% ACN containing 0.1% HCO2H over 35 min was used. The samples were dissolved at a concentration of 0.5 mg/ml in 50:50 ACN:H2O prior to injection.
Particle Size and Zeta Potential Measurements
The PR+:siRNA particle sizes, size distributions and zeta potentials were evaluated by dynamic light scattering using a particle size analyzer (Zetasizer Nano S, Malvern Instruments Ltd.) at 20 • with a scattering angle of 90°.
Atomic Force Microscopy Analysis
AFM imaging of the nanoparticles was conducted in tapping mode (MultiMode, Veeco, USA) using dry samples on mica. The AFM tips (PPP-NCH, Nanoscience Instruments, Inc., USA) had a typical radius of 7 nm or less and force constant of 46 N/m. The images were recorded at a scan rate of 0.5 or 1 Hz. Samples were prepared by dropping 2 mL of solution on a mica surface followed by overnight drying at 20 •.
Gel Retardation Assay
The complexation ability of PR+ with siRNA was determined by 4% agarose (low melting point) gel electrophoresis. The agarose gels were precast in TBE buffer with GelRed dye at 1:10,000 dilution. PR+:siRNA complexes containing 0.2 μg of siRNA at different N/P ratios were loaded onto the gel. A 1:5 dilution of loading dye was added to each well and electrophoresis was carried out at a constant voltage of 55 V for 2 h in TBE buffer. The siRNA bands were then visualized under a UV transilluminator at λ = 365 nm.
Cell Viability Assay
The cytotoxicity of the PR+ relative to PEI (25 kDa) was evaluated using the MTS assay in NIH3T3-GFP cells. The relative cell viabilities were measured as a function of amine densities of the PR+. The cells were cultured in complete DMEM medium supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity. The cells were seeded in 96-well microtiter plates (Nunc, Wiesbaden, Germany) at densities of 10,000 cells/well. After 24 h, the culture media was replaced with serum-free culture media containing increasing amine concentrations of PR+ and the cells incubated for an additional 24 h. MTS reagent (15TL) was added to each well and then incubated for 2 h before measuring the absorbance with a microplate reader (Spectra Plus, TECAN) at λ = 492 nm. The cell viability (%) relative to control cells cultured in media without polymers was calculated as [A]test/[A]control × 100%, where [A]test is the absorbance of the wells with polymers and [A]control is the absorbance of the control wells. All experiments were conducted for three samples and averaged. The median lethal dose (LD50) is the dose of a toxic material that kills 50% of the cells tested. In this study, LD50 was the concentration of a gene carrier at which the relative cell viability decreased to 50%.
Cellular Uptake Studies
NIH3T3 cells were used to study the uptake of the complexes by plating 60,000 cells per well in 24-well plates and incubating for 24 h before the experiment. Complexes of AlexaFluor555 labeled-siRNA (1 Og per well) with the PR+ at different N/P ratios were incubated with cells for 4 h at 37 °C in 10% serum-supplemented media. After 4 h, the spent media was removed and the cells were washed with PBS and trypsinized. These cells were then collected and analyzed by flow cytometry using the FL2 channel.
In Vitro Gene Knockdown Experiment
CHO-GFP and NIH3T3-GFP cells were cultured in complete F12 and DMEM media supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity, respectively, at cell densities of 60,000 cells/well in 24 well plates. After 24h, the culture media was replaced with serum-supplemented media containing the PR+:siRNA complexes. The cells were incubated with the complexes for 4 h, after which the spent media was aspirated and fresh serum-supplemented media was added. After an additional 36 h incubation, the media was aspirated and the cells were washed with PBS, trypsinized and analyzed by FACS using the FL1 channel. %GFP mean fluorescence intensity was calculated relative to the untreated samples.
Results and Discussion
Synthesis of Cationic Polyrotaxanes
Three PR+ were synthesized by threading α-CD onto 1,ω-bisamine PEG (MW = 2, 3.4, and 10 kD) in a saturated α-CD aqueous solution at 20 °C to form the corresponding pseudo-polyrotaxane precursors. The PEG bisamines were synthesized from commercially available PEG using a method previously described (Supporting Information)32. The pseudorotaxanes were endcapped with an aqueous solution of TNBS that was pre-activated with NaHCO3. The crude solution was centrifuged at 9,000 rpm and washed with 18 MΩ H2O before re-centrifugation to pellet the PR product. This operation was performed multiple times until the supernatant was clear and colorless, indicating effective removal of free α-CD and TNBS starting material. The crude PR solid was dried under vacuum before characterization by 300 MHz 1H NMR to calculate the average number of α-CDs that were threaded onto the PEG core. These intermediates were post-modified via stepwise CDI activation and DMEDA coupling in DMSO to give PR+ products with random DMEDA-carbamate modifications on the PR scaffold. The products were then dialyzed three times against H2O to remove excess DMEDA and other <6 kD molecular weight impurities. The dialyzed product was dried under vacuum and analyzed by 1H NMR to calculate both the average CD threading and average DMEDA content per CD unit in the final product. 1H NMR revealed that the 2k, 3.4k and 10k PR+ threading efficiencies were ~ 11%, 20%, and 9%, respectively, with 2, 6, and 1.7 DMEDA modifications per CD, respectively (see 1H NMR spectra, Supporting Information). HILIC analysis of the PR+ products showed no indication of either free α-CD or cationic DMEDA-carbamoyl-α-CD in the samples. The absence of any signals attributable to unmodified or modified α-CD by ESI MS further corroborated the finding that the final PR+ samples contained little or no unmodified and/or modified α-CD (chromatograms & ESI MS spectra, Supporting Information).
Characterization of Particles Formed by PR+:siRNA Complexation
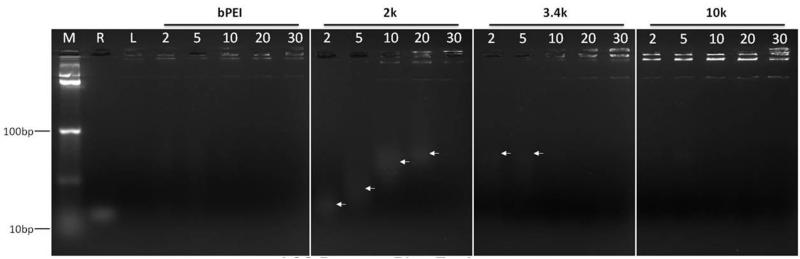
The PR+ materials were compared with 25 kD bPEI and Lipofectamine 2000 (L2k) controls with respect to siRNA complexation efficiency, ζ potential, and particle size. Gel shift assays of PR+:siRNA complexes indicate that each PR+ had complexation efficiencies similar to bPEI and L2k (Figure 2). Stable PR+:siRNA complexes formed only at high N/P ratios such as 30 for the 2k PEG derivative, whereas the 3.4k, 10k and bPEI species could effectively condense siRNA at lower N/P ratios. We attribute this to the lower overall charge on the 2k PR+ derivative relative to the 3.4k and 10k congeners, thus requiring a higher N/P ratio to effectively condense the siRNA than the higher mass species.
Figure 2.
Gel retardation assay showing the complexation properties of the PR materials. M: Marker; R: free siRNA; L: lipofectamine 2000; 2-30: N/P ratios of bPEI, 2k, 3.4k and 10k PR+ complexes with siRNA. Arrows indicate uncomplexed siRNA.
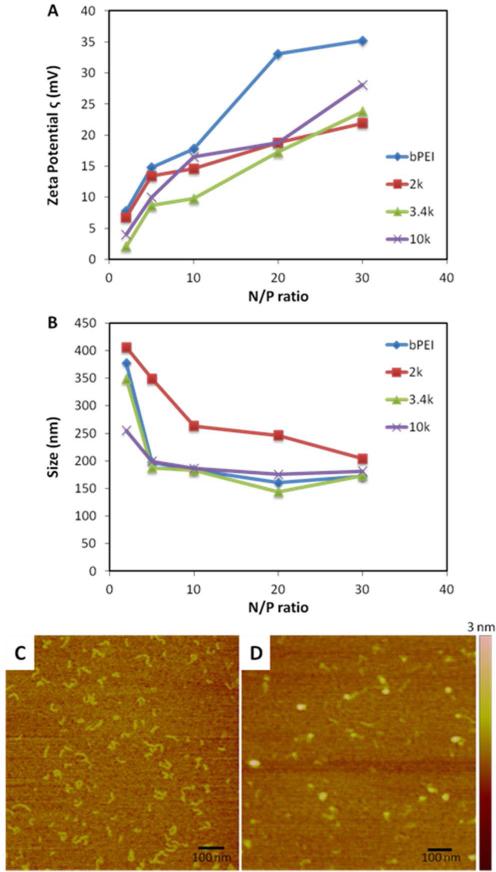
ζ potentials measurements for the PR+:siRNA complexes revealed that each of the PR+-based complexes had significantly lower surface charges than bPEI. It was observed that at lower N/P ratios, the incremental increase in charge was substantial with increasing N/P ratio, while at higher N/P ratios the charge was not affected as significantly. It also was observed that, regardless of the PR+ used, the ζ potential of the complexes were comparable at higher N/P ratios. Since transfection complexes with highly positive surface charge (Figure 3A) are known to negatively affect cell viability and plasma stability, the appearance of lower ζ potentials for PR+:siRNA complexes are encouraging.
Figure 3.
Characterization of siRNA complexes formed by 2k, 3.4k and 10k PR+. (A) ζ potentials; (B) DLS measurements; and AFM images of (C) 10k PR+; (D) 10k PR+:siRNA at N/P = 20.
Dynamic light scattering (DLS) was used to determine the complex sizes produced using the different PR+ materials (Figure 3B). We found that 3.4k and 10k PR+ both produce particles that were of comparable size to bPEI. Even though 2k also condensed siRNA to form particles of < 250 nm at high N:P ratios (≥ 20), these particles were significantly larger than those formed by 3.4k or 10k PR+ (150 – 200 nm).These results further indicate that the charge density and threading of α-CDs on the 2k PEG axle is too low for efficient siRNA complexation at lower N/P ratios.
AFM images revealed that the uncomplexed PR+ material displays a rod-like structure, however, upon complexation with siRNA, spherical particles are formed (Figure 3C). These trends were observed for all the PR+, regardless of PEG axle size (Supporting Information). The sizes determined by AFM are smaller than those measured by DLS due to the dry nature of the AFM samples. We infer from these observations that PR+ are able to condense siRNA into compact and relatively uniform particles that are small enough to be internalized by cells via endocytosis.
Biological Performance of PR+:siRNA Complexes
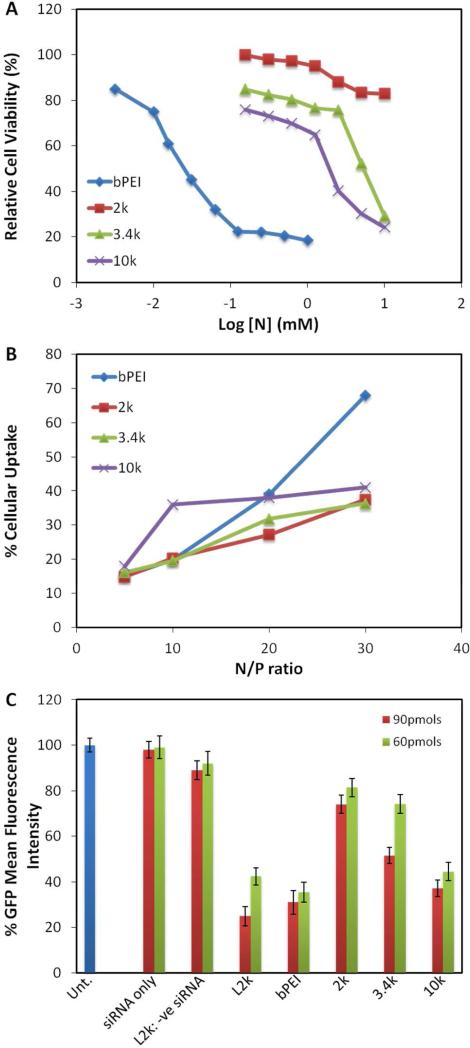
The acute in vitro cytotoxicity of transfection agents is a very important parameter to consider for evaluating their clinical translation potential. An MTS cell viability assay was performed as a function of nitrogen concentration for all the PR+ materials to enable direct comparison of the toxicity profiles of the materials based on their nitrogen content. Figure 4A shows that all the PR+ were found to be 100- to 200-times less toxic than bPEI. While 2k PR+ had little effect on cell viability, the LD50s of 3.4k and 10k PR+ were found to be 4.5 mM and 1.6 mM, respectively, compared to 0.025 mM for bPEI. Cellular uptake studies were carried out with fluorescently labeled siRNA using L2k and bPEI as controls (Figure 4B).
Figure 4.
Biological performance of PR+:siRNA complexes. (A) Relative cell viabilities PR+ and bPEI as a function of amine densities in 10% serum-supplemented media; (B) relative cellular uptakes of PR+ and bPEI with L2k as positive control; and (C) in vitro GFP knockdown efficiencies of 2k, 3.4k and 10k PR+:siRNA complexes in 10% serum-supplemented media with L2k and bPEI as controls.
Cellular uptake levels were calculated with respect to L2k as 100%. These studies revealed that all the PR+ materials had similar uptake levels, albeit lower than bPEI at N/P = 30. Interestingly, while it was observed that the uptake of bPEI:siRNA particles increased with an increase in N/P ratio, the PR+:siRNA particles plateaued with little or no increase in uptake at N/P ratios greater than 10. We infer from these finding that a lower extent of cell association occurs with the PR+:siRNA due to their lower ζ, leading to a lower amount of internalized siRNA. If correct, this suggests that the PR+:siRNA complexes may have lower off-target effects than the corresponding bPEI:siRNA complexes.
The in vitro gene knockdown efficiency of transfection complexes formed between anti-GFP siRNA and PR+ was assessed in NIH3T3-GFP cells at various N/P ratios in the presence of serum using bPEI and L2k as controls (Figure 4C). An N/P ratio of 20 was observed to be optimal for performance and cell viability of the PR+:siRNA complexes. At N/P ratios of 5 and 10, the performance was significantly lower, while at N/P = 30, the performance was good, but cell viability suffered. A polymer size dependence on pRTX+ performance was also observed, with 10k PR+ being the most active, followed by 3.4k and 2k PR+. The poor performance of the 2k PR+ can be attributed to its poor complexation ability, which was evident from the gel shift assay. Interestingly, lowering the dose from 90 pmol to 60 pmol siRNA did not affect the performance of 10k PR+ greatly, with ~60% to 70% gene suppression observed in both cases. Similar performance profiles were observed for these materials in CHO-GFP cells, indicating that the performance was independent of the cell lines used (Supporting Information). Our results show that PR+ based on 10k PEG with 1-2 cationic charges per CD may be optimal for in vitro siRNA delivery. This performance profile can be attributed to a combination of various factors such as favorable complexation ability, <200 nm particle sizes, near zero ζ potentials, good cytotoxicity properties, and facile cellular uptake. While the ζ potentials, cytotoxicities and cellular uptake levels of all the PR+ materials are comparable, the greater siRNA complexation efficiency displayed by 10k PR+ suggests that this derivative may be preferred for in vivo studies.
Conclusions
Stable and effective transfection complexes were formed upon mixing siRNA and positively charged DMEDA-modified α-CD polyrotaxanes. Efficient siRNA complexation by these PR+ can be attributed to the facile rotational and translational motion of the CDs along the PEG chain, allowing for better ion pairing in the vector complexes. The PR+ were capable of condensing the siRNA into complexes less than 200 nm in size and showed knockdown efficiencies that were comparable to bPEI and L2k, while displaying 100-200 fold lower toxicity. Additionally, the gene suppression efficiency did not change significantly even upon siRNA dose reduction or changes in cell line. Our results suggest that cationic polyrotaxanes could potentially be effective tools in RNAi-based gene therapy. Further improvements for clinical translation might also include incorporation of enzyme-responsive or pH-responsive linkages to accelerate siRNA release and/or attachment of targeting ligands to improve specificity and uptake by target cells.
Supplementary Material
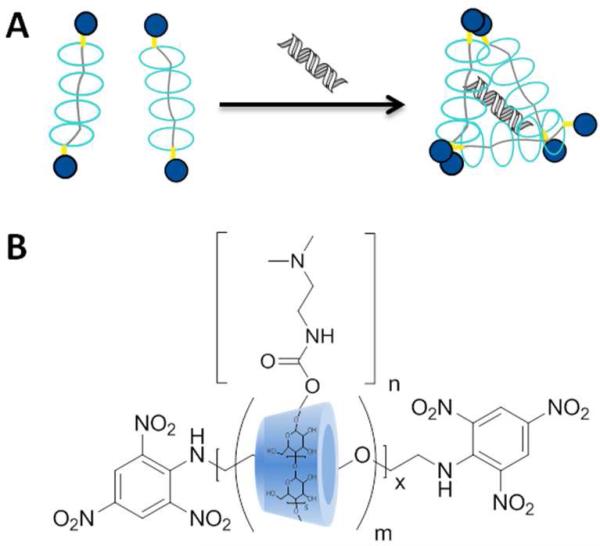
Figure 1.
(A) Conceptual diagram of PR+:siRNA complexation and (B) general structure of PR+ prepared in this study.
References
- 1.Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, Neitz J, Neitz M. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461:784. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nature Rev. Genet. 2007;8:573. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DWY, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nature Biotechnol. 2010;20:172. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 4.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasachari S, Fichter KM, Reineke TM. Polycationic β-cyclodextrin “click clusters”: monodisperse and versatile scaffolds for nucleic acid delivery. J. Am. Chem. Soc. 2008;130:4618. doi: 10.1021/ja074597v. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasachari S, Reineke TM. Versatile supramolecular pDNA vehicles via “click polymerization” of β-cyclodextrin with oligoethyleneamines. Biomaterials. 2009;30:928. doi: 10.1016/j.biomaterials.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez H, Hwang SJ, Davis ME. New class of polymers for the delivery of macromolecular therapeutics. Bioconj. Chem. 1999;10:1068. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 8.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 1. Carbohydrate size and its distance from charge centers. Bioconj. Chem. 2003;14:247. doi: 10.1021/bc025592k. [DOI] [PubMed] [Google Scholar]
- 9.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 2. Charge center type. Bioconj. Chem. 2003;14:255. doi: 10.1021/bc025593c. [DOI] [PubMed] [Google Scholar]
- 10.Sparks J, Slobodkin G, Matar M, Congo R, Ulkoski D, Rea-Ramsey A, Pence C, Rice J, McClure D, Polach KJ, Brunhoeber E, Wilkinson L, Wallace K, Anwer K, Fewell JG. Versatile cationic lipids for siRNA delivery. J. Control. Rel. 2012;158:269. doi: 10.1016/j.jconrel.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead KA, Sahay G, Li GZ, Love KT, Alabi CA, Ma M, Zurenko C, Querbes W, Langer RS, Anderson DG. Synergistic silencing: combinations of lipid-like materials for efficacious siRNA delivery. Mol. Ther. 2011;19:1688. doi: 10.1038/mt.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen DN, Mahon KP, Chikh G, Kim P, Chung H, Vicari AP, Love KT, Goldberg M, Chen S, Krieg AM, Chen J, Langer R, Anderson DG. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E797. doi: 10.1073/pnas.1121423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li SD, Huang L. Non-viral is superior to viral gene delivery. J. Control. Rel. 2007;123:181. doi: 10.1016/j.jconrel.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Lee SK, Han MS, Asokan S, Tung CH. Effective gene silencing by multilayered siRNA-coated gold nanoparticles. Small. 2011;7:364. doi: 10.1002/smll.201001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J, Xiao Z, Votruba AR, Vilos C, Farokhzad OC. Differentially charged hollow core/shell lipid-polymer-lipid hybrid nanoparticles for small interfering RNA delivery. Angew. Chem. Int. Ed. Engl. 2011;50:7027. doi: 10.1002/anie.201101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Tang G, Wang Q, Li D, Shen F, Zhou J, Yu H. Two novel non-viral gene delivery vectors: low molecular weight polyethylenimine cross-linked by (2-hydroxypropyl)-β-cyclodextrin or (2-hydroxypropyl)-γ-cyclodextrin. Chem. Commun. 2006;22:2382. doi: 10.1039/b601130f. [DOI] [PubMed] [Google Scholar]
- 17.Lee CC, Mackay JA, Frechet JMJ, Szoka FC. Designing dendrimers for biological applications. Nature Biotechnol. 2005;23:1517. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 18.Helms B, Meijer EW. Chemistry. Dendrimers at work. Science. 2006;313:929. doi: 10.1126/science.1130639. [DOI] [PubMed] [Google Scholar]
- 19.Li JJ, Zhao F, Li J. Polyrotaxanes for applications in life science and biotechnology. Appl. Microbiol. Biotechnol. 2011;90:427. doi: 10.1007/s00253-010-3037-x. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Li J. Supramolecular hydrogels based on inclusion complexation between poly(ethylene oxide)-b-poly(ε-caprolactone) diblock copolymer and α-cyclodextrin and their controlled release property. J. Biomed. Mater. Res. A. 2008;86:1055. doi: 10.1002/jbm.a.31710. [DOI] [PubMed] [Google Scholar]
- 21.Moon C, Kwon YM, Lee WK, Park YJ, Yang VC. In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide. J. Control. Rel. 2007;124:43. doi: 10.1016/j.jconrel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichi T, Watanabe J, Ooya T, Yui N. Controllable erosion time and profile in poly(ethylene glycol) hydrogels by supramolecular structure of hydrolysable polyrotaxane. Biomacromolecules. 2001;2:204. doi: 10.1021/bm005617n. [DOI] [PubMed] [Google Scholar]
- 23.Yang C, Wang X, Li H, Goh SH, Li J. Synthesis and characterization of polyrotaxanes consisting of cationic α-cyclodextrins threaded on poly[(ethylene oxide)-ran-(propylene oxide)] as gene carriers. Biomacromolecules. 2007;8:3365. doi: 10.1021/bm700472t. [DOI] [PubMed] [Google Scholar]
- 24.Yang C, Li H, Wang X, Li J. Cationic supramolecules consisting of oligoethylenimine-grafted α-cyclodextrins threaded on poly(ethylene oxide) for gene delivery. J. Biomed. Mater. Res. A. 2009;89:13. doi: 10.1002/jbm.a.31976. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Wang X, Li H, Tan E, Lim CT, Li J. Cationic polyrotaxanes as gene carriers: physicochemical properties and real-time observation of DNA complexation, and gene transfection in cancer cells. J. Phys. Chem. B. 2009;113:7903. doi: 10.1021/jp901302f. [DOI] [PubMed] [Google Scholar]
- 26.Ooya T, Choi HS, Yamashita A, Yui N, Sugaya Y, Kano A, Maruyama A, Akita H, Ito R, Kogure K, Harashima H. Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J. Am. Chem. Soc. 2006;128:3852. doi: 10.1021/ja055868+. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita A, Kanda D, Katoono R, Yui N, Ooya T, Maruyama A, Akita H, Kogure K, Harashima H. Supramolecular control of polyplex dissociation and cell transfection: efficacy of amino groups and threading cyclodextrins in biocleavable polyrotaxanes. J. Control. Rel. 2008;131:137. doi: 10.1016/j.jconrel.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y, Nomura T, Harashima H, Yamashita A, Yui N. Post-nuclear gene delivery events for transgene expression by biocleavable polyrotaxanes. Biomaterials. 2012;33:3952. doi: 10.1016/j.biomaterials.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 29.Yamada Y, Nomura T, Harashima H, Yamashita A, Katoono R, Yui N. Intranuclear DNA release is a determinant of transfection activity for a non-viral vector: biocleavable polyrotaxane as a supramolecularly dissociative condenser for efficient intranuclear DNA release. Biol. Pharm. Bull. 2010;33:1218. doi: 10.1248/bpb.33.1218. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Wang H, Wang C, Li Y, Lu W, Chen S, Luo J, Jiang Y, Chen J. Receptor-mediated, tumor-targeted gene delivery using folate-terminated polyrotaxanes. Mol Pharm. 2012;9:1067. doi: 10.1021/mp200315c. [DOI] [PubMed] [Google Scholar]
- 31.Yamada Y, Hashida M, Nomura T, Harashima H, Yamasaki Y, Kataoka K, Yamashita A, Katoono R, Yui N. Different mechanisms for nanoparticle formation between pDNA and siRNA using polyrotaxane as the polycation. Chemphyschem. 2012;13:1161. doi: 10.1002/cphc.201100800. [DOI] [PubMed] [Google Scholar]
- 32.Harris JM, Struck EC, Case MG, Paley MS, Yalpani M, VanAlstine JM, Brooks DE. J. Poly. Sci. 1984;22:341. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.