Abstract
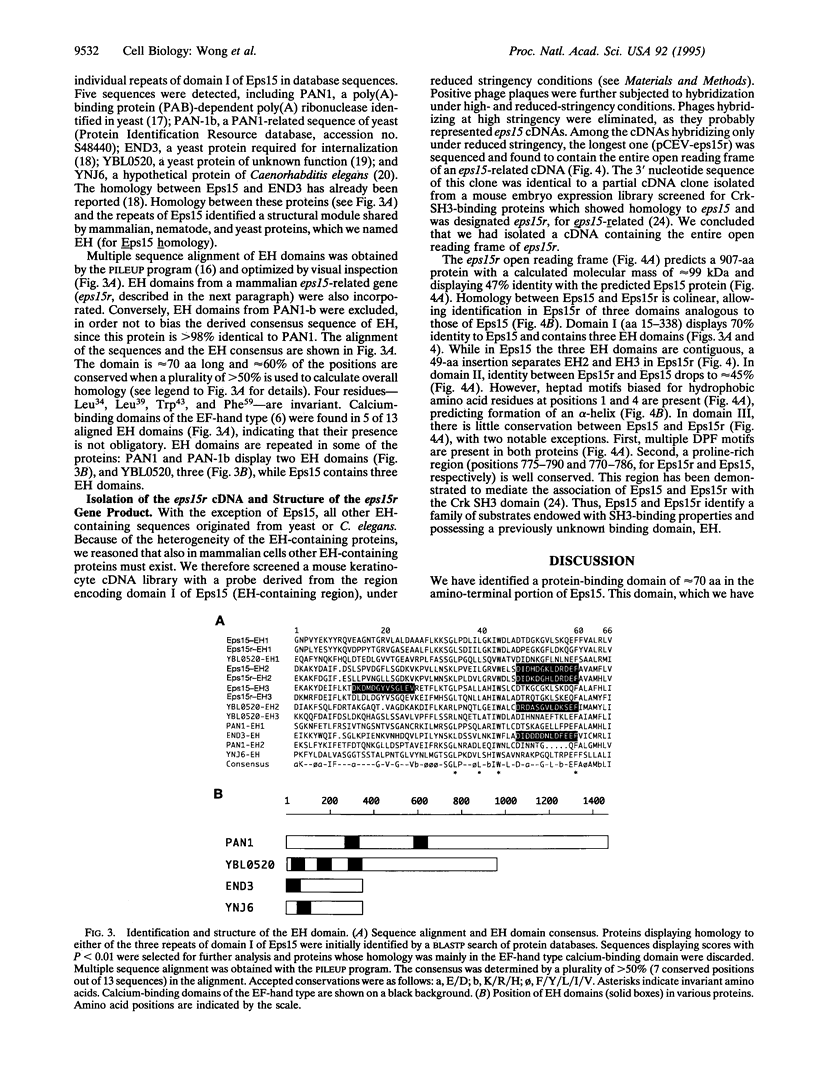
In this report we structurally and functionally define a binding domain that is involved in protein association and that we have designated EH (for Eps15 homology domain). This domain was identified in the tyrosine kinase substrate Eps15 on the basis of regional conservation with several heterogeneous proteins of yeast and nematode. The EH domain spans about 70 amino acids and shows approximately 60% overall amino acid conservation. We demonstrated the ability of the EH domain to specifically bind cytosolic proteins in normal and malignant cells of mesenchymal, epithelial, and hematopoietic origin. These observations prompted our search for additional EH-containing proteins in mammalian cells. Using an EH domain-specific probe derived from the eps15 cDNA, we cloned and characterized a cDNA encoding an EH-containing protein with overall similarity to Eps15; we designated this protein Eps15r (for Eps15-related). Structural comparison of Eps15 and Eps15r defines a family of signal transducers possessing extensive networking abilities including EH-mediated binding and association with Src homology 3-containing proteins.
Full text
PDF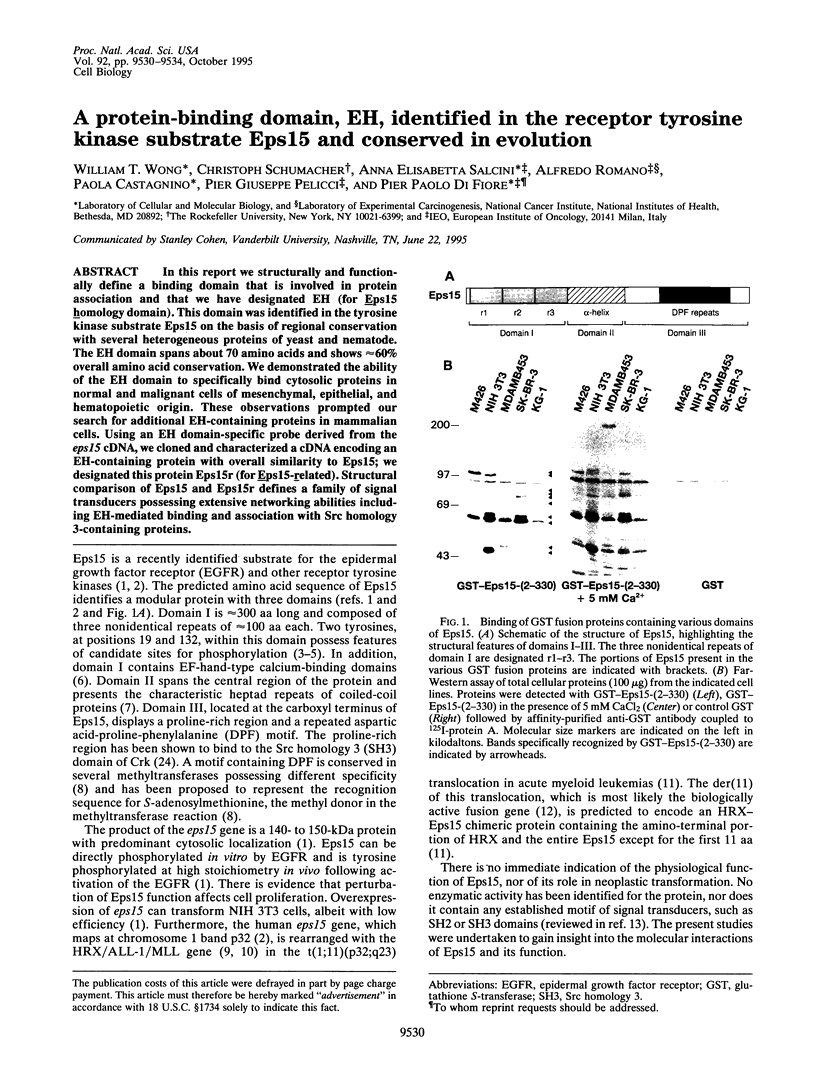



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bairoch A. The PROSITE dictionary of sites and patterns in proteins, its current status. Nucleic Acids Res. 1993 Jul 1;21(13):3097–3103. doi: 10.1093/nar/21.13.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O. A., Mauchauffe M., Mecucci C., Van den Berghe H., Berger R. A novel gene, AF-1p, fused to HRX in t(1;11)(p32;q23), is not related to AF-4, AF-9 nor ENL. Oncogene. 1994 Apr;9(4):1039–1045. [PubMed] [Google Scholar]
- Bénédetti H., Raths S., Crausaz F., Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994 Sep;5(9):1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. B., Ren R., Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995 Jan 27;80(2):237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- De Wergifosse P., Jacques B., Jonniaux J. L., Purnelle B., Skala J., Goffeau A. The sequence of a 22.4 kb DNA fragment from the left arm of yeast chromosome II reveals homologues to bacterial proline synthetase and murine alpha-adaptin, as well as a new permease and a DNA-binding protein. Yeast. 1994 Nov;10(11):1489–1496. doi: 10.1002/yea.320101113. [DOI] [PubMed] [Google Scholar]
- Engel M., Williams R. W., Erickson B. W. Designed coiled-coil proteins: synthesis and spectroscopy of two 78-residue alpha-helical dimers. Biochemistry. 1991 Apr 2;30(13):3161–3169. doi: 10.1021/bi00227a002. [DOI] [PubMed] [Google Scholar]
- Fazioli F., Kim U. H., Rhee S. G., Molloy C. J., Segatto O., Di Fiore P. P. The erbB-2 mitogenic signaling pathway: tyrosine phosphorylation of phospholipase C-gamma and GTPase-activating protein does not correlate with erbB-2 mitogenic potency. Mol Cell Biol. 1991 Apr;11(4):2040–2048. doi: 10.1128/mcb.11.4.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F., Minichiello L., Matoskova B., Wong W. T., Di Fiore P. P. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993 Sep;13(9):5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Gu Y., Nakamura T., Alder H., Prasad R., Canaani O., Cimino G., Croce C. M., Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992 Nov 13;71(4):701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W., Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci. 1991 Mar;16(3):98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- Hunter T. Synthetic peptide substrates for a tyrosine protein kinase. J Biol Chem. 1982 May 10;257(9):4843–4848. [PubMed] [Google Scholar]
- Klimasauskas S., Timinskas A., Menkevicius S., Butkienè D., Butkus V., Janulaitis A. Sequence motifs characteristic of DNA[cytosine-N4]methyltransferases: similarity to adenine and cytosine-C5 DNA-methylases. Nucleic Acids Res. 1989 Dec 11;17(23):9823–9832. doi: 10.1093/nar/17.23.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Aaronson S. A. Detection and isolation of novel protein-tyrosine kinase genes employing reduced stringency hybridization. Methods Enzymol. 1991;200:546–556. doi: 10.1016/0076-6879(91)00170-2. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983 Mar 15;164(4):605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. The der(11) chromosome contains the critical breakpoint junction in the 4;11, 9;11, and 11;19 translocations in acute leukemia. Genes Chromosomes Cancer. 1992 Oct;5(3):264–266. doi: 10.1002/gcc.2870050316. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Deardorff J. A. Translation initiation requires the PAB-dependent poly(A) ribonuclease in yeast. Cell. 1992 Sep 18;70(6):961–973. doi: 10.1016/0092-8674(92)90246-9. [DOI] [PubMed] [Google Scholar]
- Schumacher C., Knudsen B. S., Ohuchi T., Di Fiore P. P., Glassman R. H., Hanafusa H. The SH3 domain of Crk binds specifically to a conserved proline-rich motif in Eps15 and Eps15R. J Biol Chem. 1995 Jun 23;270(25):15341–15347. doi: 10.1074/jbc.270.25.15341. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Carraway K. L., 3rd, Eck M. J., Harrison S. C., Feldman R. A., Mohammadi M., Schlessinger J., Hubbard S. R., Smith D. P., Eng C. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995 Feb 9;373(6514):536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Di Fiore P. P., Carpenter G. The carboxyl terminus of epidermal growth factor receptor/erbB-2 chimerae is internalization impaired. Oncogene. 1993 Nov;8(11):3021–3028. [PubMed] [Google Scholar]
- Tkachuk D. C., Kohler S., Cleary M. L. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992 Nov 13;71(4):691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- Wilson R., Ainscough R., Anderson K., Baynes C., Berks M., Bonfield J., Burton J., Connell M., Copsey T., Cooper J. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994 Mar 3;368(6466):32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- Wong W. T., Kraus M. H., Carlomagno F., Zelano A., Druck T., Croce C. M., Huebner K., Di Fiore P. P. The human eps15 gene, encoding a tyrosine kinase substrate, is conserved in evolution and maps to 1p31-p32. Oncogene. 1994 Jun;9(6):1591–1597. [PubMed] [Google Scholar]