Abstract
Objectives
Growing evidence suggests that children who are deaf and use cochlear implants (CIs) can communicate effectively using spoken language. Research has reported that age of implantation and length of experience with the CI play an important role in a predicting a child’s linguistic development. In recent years, the increase in the number of children receiving bilateral CIs (BiCIs) has led to interest in new variables that may also influence the development of hearing, speech, and language abilities, such as length of bilateral listening experience and the length of time between the implantation of the two CIs. One goal of the present study was to determine how a cohort of children with BiCIs performed on standardized measures of language and nonverbal cognition. This study examined the relationship between performance on language and nonverbal intelligence quotient (IQ) tests and the ages at implantation of the first CI and second CI. This study also examined whether early bilateral activation is related to better language scores.
Design
Children with BiCIs (n = 39; ages 4 to 9 years) were tested on two standardized measures, the Test of Language Development and the Leiter International Performance Scale-Revised, to evaluate their expressive/receptive language skills and nonverbal IQ/memory. Hierarchical regression analyses were used to evaluate whether BiCI hearing experience predicts language performance.
Results
While large intersubject variability existed, on average, almost all the children with BiCIs scored within or above normal limits on measures of nonverbal cognition. Expressive and receptive language scores were highly variable, less likely to be above the normative mean, and did not correlate with Length of first CI Use, defined as length of auditory experience with one cochlear implant, or Length of second CI Use, defined as length of auditory experience with two cochlear implants.
Conclusions
All children in the present study had BiCIs. Most IQ scores were either at or above that found in the general population of typically hearing children. However, there was greater variability in their performance on a standardized test of expressive and receptive language. This cohort of children, who are mainstreamed in schools at age-appropriate grades, whose mothers’ education is high, and whose families’ socioecononomic status is high, had, as a group, on average, language scores within the same range as the normative sample of hearing children. Further research identifying the predictors that contribute to the high variability in both expressive and receptive language scores in children with BiCIs will provide useful information that can aid in clinical management and decision making.
Keywords: Bilateral, Children, Cochlear implants, Hearing age, Language
INTRODUCTION
Children who are deaf and experience severe or profound sensorineural hearing loss (SNHL) at birth or early in life demonstrate an inability to access the acoustic and phonetic cues that occur in speech. As a result, these children typically experience delayed and disordered development of spoken language (e.g., Yoshinaga-Itano et al. 1998). Over the past two decades, increases in newborn hearing screenings and loosening candidacy criteria have resulted in greater numbers of infants and young children with SNHL receiving cochlear implants (CIs). CIs are surgically implantable devices that provide deaf individuals access to auditory information by converting acoustic waves into electrical signals that directly stimulate the auditory nerve. With experience, the brain learns to interpret the signal; however, it is well known that the signal delivered to the auditory nerve is spectrally degraded relative to the acoustic input. Despite this issue, advancing CI technology effectively facilitates the development of oral language in most deaf individuals.
An essential part of the evaluation of CI benefit is the improvement in language skills with CI users relative to hearing aid users with similar degrees of hearing loss (Fink et al. 2007). It is well documented that early unilateral implantation is highly correlated with improvements in language development when compared with improvements in children who receive CIs later in childhood (e.g., Kirk et al. 2002). Children with unilateral CIs have been shown to improve on both receptive and expressive language skills as they grow older and gain more listening experience (Hay-McCutcheon et al. 2008). Using a developmental trajectory analysis it has also been shown that a group of children who were implanted before 12 months of age performed better on a speech perception task than a group of children implanted between 13 and 36 months, suggesting that there is a clear advantage for early implantation (Tajudeen et al. 2010).
Many children who are implanted with a CI develop language skills that enable them to communicate verbally and to function in mainstream environments along with their normally hearing peers (Geers et al 2009). The development of language skills in CI users compared with that of typically developing, normal-hearing (NH) age-matched peers has been a topic of great interest. Thus, studies often tend to compare performance in CI users and NH peers on standardized measures, usually normed on typically developing NH populations. Whereas children implanted at 3 years of age or older have been more likely to show language delays on standardized tests relative to their NH peers, children implanted at a younger age are more likely to perform at a level that is closer to the age-matched NH peers (i.e., Houston & Miyamoto 2010; Colleti et al. 2011). It is important to note that speech and language skills assessed by standardized tests are not uniform across the hearing-impaired population, and in fact, large individual variability is typically observed (e.g., Sarant et al. 2001; Hayes et al. 2009; Niparko et al. 2010).
An additional area of focus on this population has been that of cognitive development, in particular in deaf children with and without CIs, where measures are typically made using nonverbal tasks designed to minimize linguistic demands. Geers and colleagues have shown that performance on non-verbal intelligence quotient (IQ) measures predicts significant variance in speech perception, production, and language (e.g., Geers et al. 2003; Tobey et al. 2003; Geers 2006). The majority of studies that have investigated nonverbal intelligence in children with CIs (without any other developmental disabilities) report performance within normal limits regardless of variables such as Length of first CI Use or age of implantation (e.g., Niparko et al. 2010). However, Colleti et al. (2011) found that on three subtests of a nonverbal intelligence test, children who were implanted before 12 months of age performed significantly better than children who were implanted between 24 and 35 months. They hypothesized that early auditory stimulation may contribute to the development of higher cognitive functions, perhaps due to improved ability to multisensory augmentation of the ability to understand the relationship between stimuli. Similar to language outcome measures, the results from nonverbal IQ tests show wide variability in this population.
The initial success observed by many CI users in the areas of spoken language and cognition was based on tests conducted in quiet listening situations, however, there has been increasing interest in investigating the ability of CI users to function in noisy, complex auditory environments. Growing evidence suggests that unilateral CI (UCI) users generally perform poorly when listening to speech in noise, and that bilateral CIs (BiCIs) can provide a benefit for many children. For example, Litovsky et al. (2006) and Mok et al. (2010) demonstrated that children who are fitted with BiCIs exhibit better speech perception in noise when tested in spatially separated conditions than children who use a bimodal condition of UCI plus a hearing aid in the opposite ear. In fact, it has become increasingly more common for young children and infants to receive BiCIs, in an effort to provide better speech understanding in noise and spatial hearing skills (for review see Litovsky 2011; Litovsky et al. 2012).
There is growing evidence to suggest that BiCIs can, in many children, lead to improved performance on spatial hearing tasks (e.g., Litovsky et al. 2004, 2006; Godar & Litovsky 2010; Grieco-Calub & Litovsky 2010, 2012; van Deun et al. 2010). However, there continues to be a gap in performance relative to age-matched peers with NH. For example, children with BiCIs who are mainstreamed in age-appropriate grade levels perform significantly worse than their NH peers on speech-in-noise segregation (Misurelli & Litovsky 2012). Furthermore, subjective measures, such as questionnaires and parental reports designed to evaluate “ease of listening” situations suggest that children with BiCIs receive higher scores than children with UCIs (Winkler et al. 2002; Tait et al. 2010).
While the benefits of BiCIs for spatial hearing are becoming more evident, our understanding of speech and language skills in this growing population is generally limited. One study reported a higher incidence of transitioning from total communication to auditory–oral communication after 18 months of bilateral implant use in children receiving their second CI before 6 years of age (Scherf et al. 2007). Another study measured language scores in 25 Dutch-speaking children who received either sequential or simultaneous BiCIs before 5 years of age. They found a negative correlation between language scores and the interval of time between the first and second implant (Boons et al. 2012). Nonetheless, it remains unclear as to whether oral communication abilities are further promoted through the use of a second CI. Given that auditory signals in each CI are relatively degraded, having access to sound in both ears might provide children with multiple “looks” at the auditory signal, perhaps leading to speech information being clearer or more easily intelligible.
The question of whether two CIs aid in speech and language acquisition may have significant clinical implications. Parents of children born with profound hearing loss who decide to pursue cochlear implantation typically seek guidance regarding ideal time of implantation and potential benefits of BiCIs. Decision making regarding whether or not to implant a child with a second CI may be influenced by findings showing that the development of speech and language is different in a cohort of bilaterally implanted children relative to unilaterally implanted children.
The purpose of the present study was twofold. First, we aimed to investigate receptive and expressive language abilities, as well as nonverbal intelligence, in a relatively large group of children all of whom were bilaterally implanted. We were specifically interested in whether these children’s performance was within the range of scores reported in the literature for age- matched typically developing peers. In Table 1 we summarize published findings on language skills that include performance of children with UCI or BiCI (Geers et al. 2009; Geers & Nicholas 2013; Holt et al. 2013; Tobey et al. 2013). These studies were selected due to their relatively large sample sizes. Notably, each study used different inclusionary criteria regarding the age of testing, age of implantation, and amount of listening experience at time of testing. As shown in Table 1, nonverbal tests of intelligence from three studies (Geers et al. 2009; Geers & Nicholas 2013; Tobey et al. 2013) show mean scores that are within normal limits. However, mean language scores are more variable and are, on average, 1 SD below the mean for the normed populations. The only exception to this is children with a mean age of 10.5 years in one study (Geers & Nicholas 2013). Authors of these four studies conclude that, despite large variability, earlier age of implantation and increased listening experience with CIs result in higher language scores for most children.
TABLE 1.
Review of standardized nonverbal IQ and language measures from existing studies on children with cochlear implants
| Authors and Year of Publication |
N Size (No. Bilateral) |
Age of Implantation (yrs; mos) |
Age at Testing (yrs; mos) |
Standardized Nonverbal IQ Measure |
Mean (SD) | Standardized Language Measure |
Mean (SD) |
|---|---|---|---|---|---|---|---|
| Geers et al. (2009) | 153 (not reported) | <5;0, range not reported | 5;0–6;11 | Weschler performance IQ | 105.6 (15.5) | CELF-P and CELF-P2 | Receptive language: 82.95 (20.09) Expressive language: 79.11 (20.96) |
| Holt et al. (2013) | 59 (18) | 1;0–3;0 | 1;0–18;0, | N/A | N/A | PLS-4 CELF-4 |
Total language: 73.42 Core language: 85 |
| Geers and Nicholas (2013) | 60 (29) | 4;6 and 10;6 | Weschler Intelligence Scale for Children-Perceptual Reasoning | 105.2 (12.9) | PLS-3 (at age 4.5 yrs) | Auditory comprehension: 82.33 (19.32) Expressive language: 74.22 (21.06) |
|
| CELF-4 (at age 10.5 yrs) | Core language: 89.19 (20.19) Receptive language: 88.42 (17.01) Expressive language: 92.48 (20.38) |
||||||
| Tobey et. al (2013) | 160 (34) | 0;6–4;11 | Children tested annually and results are reported from 4- and 6-yr follow-up visits | Bayley PDI | 94.3 (18.5) | CASL (4-yr follow-up) | Core composite: 76.4 (22.6) |
| Leiter-R | 105.1 (20.0) | CASL (6-yr follow-up) | Core composite: 77.6 (24.3) |
CELF-P indicates Clinical Evaluation of Language Fundamentals-Preschool
CELF-4, Clinical Evaluation of Language Fundamentals- 4th edition; CASL, Comprehensive Assessment of Spoken Language; IQ, intelligence quotient; PLS, Preschool Language Scale; Bayley PDI, Bayley Scales Psychomotor Developmental Index; N/A, not applicable.
The goal of the present study was to focus on measures of nonverbal intelligence and language outcomes in a cohort of children who were selected because they all received their first CI by age 3;6 and were bilaterally implanted by age 7;0. The purpose was not to directly compare with prior studies, because data for children with UCI and BiCI were not treated separately. Rather, here we focus on a cohort of children with BiCIs, who are educated in mainstream oral communication settings, who do not have any other impairments, and whose mothers have an average of 16.6 years of education. Measures of nonverbal IQ showed that these children scored within or above the range of scores seen in the normed typically developing population. Thus, we sought to evaluate their language outcomes for expressive and receptive language skills. To the extent that this cohort of children with BiCIs show a trend toward higher scores, this would provide an indication that the criteria used for recruitment into our study focused on factors that may maximize language outcomes.
The second goal of this study was to focus more closely within the cohort of children studied regarding the potential role of two factors, Length of first CI Use and Length of second CI Use, in predicting cognitive and linguistic development. Given the relatively narrow range of age at activation of the first CI in our sample, this factor was not predicted to be a strong variable for language outcomes. However, the Length of second CI Use was predicted to be associated with better performance, in particular on receptive language skills, which may depend on access to clearer speech as discussed earlier. Beyond the inherent scientific interest, there is clear clinical relevance, as caregivers and clinicians attempt to make informed decisions about intervention strategies for children who are deaf.
PARTICIPANTS AND METHODS
Participants
Participants consisted of 4- to 9-year-old children (n = 39, mean age 5;9) with varying amounts of bilateral listening experience. Inclusion criteria required that participants should be native English speakers and have no reported developmental disabilities. In addition, age at implantation of the CIs was to be no later than age 3;6 years for the first CI and 7;0 years for the second CI. Finally, participants were to have at least 1 year of listening experience with their first CI (see Tables 2 and 3 for detailed information) and their primary mode of communication was to be oral. All children were either mainstreamed in school or enrolled in a school for children who have hearing loss with auditory (oral) communication. Device manufacturer was not a controlled variable in this study, as children with any device type were recruited based on the aforementioned criteria; device types for each child are shown in Table 3. Each child’s audiologist completed CI programming during regularly scheduled appointments. As is common clinical protocol for BiCIs, each ear was programmed independently. During testing, the setting most used in everyday listening by the children, as indicated by parent report and audiologist recommendation, was the one used for all aspects of data collection.
TABLE 2.
Summary of demographic data for n = 39 children with bilateral CIs (yr; mo)
| Mean | Minimum | Maximum | SD | |
|---|---|---|---|---|
| Chronological age | 5; 9 | 4; 1 | 8; 5 | 1; 4 |
| Length of first CI use | 4; 2 | 1; 11 | 6; 8 | 1; 4 |
| Length of second CI use | 2; 4 | 0; 4 | 6; 4 | 1; 6 |
| Chronological age at CI1 | 1; 6 | 0; 8 | 3; 7 | 0; 8 |
| Chronological age at CI2 | 3; 5 | 0; 8 | 6; 2 | 1; 5 |
| Interval between CI1 and CI2 | 1; 11 | 0; 0 | 4; 11 | 1;5 |
CIs, cochlear implants.
TABLE 3.
Complete demographic data for n=39 children with bilateral Cis
| Subject | Sex | CA at C11 (yr; mo) |
Device Type |
CA at Cl2 (yr; mo) |
Device Type |
Interval (yr; mo) |
CA at Test (yr; mo) |
Length of First CI Use (yr; mo) |
Length of Second CI Use (yr; mo) |
Leiter-R Brief IQ |
Leiter-R Memory Screen |
TOLD P;4 Core Lang |
TOLD P;4 Listening Comp |
TOLD P;4 Speech Comp |
Maternal Education |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CICA | M | 2; 5 | MedEl PULSARci100 | 2; 5 | MedEl PULSARci100 | 0; 0 | 4; 5 | 2; 0 | 2; 0 | 87 | 96 | 64 | 83 | 55 | 20 |
| CIEH | M | 1; 1 | Cochlear Freedom | 1; 1 | Cochlear Freedom | 0; 0 | 4; 1 | 3; 0 | 3; 0 | 105 | 109 | 76 | 91 | 73 | 18 |
| CIEJ | M | 1; 3 | Cochlear Freedom | 1; 3 | Cochlear Freedom | 0; 0 | 4; 7 | 3; 4 | 3; 4 | 105 | 125 | 62 | 80 | 58 | 15 |
| CIBB | F | 0; 8 | Cochlear Nucleus24 | 0; 8 | Cochlear Nucleus24 | 0; 0 | 7; 0 | 6; 4 | 6; 4 | 100 | 74 | 87 | 91 | 82 | 16 |
| CICK | M | 1; 1 | Adv Bionics HiRes 90K/HiFocus |
1; 3 | Adv Bionics HiRes 90K/HiFocus |
0; 2 | 4; 7 | 3; 7 | 3; 5 | 115 | 87 | 108 | 122 | 100 | 22 |
| CIEC | M | 2; 5 | Cochlear Nucleus24 Contour |
2; 10 | Cochlear Nucleus24 Contour |
0;5 | 6;11 | 4;7 | 4;2 | 127 | 112 | 88 | 102 | 91 | 16 |
| CIEB | F | 3; 7 | Cochlear Nucleus24 Contour |
4; 0 | Cochlear Nucleus24 Contour |
0; 5 | 8; 3 | 4; 8 | 4; 3 | 98 | 96 | 108 | 117 | 100 | 16 |
| CIBI | F | 1; 1 | Cochlear Nucleus24 | 1; 6 | Cochlear Freedom | 0; 5 | 7; 3 | 6; 2 | 5; 9 | 95 | 115 | 103 | 117 | 100 | 19 |
| CIBV | M | 1; 5 | Adv Bionics HiRes 90K/HiFocus |
1; 11 | Adv Bionics HiRes 90K/HiFocus |
0; 6 | 5; 0 | 3; 7 | 3; 1 | 129 | 115 | 91 | 94 | 94 | 16 |
| CIDR | F | 1; 10 | Adv Bionics HiRes 90K/HiFocus |
2; 8 | Adv Bionics HiRes 90K/HiFocus |
0; 10 | 4; 6 | 2; 8 | 1; 10 | 100 | 74 | 78 | 94 | 76 | 18 |
| CICF | F | 1; 6 | Cochlear Freedom | 2; 5 | Cochlear Freedom | 0; 10 | 4; 6 | 3; 0 | 2; 1 | 124 | 137 | 104 | 108 | 103 | 16 |
| CIED | F | 2; 3 | Adv Bionics HiRes 90K/HiFocus |
3; 2 | Adv Bionics HiRes 90K/HiFocus |
0; 11 | 6; 5 | 4; 2 | 3; 3 | 119 | 122 | 118 | 119 | 115 | 20 |
| CIEE | M | 2; 11 | Cochlear Freedom | 4; 0 | Cochlear Freedom | 1; 1 | 4; 3 | 1; 5 | 0; 4 | 124 | 84 | 70 | 94 | 61 | 15 |
| CIDX | M | 1; 5 | Cochlear Nucleus24 Contour |
2; 7 | Cochlear Nucleus24 Contour |
1; 2 | 6; 10 | 5; 5 | 4; 3 | 139 | 131 | 115 | 117 | 103 | 12 |
| CICB | F | 0; 11 | Cochlear Nucleus24 Contour |
2; 2 | Cochlear Freedom | 1;2 | 4;3 | 3; 4 | 2; 2 | 127 | 90 | 113 | 108 | 112 | 20 |
| CIES | M | 1; 7 | Cochlear Freedom | 2; 10 | Cochlear Freedom | 1;3 | 4; 3 | 2; 8 | 1;4 | 97 | 112 | 94 | 102 | 88 | 13 |
| CICL | M | 1; 5 | Cochlear Freedom | 2; 9 | Cochlear Freedom | 1;4 | 4; 10 | 3; 5 | 2; 1 | 115 | 115 | 112 | 119 | 106 | 18 |
| CIDV | F | 2; 2 | Cochlear Nucleus24 Contour |
3; 6 | Cochlear Freedom | 1;4 | 6; 6 | 4; 4 | 3; 0 | 95 | 96 | 81 | 86 | 82 | 16 |
| CICN | F | 1; 3 | Cochlear Freedom | 2; 8 | Cochlear Freedom | 1;6 | 4; 1 | 2; 9 | 1;2 | 113 | 109 | 108 | 111 | 106 | 16 |
| CIDY | M | 1; 2 | MedEl Combi 40+ | 2; 9 | MedEl PULSARci100 | 1;7 | 5; 11 | 4; 10 | 3; 2 | 115 | 93 | 58 | 80 | 55 | 16 |
| CIEI | M | 1; 1 | Adv Bionics HiRes 90K/HiFocus |
2; 9 | Adv Bionics HiRes 90K/HiFocus |
1;7 | 5; 11 | 4; 9 | 3; 2 | 123 | 103 | 72 | 94 | 64 | 15 |
| CIDP | F | 0; 11 | MedEl Combi 40+ | 2; 8 | MedEl PULSARci100 | 1; 9 | 4; 11 | 4; 0 | 2; 2 | 121 | 112 | 100 | 102 | 82 | 20 |
| CICM | M | 1; 1 | Adv Bionics HiRes 90K/HiFocus |
3; 2 | Adv Bionics HiRes 90K/HiFocus |
2; 1 | 4; 4 | 3; 3 | 1; 2 | 105 | 96 | 109 | 117 | 103 | 18 |
| CIEL | F | 1; 1 | Cochlear Nucleus24 Contour |
3; 4 | Cochlear Freedom | 2; 3 | 7; 7 | 6; 5 | 4; 2 | 102 | 90 | 87 | 97 | 79 | 12 |
| CIBT | M | 2; 3 | Cochlear Nucleus24 Contour |
4; 7 | Cochlear Freedom | 2; 4 | 6; 9 | 4; 5 | 2; 1 | 93 | 71 | 82 | 83 | 85 | 15 |
| CICQ | M | 1; 2 | Cochlear Freedom | 3; 7 | Cochlear Freedom | 2; 4 | 4; 2 | 2; 11 | 0; 6 | 143 | 106 | 73 | 88 | 61 | 18 |
| CIDW | M | 2; 3 | Adv Bionics HiRes 90K/HiFocus |
5; 0 | Adv Bionics HiRes 90K/HiFocus |
2; 9 | 5; 4 | 3; 1 | 0; 4 | 98 | 106 | 82 | 80 | 88 | 18 |
| CIDG | F | 1; 2 | Cochlear Freedom | 3; 11 | Cochlear Freedom | 2; 9 | 4; 3 | 3; 2 | 0; 5 | 117 | 106 | 73 | 83 | 70 | 13 |
| CI BW | F | 1; 1 | Cochlear Nucleus24 Contour |
3; 10 | Cochlear Freedom | 2; 9 | 5; 11 | 3; 11 | 1; 2 | 105 | 100 | 91 | 100 | 82 | 16 |
| CIDZ | F | 2; 2 | Cochlear Nucleus24 Contour |
6; 0 | Cochlear Freedom | 3; 0 | 8; 5 | 6; 3 | 3; 3 | 97 | 137 | 104 | 117 | 103 | 19 |
| CIEG | M | 1; 6 | Adv Bionics HiRes 90K/HiFocus |
4; 11 | Adv Bionics HiRes 90K/HiFocus |
3; 5 | 6; 1 | 4; 7 | 1; 2 | 111 | 103 | 95 | 105 | 97 | 20 |
| CIDJ | F | 1; 8 | Cochlear Nucleus24 Contour |
5; 1 | Cochlear Freedom | 3; 5 | 8; 1 | 5; 5 | 2; 0 | 115 | 109 | 87 | 91 | 91 | 18 |
| CIDQ | F | 0; 9 | Cochlear Nucleus24 Contour |
4; 3 | Cochlear Freedom | 3; 5 | 6; 7 | 5; 10 | 2; 4 | 121 | 109 | 104 | 94 | 97 | 12 |
| CIEF | F | 1; 3 | Cochlear Nucleus24 Contour |
4; 10 | Cochlear Freedom | 3; 6 | 5; 10 | 4; 7 | 1; 0 | 95 | 103 | 86 | 97 | 88 | 16 |
| CICY | M | 1; 0 | Adv Bionics HiRes 90K/HiFocus |
4; 8 | Adv Bionics HiRes 90K/HiFocus |
3; 8 | 5; 2 | 4; 2 | 0; 6 | 103 | 100 | 91 | 97 | 88 | 15 |
| CIBU | M | 1; 2 | MedEl Combi 40+ | 5; 1 | MedEl PULSARci100 | 3; 11 | 6; 3 | 5; 1 | 1; 1 | 127 | 118 | 74 | 74 | 88 | 19 |
| CIAW | M | 1; 2 | Cochlear Nucleus24 Contour |
5; 4 | Cochlear Freedom | 4; 2 | 7; 8 | 6; 5 | 2; 3 | 107 | 106 | 101 | 114 | 94 | 17 |
| CIDF | F | 1; 2 | Cochlear Nucleus24 Contour |
5; 6 | Cochlear Freedom | 4; 4 | 5; 11 | 4; 9 | 0; 5 | 109 | 115 | 81 | 91 | 82 | 13 |
| CIDN | M | 1; 2 | MedEl Combi 40+ | 6; 2 | MedEl PULSARci100 | 4; 11 | 7; 0 | 5; 10 | 0; 11 | 127 | 115 | 118 | 114 | 119 | 16 |
CA, chronological age; Cls, cochlear implants.
The linguistic and cognitive data presented here are part of a prospective, longitudinal study in which participants were recruited from across the United States by referrals from their audiologists, surgeons, or were self-referred, and came to Madison, Wisconsin, to participate in a battery of tests. This type of recruitment may have led to biased sampling because the families who enrolled in the study were highly motivated and often traveled long distances to participate in research. Children typically completed 2 to 3 days of testing during which they participated in various tasks that included left/right discrimination, speech in noise, and sound source identification, as well as a standardized language and cognition assessment. Here we present results from each child’s first visit to our lab.
Outcome Measures
Participants were tested with the Leiter International Performance Scale-Revised (Leiter-R; Roid & Miller 1997) and the Test of Language Development–Primary 4th Edition ( TOLD-P:4; Newcomer & Hammill 2008). Testing was administered by a trained experimenter, and conducted in a quiet carpeted room. The Leiter-R test battery was used to evaluate the nonverbal intelligence and memory abilities of the participants. Six subtests from the Visualization and Reasoning, and Attention and Memory batteries were administered, including repeated patterns, figure-ground, form completion, and sequential order subtests to assess the child’s visualization, and the associative pairs and forward memory subtests to assess memory. Performance on these tests yields standard scores for Leiter-R IQ and Memory Screening.
Six subtests administered from the TOLD-P:4 included picture vocabulary, relational vocabulary, oral language, syntactic understanding, sentence imitation, and morphological completion, and yielded standard scores for receptive and expressive language. Standard scores were calculated for each composite and the overall core language score. The use of standardized scores allowed us to control for chronological age and to compare performance of the children with BiCIs with performance of the normative sample of hearing peers. For each participant, total testing time was approximately 90 to 120 minutes for each test, depending on breaks.
Predictor Variables
Two variables inherent to each child at time of testing were targeted as potentially important in predicting linguistic and cognitive development. All information was provided by the parents, and if necessary, confirmed by the children’s audiologist. The first variable was Length of first CI Use, operationally defined as the amount of time that the listener had been unilaterally exposed to sound and was defined as length of listening experience since activation of the first CI. The second variable, Length of second CI Use was defined as the amount of time that each child had been listening with two CIs at the time of testing. All children in the study were congenitally deaf (n=39) so their Length of first CI Use and Length of second CI Use were calculated by subtracting the date of activation for each CI from the date of their testing session at the Binaural Hearing and Speech Lab.
RESULTS
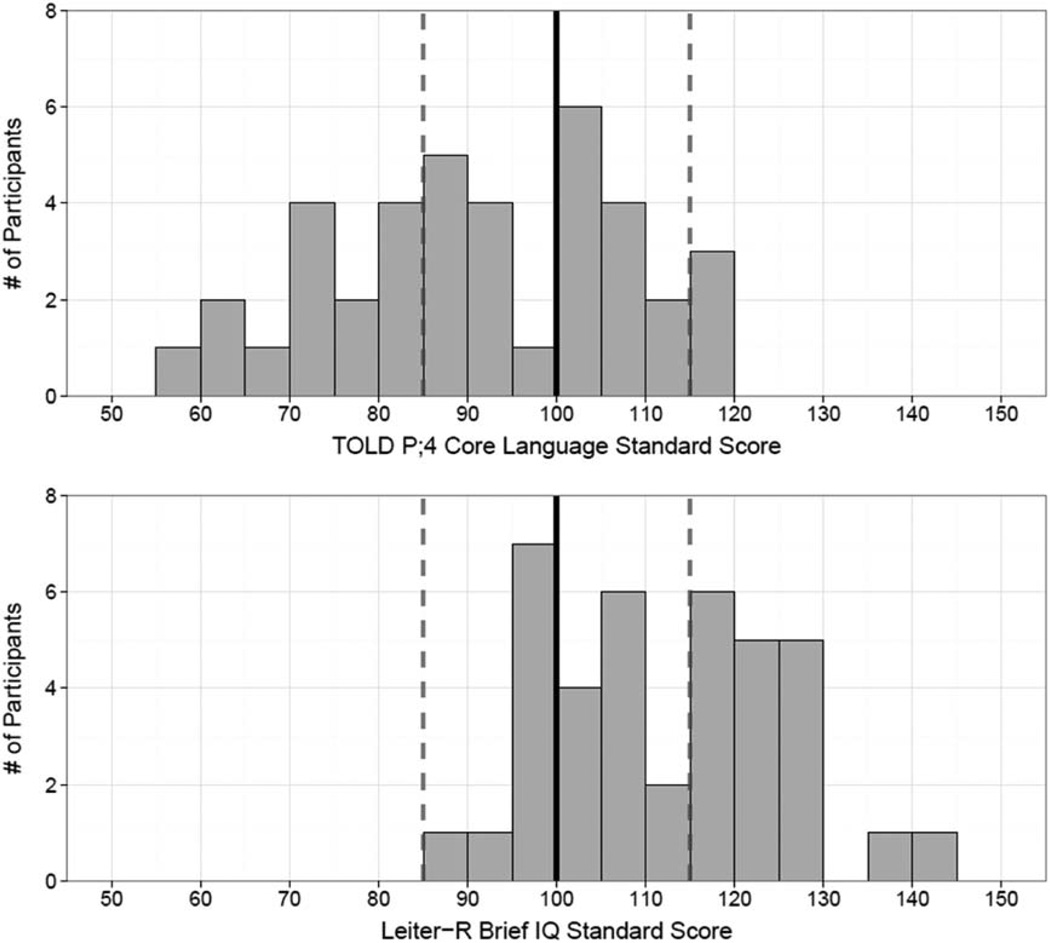
Table 4 displays the mean standard scores on the outcome measures obtained from the Leiter-R and the TOLD-P;4 for the 39 participants tested in this study. Figure 1 shows histograms of the Leiter-R Brief IQ scores (A) and Core Language scores (B) for the same children. Performance is shown relative to the mean (±1 SD) of mean scores reported for the population of typically developing, NH peers. These normed values correspond to a standard score of 100 (solid vertical line) and standard deviation of ±15 (dotted vertical lines), or percentiles ranging from 15th to 85th. All 39 participants in the present study had Leiter-R Brief IQ scores within or above 1 SD of the NH population (21 of 39 were within 1 SD, and 18 of 39 were above 1 SD from the mean). Core Language scores were distributed differently: 23 of 39 fell within 1 SD from the mean, 14 of 39 were below 1 SD from the mean, and 2 of 39 were above 1 SD from the mean.
TABLE 4.
Mean standard score (± SD) and range of scores for n = 39 children with bilateral CIs
| Brief IQ | Memory Screen | Core Language | Listening Composite | Speaking Composite | |
|---|---|---|---|---|---|
| Mean (SD) | 111.49 (13.52) | 105.05 (15.49) | 90.97 (16.42) | 99.31 (13.50) | 87.72 (16.68) |
| Range | 87–139 | 71–137 | 58–118 | 74–122 | 55–119 |
CIs, cochlear implants; IQ, intelligence quotient.
Fig. 1.
Histograms showing frequency of standard scores for Leiter-R Brief IQ (A) and TOLD P;4 Core Language (B). TOLD indicates Test of Language Development- Primary 4th Edition.
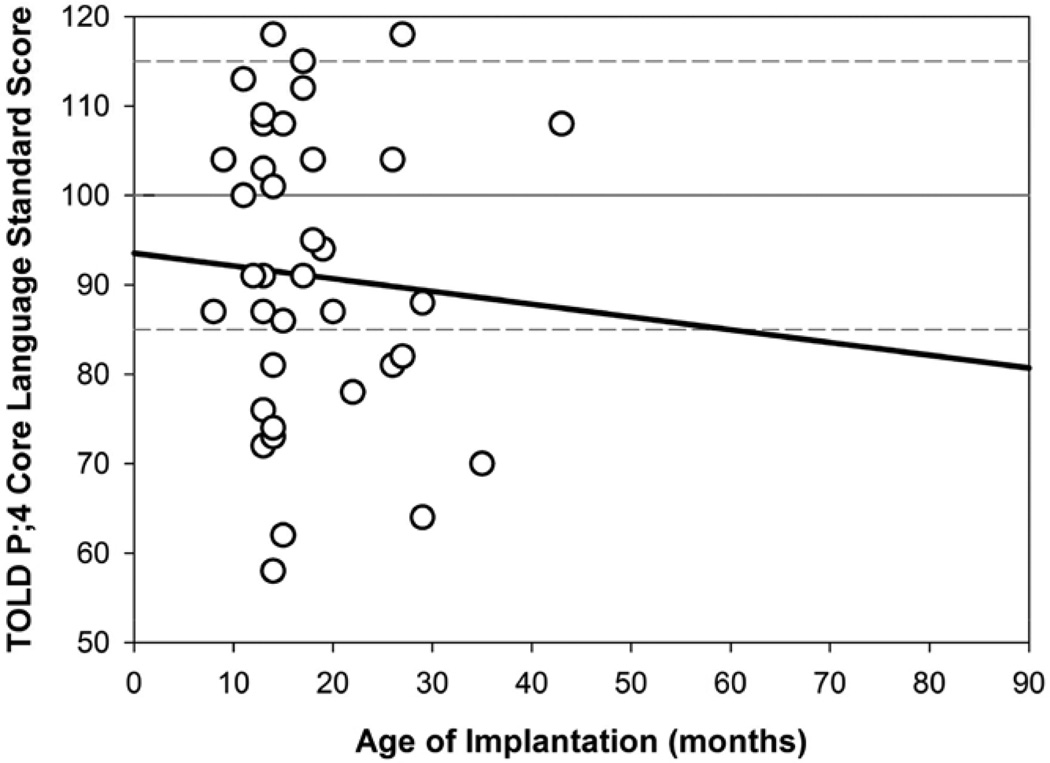
Figures 2 through 4 show results from simple linear regressions for Core Language scores, where the variability in performance can be observed. Figure 2 shows results as a function of Age of Implantation for CI1. A negative nonsignificant correlation (r2=0.004; p=0.69) between age of implantation of CI1 and Core Language scores was observed. The lack of effect is likely due to the relatively tight clustering of the age of activation of the first CI in our sample, with heavy clustering between 12 and 24 months of age. An additional analysis was conducted by subdividing the participants into groups according to whether they received the first CI before or after 18 months of age. A t test revealed a nonsignificant age of implantation effect (t[38] = 0.507, p > 0.07); however, there was a trend toward higher Core Language scores for children who received their first CI before 18 months.
Fig. 2.
Simple linear regression showing the relationship between Core Language standard scores and age of implantation (CI1; mos) for n=39 children with bilateral CIs. CI indicates cochlear implant.
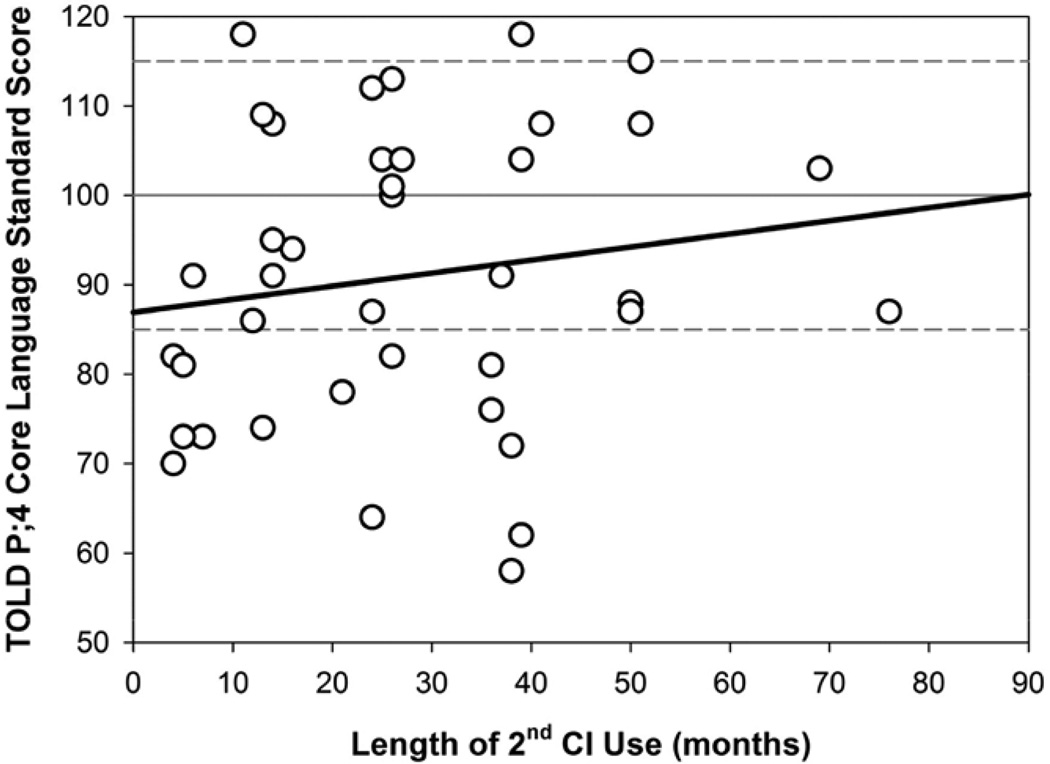
Fig. 4.
Simple linear regression showing the relationship between Core Language standard scores and length of second CI use (mos) for n=39 children with bilateral CIs. CI indicates cochlear implant.
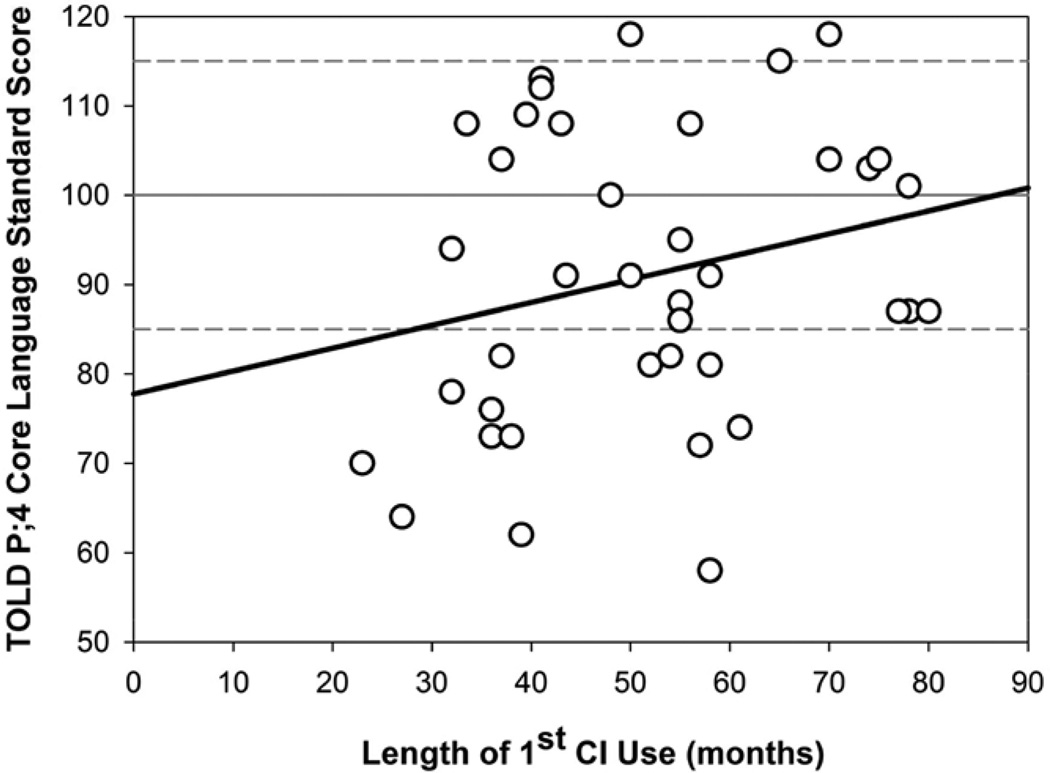
Figure 3 shows the Core Language scores as a function of Length of first CI Use, whereby more experience with the first CI is weakly, but again not significantly related to higher Core Language scores (r2=0.060; p=0.133). Figure 4 shows the Core Language scores as a function of Length of second CI Use, whereby more experience listening with bilateral stimulation is not significantly related to Core Language scores (r2=0.025; p=0.338).
Fig. 3.
Simple linear regression showing the relationship between Core Language standard scores and length of first CI use (mos) for n=39 children with bilateral CIs. CI indicates cochlear implant.
To evaluate the extent to which Length of second CI Use may have contributed to children’s performance on these tests, over and above Length of first CI Use, hierarchical regression analyses were conducted. Standardized regression coefficients and R2 values for each regression are presented in Table 5. The multiple regression analysis of participants’ standardized scores showed that Length of first CI Use did not significantly predict the Speaking Composite (p = 0.08), Core Language (p = 0.129), and Listening Composite (p = 0.305) measures obtained from the TOLD-P;4. The independent effects of BiCI experience on each of the three language measures did not significantly predict performance (all p > 0.05) over and above effects of Length of first CI Use. These findings suggest that, in this cohort of children with BiCIs, the use of a second CI does not significantly impact overall language scores.
TABLE 5.
Hierarchic regression statistics for the sample of bilateral CI children (n = 39)
| Standardized Regression Coefficients (β) for Variables in the Equations |
||
|---|---|---|
| Condition | Step 1: Length of First CI Use | Step 2: Length of Second CI Use |
| Memory screen | 0.198 | −0.113 |
| Core language | 0.253 | 0.035 |
| Listening composite | 0.084 | 0.211 |
| Speaking composite | 0.351 | −0.079 |
| Proportion of variance accounted for (R2) for variables in the equations (ΔR2 in parentheses) |
||
| Condition | Step 1: Length of first CI use | Step 1 + 2: Length of first + second CI use |
| Memory screen | 0.020 (0.020) | 0.030 (0.010) |
| Core language | 0.073 (0.073) | 0.074 (0.001) |
| Listening composite | 0.036 (0.036) | 0.069 (0.033) |
| Speaking composite | 0.097 (0.097) | 0.101 (0.004)U |
CIs, cochlear implants.
DISCUSSION
This study was one of the first, and the most comprehensive study, in which expressive and receptive language outcomes are shown for a cohort of children with BiCIs. The results provided here suggest three major findings. First, on measures of nonverbal cognition, all 39 children performed within or above age- level expectation. Second, on measures of core language, 25 of 39 children (64%) scored within or above age expectations.
Despite significant variability in the group, children with BiCIs tested here are high performers on measures of nonverbal cognition. Svirsky et al. (2004) found that children implanted with one CI before 3 years of age can acquire both language and cognitive skills close to those of NH children. Our results showed that many children in the sample, whether implanted with their first CI before or after 18 months, achieved similar language skills as their NH peers. These results also corroborate the results from Wie (2010), who found children with BiCIs showed positive associations between early implantation (between 5 and 18 months) and expressive and receptive language scores at follow-up testing 3 to 12 months postactivation, but diminished, nonsignificant age of implantation effects after 36 months of BiCI use. It is important to emphasize the fact that, while results from the present study, as well as other studies that use standardized measures, show overlap in outcomes between early implanted children and normally hearing peers, these measures cannot be taken to further demonstrate that the CI users have “normal language.” The results can only be interpreted to mean that the children have age-appropriate skills as measured by the standardized language measures. For example, Todd et al. (2011) showed that productions of phonemes by children with CIs had less contrast than those of NH children of the same chronological age, and NH children with the same duration of auditory experience. The authors concluded that reduced contrast may explain in part why the speech of children with CIs is less intelligible than that of their peers with NH.
The third major finding, that Length of first CI Use accounts for more of the variance in language score performance than Length of second CI use, is consistent with previous research showing that increased exposure to auditory stimulation leads to increased expressive and receptive language development (Niparko et al. 2010). The prediction that receptive language, or the Listening Composite score, would be more influenced by the Length of second CI Use than expressive language, or the Speaking Composite score, was verified as Length of second CI Use accounted for 3.3% of the variance in Listening Composite scores over and above Length of first CI Use as compared with the 0.4% accounted for by the Speaking Composite score. One possible explanation for this finding is that children with CIs often receive speech and language therapy focusing on listening and auditory development as soon as their CI is activated. They are often explicitly taught to listen, and in this cohort of children tested, their receptive language scores are considerably higher than their spoken language scores. Another possible explanation is that children with BiCIs are on a delayed developmental trajectory in terms of auditory development; receptive language comes in first and with more experience it could be expected that these children will start to look more similarly to NH peers. This theory is supported by the differences in standard deviations between the Speaking and Listening Composite measures.
Similar to findings in the study by Hayes et al. (2009), we may not have captured the increase in receptive language skills by testing children more than a year after they received their CIs. It is likely of course that other factors account for additional variability, including factors inherent to the subjects, clinical interventions, and environmental factors. A study with a much larger sample size and greater variation on these factors might shed light on these particular issues.
There are two important caveats to raise concerning results from the present study. The first is that our participants are not a representative sample of all children with BiCIs. Most of the families of these children were able and willing to travel from outside the state to participate in this study. While this motivation to participate in our research study cannot be objectively quantified, anecdotal reports from parents and caregivers indicate that many of these adults exercise a high level of motivation when implementing family-based therapy strategies designed to promote both listening and spoken language skills in their child. Because of this, the sample of BiCI users in this study likely oversamples children whose home situation promotes an environment that facilitates auditory habilitation. If this is the case, the wide range of linguistic performance paired with the relatively small range of nonverbal IQ scores of these children who use BiCIs underscores the importance of considering multiple factors that likely contribute to overall performance. Some of the other factors that might be associated with the results of this study include communication mode, familial support, type and frequency of auditory habilitation, and educational placement (Geers 2006). Evolution of CI technology and device adjustments (e.g., number of electrodes activated, accuracy of the mapping) have also been cited as sources of variability (Geers et al. 2007). None of the abovementioned factors were controlled for in this study. Therefore, the generalizability of the results is unclear as the BiCI participants were self-selected and motivated to participate in this research.
The second caveat is the absence of control groups of children with unilateral CIs or children with profound SNHL who are not fitted with CIs. The presence of such control groups could potentially provide a more definitive exploration of the efficacy of bilateral cochlear implantation relative to other groups of children. In addition, due to the cross-sectional nature of the study, baseline measure of preimplantation performance on the standardized measures across subjects that could provide an estimate of effects that might be augmented by addition of a second ear is lacking. A recent study by Boons et al. (2012), where 25 children with UCIs were matched to 25 children with BiCIs, found that spoken language expression and comprehension scores were significantly better in the children who received simultaneous BiCIs. One of the strengths of that study was the controlled match between the UCI and BiCI groups for age at first CI, HA at time of testing, sex, and cause of deafness. All the participants in that study received their first CI by 2 years of age, and testing was conducted starting 3 years after the first implantation. It is possible that in the sample of BiCI children reported here, the variability and relatively large range of listening experience with both one and two CIs at the time of testing may have obfuscated or diluted any significant findings. Hayes et al. (2009) reported that in the first year after implantation, receptive vocabulary in children with UCIs improved at a rate greater than a year’s worth of progress typically seen in normally hearing peers, but that this growth tapered off and plateaued over time. While these results were reported for children with UCIs, it could be the case that in the BiCI cohort tested in this study, we did not observe any effects of Length of second CI Use over and above that of Length of first CI Use because the rate of improvement had tapered off by the time they were tested. These data are part of a prospective, longitudinal study that will allow for further analysis and tracking of development with BiCIs across annual visits in which the same language measures are collected.
Despite the noted limitations, results from this study have significant implications for children with BiCIs. In clinical treatment of children who are deaf and who receive BiCIs, factors related to language development should be considered carefully in combination with knowledge regarding costs, risks, auditory skill development, and social and emotional outcomes. Given that the TOLD-P;4 only assesses aspects of language at the word or sentence level (rather than connected discourse level) and taps “off-line” language processes, these findings emphasize the need to develop more sensitive “online” language- processing measures that might reveal some subtle speed of processing deficits, monitor the benefit of amplification in supporting spoken language and acquisition of listening skills, and guide intervention in children with BiCIs. For example, Grieco-Calub et al. (2009) used eye-gaze measures to study word recognition in toddlers, and found that CI users not only are less accurate than NH age-matched peers, but their responses are slower too. In addition, identifying the predictors that contribute to the high variability in both expressive and receptive language development in BiCI users will provide useful information.
As increasingly more infants receive bilateral CIs at younger ages, it will be important to identify potential benefits for language acquisition and language processing in these children at younger ages. Further research will be done to compare our bilateral cohort with a unilateral cohort and to monitor the linguistic development of the children in this study longitudinally so that both group- and individual-growth curves can be established.
ACKNOWLEDGMENTS
The authors thank the members of the Binaural Hearing and Speech Lab who have helped with data collection, and the families of the participants. In addition, the authors are grateful to many clinics that have helped with recruitment of participants, including Center for Communication, Hearing and Deafness, Children’s Healthcare of Atlanta, Children’s Hospital of Chicago, Dallas Otolaryngology Associates, Mass Eye & Ear, Mayo Clinic, University of Minnesota Medical Center—Fairview, UNC Pediatric Cochlear Implant Program, and UW Children’s Hospital Audiology.
This research is supported by National institutes of Health-National Institute on Deafness and other Commuication Disorders Grant No. R01 DC008365 (R.Y.L., PI) and in part by a core grant to the Waisman Center from the National Institute on Deafness and other Commuication Disorders (P30 HD03352).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- Boons T, Brokx JP, Frijns JH, et al. Effect of pediatric bilateral cochlear implantation on language development. Arch Pediatr Adolesc Med. 2012;166:28–34. doi: 10.1001/archpediatrics.2011.748. [DOI] [PubMed] [Google Scholar]
- Colleti L, Mandal M, Zoccante L, et al. Infants versus older children fitted with cochlear implants: Performance over 10 years. Int J Pediatr Otorhinolaryngol. 2011;75(4):504–509. doi: 10.1016/j.ijporl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Fink NE, Wang NY, Visaya J, et al. CDACI Investigative Team. Childhood Development after Cochlear Implantation (CDaCI) study: Design and baseline characteristics. Cochlear Implants Int. 2007;8:92–116. doi: 10.1179/cim.2007.8.2.92. [DOI] [PubMed] [Google Scholar]
- Geers AE. Factors influencing spoken language outcomes in children following early cochlear implantation. Adv Otorhinolaryngol. 2006;64:50–65. doi: 10.1159/000094644. [DOI] [PubMed] [Google Scholar]
- Geers AE, Brenner C, Davidson L. Factors associated with the development of speech perception skills in children implanted by age five. Ear Hear. 2003;24(1 Suppl):24S–36S. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- Geers AE, Moog JS, Biedenstein J, et al. Spoken language scores of children using cochlear implants compared to hearing age- mates at school entry. J Deaf Stud Deaf Educ. 2009;14:371–385. doi: 10.1093/deafed/enn046. [DOI] [PubMed] [Google Scholar]
- Geers AE, Nicholas JG. Enduring advantages of early cochlear implantation for spoken language development. J Speech Lang Hear Res. 2013;56:643–655. doi: 10.1044/1092-4388(2012/11-0347). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AE, Nicholas JG, Moog JS. Estimating the Influence of Cochlear Implantation on Language Development in Children. Audiol Med. 2007;5:262–273. doi: 10.1080/16513860701659404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SP, Litovsky RY. Experience with bilateral cochlear implants improves sound. Otol Neurotol. 2010;31(8):1287–1292. doi: 10.1097/MAO.0b013e3181e75784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco-Calub TM, Litovsky RY. Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing. Ear Hear. 2010;31:645–656. doi: 10.1097/AUD.0b013e3181e50a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco-Calub TM, Litovsky RY. Spatial acuity in 2-to-3-year-old children with normal acoustic hearing, unilateral cochlear implants, and bilateral cochlear implants. Ear Hear. 2012;33:561–572. doi: 10.1097/AUD.0b013e31824c7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco-Calub TM, Saffran JR, Litovsky RY. Spoken word recognition in toddlers who use cochlear implants. J Speech Lang Hear Res. 2009;52:1390–1400. doi: 10.1044/1092-4388(2009/08-0154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-McCutcheon MJ, Kirk KI, Henning SC, et al. Using early language outcomes to predict later language ability in children with cochlear implants. Audiol Neurootol. 2008;13:370–378. doi: 10.1159/000148200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes H, Geers AE, Treiman R, et al. Receptive vocabulary development in deaf children with cochlear implants: Achievement in an intensive auditory-oral educational setting. Ear Hear. 2009;30:128–135. doi: 10.1097/AUD.0b013e3181926524. [DOI] [PubMed] [Google Scholar]
- Holt RF, Beer J, Kronenberger WG, et al. Developmental effects of family environment on outcomes in pediatric cochlear implant recipients. Otol Neurotol. 2013;34:388–395. doi: 10.1097/MAO.0b013e318277a0af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DM, Miyamoto RT. Effects of early auditory experience on word learning and speech perception in deaf children with cochlear implants: Implications for sensitive periods of language development. Otol Neurotol. 2010;31:1248–1253. doi: 10.1097/MAO.0b013e3181f1cc6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KI, Miyamoto RT, Lento CL, et al. Effects of age at implantation in young children. Ann Otol Rhinol Laryngol Suppl. 2002;189:69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- Litovsky RY. Review of recent work on spatial hearing skills in children with bilateral cochlear implants. Cochlear Implants Int. 2011;12(Suppl 1):S30–S34. doi: 10.1179/146701011X13001035752372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Goupell MJ, Godar S, et al. Studies on bilateral cochlear implants at the University of Wisconsin’s Binaural Hearing and Speech Laboratory. J Am Acad Audiol. 2012;23:476–494. doi: 10.3766/jaaa.23.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Johnstone PM, Godar SP. Benefits of bilateral cochlear implants and/or hearing aids in children. Int J Audiol. 2006;45(Suppl 1):S78–S91. doi: 10.1080/14992020600782956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J, et al. Bilateral cochlear implants in adults and children. Arch Otolaryngol Head Neck Surg. 2004;130:648–655. doi: 10.1001/archotol.130.5.648. [DOI] [PubMed] [Google Scholar]
- Misurelli SM, Litovsky RY. Spatial release from masking in children with normal hearing and with bilateral cochlear implants: Effect of interferer asymmetry. J Acoust Soc Am. 2012;132:380–391. doi: 10.1121/1.4725760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M, Galvin KL, Dowell RC, et al. Speech perception benefit for children with a cochlear implant and a hearing aid in opposite ears and children with bilateral cochlear implants. Audiol Neurootol. 2010;15:44–56. doi: 10.1159/000219487. [DOI] [PubMed] [Google Scholar]
- Newcomer P, Hammill D. Test of Language Development—Primary. 4th ed. Austin, TX: Pro-ed; 2008. [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, et al. CDaCI Investigative Team. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid G, Miller L. Leiter International Performance Scale—Revised. Wood Dale, IL: Stoelting Press; 1997. [Google Scholar]
- Sarant JZ, Blamey PJ, Dowell RC, et al. Variation in speech perception scores among children with cochlear implants. Ear Hear. 2001;22:18–28. doi: 10.1097/00003446-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Scherf F, van Deun L, van Wieringen A, et al. Hearing benefits of second-side cochlear implantation in two groups of children. Int J Pediatr Otorhinolaryngol. 2007;71:1855–1863. doi: 10.1016/j.ijporl.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9:224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- Tait M, Nikolopoulos TP, De Raeve L, et al. Bilateral versus unilateral cochlear implantation in young children. Int J Pediatr Otorhinolaryngol. 2010;74:206–211. doi: 10.1016/j.ijporl.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Tajudeen BA, Waltzman SB, Jethanamest D, et al. Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otol Neurotol. 2010;31:1254–1260. doi: 10.1097/MAO.0b013e3181f2f475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey EA, Geers AE, Brenner C, et al. Factors associated with development of speech production skills in children implanted by age five. Ear Hear. 2003;24(1 Suppl):36S–45S. doi: 10.1097/01.AUD.0000051688.48224.A6. [DOI] [PubMed] [Google Scholar]
- Tobey EA, Thal D, Niparko JK, et al. CDaCI Investigative Team. Influence of implantation age on school-age language performance in pediatric cochlear implant users. Int J Audiol. 2013;52:219–229. doi: 10.3109/14992027.2012.759666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AE, Edwards JR, Litovsky RY. Production of contrast between sibilant fricatives by children with cochlear implants. J Acoust Soc Am. 2011;130:3969–3979. doi: 10.1121/1.3652852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deun L, van Wieringen A, Scherf F, et al. Earlier intervention leads to better sound localization in children with bilateral cochlear implants. Audiol Neurootol. 2010;15:7–17. doi: 10.1159/000218358. [DOI] [PubMed] [Google Scholar]
- Wie OB. Language development in children after receiving bilateral cochlear implants between 5 and 18 months. Int J Pediatr Otorhinolaryngol. 2010;74:1258–1266. doi: 10.1016/j.ijporl.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Winkler F, Schön F, Peklo L, et al. The Würzburg questionnaire for assessing the quality of hearing in CI-children (WH-CIK) Laryngorhinootologie. 2002;81:211–216. doi: 10.1055/s-2002-25042. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Sedey AL, Coulter DK, et al. Language of earlyand later-identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]