Abstract
Extracellular vesicles (EVs) are released from many cell types, including normal and pathological cells, and range 30-1000 nm in size. Once thought to be a mechanism for discarding unwanted cellular material, EVs are now thought to play a role in intercellular communication. Evidence is accruing that EVs are capable of carrying mRNAs, miRNAs, noncoding RNAs, and proteins, including those associated with neurodegenerative diseases and cancer, which may be exchanged between cells. For this reason, neurodegenerative diseases and cancers may share a common mechanism of disease spread via EVs. Understanding the role EVs play in disease initiation and progression will aid in the discovery of new clinically relevant biomarkers and the development of better targeted molecular and biological therapies.
Keywords: extracellular vesicles, microvesicle, exosome, stem cell, cancer, neurodegeneration
Intercellular communication
Within the central nervous system (CNS), communication between cells has been widely explored. Intercellular communication is required for the nervous system to function, including synaptic plasticity, trophic support, ion regulation, and electrical activity [1]. Much of this communication has been studied at the synapse, where neurotransmitter release from synaptic vesicles and receptor-mediated signaling cascades play a major role in neural communication. Synaptic signaling occurs over a very short distance, requiring the postsynaptic cell to have receptors for the neurotransmitter released into the synapse from the presynaptic cell. In neurons, chemical signals trigger electrical signals that travel quickly throughout the CNS, where circuits are in place for communication to occur across brain structures [2]. In the absence of electrical signaling, chemical communication can occur locally using autocrine or paracrine signaling, or over long distances as in the case of the endocrine system [3]. However, long-distance signaling places free-floating proteins and nucleic acids at risk for degradation.
Intercellular transport of macromolecules is known to utilize well-characterized exocytic and endocytic release and uptake machinery, including multivesicular bodies (see Glossary). The intercellular transfer of viruses (e.g. pseudorabies), lectins, toxins (e.g. tetanus, cholera), and macromolecules in the CNS has been studied as a means for understanding mechanisms of axonal transport as well as recently being exploited for uncovering the CNS connectome [2-4]. Certain diseases are capable of taking advantage of neuronal circuitry by infecting connected brain structures (e.g. the basal ganglia in Parkinson’s disease), leading to multisystem spread, atrophy of associated cellular networks, and loss of tissue integrity and function [2]. There is mounting evidence that extracellular vesicles (EVs) are involved in intercellular communication in many systems throughout the body, including the CNS. EVs are released by many cell types and have been found to carry cargoes including DNA, mRNA, miRNA, lipids, and/or proteins (Figure 1) [3, 5, 6]. EVs from brain-derived cells have been shown to carry molecules associated with neurodegenerative diseases [7-10]. Cancer cells have been shown to release EVs carrying oncoproteins and RNAs that support their viability [5, 11-13]. We propose that neurodegenerative diseases and cancer share a common route of transport of disease-related proteins and nucleic acids that could mirror normal neurocytological transport mechanisms. In this way, we hypothesize that affected cells can take up and release a myriad of molecular and biological agents that have the potential to affect, as well as potentially infect, large numbers of other cells in distant circuitries and spread degenerative as well as neoplastic disease throughout the brain or the body.
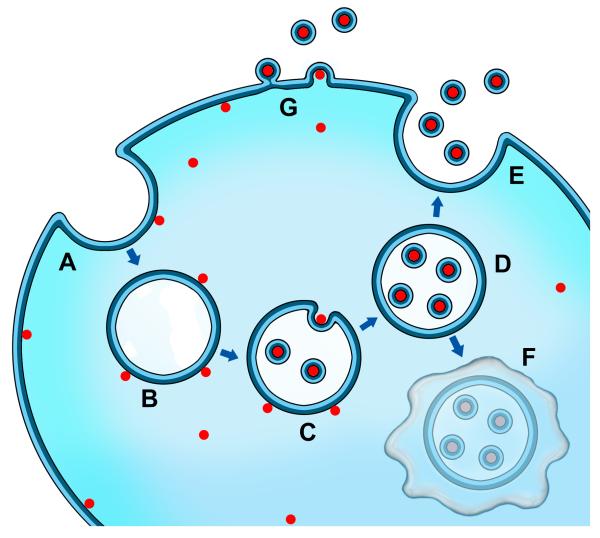
Figure 1.
Extracellular vesicle (EV) biogenesis and transfer. Depiction of cargo loading into EVs such as nucleic acids and intracellular proteins where endocytosis of the plasma membrane (A) results in the uptake of proteins, nucleic acids, and membrane-associated molecules (red dots), and formation of the early endosome (B). Upon transformation of the early endosome into the late endosome (C), exosomes are formed by inward budding of the late endosome/multivesicular body (MVB) with the content, including mRNA, miRNA, DNA, and protein, in a similar orientation as at the plasma membrane (D). Fusion of the MVB with the plasma membrane allows for the release of exosomes into the extracellular space (E). Alternatively, the MVB may fuse with the lysosome for degradation (F). Other EVs, such as ectosomes, can be formed directly at the plasma membrane (G). Released EVs, along with their content, may be taken up by an adjacent or distant cell.
Extracellular vesicles
Ranging from 30-1000 nm in size, EVs include distinct subtypes such as exosomes, ectosomes, microvesicles, oncosomes, and shedding bodies [3, 14]. Exosomes arise from inward budding of the late endosome, and are thus bound by a lipid bilayer similar in composition to the cell membrane from which the vesicle derived (Figure 1). Held within the cell by multivesicular bodies (MVBs), exosomes face degradation if the MVB fuses with a lysosome [15]. Alternatively, exosomes can be released at the plasma membrane when association of an MVB at the cell membrane occurs at lipid rafts. Other types of EVs, such as ectosomes, can be shed directly from the plasma membrane (Figure 1G) [16]. Endocytosis of an EV by another cell is thought to occur through clathrin- and receptor-mediated processes [3]. Exosomes are derived from the late endosome, and thus can be identified using markers for components of the late endosome, including for example CD63, ALG-2-interacting protein (Alix), and Tumor susceptibility gene 101 (Tsg101), in combination with their size (30-100 nm), morphology (saucer shape upon fixation for transmission electron microscopy), and density (1.15-1.19 g/mL in sucrose) [3]. Apart from these identification methods, exosomes are difficult to distinguish from other subtypes experimentally, and much of the literature regarding this topic utilizes different terms and definitions to describe this population of vesicles. We will therefore refer to the whole population of secreted and released vesicles as extracellular vesicles (EVs) throughout this article.
By enclosing their cargo within a lipid bilayer, EVs provide a way for cells to communicate long distances as the cargo is protected from extracellular degradative proteases and RNases. The generation and release of EVs can be an extremely rapid cellular process that reflects dynamic cellular state and specific aspects of disease [17]. EVs have been found in many biological fluids including plasma, cerebral spinal fluid (CSF), and urine [3], making them ideal candidates for biomarker studies.
While EVs have been studied for many years, especially in the field of immunology, there has been a recent surge of attention devoted to EVs in other fields [16]. Recent reports show that EVs are secreted from cells in the brain [18-22], and in addition to a functional role in the CNS under normal conditions, may contribute to pathogenesis and disease progression. Given the recent attention to brain-derived EVs, it is important to investigate their normal function and how they might contribute to disease progression.
Normal EV communication in the brain
EVs are produced by several cell types in the brain, including neurons, oligodendrocytes, astrocytes, and microglia [18-22]. Neuronal interactions with glial cells are mediated by EVs through the transfer of proteins, mRNAs, and miRNAs, where vesicle release into the extracellular space is taken up by recipient cells [18, 23-25]. Locally, these cargoes have so far been shown to regulate neurite growth and synapse formation [20, 23, 26]. In addition, because EVs shed from primary neurons contain the adaptor protein Ndfip1 (neural precursor cell expressed developmentally down-regulated protein 4 family-interacting protein 1), they are hypothesized to play a role in protein removal and trafficking [27].
Normal cells release EVs constitutively, but increase their release in response to stress, such as hypoxia, DNA damage, exposure to a bacterium or virus, and cellular senescence [3, 28]. One putative functional role for this stress response is to alert the immune system via activation of heat shock proteins (Hsp), as in the case of exosome-mediated Hsp70 release from astrocytes [29]. Additionally, ATP release stimulates EV shedding from microglia via P2X7 receptor activation, which is important for cytokine circulation and antigen presentation [30]. Therefore, EVs appear to play a key role in neuronal communication under non-pathological conditions including development, homeostasis, and immune surveillance/pathogen response.
Role of EVs in neurodegenerative diseases
When a state of stress progresses into pathology, cells have been shown to release EVs that carry proteins, mRNAs, and miRNAs that play a direct role in disease progression. As EV cargoes are protected from extracellular degradative proteases and RNases, EVs have the capacity to transfer disease-associated nucleic acids and proteins from affected to naïve cells. This could be potentially precarious for cells that are already at risk of degeneration from pre-existing genetic mutations, environmental toxins, and mutagens that alter cellular machinery and function. EVs investigated in cell culture models of neurodegeneration have been shown to carry proteins prone to aggregation, a hallmark of many neurodegenerative disorders [31]. Transfer of protein from a diseased cell to a healthy cell may lead to accumulation and aggregation of the protein in the target cell, where accumulation and aggregation of these proteins is linked to pathogenesis of neurodegenerative diseases. In the case of RNA transfer, alteration of gene expression and protein translation may occur in target cells which may alter their viability in addition to cellular function [3].
The origination of EVs from multivesicular bodies suggests that they are involved in the clearance of toxins or misfolded proteins by the lysosomal storage pathway. Lysosomal dysfunction has been implicated in several neurodegenerative diseases including amyotrophic lateral sclerosis (ALS) as well as Alzheimer’s, Parkinson’s, and Huntington’s diseases [32]. These neurodegenerative diseases are all known as ’proteinopathies’, and are characterized by the accumulation of aggregated proteins such as β-amyloid (Aβ) and tau in Alzheimer’s disease (AD), α-synuclein in Parkinson’s disease (PD), TDP-43 in ALS, and huntingtin and polyglutamine in Huntington’s disease (HD). Interestingly, several proteins prone to aggregation have also been shown to be secreted in EVs, including Aβ [9], tau [10], α-synuclein [7], TDP-43 [8], and prion protein [33]. In the presence of neuron-derived EVs, the addition of soluble forms of Aβ resulted in targeting Aβ to the lysosome and prevented oligomeric formation of Aβ as well as neuronal death [34]. In PD, α-synuclein protein is the major component of cellular protein aggregates called Lewy bodies and Lewy neurites. Dysfunction of the lysosome in cells that over-express α-synuclein results in increased EV-mediated α-synuclein release [35]. Cell-to-cell transmission of α-synuclein oligomers via EVs has been demonstrated, where it has been hypothesized that this represents an attempt by cells to clear toxic protein components when autophagy is insufficient to deal with the toxic protein accumulation [36]. Thus, lysosomal dysfunction may overwhelm the ability of the cell to clear aggregated protein, and lead the cell to release aggregated protein packaged in EVs. Consequently, other cells take up the shed EVs as well as the toxic proteins within them.
There have been several reports implicating viruses in the onset and transmission of neurodegenerative diseases. As far as potential routes of entry for initial degenerative disease, infections that might begin as respiratory or gastrointestinal could eventually reach the brainstem, and subsequently the forebrain, in advanced disease via cranial nerve connections, and contribute to neurodegeneration (Figure 2) [37]. For example, intranasal infection of the H5N1 influenza virus in wild-type mice has been shown to transmit to the CNS, whereupon aggregation and phosphorylation of α-synuclein has been observed, along with dopaminergic neuronal loss and the expression of PD-like symptoms [38]. Viruses are also capable of using EVs to enhance their transmissibility, for example where virulence factors are concentrated in vesicles to increase the potency of their delivery over simple diffusion [39]. Viral infection has also been shown to result in increased EV release and altered molecular cargoes [40]. In this way, viruses utilize a transcellular communication network that might take advantage of specific synaptic associations, as was recently shown for caudorostral propagation of synucleinopathy in an animal model of PD following intravagal nerve injections of viral vectors [41]. In addition, neurotropic viruses have been shown to transcellularly transport to distant CNS sites following nasal administration in rodent models [42]. As already alluded to, the presence of disease has the potential to stimulate EV release as a response to stress [39], where disease-associated macromolecules could be spread to the CNS as well as other somatic systems that then become susceptible to the same disease processes. Brainstem raphe nuclei interconnections involving the basal ganglia and the vagal nerve nuclear complex, as well as other brainstem and forebrain sites, may act as a conduit for EV-mediated spread of disease (see Figure 2) [43]. Some high resolution images of neuronally transported lectins [44], for example, show cellular vesicle release and uptake similar to that known to involve EVs for viral trafficking and spread of infection [15, 39]. Thus there appears to be shared cellular and molecular mechanisms between viruses and neural cells, with regard to the packaging and expulsion of toxic proteins in EVs. This is an unexplored area of research that could unveil the simultaneous reduction of the viral or proteinopathic burden, as well as expose a transcellular network of naïve cells to infection and proteinopathy (Box 2). Viruses in the brain can induce neurodegenerative disease, and also increase the secretion of EVs thereby spreading both viral and neurotoxic proteins more efficiently throughout the brain.
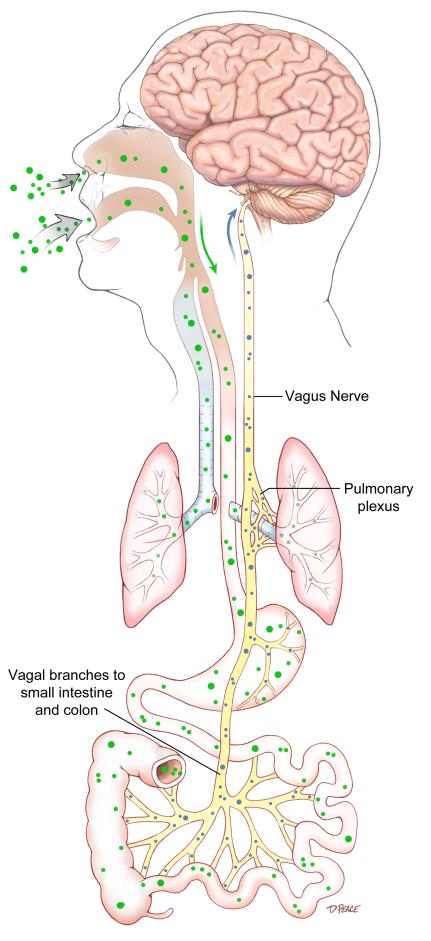
Figure 2.
Hypothesis of disease origin and spread by extracellular vesicles (EVs). Disease may ensue and progress by inhalation or ingestion of a virus, which could be transported to the lungs or intestinal tract (green dots). Release of EVs (small blue dots) by a virus could be then transported via the vagus nerve to brainstem nuclei, including the vagal nerve complex and then the midline raphe nuclei which connect to and interrelate synaptically-related structures of the brain (e.g. raphe and vagal nerve complex, substantia nigra, and the caudate-putamen).
Box 2. Outstanding Questions.
Are virus-derived EVs involved in the spread of neurodegenerative disease and cancer pathology?
Is progenitor cell fate, and overall neurogenesis in the adult brain, affected by EVs shed from diseased cells?
EVs and cancer progression
EVs shed from cancer cells also carry proteins, mRNAs, miRNAs, and DNA. These protein and RNA species appear to be preferentially packaged into EVs, where they are enriched in the EV as compared to the parent cancer cell [5, 11]. Cancer-derived EVs can carry proteins such as oncoproteins (e.g. epidermal growth factor receptor varient III (EGFRvIII), and the receptor tyrosine kinase MET) [11-13], ephrins and their receptors, and chemokine receptors [14]. In addition, EVs from cancer cells carry mRNAs, miRNAs, and other small non-coding RNAs [5, 6]. Glioma-derived EVs containing coding and noncoding RNAs, when cocultured with endothelial cells, induced changes in gene expression in the endothelial cells [6]. It is well known that viruses have also been linked with the onset of certain types of cancer, including human papillomavirus (HPV), human immunodeficiency virus (HIV), Epstein–Barr virus, human T-cell lymphothropic (T-cell leukaemia/lymphoma) virus (HTLV)-1, hepatitis’ B and C, and cytomegalovirus (CMV) [45, 46]. As discussed for the spread of neurodegenerative disease, EVs released from viruses known to be linked to certain cancers may also spread the pathology (Figure 2; Box 2). Thus, through EVs, both cancer cells and degenerating neurons have the ability to send pathogenic molecules from one cell to another, and the diseases associated with them could be initiated in a similar fashion.
Just as neural-derived EVs can have local effects on nearby cells, EVs shed from cancer cells may induce tumor-promoting effects in nearby cells. EVs secreted from tumor cells can have a self-promoting effect, for example EVs derived from glioblastoma and multiple myeloma have been shown to stimulate the proliferation of cultured cells from which they were derived [11, 47]. Additionally, there is increasing evidence that primary tumors release EVs that suppress immune cell activation as well as prepare other tissue and organ sites for metastasis. EVs derived from ovarian cancer patients decreased the expression of CD-3ζ and Janus kinase 3 (JAK 3), which normally play a role in immune cell activation, in cultured human T lymphocyte Jurket cells, and induced apoptosis [48]. In another study, mammary tumor derived EVs inhibited natural killer (NK) cell cytotoxic activity [49]. EVs from glioblastoma and melanoma cells have been shown to induce angiogenesis through modulation of endothelial cells [50-52]. In addition, melanoma-derived EVs condition sentinel lymph nodes for metastasis, as well as educate bone marrow progenitors toward a pro-vasculogenic phenotype via receptor tyrosine kinases [12, 53]. Enhancement of the tumor microenvironment by EVs has also been observed from both the primary cancer site as well as from bone marrow for breast, ovarian, and lung cancers [54-56].
Whereas cancer-derived EVs are able to alter the bone marrow progenitor cell phenotype, as well as the phenotypes of other types of cells in healthy tissue, it remains to be determined if EVs from diseased neuronal cells affect normal neural stem/progenitor cells in the adult brain (Box 2). A compromised stem/progenitor cell may not be able to repair or replace lost neural circuitry components, which would lead to a stem cell pathology [57]. One of the major defining attributes of an adult stem/progenitor cell, in addition to its residence in adult poietic niches for maintenance of tissues homeostasis during normal adult neurogenesis (Box 1), is its ability to repair and replace at-risk and lost cells following tissue injury or disease. Examples have been reported of human neural stem/progenitor cells with altered growth processes, which are indicative of regeneration failure in neurodegenerative diseases such as PD [58]. Further, adult stem cells can generate too much tissue and contribute to an abnormal growth milieu in CNS neoplasias including glioblastoma [59]. It remains to be explored how EVs contribute to the pathology of neural stem/progenitor cells observed in these diseases (Box 2).
Box 1. Neurogenesis in the adult human brain.
Neurogenesis occurs in the human brain throughout adulthood. The well-studied regions known to harbor neural stem and progenitor cells are the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus. However, other regions within the brain have been shown to regenerate new mature cells. Neural stem and progenitor cells have been shown to give rise to neurons, astrocytes, and oligodendrocytes. Adult neurogenesis can be triggered by injury or disease in an effort to replace, repair, or salvage affected brain tissue. However, with disease, neurogenic attempts often fail to fully recover normal brain function damaged by pathogenesis. In Huntington’s disease (HD), there is increased proliferation of stem/progenitor cells in the SVZ yet the increase is insufficient to compensate for the cells that have degenerated. In Parkinson’s disease (PD), satellite cells within the substantia nigra maintain the potential for neurogenesis, yet there appear to be too few without the right signals to stimulate proliferation or differentiation. In the case of glioma, an over-abundance of stem/progenitor cells may potentiate the cancer itself rather than protect the normal tissue of the brain. Induction of neurogenesis in Alzheimer’s disease (AD) patients appears to have a beneficial impact on disease progression. While mechanisms for brain repair appear to be intrinsic, they fail to fully recover the diseased brain from degeneration [57].
EVs may thus provide a common mechanism for disease propagation between neurodegenerative diseases and cancers. There are many contributors to disease initiation and propagation, including genetic susceptibility, environmental exposure, aging, mutagens, and/or epigenetic factors. We propose that EVs could also play a role in the initiation and/or spread of disease, making these seemingly distinct diseases similar in nature to one another. In this way, we suggest that cancers and neurodegenerative diseases could be similar in onset and propagation, via our growing understanding of EVs and their role in initiating or enhancing disease spread. We believe that parallels can be drawn in consideration to the treatment of neurodegenerative diseases and cancer, as well as the identification of biomarkers for each disease.
Therapeutic implications for EVs
Discovery of disease-specific biomarkers housed on and within EVs is essential to better facilitate real-time monitoring of patient disease risk, course, and response to therapy. Dilution and mixing of EVs derived from other tissues and organs currently complicates such attempts, thus it is also essential to identify markers on the disease-derived EVs themselves. Identification of EVs may include the discovery of a molecular signature, as in the finding of a conserved membrane glycan signature observed for a variety of EVs [60]. Comparisons of EVs to their cell of origin have shown that diseased cells preferentially package particular molecules in EVs. Studies performed using tumor cells show some mRNAs and miRNAs are found at higher levels in EVs than in their cells of origin, suggesting that not only does their release act to modify the tumor microenvironment, but also may be a distinct molecular signature of the tumor itself [5, 6, 11]. Experimental studies have confirmed that glioblastoma and control patients can be distinguished by the RNA profiles in their circulating EVs [5, 61], and that a specific “zipcode” sequence in the 3′ untranslated region (UTR) of mRNAs is enriched in EVs derived from glioblastomas [62]. As discussed above, detection of aggregated protein or oncoproteins localized to EVs could certainly signal disease presence. These disease- and patient-specific addresses may exist for a variety of disease states beyond the cancers and neurodegenerative diseases.
In addition to EV detection, methods of isolation and storage need to be optimized before EVs can be utilized as biomarkers. Due to the variability in isolation techniques, methods need to be standardized for the isolation of EVs. Current methods are quite laborious, therefore there is a need to develop quicker methods for use in a clinical laboratory setting. It will also be important to identify reference points or controls which to measure against when utilizing EVs as biomarkers. Nevertheless, EVs isolated from newly collected patient plasma are stable under various storage conditions for up to 90 days and are biologically active [63]. Urine that was frozen at −80°C for 1 week had 86-100% recovery of EVs compared to controls, indicating that EVs are also stable in frozen biological fluids for up to one week [64]. The stability of EVs in biological fluids suggests that they are a good tool for use as biomarkers.
Patient-specific EVs could be used themselves as therapeutics in new regenerative medicine protocols for both neurodegenerative diseases and cancer. EVs from activated stem cells of a variety of tissue sources inhibit apoptosis of cells, and have proregenerative effects [65]. EVs derived from bone marrow mesenchymal stem cell niches have been reported to inhibit growth and survival of tumor cells [66], and EVs from normal bone marrow mesenchymal stromal cells inhibit multiple myeloma cell growth [47]. Taken together, these studies suggest that EVs released from normal cells contain within them protective agents that either save or restore at-risk tissue in both neurodegeneration and cancer. Future studies utilizing transplantation of stem cell-derived EVs may circumvent some of the problems associated with stem cell transplantation while maintaining the beneficial therapeutic effects offered by these cells.
A better understanding of pharmacological manipulation of EV generation and release is necessary to develop new therapeutics to moderate or deter their pathogenetic roles in disease course. For example, sphingomyelinase is required for the biosynthesis of ceramide, a lipid that triggers budding of exosomes into MVBs. Inhibition of neutral sphingomyelinases reduces the release of exosomes and ectosomes [67, 68], as well as the secretion of miRNAs [69]. In addition, sphingomyelinase overexpression results in increased extracellular miRNA content, contained within EVs, with concomitant gene silencing in the recipient cells [69]. These studies emphasize the value of investigating pharmacological approaches to inhibit EV release for treatment of disorders in which EVs could be involved in disease transmission.
Interestingly, EVs themselves also can be used as disease-modifying agents by way of specifically engineering them to carry gene therapeutics. For example, EVs have been shown to associate with AAV vectors, whereby transduction efficiency and gene delivery are improved [70]. EVs may be engineered to transfer proteins between cells [71], as well as serve to deliver suicide mRNA and proteins that contribute to tumor regression [72]. In one study, EVs electroporated with exogenous siRNA to BACE1, a therapeutic target in AD, were directed to the brain using the neuron-specific RVG peptide, with subsequent successful knockdown of BACE1 mRNA and protein levels [73]. Therapeutic treatments might also take advantage of EV size as a means to deliver a drug or other therapeutic across the blood-brain barrier. It has been shown in mouse models that EVs containing a Stat3 inhibitor delivered intranasally were able to reach the brain, and protected against lipopolysaccharide (LPS)- and experimental autoimmune encephalitis (EAE)-mediated brain inflammation, as well as tumor growth [74]. Future therapeutic treatments could be aimed at harnessing the innate delivery and protective attributes of EVs. Understanding the precise environmental, cellular, and molecular interactions that contribute to dynamic changes in cellular phenotype [75] could be quite insightful for the design of novel therapies based on the newly appreciated potential of EVs to uniquely modify disease transmission, progression, and potential remission.
Concluding remarks
Understanding the biology of EVs seems crucial toward a better understanding of the nature and course of a variety of diseases with so many unmet critical needs. It is becoming apparent that many diseases, such as cancer and neurodegenerative diseases, could owe their most aggressive courses to a nanosized extracellular vesicle that is only now beginning to become appreciated as a transmitter of both normal and pathologic cellular information. A coming together of diverse investigative fields including immunology, neurology, oncology, and infectious disease could soon help the translation of the distinctive molecular and cellular biology of EVs to a better understanding of the initiation, spread, and management of many of our most challenging human diseases.
Highlights.
Exploration of the potential infectious nature of extracellular vesicles (EVs)
Review of basic EV nature and function
Examination of the ability of EVs to carry pathologic species
Comparison of similar roles for EVs in neurodegenerative disease and cancer
Acknowledgements
The authors would like to thank Joan Samuelson, the ABC2 Foundation, as well as Michael Freeman, Dolores DiVizio, Samantha Morley, Stephen Gould, Jim Olson, Johan Skog, Leonora Balaj, and Xandra Breakefield for their mentoring and sharing of insights into EV biology. Illustrations were expertly drawn by David Peace of the Department of Neurological Surgery, University of Florida College of Medicine. Support for the authors was provided by NIH/NINDS grant NS055165, The Michael J. Fox Foundation for Parkinson’s Research, and the McKinney, Maren, and Thompson Regenerative Medicine Funds.
Glossary
- AAV (Adeno-associated virus)
a small virus which infects human cells used as a viral vector to drive gene expression in infected cells in the laboratory and for gene therapy.
- Autophagy
a process by which a cell digests parts of its own contents for turnover or removal via degradation by the lysosome. The role of autophagy is to support cellular maintenance and protect cells from undergoing apoptosis by removing damaged or excess organelles.
- Blood-brain barrier (BBB)
the BBB separates circulating blood from the central nervous system, and is composed of endothelial cells, astrocytic end feet, and pericytes. The BBB restricts large molecules from entering the CNS, making it difficult to design drugs that are small enough to cross the BBB and have their actions on the brain.
- Connectome
is a comprehensive map of the brain which diagrams cellular connections. The connectome is used to understand how cells and structures are organized and related to each other and contribute to diverse nervous system functions and behaviors.
- Glial cells
Glial cells are unique to the nervous system, and are distinct from neurons. Microglia are found within the central nervous system and are involved in the immune response. Macroglia include oligodendrocytes and astrocytes in the CNS, and Schwann cells in the peripheral nervous system. Astrocytes are known to play a role in growth factor and ion exchange, whereas oligodendrocytes and Schwann cells are responsible for myelination of nerve axons.
- Lysosome
an organelle responsible for the degradation of macromolecules using a low pH.
- MicroRNA (miRNA)
non-coding RNAs involved in post-transcriptional regulation of gene expression.
- Multivesicular body/endosome
Endosomes are membrane-bound compartments within the cell that are involved in the sorting and recycling of molecules. The early endosome can mature into a late endosome (also known as a multivesicular body; MVB) in which molecules are sorted into vesicles. Contents within the MVB will either be degraded or recycled back to the plasma membrane.
- Neurodegenerative disease
a disease that causes cell death within the nervous system.
- Neoplasia
abnormal cellular growth or division.
- Prion disorder
prion disorders occur with the conversion of normal protein into a disease-causing isoform which mimics viral and bacterial pathogens. Accumulation of mutant prion protein may cause neurodegeneration and can be lethal.
- Proteinopathy
a cellular pathology caused by a misregulated protein.
- Virulence factor
molecule expressed and secreted by a pathogen that facilitates the ability of the pathogen to spread disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fuxe K, et al. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, et al. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pant S, et al. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steindler DA, Bradley RH. N-[acetyl-3H] wheat germ agglutinin: anatomical and biochemical studies of a sensitive bidirectionally transported axonal tracer. Neuroscience. 1983;10:219–241. doi: 10.1016/0306-4522(83)90095-7. [DOI] [PubMed] [Google Scholar]
- 5.Manterola L, et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro Oncol. 2014;16:520–527. doi: 10.1093/neuonc/not218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CC, et al. Glioma microvesicles carry selectively packaged coding and noncoding RNAs which alter gene expression in recipient cells. RNA Biol. 2013;10:1333–1344. doi: 10.4161/rna.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emmanouilidou E, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nonaka T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Rajendran L, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 14.Breakefield XO, et al. Gesicles: Microvesicle “cookies” for transient information transfer between cells. Mol Ther. 2011;19:1574–1576. doi: 10.1038/mt.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould SJ, et al. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi DS, et al. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev. 2014 doi: 10.1002/mas.21420. In press. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 17.Koumangoye RB, et al. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS One. 2011;6:e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fruhbeis C, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guescini M, et al. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 20.Lachenal G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Faure J, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Potolicchio I, et al. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175:2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31:7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morel L, et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288:7105–7116. doi: 10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruhbeis C, et al. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182. doi: 10.3389/fncel.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smalheiser NR. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct. 2007;2:35. doi: 10.1186/1745-6150-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putz U, et al. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J Biol Chem. 2008;283:32621–32627. doi: 10.1074/jbc.M804120200. [DOI] [PubMed] [Google Scholar]
- 28.Xu D, Tahara H. The role of exosomes and microRNAs in senescence and aging. Adv Drug Deliv Rev. 2013;65:368–375. doi: 10.1016/j.addr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Taylor AR, et al. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol. 2007;67:1815–1829. doi: 10.1002/dneu.20559. [DOI] [PubMed] [Google Scholar]
- 30.Bianco F, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 31.Funk KE, Kuret J. Lysosomal fusion dysfunction as a unifying hypothesis for Alzheimer’s disease pathology. Int J Alzheimers Dis. 2012;2012:752894. doi: 10.1155/2012/752894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, et al. The lysosome and neurodegenerative diseases. Acta Biochim Biophys Sin (Shanghai) 2009;41:437–445. doi: 10.1093/abbs/gmp031. [DOI] [PubMed] [Google Scholar]
- 33.Fevrier B, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuyama K, et al. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Erviti L, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danzer KM, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braak H, et al. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Jang H, et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A. 2009;106:14063–14068. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 2011;13:1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 40.Meckes DG, Jr., Raab-Traub N. Microvesicles and viral infection. J Virol. 2011;85:12844–12854. doi: 10.1128/JVI.05853-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulusoy A, et al. Caudo-rostral brain spreading of alpha-synuclein through vagal connections. EMBO Mol Med. 2013;5:1119–1127. doi: 10.1002/emmm.201302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Pol AN, et al. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J Virol. 2002;76:1309–1327. doi: 10.1128/JVI.76.3.1309-1327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai H, et al. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- 44.Steindler DA, Cooper NG. Wheat germ agglutinin binding sites in the adult mouse cerebellum: light and electron microscopic studies. J Comp Neurol. 1986;249:170–185. doi: 10.1002/cne.902490205. [DOI] [PubMed] [Google Scholar]
- 45.Cobbs CS. Evolving evidence implicates cytomegalovirus as a promoter of malignant glioma pathogenesis. Herpesviridae. 2011;2:10. doi: 10.1186/2042-4280-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Flora S, Bonanni P. The prevention of infection-associated cancers. Carcinogenesis. 2011;32:787–795. doi: 10.1093/carcin/bgr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roccaro AM, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 50.Sheldon H, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 51.Kucharzewska P, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hood JL, et al. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hood JL, et al. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 54.Cho JA, et al. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 55.Cho JA, et al. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol. 2011;123:379–386. doi: 10.1016/j.ygyno.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Del Tatto M, et al. Marrow cell genetic phenotype change induced by human lung cancer cells. Exp Hematol. 2011;39:1072–1080. doi: 10.1016/j.exphem.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steindler DA, et al. Stem cell pathologies and neurological disease. Mod Pathol. 2012;25:157–162. doi: 10.1038/modpathol.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, et al. Neurogenic potential of progenitor cells isolated from postmortem human Parkinsonian brains. Brain Res. 2012;1464:61–72. doi: 10.1016/j.brainres.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ignatova TN, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 60.Batista BS, et al. Identification of a conserved glycan signature for microvesicles. J Proteome Res. 2011;10:4624–4633. doi: 10.1021/pr200434y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noerholm M, et al. RNA expression patterns in serum microvesicles from patients with glioblastoma multiforme and controls. BMC Cancer. 2012;12:22. doi: 10.1186/1471-2407-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolukbasi MF, et al. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalra H, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354–3364. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 64.Zhou H, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69:1471–1476. doi: 10.1038/sj.ki.5000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ratajczak MZ, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 66.Bruno S, et al. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013;22:758–771. doi: 10.1089/scd.2012.0304. [DOI] [PubMed] [Google Scholar]
- 67.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 68.Bianco F, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kosaka N, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maguire CA, et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther. 2012;20:960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mangeot PE, et al. Protein transfer into human cells by VSV-G-induced nanovesicles. Mol Ther. 2011;19:1656–1666. doi: 10.1038/mt.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mizrak A, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 2013;21:101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alvarez-Erviti L, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 74.Zhuang X, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steindler DA. Redefining cellular phenotypy based on embryonic, adult, and cancer stem cell biology. Brain Pathol. 2006;16:169–180. doi: 10.1111/j.1750-3639.2006.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]