Abstract
Advanced lung diseases such as pulmonary arterial hypertension (PAH) and interstitial lung diseases (ILD) are chronic diseases that cause significantly high morbidity and mortality. As a result, patients can undergo some psychological changes leading to a poor quality of life and depression. Diagnosis of depression is often obscured because fatigue and apathy, two common symptoms of depression, frequently overlap with PAH and ILD. Healthcare providers are sometimes reluctant to ask or mistakenly believe that these symptoms are part of the ongoing disease process, rather than a serious condition like depression. Screening tools are available for physicians to be well positioned in recognizing clinical depression in PAH and ILD. A MedLine/PubMED search was performed identifying all relevant articles with “PAH”, “ILD”, “screening tools” and/or “Depression” in the title. The aim of this review is to provide a brief description of some of the instruments used to screen patients and classes of psychotropic medications accessible to physicians. While pulmonary rehabilitation programs can have a positive impact on patients, physicians should also utilize cognitive behavioral therapy (CBT) as part of regular care.
Keywords: Depression, Interstitial lung diseases, Medications, Pulmonary arterial hypertension, Screening tools
Introduction
Chronic diseases are lifelong disorders that can affect a person's ability to function.[1] They are slow progressive diseases that can incite changes in afflicted patients and their families. The reality of chronic disease brings with it challenges to cope and adjust to unforeseen lifestyle changes. In fact, expectations of a cure can make it difficult for patients to cope with their chronic condition. Nevertheless many patients are successful in making adjustments imposed upon them by their illness, there are instances where patients are unable to completely adjust and depression can certainly set in.
Pulmonary arterial hypertension (PAH) and interstitial lung diseases (ILD) are two diverse groups of lung conditions that cause significant morbidity and mortality. Both PAH and ILD are rare and debilitating in nature leading to a stepwise decline in physical health as well as decline in psychological health. The chronic nature of these conditions provides a major challenge to both patients and their treating physicians, because of a lack of affective treatment strategy. Hence, a concrete emphasis should be made by physicians to address the psychological impact as part of their comprehensive treatment plan. Evidence-based research has shown that depression is common in lung conditions such as chronic obstructive pulmonary disease (COPD), asthma and cystic fibrosis [Table 1].[2,3] In this review we will focus on the impact of depression in patients with PAH and ILD.
Table 1.
Pulmonary conditions associated with depression

Depression is a condition defined by persistent, pervasive low mood, anergia and loss of interest in activity.[4] It is an imbalance between normal emotions that everyone goes through at any juncture. There are occasions when these “normal blues” take control of a person. If not corrected, these “normal blues” have a substantial impact on individuals by affecting their work, family and social relationships and quality of life. Diagnosis of depression begins with a clinical assessment of parameters within time frame of 2 weeks.[4] It requires five or more parameters to meet a clinical definition: Insomnia, excessive guilt, worthless, lack of energy, anhedonia (lack of interest), poor concentration, suicide ideations and weight changes.[4] As a matter of fact, some of these depressive symptoms can overlap with many pulmonary conditions [Figure 1].
Figure 1.
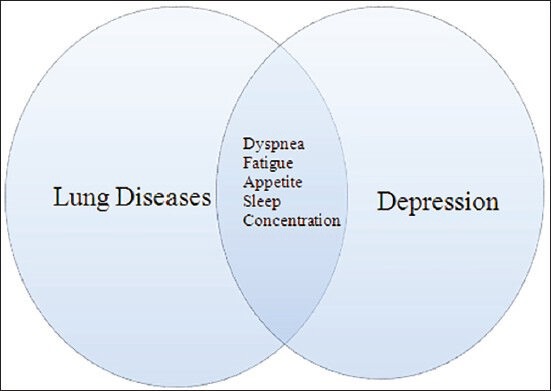
Overlap of depressive symptoms in lung diseases
Pathophysiology of depression
Depression is multi-factorial disorder with an unpredictable course. Numerous studies in the past have shown variable levels of brain activity in depression and therefore leading to a search for “biochemical explanation” for this pathophysiological process. There is no doubt that psychological theories provide a rationale for depression, but the thought of having a neurohormonal or chemical imbalance in the brain cannot be ignored. The monoamine deficiency hypothesis (MDH) and the hypothalamic-pituitary axis (HPA) dysregulation have been speculated to clarify the underlying biological basis of depression.[5] MDH explores the association between depleted levels of neurotransmitters (NTRs) serotonin (5-HT), norepinephrine (NE) and dopamine (DA) in the central nervous system (CNS) with subsequent depression.[5] The imbalance is partly corrected by antidepressant medications, thus forming a platform for this theory. The development of neuroimaging techniques such as positron emission tomography (PET) scans along with molecular evidence opens an exciting new opportunity to offer support for these three NTRs theories.[6]
Serotonin (5-HT) is a potent modulator of feeling and behavior such as anxiety, irritability, sleep, appetite and sexuality. NE is responsible for attention, drive and motivation. Evidence of these compounds came to light with fortuitous discovery of using Reserpine (previously used hypertensive medication) that caused depletion of NE, 5-HT and DA stores resulting in depressive symptomatology.[7] The use of Iproniazaid as an anti-TB drug, on the other hand, showed an opposite effect of increasing NE, 5-HT and DA levels resulting in euphoria.[7]
The HPA involving abnormal levels of cortisol in response to stress has been theorized as a pathway leading to depression.[5] In general, cortisol-releasing hormone (CRH) increases adrenal corticotropic hormone (ACTH) that further increases cortisol. The full function of HPA can be examined by dexamethasone suppression test (DST). In this test, a small dose of synthetic glucocorticoid (dexamethasone) is given at night, cortisol is measured in morning and any inhibitory response is considered as normal response. Depressed patients tend to have an overactive HPA with high level of cortisol and commonly referred to as dexamethasone non-suppression.[6]
Others have proposed a more sensitive test referred to as DEX-CRF test that combines dexamethasone (DEXA) with cortisol-releasing factor (CRF).[6] Patients are treated with oral DEXA at night and CRF the next day. Depressed patients show increased levels of ACTH and cortisol, compared to normal patients indicating hypersecretion of CRF. There is mounting evidence that CRH produced in limbic region is a pathological player in depression. Evidence of decreased serotonergic activity in cerebrospinal fluid and an decreased density of serotonin receptors have been found in post partum depressed patients.[6] CRH is responsible for behavioral changes that can be disruptive such as decrease sexual behavior, decreased sleep and eating. Some of these disruptive factors are also present in depression.[6] The HPA dysfunction is also reflected as altered responsiveness to thyrotropin-releasing hormone (TRH) infusion test. Even HPA modulation has shown some beneficial effect in depression.
It is also believed that high levels of pro-inflammatory cytokines can also precipitate depression.[1] Like any chronic illness, depression tends to increase the level of cytokines such IL-1, TNF α, INF γ etc.[1] This increase in cytokines modulates the levels of neurotransmitters in the brain and activate HPA axis causing depressive symptoms.[1,8] This so-called sickness behavior, is a result of increased inflammatory response and the adaptive changes individuals go through when ill. In response to illness, patients feel fatigued, have a loss appetite and suffer from impaired cognition, also seen in depression.[8]
The association between depression and medical illness is intricate and many factors can lead a patient to have some depressive symptoms. They include age, history of prior episode of depression, poor family support and severity of condition.[9] Depression can also be a result of medical illness such as thyroid dysfunction or as consequence of side effects of medications such as corticosteroids or beta blockers. These complexities point out to the fact that secondary causes must be ruled out via a meticulous history and physical examination, appropriate biochemical and pathological tests or neuroimaging.
Screening and depression
Depression in PAH and ILD share a unique notion with other chronic diseases, particularly with congestive heart failure (CHF). Studies in patients with CHF have highlighted the significance of under-recognition of depression that consequently results in under-utilization of antidepressants.[10] Jacob et al. found, retrospectively, that nearly 78% of patients had at least one admission to a non-psychiatric community hospital, with only 7.9% of patients on any type of antidepressants.[10] The authors concluded that depression was overlooked in CHF due to confounding signs and symptoms such as apathy and fatigue.[10] Similarly, depression in lung disease remains a challenge for physicians, to a certain extent because of some overlapping symptoms between depression and somatic complaints. Physicians allocate ample amount of time towards addressing medical complaints, however they neglect the psychological impact perhaps because of a lack of proper training in detecting underlying depression.
Over the years, several depression screening tools have been developed for physicians to assess patients for depression. Though these scales are not used in diagnosing depression, they provide comprehensive analysis of the symptomatic burden and are adjunct for diagnosis. Several variables go into selecting a rating scale for patient.[11] A rating scale is selected based on its validity, reliability and user-friendliness.[11] A screening tool used should also have high sensitivity (low false negatives) and high specificity (low false positives).[11] The scale selected by clinicians should be of practical value, providing information they need to evaluate their patients. The U.S. Preventive Services Task Force (USPSTF) has also issued recommendations supporting screening for depression in a primary care setting with adequate support staff to assure accurate diagnosis, treatment and follow-up.[12] A literature review by Williams et al. found 16 instruments that can be used to evaluate for depression in a primary care setting.[13] Hamilton Rating Scale for Depression (HAM-D/HRSD), Montgomery–Åsberg Depression Rating Scale (MADRS), Beck Depression Inventory (BDI), Center for Epidemiologic Studies Depression Scale (CES-D) and SDS-Zung Depression Scale are some of the most commonly used to estimate severity and response to treatment in patients with depression.[13,14] All questionnaires address questions about anhedonia (lack of interest) as it is a major DSM- IV criterion in diagnosis of major depression. Each tool has its own exclusive scoring scheme; higher scores can be a sign of severe depression suggesting further assessment by the treating physician [Table 2].[15]
Table 2.
Summary of scales used for screening depression
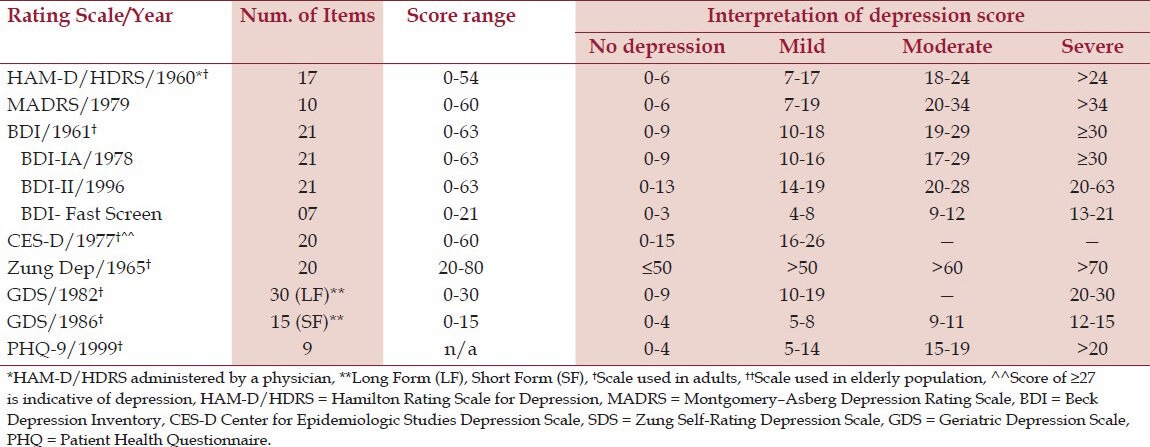
Depression screening tools
There are several scales available for screening patients for depression. A few of those scales are reviewed below.
Hamilton Rating Scale for Depression (HAM-D/HDRS)
The scale was one of the earliest scales to be developed in 1960 to measure severity of depression in inpatient population.[16] Initially the scale had 21 questions, with the last 4 questions (diurnal variation, de-realization, paranoid symptoms, and obsessive symptoms) not necessarily reflecting common features of depression or its severity.[16,17] Therefore, the 17-item scale is commonly used in clinical practice and clinical trials. HAM-D is a clinician-administered scale, thus requires special clinician training and assessment time is approximately 20-30 minutes. Eight items are scored on a 5-point scale (0-4) and 9 items are scored on 3-point scale (0-2). The scale emphasizes on comparatively large amount of somatic symptoms and less on cognitive symptoms.[13,16,18]
Montgomery–Åsberg Depression Rating Scale (MADRS)
The scale was developed in 1979 and is the most commonly scale used to measure the severity of depressive episodes in patients with mood disorders.[14] It is based on a clinical interview by the physician. A higher MADRS score indicates more severe depression, and each item yields a score of 0 to 6. There are 10 items with an overall score ranging from 0 to 60.[14] It is used in most of the clinical trials to see treatment response.
Beck Depression Inventory (BDI)
Initially developed in 1961, this scale is based on symptoms noted in depressed patients. It is a self-reported tool with 21 questions that reflects emotional, behavioral as well as somatic complaints in patients older than 13 years old.[19,20] BDI-IA was a modified version of the original BDI and that was subsequently replaced by BDI-II and BDI-Fast Screen (previously known as BDI- Primary Care).[15,17,20] The BDI-II is the modified version that evokes DSM-IV criteria of depression.[15,20] While the original BDI reflected symptoms in time frame of “today” consequent versions included time frame of the “past weeks, including today.”[15,20] The BDI-PC is a 7-item scale that addresses cognitive and affective items.[20] All four versions of this scale use a 4-point scaling system with a score range of 0-63.[20]
Center for Epidemiologic Studies Depression Scale (CES-D)
This scale was first published in 1977 and is designed to identify depression amongst the general population.[21] The scale is predominantly used for population surveys requiring a large sample size, measuring affective and somatic aspect of depression.[18] Somatic factors, affect (positive and negative) and interpersonal problems are analyzed with 20 items in a time frame of “within the past week.”[13,18,20] Each item achieves a score range of 0-3 and scores are tallied from 0 to 60, with higher scores (≥16) indicative of more severe depressive symptomatology. A revised version of this scale reflects DSM-VI criteria and referred as CESD-R. This scale is used rarely.
Zung Self-Rating Depression Scale (SDS)
This scale is a self-reporting 20-item instrument used to promptly assess severity of depression in patients, with half of the items stated negatively and the other half stated positively.[18,22] Each item score range is from 1 through 4. A total score of 50 and greater indicates some severity of depression. The test is quick, simple and easy to administer to patients. It is primarily used in clinical research to monitor treatment.[18]
Geriatric Depression Scale (GDS)
The GDS is a self-reported depression scale developed in 1982 specifically for the geriatric population (>60 years) with a time frame of “past week.”[15,23] It is used for screening depression in elderly population and therefore distinguishing dementia from depression in patients between 65 and 85 aged patients.[20] Although the items on scale address cognitive and affective domains, somatic domains are not included.[20] Originally developed as 30-item scale, the GDS has been modified (15 questions) and validated.[24] On both versions of the scale, GDS questions were answered in a “yes” or “no” format with one point assigned to items answered “yes” within a time frame of “week” and patient comprehension simplified.[15,20] A GDS ≥ 11 is considered a sign for possible depression.[20] The scale can be used in patients with mild dementia however, it cannot be used in severely impaired demented patients.[20,21] A 5-item GDS is also found to be equally effective as compared to 15-item GDS with a sensitivity of 0.94 and specificity of 0.81, respectively.[24]
Patient Health Questionnaire (PHQ)
The Patient Heath Questionnaire 9 PHQ (9) was developed to assist primary care physicians in evaluating patients for depression within the time frame of the “past 2 weeks”. It is self-administered scale validated by clinical trials.[25] It has nine questions akin to the nine criteria of depression according to DSM VI.[25] It has been used extensively in other medical conditions and serves a dual purpose of providing diagnosis as well as a gradient of depression.[26] A score above 10 is indicative of some degree of depression and higher score indicate more severe depression.[25] As an alternative to PHQ-9, which contains questions about “risk of suicide”, PHQ-8 can also be used as an instrument to measure depression. It is practicable to use PHQ-8 instead of PHQ-9 because studies have shown that difference in results using either scale is only minor and has negligible effects.[25,26,27]
Depression and pulmonary arterial hypertension
PAH is a relatively rare and debilitating phenomenon that encompasses both pulmonary and cardiac vasculatures simultaneously. Patients present with marked dyspnea on exertion that is identical to dyspnea from other conditions. Patients exhibit features of heart failure due to progressive remodeling of pulmonary artery leading to high pulmonary artery pressure, high pulmonary vascular resistance and right ventricular strain.[27,28] Over the past 20 years, new pharmacological modalities have been introduced such as prostanoids, phosphodiesterase type-5 inhibitors (PDE-5), endothelin receptor antagonists (ERAs) and most recently stimulators of soluble guanylate cyclase (sGC).[28,29] Aside from their side effect profile, these modalities are certainly trying to help patients achieve some stability with limited success, though they do not offer permanent cure. As an adjunct to medication, exercise and respiratory training have also been evaluated. Mereles et al. reported in a study of 30 patients, who completed 15 weeks of exercise and respiratory training, an improvement in 6 minute walk test (6 MWT) and quality of life scores.[30]
Patients with PAH often report of restricted ability in carrying out their activities of daily livings: such as shopping, conducting household chores, social isolation and loss of work.[31] This burden is undesirablely shared by patients’ caregivers.[31] A recently concluded survey found that nearly 57% of caregivers felt physically drained due to the additional responsibilities entrusted upon them.[31] The constant worry about paying for treatment creates a setting for these patients to develop depression.[27]
Lowè et al. found an association between declining functional class with increased prevalence of mental disorder, mainly depression in 15.6% of patients.[32] The study highlighted under utilization of psychiatric treatment regularly in patients with pulmonary hypertension.[32] Though 70% of patients received medical therapy for pulmonary hypertension, only 24% received any therapy for mental illness.[32] McCollister et al. used PHQ 8 questionnaire to analyze the psychological effects of depression and concluded that 15% had a score of >10, suggestive of symptoms of depressions, 40% had mild to moderate symptoms (PHQ-8 score 4-9), and 45% had no depression symptoms (PHQ-8 score 0-3).[27] They also reported an inverse relationship between functional class, depression and 6 MWT.[27] A higher functional class was related to lower 6 MWD and high depressive symptoms. The study concluded with only 25 % of patients were on any type of antidepressants, further denoting that depression was being under diagnosed and therefore untreated.[27]
White et al. evaluated 46 PAH patients and found that 26% had some symptoms of moderate to severe depression as well as 19.6% had moderate to severe anxiety.[33] This cohort used Beck depression inventory as their instrument of measuring depression with a mean BDI score of 13 ± 8.3.[33] Study further investigated effects on cognition and intellectual function in patient and found profound impairment in motor ability (57%), executive dysfunction (15%), and impaired attention in 13%.[33]
In treatment of depression, SSRIs remain the primary drug of choice. Ideally, selective serotonin reuptake inhibitors (SSRIs) would also be the primary choice of treatment of depression in patients with PAH as well. Sadly, this ideology has come under some scrutiny. There is conflicting data that suggests that SSRIs may not be as safe as previously presumed.[34] Sadoughi et al. observed high risk of fatality with SSRIs in patients with pulmonary hypertension, with worsened New York Heart Association (NYHA) functional classification class and shorter 6 MWT.[34] These findings, however, were inconsistent and more research needs to be done to evaluate the association between with SSRIs and PAH.[35]
Depression and interstitial lung diseases
Interstitial lung diseases are a diversified group of chronic debilitating conditions that result in progressive scarring and fibrosis of lung parenchyma.[36] It is a disease of middle aged to older patient population and its chronic nature leads to a high morbidity and mortality.[37] As a result of continuous fibrosis of parenchyma, the major physiological modification in ILD is reduced gas exchange.[37,38] Patients with ILD present with non-specific symptoms of dyspnea and dry cough. These symptoms can be overlooked for other common ailments such as smoking or old age.[39] Diagnosis begins with thorough history and physical examination along with diagnostic tests available.[37] ILD is classified into known and unknown causes.[37] Some of the known causes of ILD include inorganic substance exposure, radiation, medication side effects, hypersensitivity pneumonitis and connective tissue diseases.[37] A list of unknown causes included interstitial pulmonary fibrosis (IPF), sarcoidosis, cryptogenic organizing pneumonia and eosinophilic pneumonia amongst others.[37]
Interstitial Pulmonary Fibrosis, presents with poor prognosis and declining lung function.[36,38,39] There are no therapies that can cure or reverse the damage to the lungs. Current medical regimen of immunosuppressants, corticosteroids and bronchodilator therapy have not shown to improve survival.[36,38,39] Nevertheless, they are available in hopes of slowing down progression of ILD. As the disease progresses, patients are placed on long-term oxygen therapy. Lung transplant remains the ultimate option for patients who meet an established criterion.[36,38,39]
The progressive feature of this disease with marked dyspnea renders these patients to limited mobility and decline in physical activities. Patients with IPF, face an uphill losing battle in efforts to maintain their independence. Patients require additional planning and time to carry out their simple activities. The sudden and profound lifestyle changes and paucity of medical options have a negative effect on the quality of life which led to concurrent symptoms of depression. Data from research in COPD has provided evidence that depression can predict survival and treatment of depression can improve quality of life.[40,41,42,43,44] Similar data is available for patients with ILD. Ryerson et al. found that 23% of patients showed signs of clinical depression and it strongly co-related with dyspnea.[45] Depressed subjects had more severe dyspnea, poor sleep quality and pain, therefore, suggesting that treating these symptoms can improve depression.[36] Tomioka et al. used SF-36 questionnaire, to show that patients with IPF had worsening physical and psychological parameters than the general population.[40] The study also reported that patients who required supplement oxygen deteriorated more severely than those who did not.[40]
Pulmonary rehabilitation (PR) programs are a vital part of clinical management of patients. PR programs in various centers are part of standard of care in COPD.[46,47] PR programs involve various levels of aerobic exercises to improve breathing, educate about patient's condition, improving muscle strength and provide psychological support.[47] Paz-Diaz et al. demonstrated that in 24 patients with COPD randomized to either PR or control, there was significant improvement in dyspnea, depression and improvement in health related quality of life.[48] With the same rationale, PR programs can be argued to be affective in patient with ILD. Ferreira et al. published data showing a strong correlation between PR and ILD in 99 patients.[46] The study showed both statistical and clinical significant improvement in dyspnea (measured by Borg score) and functional status (measured by 6 MWD) after completing 6-8 weeks of PR program.[46] Our own study of 21 patients found that there was a significant decrease in fatigue (based on Fatigue Severity Scale) after completing a PR program.[49] Patients reported a mean fatigue score of 4.6 ± 1.7 before starting PR and mean score of 3.9 ± 1.6 after completing PR.[49]
Treatment choices
Treating symptoms of depression in advanced lung disease should be considered, if the illness accounts for any functional impairment.[50] Though medications are available, cognitive approach should also be utilized.[50] In general, the majority of patients with major depression will have an adequate response to therapy.[50] Although medication may only be needed for a short-term basis, the chronic impact of lung disease may require long-term therapy.[50] Selecting a medication depends not only on the side effect profile, but cost and drug-drug interactions as well. A patient's response should be evaluated frequents to observe the effectiveness and dose changed accordingly.[51]
There are several classes of medication available for depression and each have a different pathway to restore balance of different neurotransmitters. These medications act by:
Figure 2.
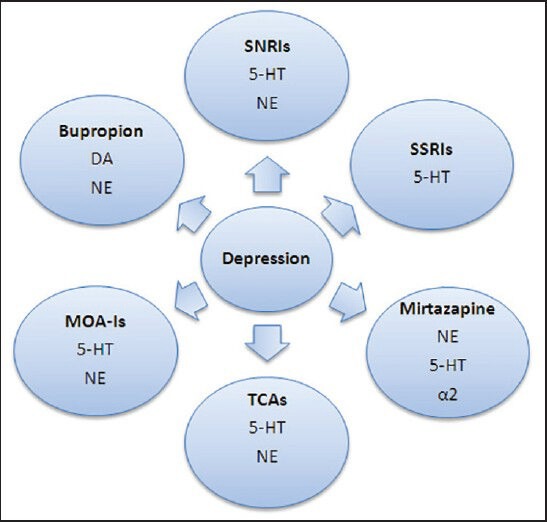
Various medications used to treat depression and the neurotransmitters involved.
‡SNRIs = selective norepinephrine reuptake inhibitor, SSRIs = selective serotonin reuptake inhibitors, TCAs = tricyclic antidepressants, MAO-Is = mono-amine oxidase inhibitors. ‡‡5-HT = serotonin, NE = norephinepherine, DA = dopamine, α2 = alpha 2 receptors
Along with, selective serotonin reuptake inhibitors (SSRIs), other classes of medications available are tricyclic antidepressants (TCAs), mono-amine oxidase inhibitors (MOIs), selective norepinephrine reuptake inhibitor (SNRIs), and atypical antidepressants such as bupropion and mirtazapine.
Selective serotonin reuptake inhibitors (SSRIs)
Selective serotonin reuptake inhibitors (SSRIs) are the mainstream therapy for depression. These medications preferably affect levels of 5-HT in the brain by slowing down its removal from brain synapses, thereby increasing serotonergic activity. Though 5-HT reuptake starts immediately, physicians should inform patients that full therapeutic effects may not appear until 3-4 weeks of consistent treatment and may increase dose if no improvement is observed.[53] Adverse effects with SSRIs are few and better tolerated than other antidepressants.[53] Some common side effects reported include sexual dysfunction, insomnia, headaches, gastritis and bleeding.[53] Of note, fluoxetine, a commonly used SSRI, has been linked to hypersensitivity pneumonitis, a known type of ILD.[54]
Tricyclic antidepressants (TCAs)
This type of antidepressant prevents reuptake of NE and 5-HT in the brain with some effects on acetylcholine and histamine.[55,56] Due to a better safety profile of SSRIs, TCAs have fallen out of favor as a primary treatment options. They are, however, used in chronic pain syndrome and tension headache. The varying properties of TCAs on acetylcholine and histamine can lead to potent and undesirable adverse effects, especially in older population. Commonly seen side effects are dry mouth, blurry vision, urinary retention and constipation. In contrast to SSRIs, TCAs can be lethal in accidental overdose, therefore, caution is necessary when these medications are prescribed to patients.[55,56]
Mono-amine oxidase inhibitors (MAO-Is)
They inhibit the activity of monoamine oxidase, thus preventing breakdown of 5-HT and NE.[55] Dietary restrictions and drug–drug interaction have prevented these medications from being used on a regular basis. Side effects associated with MAOIs are headaches, insomnia and dry mouth.[55]
Selective norepinepherine reuptake inhibitors (SNRIs)
Selective norepinephrine reuptake inhibitor (SNRIs) are drugs that block monoamine oxidase. They increase the level of 5-HT and NE, in contrast to SSRIs which only increase 5-HT.[51] The side effect profile of SNRIs is similar to that of SSRI, but to a lesser degree. These drugs should be used with caution in patients with hypertension. Venlafaxine, a type of SNRI, is linked with hypersensitivity pneumonitis.[57]
Atypical antidepressants
Atypical antidepressants are medications that are distinct from other classes of antidepressant medications such as bupropion and mirtazapine. These medications are used as an adjunctive therapy to first-line treatments. Bupropion is a unique class of medication that inhibits DA reuptake and to some extent NE. It has a stimulatory affect and can be used as an alternative therapy to SSRI.[50] The main side effect associated with bupropion is decreased seizure threshold.[50,51] Bupropion has the advantage of causing less sexual side effects or weight gain when compared to other antidepressants. Mirtazapine enhances NE and 5-HT transmission by blocking the alpha 2 (α2) receptors.[55] A common side effect associated with mirtazapine is weight gain.[55,56] Two other atypical medications that are available, trazodone and nefazodone, that have similar effects to SSRIs.
Cognitive behavior therapy
Cognitive behavioral therapy (CBT) is a type of psychotherapy that combines the principles of behavioral and cognitive therapy that is tailored toward the respective physical illness. It allows patients to understand and challenge their negative thoughts that are causing depression and replacing them with positive statements.[58] Some of the common reasons to use CBT in patients are:
Co-morbid psychiatric disorders
Difficulties in adjustment to illness
Difficulties in adherence to treatment
Problems related to illness behaviors[58]
The application of CBT can be successfully included as part of PR programs that can allow patients to confront their irrational thoughts and simultaneously improving their ability to function and their overall well-being.
Role of tele-healthcare (THC) management
Tele-healthcare is a new and innovative way to provide personalized health care to patients after they submit data about their illness (symptoms, oxygen saturation and sputum) electronically to a health care professional.[58] In return, the health care professional uses clinical skills and judgment to provide personalized feedback to the patient on real time.[59] The benefits of this approach is that the patient is continuously stimulated to monitor his/her own condition and symptoms, encourage to be adherent to drug therapy, and creates a platform for early detection and intervention, thus, reducing hospital readmission and costs to the health system.[59]
In a recent published Cochrane review, this approach was associated with a clinically significant increase in health-related quality of life (HRQoL) and a significant reduction in emergency visit and hospitalizations, without differences in 1-year survival.[60] However, there is currently limited evidence to date to generalize its use in the different subtypes of population with advanced lung disease. Therefore, more studies are needed to confirm its utility, particularly in patients with advanced severe pulmonary diseases like PAH and ILD, who usually suffer of limited mobility and are unlikely to attend and benefit of CBTs meetings or enroll into PR programs.
Conclusion
Advance lung diseases like PAH and ILD continue to cause significant morbidity and mortality. The presence of symptoms of depression suggests that they are more persistent than transient in nature. The complex interplay between lung disease and depression continues to be under recognized and untreated. In addition to physical burden, decline in functional capacity, decrease exercise capacity and decrease quality of life have a cumulative effect making patient vulnerable to depression. In clinical practice, physicians use non-invasive tools such as 6WMD, the NYHA functional classification, Borg Dyspnea Index (BDI) along with various invasive laboratory parameters to measure disease status or progression. These aforementioned measurements are good to assess physical impairment and disabilities associated with PAH and ILD, nevertheless, currently they fail to address the psychological issues patients confront daily. More research is needed for these parameters to be useful in assessing depression.
There is a two way relationship between depression and chronic illness, with both exacerbating each other. This certainly can apply to depression and advanced lung diseases. Patients have difficulty adjusting to their illness, thus feel depressed. Depression, on the other hand, can impede any progress accomplished and therefore making it difficult to manage condition [Figure 3]. The feeling of poor future outlook, feeling of guilt and self doubt can make patients non-compliant.[61] The non-compliance attitude of patients can drive up health care costs with increase emergency room visits, hospital admissions and may result in adverse outcomes. With many validated tools for a physician to administer, assessing depression can be part of providing appropriate care. If any signs of clinical depression are apparent to the treating physician, then they should be referred to psychiatrist or psychologist. An instance where a patient mentions “thoughts of suicide” warrants further investigation.
Figure 3.
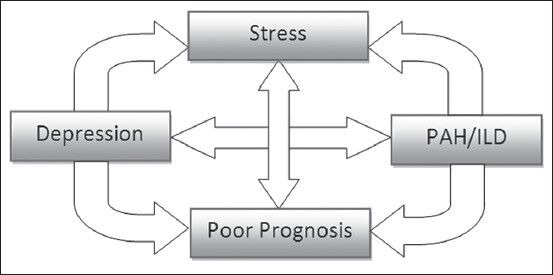
Co-relationship between depression and PAH/ILD
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.de Ridder D, Geenen R, Kuijer R, van Middendorp H. Psychological adjustment to chronic disease. Lancet. 2008;372:246–55. doi: 10.1016/S0140-6736(08)61078-8. [DOI] [PubMed] [Google Scholar]
- 2.Shanmugam G, Bhutani S, Khan DA, Brown ES. Psychiatric considerations in pulmonary disease. Psychiatr Clin North Am. 2007;30:761–80. doi: 10.1016/j.psc.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Ain A, Lolak S. Psychiatric aspects of chronic lung disease. Curr Psychiatry Rep. 2009;11:219–25. doi: 10.1007/s11920-009-0034-9. [DOI] [PubMed] [Google Scholar]
- 4.Text Revision. 4th ed. Washington DC: American Psychiatric Association; 2000. American Psychiatric Association. Diagnostic and statistical manual for mental disorders. [Google Scholar]
- 5.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 6.Nemeroff CB. Recent findings in the Pathophysiology of Depression. FOCUS. 2008;6:3–14. [Google Scholar]
- 7.Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002;4:7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasler G. Pathophysiology of depression: Do we have any solid evidence of interest to clinicians? World Psychiatry. 2010;9:155–61. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinshaw DB, Carnahan JM, Johnson DL. Depression, anxiety, and asthenia in advanced illness. J Am Coll Surg. 2002;195:271–7. doi: 10.1016/s1072-7515(02)01191-2. [DOI] [PubMed] [Google Scholar]
- 10.Jacob S, Sebastian JC, Abraham G. Depression and congestive heart failure: Are antidepressants underutilized? Eur J Heart Fail. 2003;5:399–400. doi: 10.1016/s1388-9842(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JE, Michalak EE, Lam RW. Depression in primary care: Tools for screening, diagnosis, and measuring response to treatment. British Columbia Medical Journal. 2002;44:415–9. [Google Scholar]
- 12.U.S. Preventive Services Task Force. Screening for Depression in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;151:784–92. doi: 10.7326/0003-4819-151-11-200912010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Williams JW, Jr, Pignone M, Ramirez G, Perez Stellato C. Identifying depression in primary care: A literature synthesis of case-finding instruments. Gen Hosp Psychiatry. 2002;24:225–37. doi: 10.1016/s0163-8343(02)00195-0. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 15.Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001–8. [PubMed] [Google Scholar]
- 16.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cusin, et al. Rating scales for depression. In: Baer L, Blais M, editors. Handbook of Clinical rating scales and assessment in psychiatry and mental health 2010. Totowa: Humana Press; 2010. pp. 7–35. [Google Scholar]
- 18.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–46. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S454–66. doi: 10.1002/acr.20556. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 22.Zung WW. A Self-Rating Depression Scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 23.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 24.Rinaldi P, Mecocci P, Benedetti C, Ercolani S, Bregnocchi M, Menculini G, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694–8. doi: 10.1034/j.1600-0579.2003.00216.x. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL. The PHQ-9: A new depression and diagnostic severity measure. Psychiatric Ann. 2002;32:509–21. [Google Scholar]
- 26.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 27.McCollister DH, Beutz M, McLaughlin V, Rumsfeld J, Masoudi FA, Tripputi M, et al. Depressive symptoms in pulmonary arterial hypertension: Prevalence and association with functional status. Psychosomatics. 2010;51:339–8. doi: 10.1176/appi.psy.51.4.339. [DOI] [PubMed] [Google Scholar]
- 28.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 29.Galié N, Ghofrani AH. New horizons in pulmonary arterial hypertension therapies. Eur Respir Rev. 2013;22:503–14. doi: 10.1183/09059180.00006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114:1482–9. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- 31.Guillevin L, Armstrong I, Aldrighetti R, Howard LS, Ryftenius H, Fischer A, et al. Understanding the impact of pulmonary arterial hypertension on patients’ and carers’ lives. Eur Respir Rev. 2013;22:535–42. doi: 10.1183/09059180.00005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Löwe B, Gräfe K, Ufer C, Kroenke K, Grünig E, Herzog W, et al. Anxiety and depression in patients with pulmonary hypertension. Psychosom Med. 2004;66:831–6. doi: 10.1097/01.psy.0000145593.37594.39. [DOI] [PubMed] [Google Scholar]
- 33.White J, Hopkins RO, Glissmeyer EW, Kitterman N, Elliott CG. Cognitive, emotional, and quality of life outcomes in patients with pulmonary arterial hypertension. Respir Res. 2006;7:55. doi: 10.1186/1465-9921-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadoughi A, Roberts KE, Preston IR, Lai GP, McCollister DH, Farber HW, et al. Use of selective serotonin reuptake inhibitors and outcomes in pulmonary arterial hypertension. Chest. 2013;144:531–41. doi: 10.1378/chest.12-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawut SM, Horn EM, Berekashvili KK, Lederer DJ, Widlitz AC, Rosenzweig EB, et al. Selective serotonin reuptake inhibitor use and outcomes in pulmonary arterial hypertension. Pulm Pharmacol Ther. 2006;19:370–4. doi: 10.1016/j.pupt.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Ryerson CJ, Arean PA, Berkeley J, Carrieri-Kohlman VL, Pantilat SZ, Landefeld CS, et al. Depression is a common and chronic comorbidity in patients with interstitial lung disease. Respirology. 2012;17:525–32. doi: 10.1111/j.1440-1843.2011.02122.x. [DOI] [PubMed] [Google Scholar]
- 37.Ryu JH, Daniels CE, Hartman TE, Yi ES. Diagnosis of interstitial lung diseases. Mayo Clin Proc. 2007;82:976–86. doi: 10.4065/82.8.976. [DOI] [PubMed] [Google Scholar]
- 38.du Bois RM. An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;21:141–6. doi: 10.1183/09059180.00000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spagnolo P, Tonelli R, Cocconcelli E, Stefani A, Richeldi L. Idiopathic pulmonary fibrosis: Diagnostic pitfalls and therapeutic challenges. Multidiscip Respir Med. 2012;7:42. doi: 10.1186/2049-6958-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomioka H, Imanaka K, Hashimoto K, Iwasaki H. Health-related quality of life in patients with idiopathic pulmonary fibrosis--cross-sectional and longitudinal study. Intern Med. 2007;46:1533–42. doi: 10.2169/internalmedicine.46.6218. [DOI] [PubMed] [Google Scholar]
- 41.Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: Effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167:60–7. doi: 10.1001/archinte.167.1.60. [DOI] [PubMed] [Google Scholar]
- 42.Lamers F, Jonkers CC, Bosma H, Chavannes NH, Knottnerus JA, van Eijk JT. Improving quality of life in depressed COPD patients: Effectiveness of a minimal psychological intervention. COPD. 2010;7:315–22. doi: 10.3109/15412555.2010.510156. [DOI] [PubMed] [Google Scholar]
- 43.Omachi TA, Katz PP, Yelin EH, Gregorich SE, Iribarren C, Blanc PD, et al. Depression and health-related quality of life in chronic obstructive pulmonary disease. Am J Med. 2009;122:778.e9–15. doi: 10.1016/j.amjmed.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanania NA, Müllerova H, Locantore NW, Vestbo J, Watkins ML, Wouters EF, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183:604–11. doi: 10.1164/rccm.201003-0472OC. [DOI] [PubMed] [Google Scholar]
- 45.Ryerson CJ, Berkeley J, Carrieri-Kohlman VL, Pantilat SZ, Landefeld CS, Collard HR. Depression and functional status are strongly associated with dyspnea in interstitial lung disease. Chest. 2011;139:609–16. doi: 10.1378/chest.10-0608. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira A, Garvey C, Connors GL, Hilling L, Rigler J, Farrell S, et al. Pulmonary rehabilitation in interstitial lung disease: Benefits and predictors of response. Chest. 2009;135:442–7. doi: 10.1378/chest.08-1458. [DOI] [PubMed] [Google Scholar]
- 47.Garvey C. Interstitial lung disease and pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2010;30:141–6. doi: 10.1097/HCR.0b013e3181c56b66. [DOI] [PubMed] [Google Scholar]
- 48.Paz-Diaz H, Montes de Oca M, Lopez JM, Celli BR. Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am J Phys Med Rehabil. 2007;86:30–6. doi: 10.1097/phm.0b013e31802b8eca. [DOI] [PubMed] [Google Scholar]
- 49.Cardenas-Garcia J, Verma S, John S, Gill P, Tsang D, Kohn N, et al. San Diego: Abstract to be presented at American Thoracic Society Conference; 2014. May 16-21, Effects of Pulmonary Rehabilitation on Fatigue Severity Scale In Patients With Advanced Lung Disease. [Google Scholar]
- 50.Wingate BJ, Hansen-Flaschen J. Anxiety and depression in advanced lung disease. Clin Chest Med. 1997;18:495–505. doi: 10.1016/s0272-5231(05)70397-x. [DOI] [PubMed] [Google Scholar]
- 51.Mann JJ. The medical management of depression. N Engl J Med. 2005;353:1819–34. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- 52.To SE, Zepf RA, Woods AG. The symptoms, neurobiology, and current pharmacological treatment of depression. J Neurosci Nurs. 2005;37:102–7. [PubMed] [Google Scholar]
- 53.Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med. 2000;343:1942–50. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Rothi RJ, Zander DS, Ros PR. Fluoxetine hydrochloride (Prozac)-induced pulmonary disease. Chest. 1995;107:1763–5. doi: 10.1378/chest.107.6.1763. [DOI] [PubMed] [Google Scholar]
- 55.Adams SM, Miller KE, Zylstra RG. Pharmacologic management of adult depression. Am Fam Physician. 2008;77:785–92. [PubMed] [Google Scholar]
- 56.Khouzam HR. Depression in the Elderly: How to Treat. Consultant. 2012;49:267–78. [Google Scholar]
- 57.Borderias Clau L, Marigil Gomez MA, Val Adan P, Marcen Letosa M, Biescas Lopez R, Garrapiz Lopez FJ. Hypersensitivity pneumonitis due to venlafaxine. Arch Bronconeumol. 2008;44:571–3. [PubMed] [Google Scholar]
- 58.Halford J, Brown T. Cognitive behavioural therapy as an adjunctive treatment in chronic physical illness. Adv Psychiatr Treat. 2009;15:306–17. [Google Scholar]
- 59.Yohannes AM. Telehealthcare management for patients with chronic obstructive pulmonary disease. Expert Rev Respir Med. 2012;6:239–42. doi: 10.1586/ers.12.28. [DOI] [PubMed] [Google Scholar]
- 60.McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease: Cochrane Review and meta-analysis. Br J Gen Pract. 2012;62:e739–49. doi: 10.3399/bjgp12X658269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]


