Abstract
Introduction:
Solid lipid nanoparticles (SLNs) are the new generation of submicron sized lipid emulsions where liquid lipid (oil) has been substituted by solid lipid. Lipids used in the formulation are safe, stable and biodegradable in nature. SLNs offer various advantages for topical drug delivery like ability of deposition into skin with the reduced systemic exposure and reduced local side-effects along with providing sustained release of drug. Mometasone furoate (MF) is a topical glucocorticoid having anti-inflammatory, anti-pruritic, anti-hyper proliferative activity. Owing to these properties it is recommended in chronic inflammation and psoriasis. In market, MF cream and lotion (0.1%) are available, which show slight skin irritation, burning and common side-effects due to steroids.
Experimental:
To overcome the shortcomings of conventional formulations, there is a need to develop a novel formulation that can reduce these side-effects and show maximum desired effects. Thus, SLN of MF can be prepared, which would help in increasing skin deposition as well as provide sustained release. In this study, SLNs were prepared by solvent - injection method.
Results:
The F8 batch had shown maximum entrapment up to55.59% and sustained drug release for more than 8 h. The skin permeability of SLN loaded gel was found to be 15.21times more than that of marketed cream. SLN loaded gel showed 83.52% of skin deposition which was 2.67 times more than marketed cream and 20 times more than plain drug loaded gel. The scanning electron microscopy and zeta potential study showed formation of good SLN dispersion. The stability study showed successful formation of stable SLNs. Thus, SLNs proved the potential for topical delivery of corticosteroid drug over the conventional formulations.
Experimental:
To overcome the shortcomings of conventional formulations, there is a need to develop a novel formulation that can reduce these side-effects and show maximum desired effects. Thus, SLN of MF can be prepared, which would help in increasing skin deposition as well as provide sustained release. In this study, SLNs were prepared by solvent - injection method.
Keywords: Entrapment efficiency, gel, lipid, mometasone furoate, solid lipid nanoparticles
INTRODUCTION
Psoriasis is one of the most common human skin diseases and is considered to have key genetic underpinnings. It is characterized by excessive growth and aberrant differentiation of keratinocytes.[1,2] Mometasone furoate (MF) is a medium potent glucocorticoid for dermatological use. MF is used for the treatment of glucocorticoid-responsive dermatoses, in the management of patients with atopic dermatitis, seborrheic dermatitis, eczema, allergic contact dermatitis, scalp psoriasis and psoriasis vulgaris.[3] However, there is still a need to develop novel systems formulated with non-irritating components that can be applied to efficiently treat psoriasis.
Nanotechnology has shown vast applications in pharmaceuticals. These applications have also been extended to many topical preparations for the treatment of cutaneous manifestations of diseases like psoriasis with the intent of containing the pharmacological effect of the drug to the surface of the skin or within the skin.[4] In such cases, the formulator should select a proper vehicle, which should increase the drug deposition and flux as well as it should protect drugs or actives from degradation.[5]
Solid lipid nanoparticles (SLN) are the new generation of nanoparticulate active-substance vehicles and are attracting major attention as novel colloidal drug carriers for topical use.[6] SLNs are in the submicron size range and they are composed of physiologically tolerated lipid components which at room temperature remain in the solid state. SLNs combine the advantages of and simultaneously avoid the limitations of polymeric nanoparticles, fat emulsions and liposomes. SLN are biocompatible and biodegradable and have been used for controlled drug delivery and specific targeting.[7,8] Compared with other vehicles such as creams, tinctures and emulsions, SLN combine advantages such as controlled release, negligible skin irritation and protection of active compounds. Especially, SLN can favor drug penetration into the skin, maintain sustained release to avoid systemic absorption, act as a ultraviolet (UV) sunscreen system and reduce skin irritation.[8] SLN were also found to have a skin targeting effect.[9]
Due to the lipophilic nature of their matrices, SLNs are considered particularly useful for the administration of lipophilic topical glucocorticoid such as MF.
The aim of this study was to develop SLNs with increased skin deposition and sustained release properties for MF.
MATERIALS AND METHODS
Materials
MF was gifted by Centaur Pharmaceuticals, Mumbai. Glycerol monostearate (GMS), Compritol 888 ATO, Apifil, Tefose-63 and Emulcire were gifted by Gattefosse India Pvt. Ltd., Mumbai. Cetyl palmitate, Syncrowax-HRC, Syncrowax-HGL were gifted by Croda Chemicals, Mumbai. Stearic acid, Tween-80 from Lubrizol, Mumbai and Phospholipon-80 H, Lipoid S-75 (Soya Lecithin) from Lipoid, Germany were gift samples. Ethanol, Acetone and Carbopol 974p were purchased from Research Lab Fine Chem., industries, Mumbai. Methyl paraben, Propyl paraben, Triethanolamine were purchased from Loba Chemie Pvt. Ltd, Mumbai.
Methods
Screening of components (solubility studies)
The saturation solubility of MF in different lipids and surfactants were determined. For saturation solubility study of MF, a fixed amount of MF was taken in a test tube. The solid lipid was added in increments of 0.1 g and the test tube was heated in a water bath with shaking at a temperature above the melting point of solid lipid. The amount of lipid in molten state required to solubilize the drug was noted. The complete dissolution was confirmed by formation of clear, transparent solution. The same method was used for the saturation solubility study of MF in lipophilic surfactants.[10]
Preparation of SLN
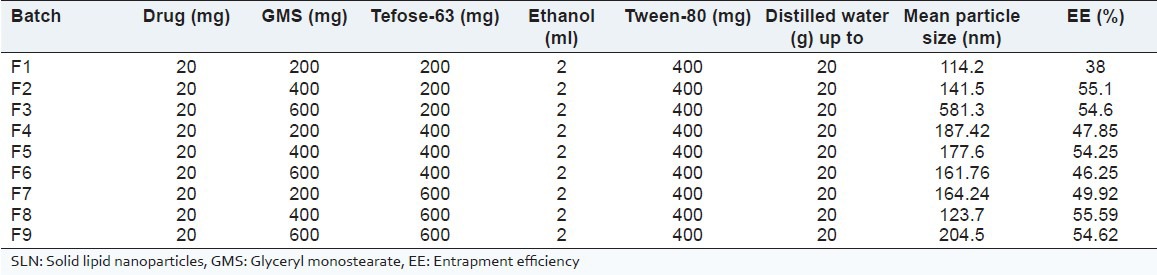
The MF loaded SLN dispersions were prepared using solvent-injection method.[11,12] The lipid phase was prepared by melting lipid and lipophilic surfactant together, i.e., GMS and Tefose-63 respectively. The solution of MF in ethanol was added to the melted lipid phase. This solution was taken in a glass syringe and was injected rapidly into the aqueous phase containing Tween 80 (having same temperature as that of lipid phase) and sonicated for 3 min using Probe Sonicator (Sonapros PR-250, Oscar Ultrasonics, Mumbai). The formed dispersion was stirred under mechanical stirrer (Remi Motors, Mumbai) for 30 min at 400 rpm, to get nanoparticulate dispersion. The composition of different batches is shown in Table 1.
Table 1.
Composition of batches of SLN
Characterization of SLN dispersions
Particle size
Mean particle size and size distribution of MF loaded SLN were determined by photon correlation spectroscopy using Nanophox (Sympatec, Germany) at room temperature.
Entrapment efficiency (EE)
The free drug (unentrapped) in the SLN dispersion was sedimented by controlled centrifugation at 2000 rpm for 30 min using Remi centrifuge and the SLN dispersion was decanted without disturbing the drug pellet. SLN dispersion and MF pellet were used for estimation of entrapped and unentrapped drug content, respectively. Fixed volume of SLN dispersion and MF pellet were dissolved separately in methanol and analyzed for entrapped and unentrapped drug content using UV spectrophotometry at 248 nm.[13]
Surface morphology
Surface Morphology of MF loaded SLNs was studied using scanning electron microscopy (SEM) (Model: JEOL JSM-6360) with an accelerating voltage of 10 kV.
Zeta potential (ZP)
Charge on surface of MF loaded SLN droplet was determined using Zetasizer 300 HAS.
Formulation of SLN loaded gel
Carbopol 974p was soaked in distilled water under mechanical stirring. This dispersion was kept overnight for complete hydration of the polymer. SLN Batch F8 (quantity equivalent to 0.1% w/w of MF) was added with stirring to ensure uniform mixing of drug in dispersion. Triethanolamine was slowly added drop by drop to form a fine gel after complete dispersion. Similar procedure was followed for preparation of the plain MF loaded gel.
Evaluation of SLN loaded gel containing MF
The formulations were observed for their visual appearance, odor, color and feel upon application such as grittiness and consistency and pH.
In vitro drug diffusion
The SLN loaded gel, plain MF loaded gel and marketed cream formulation (MOMATE®) were studied for their diffusion through dialysis membrane LA-395 (Mol. Wt. cut off 12,000-14,000 Daltons, Hi-media) using ethanol: water (1:1) as release media and USP apparatus II paddle type. The membrane was soaked in dist. water and kept overnight. A fixed quantity of formulations, i.e., SLN loaded gel, plain MF loaded gel and marketed cream (each containing 0.1% w/w of MF) were placed in different bags of dialysis membrane. The media was continuously stirred with rotating shaft at a speed of around 50 rpm. The temperature was maintained at 37°C ± 0.5°C with the help of heater. The dissolution was carried out for 8 h. At pre-determined time intervals (0.5 h), sample was withdrawn and replaced with the same volume of release media. The percent cumulative drug released or diffused at different time intervals analyzed by using UV spectrophotometry at 248 nm.[14]
Ex vivo skin permeation
The permeation study was performed using the modified Franz-diffusion cell apparatus following previously reported method.[15,16,17,18] The marketed cream, plain MF loaded gel and SLN loaded gel, all were studied for the permeation through rat skin. The receptor chamber with cross-sectional area of 4.32 cm2 was filled with receptor medium (ethanol:water 1:1). The gel or cream was applied uniformly on dorsal side of rat skin and donor compartment was placed. At set interval of 1-8 h, 0.5 ml of the sample was removed and immediately replaced by the equal volume of receptor phase solution. The amount of MF diffused into the receptor phase from the formulations was determined by UV spectrophotometer at 248 nm. By determining the cumulative drug permeated at various time intervals, the cumulative % of drug diffused versus time (in h) was plotted for plain MF loaded gel, MF-SLN loaded gel and also marketed cream.
Skin deposition
This study was performed after the completion of permeation study. For determination of drug deposited in skin, Franz diffusion cell was dismantled after a period of 8 h and the skin (from diffusion study) was carefully removed from the cell. The formulation applied on skin surface was swabbed with phosphate buffer pH 6.8 and followed by methanol. The procedure was repeated twice to ensure no traces of formulation are left onto skin surface. The skin was then cut into small pieces and kept in methanol to extract the drug present in skin for 48 h. Then, it was analyzed spectrophotometrically after suitable dilution and filtration. The standard calibration curve equation was used to determine the amount of drug deposited in the skin.[19,20]
RESULTS AND DISCUSSION
Formulation development
Screening of components: The excipients showing more solubilization potential for MF were selected for the preparation of SLNs. From saturation solubility of MF in different lipids [Table 2], it was concluded that GMS showed higher solubilization potential for MF as compared to other lipids. Thus, it was selected for further study. Similarly, solubility data of MF in different lipophilic surfactants is shown in Table 3. It was observed that, MF is more soluble in Tefose-63 when compared to other lipophilic surfactants. Therefore, it was further used for preparation of SLNs.
Table 2.
Saturation solubility of MF in different lipids
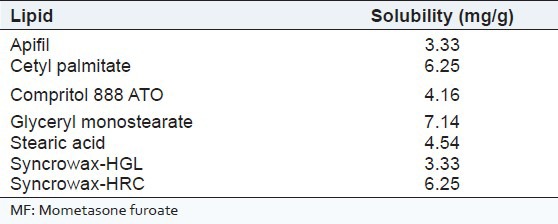
Table 3.
Saturation solubility of MF in different lipophilic surfactants

Characterization of SLN dispersions
Particle size: The amount of lipid has a great effect on particle size, since with small increase in amount of lipid, the particle size increases drastically (as seen with batches F1-F3).
EE: The EE was found to increase with increasing concentration of both lipid and surfactant [Table 1]. However an increase in lipid from 400 to 600 mg does not lead to further increase in % EE. Thus, there was no benefit of increasing lipid concentration further. Surfactant amount above 600 mg lead to formation of sticky solutions so the higher limit of surfactant was fixed to 600 mg.
SEM analysis: Figure 1 shows the SEM micrographs of MF loaded SLNs; it clearly reveals that all the SLNs were spherical in shape and smooth in nature.
Figure 1.
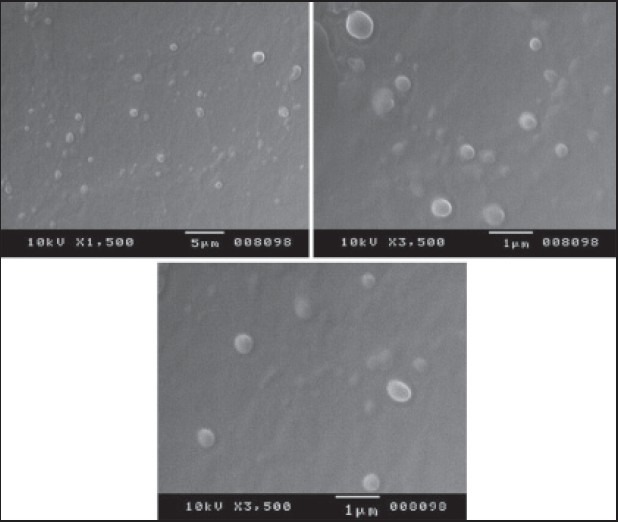
Scanning electron microscopy micrographs of mometasone furoate loaded solid lipid nanoparticles
ZP: ZP was found to be 5.62 mV of MF loaded SLN dispersion. The positive value reveals the stability of nanoparticles against aggregation potential. The repulsion of positively charged particles leads to behave as separate entities in a nanoparticulate suspension.
Evaluation of SLN loaded gel
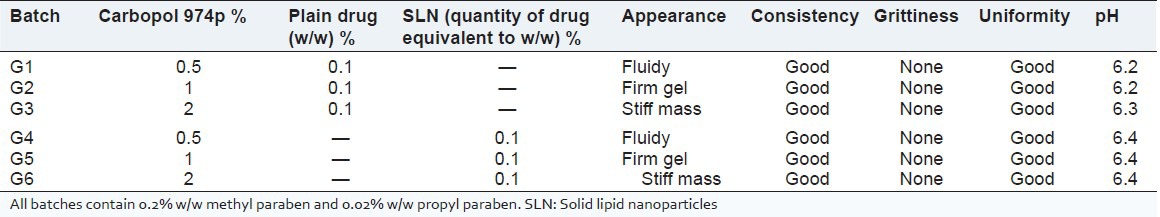
Results of physical evaluation are enlisted in Table 4. Batches G2 and G5 showed formation of a firm gel for topical application with pH near to skin pH.
Table 4.
Physical evaluation of gel formulations
In vitro drug diffusion
Figure 2 shows the comparative study of in vitro drug diffusion of SLN loaded gel (G5), marketed cream and plain drug loaded gel (G2). The ideal topical formulation should show a release or diffusion for sufficiently longer period of time so as to avoid frequent application with better patient compliance. The marketed cream showed comparatively more sustained release than plain gel while SLN loaded gel (G5) showed slower diffusion of the drug through dialysis membrane and followed a sustained release profile.
Figure 2.
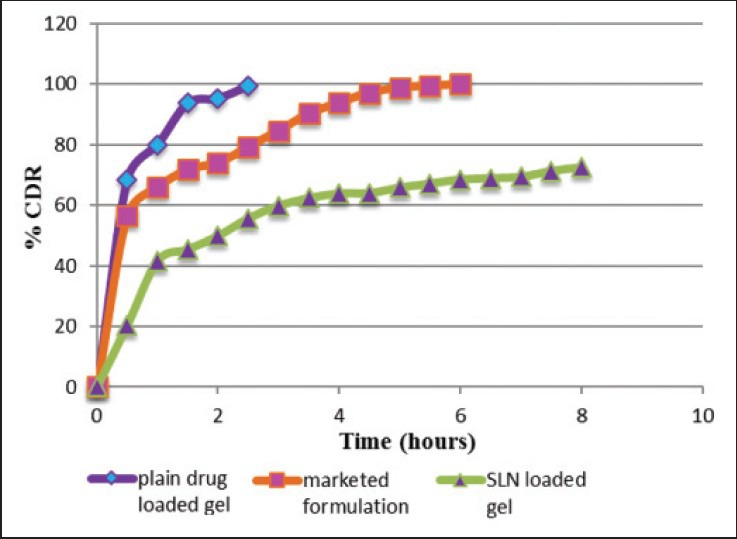
In vitro drug diffusion/release (percent cumulative drug released or diffused) of plain drug loaded gel, marketed cream and solid lipid nanoparticle loaded gel
Ex vivo skin permeation
The ideal formulation should give minimum permeation and maximum skin deposition for topical formulation of corticosteroids used in psoriasis. The results of ex vivo skin permeation study [Figure 3] showed percent cumulative drug permeation (% CDP) where plain drug loaded gel gives 95.77% permeation of MF in 5 h while marketed cream gives 65% and SLN loaded gel (G5) gives only 4% of permeation (% CDP) of MF in 8 h. The results of skin permeation study made it essential to go for the skin deposition study of the formulations since the remaining drug from the formulation had to be analyzed.
Figure 3.
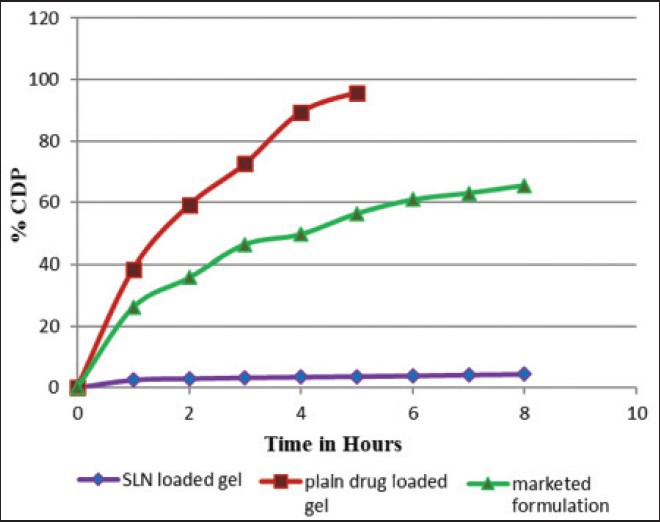
Ex vivo skin permeation (percent cumulative drug permeated) of solid lipid nanoparticle loaded gel; Plain drug loaded gel and marketed cream formulation
Skin deposition study
Table 5 shows the skin accumulation data of SLN loaded gel, plain drug loaded gel, marketed cream formulation in excised skin of Wistar albino rat. The % deposition profile is shown in Figure 4. SLN loaded gel formulation shows highest, i.e., 83.52% skin deposition of MF when compared to marketed cream formulation which shows 31.55% MF accumulation in the skin. The plain MF loaded gel shows minimum accumulation in the rat skin which is only 4.06% of applied gel formulation. Psoriatic skin is very critical to treat, difficult for application of medication and there is hyper-proliferation of the epidermal keratinocytes, thus needs a topical formulation and not transdermal. As per the requirement of an ideal formulation, higher deposition of drug in the epidermal skin and minimal penetration through skin is able to release the drug for a longer period of time after application. Thus, the SLN loaded gel formulation was found to be the ideal formulation for the topical treatment of psoriasis.
Table 5.
Percent skin deposition

Figure 4.
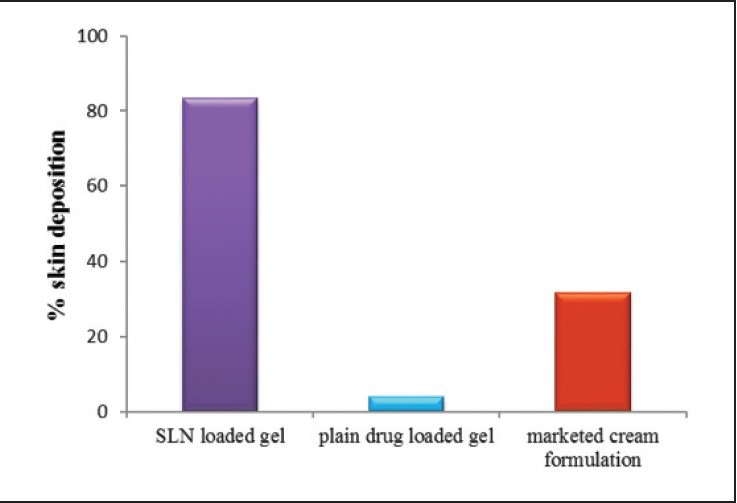
Percent skin deposition
CONCLUSION
The present research work could be concluded as successful production of SLNs using GMS as a solid lipid by solvent - injection technique. Further, developed SLNs were meaningfully utilized for the topical delivery of lipophilic, anti-psoriatic drug, MF. Greater skin deposition and slow drug release was observed with the developed SLN. SLN topical gel containing MF would be advantageous over the marketed cream product. Production of MF loaded SLNs and its formulation as a topical gel could be a new, cost-effective and commercially viable alternative to the marketed product.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 2.Mikhail M, Scheinfeld N. Evidence based review of Topical treatments for psoriasis. Dermatology. 2004;4:420–9. [Google Scholar]
- 3.Prakash A, Benfield P. Topical mometasone. A review of its pharmacological properties and therapeutic use in the treatment of dermatological disorders. Drugs. 1998;55:145–63. doi: 10.2165/00003495-199855010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer-Korting M, Mehnert W, Korting HC. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev. 2007;59:427–43. doi: 10.1016/j.addr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Severino P, Andreani T, Macedo AS, Fangueiro JF, Santana MH, Silva AM, et al. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J Drug Deliv 2012. 2012 doi: 10.1155/2012/750891. 750891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang JY, Fang CL, Liu CH, Su YH. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur J Pharm Biopharm. 2008;70:633–40. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Mehnert W, Mäder K. Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Hu W, Chen H, Ni Q, Xu H, Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328:191–5. doi: 10.1016/j.ijpharm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Chang X, Du D, Liu W, Liu J, Weng T, et al. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J Control Release. 2006;110:296–306. doi: 10.1016/j.jconrel.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 10.Mandawgade SD, Patravale VB. Development of SLNs from natural lipids: Application to topical delivery of tretinoin. Int J Pharm. 2008;363:132–8. doi: 10.1016/j.ijpharm.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Schubert MA, Müller-Goymann CC. Solvent injection as a new approach for manufacturing lipid nanoparticles —Evaluation of the method and process parameters. Eur J Pharm Biopharm. 2003;55:125–31. doi: 10.1016/s0939-6411(02)00130-3. [DOI] [PubMed] [Google Scholar]
- 12.Parmar B, Mandal S, Petkar K, Sawant K. Valsartan loaded solid lipid naoparticles: development, characterization, in-vitro and ex-vivo evaluation. Int J Pharm Sci Nanotechnol. 2011;4:1483–90. [Google Scholar]
- 13.Agrawal Y, Petkar KC, Sawant KK. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int J Pharm. 2010;401:93–102. doi: 10.1016/j.ijpharm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Sanap GS, Mohanta GP. Design and evaluation of miconazole nitrate loaded Nlc for improving antifungal therapy. J App Pharm Sci. 2013;3:46–54. [Google Scholar]
- 15.Ana C, Alexandra M. Development, stability and in vitro permeation studies of gels containing mometasone furoate for the treatment of dermatitis of the scalp. Braz J Pharm Sci. 2010;46:109–14. [Google Scholar]
- 16.Jain AK, Thomas NS, Panchagnula R. Transdermal drug delivery of imipramine hydrochloride. I. Effect of terpenes. J Control Release. 2002;79:93–101. doi: 10.1016/s0168-3659(01)00524-7. [DOI] [PubMed] [Google Scholar]
- 17.Wang YY, Hong CT, Chiu WT, Fang JY. In vitro and in vivo evaluations of topically applied capsaicin and nonivamide from hydrogels. Int J Pharm. 2001;224:89–104. doi: 10.1016/s0378-5173(01)00755-4. [DOI] [PubMed] [Google Scholar]
- 18.Kumari K, Sara UV, Sachdeva M. Formulation and evaluation of topical hydrogel of mometasone furoate using different polymers. Int J Pharm Chem Sci. 2013;2:89–100. [Google Scholar]
- 19.Jenning V, Gysler A, Schäfer-Korting M, Gohla SH. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2000;49:211–8. doi: 10.1016/s0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 20.Niyaz B, Kalyani P, Divakar G. Formulation and evaluation of gel containing fluconzole-antifungal agent. Int J Drug Dev Res. 2011;3:109–28. [Google Scholar]


