Abstract
Introduction:
The present study aims at developing a gastroretentive swellable and floating matrix tablet formulation of ciprofloxacin hydrochloride (HCl) for the effective treatment of infections caused by susceptible organisms. Ciprofloxacin HCl is a fluoroquinoline antibiotic drug. Ciprofloxacin HCl is more stable in acidic medium and it has a narrow absorption window which is sited at the stomach and proximal portions of the small intestine, so it covers the required criteria for selection of drug for gastroretentive dosage form.
Materials and Methods:
Ciprofloxacin HCl gastroretentive tablets were formulated by using direct compression method and different grades of hydroxypropyl methylcellulose as suspending and stabilizing agent (polymer), sodium starch glycolate (SSG), crospovidone as disintegrates, sodium bicarbonate as alkalizing agent and magnesium stearate as lubricant.
Results:
The tablets were evaluated for post compression parameters. All the parameters were within the pharmacopoeial limits.
Conclusion:
The in vitro dissolution studies showed that the drug release was fast in formulations F2, F4 and F6 containing SSG as super disintegrant when compared with all other formulations. In SEM study of F2 formulation shows maximum swelling and porosity observed after 12 h. Hence, formulation F2 shows the best formulation among the six formulations containing different binders and super disintegrants.
Keywords: Ciprofloxacin hydrochloride, direct compression, floating tablet, gastroretentive dosage form
INTRODUCTION
Drug is an active chemical entity used for diagnosis, prevention and treatment of disease; they also modify physiological state of the body. The oral route of drug administration is the most important method of administrating drugs for systemic effects. Oral route of drug administration has wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular and most preferred route due to its advantages. Tablets are solid dosage forms containing medicinal substances with or without suitable diluents. They offer safe and convenient ways of active pharmaceutical ingredients (API) administration with excellent physiochemical stability in comparison to some other dosage forms and provide accurate dosing. The control of gastrointestinal transit of orally administered dosage forms using gastroretentive drug delivery systems (GRDDS) can improve the bioavailability of drugs that exhibit site-specific absorption. Prolonged gastric retention can be achieved by using floating, swelling, bio-adhesive or high-density systems.[1]
A controlled drug delivery system with prolonged residence time in the stomach is of particular interest for drugs, which are locally active in the stomach (misoprostol, antacids antibiotics against Helicobacter pylori).[2] GRDDS is useful for drugs which have an absorption window in the stomach or in the upper small intestine (e.g., L-dopa, P-amino benzoic acid, furosemide), drugs which are unstable in the intestine or colonic environment (e.g., captopril), exhibit low solubility at high pH values (diazepam, verapamil) and drugs which alter normal flora of the colon (antibiotics). Various techniques used in the preparation of control release tablets such as molding technique, direct compression technique, mass extrusion technique. In the present investigation, direct compression method was taken as it was a robust process which helps in reducing elasticity problems and imparts flow ability to a formulation.[3,4] The commonly used super disintegrants are sodium starch glycolate (SSG) and crospovidone (CPD). The use of various binders and super disintegrants affects the disintegration time and dissolution studies.[5]
The bacterial action of ciprofloxacin results from inhibition of enzymes topoisomerase-II and topoisomerase-IV, which are required for bacterial deoxyribonucleic acid replication, transcription repair and recombination and used in the urinary tract infection, lower respiratory tract infection, acute sinusitis, complicated intra-abdominal infections. The objective of the present investigation was to prepare ciprofloxacin hydrochloride (HCl) gastroretentive tablets by direct compression method using SSG, CPD, hydroxypropyl methylcellulose (HPMC) K100 LV, HPMC K15M, HPMC K100M, sodium bicarbonate (SBC) and magnesium stearate as excipients in the formulation.[6]
MATERIALS AND METHODS
Ciprofloxacin HCl was obtained as a gift sample from Aurobindo Pharma Ltd., different grades of HPMC were obtained as gift samples from Torrent Pharmaceutical Ltd., (Ahmedabad, India). Moreover all other excipients and chemicals used were of analytical grade, which were purchased from local laboratories.
Preparation of floating tablets
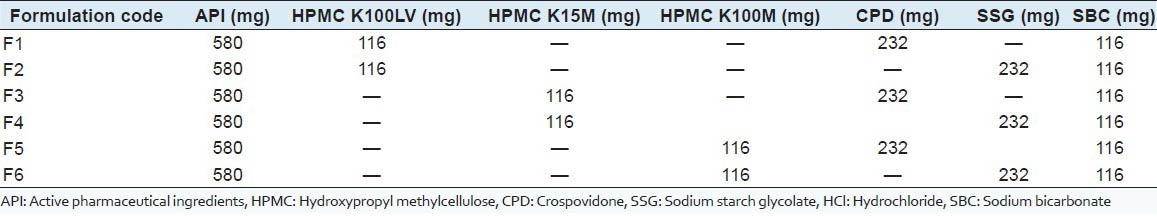
The method used in the formulation of ciprofloxacin HCl gastroretentive tablets was direct compression method. All the batch formulations are formulated by direct compression method. Accurately weigh specified quantity of raw materials such as ciprofloxacin, super disintegrants, HPMC, magnesium stearate in a weighing balance. Floating matrix tablets containing ciprofloxacin HCl were prepared by direct compression technique using varying concentrations of different grades of HPMC polymers with SSG, CPD, SBC and magnesium stearate as shown in Table 1. API, polymer, swelling agent and SBC were weighed and passed through sieve no. 40 mixed homogeneously in a glass mortar for about 5–10 min and before compression all powder/granules were lubricated with the help of magnesium stearate. The well-mixed powder was compressed by direct compression technique.[7] Tablets were compressed on Shakti multi station punching machine using 13 mm flat punches, with the hardness of 5 kg/cm2.
Table 1.
Formulation batches of ciprofloxacin HCl gastroretentive tablets
Physical parameters
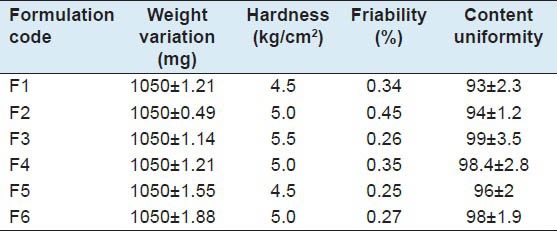
The formulated tablets were evaluated for the following parameters such as weight variation, hardness, friability, content uniformity the results has been tabulated.[8]
Floating study
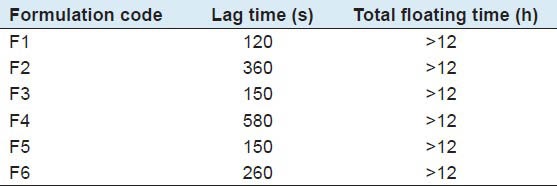
The time taken for dosage form to emerge on the surface of medium is called buoyancy lag time. Duration of time by which the dosage form constantly emerges on the surface of medium is called total floating time. Tablets were placed in a 400 ml flask of 0.1 N HCl solution (pH 1.2), time needed to go upward and float on the surface of the liquid and floating duration were determined which is shown in Table 2.
Table 2.
Summary for floating studies
Swelling index (SI) study
The swelling of the polymers can be measured by their ability to swell and absorb water. The swelling property of the formulation was determined by various techniques. The SI of the tablet was done using USP dissolution apparatus II. The medium used was distilled water, 900 ml rotated at 50 rpm. The medium was maintained at 37 ± 0.5°C throughout the study. After a selected time intervals, the tablets were withdrawn, blotted to remove excess water and weighed. Swelling characteristics of the tablets were expressed in terms of SI which is shown in:[9]

Scanning electron microscopy (SEM)
The SEM analysis was conducted using Jeol, Japan (Model-JSM 5610 LV) SEM for the optimized formulation F2 at: Dry tablet surface and tablets after swelling of 4, 8 and 12 h.
As with SEM high vacuum is required for image formation and samples must be thoroughly desiccated before entering the vacuum chamber, therefore samples were thoroughly dried after swelling for analysis. The dried samples were mounted on the sample holder using double-sided adhesive carbon tape.[10]
The SEM was operated at 15 KV. The condenser lens position was maintained at a constant level. The photomicrographs were recorded at ×500.
RESULTS AND DISCUSSION
Oral drug delivery system represents one of the frontier areas of controlled drug delivery system; such dosage forms are having a major advantage of patient compliance. Floating drug delivery system belongs to oral controlled drug delivery system group that are capable of floating in the stomach by bypassing the gastric transit. These dosage forms are also defined as gastro retentive drug delivery system or hydro dynamically balanced dosage form or gastric floating drug delivery system, which can float in the contents of the stomach and release the drug in a controlled manner for a prolonged period. The release rate will be controlled depending upon the type and concentration of the polymer that swells, leading to diffusion and erosion of the drug.[11,12]
Drug excipients compatibility studies
Physical compatibility studies were assured by Fourier transform infrared spectroscopy (FT-IR) studies. The crude drug sample and the complete formula of the final formulation were chosen for the study. The FT-IR spectra's of the above samples were studied after a period of 30 days from preparation of the mixtures, to facilitate prompt detection of incompatibility. Based on the spectral data it has been observed that there are no shift of any major peaks in appearance or disappearance of the peak.
Evaluation of post compression parameters
The post-compression parameters such as weight variation, hardness, friability, content uniformity were evaluated. All the parameters were within the pharmacopoeial limits [Table 3].
Table 3.
Evaluation of post-compression parameters
Floating test
The time between the introduction of dosage form and its buoyancy in 0.1N HCl medium and floating lag time, total floating time were measured. All formulations floated more than 12 h with a lag time of up to 2-9.66 min. During floating duration, formulations maintained matrix integrity. Swelling of the tablets was observed, which gave floating ability to formulations. Floating duration and lag time were found to be dependent to the amounts of polymers incorporated in formulations. Formulation F1, F3 and F5 shows good floating lag time as compared to other formulations may be because of CPD which is common in these three formulations.[13]
In vitro dissolution studies
The in vitro dissolution studies were conducted by using USP Type II Apparatus, 900 ml of pH 0.1N HCl was used as dissolution medium. Speed was maintained at 75 rpm and temperature maintained at 37°C ±0.5°C. The samples were withdrawn up to 12 h and measured by UV Spectrophotometer at 276 nm. The results were tabulated in Table 4.
Table 4.
In vitro dissolution studies
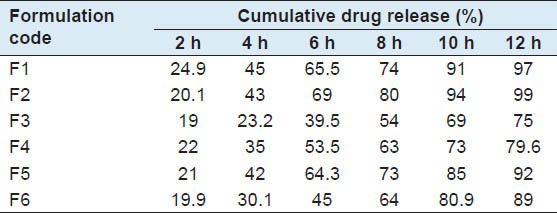
The release of ciprofloxacin was found to be a function of the polymer concentration and super disintegrants. All formulations retarded the release of drug for 12 h. The effect of SSG and CPD at different concentrations on the release of ciprofloxacin from tablet matrices was studied.[14]
Figure 1 shows the drug release profile of drug from SSG and CPD, at different concentrations of polymer. Among all six batches, batch F2 shows good cumulative drug release (%) after 12 h which contains a combination of HPMC and SSG.
Figure 1.
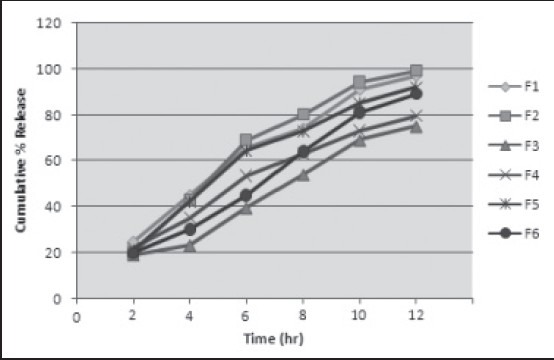
In vitro dissolution profile of formulations F1 to F6
Percentage swelling
The swelling behavior of dosage form can be done in dissolution apparatus-II by using 900 ml of distilled water as medium rotated at 75 rpm at 37°C and measured its dimensional changes, weight gain or water uptake at regular intervals.[15]
The results were tabulated in Table 5 and shown in Figure 2. Among all formulations, F2, F4 and F6 show good swelling properties after 10 h combination of HPMC and SSG helps to improve swelling properties.
Table 5.
Summary for swelling property
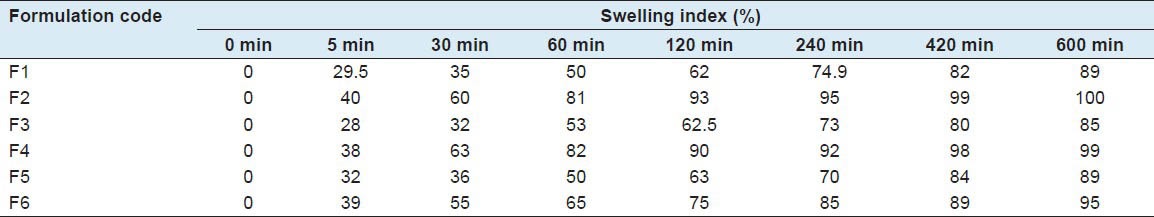
Figure 2.
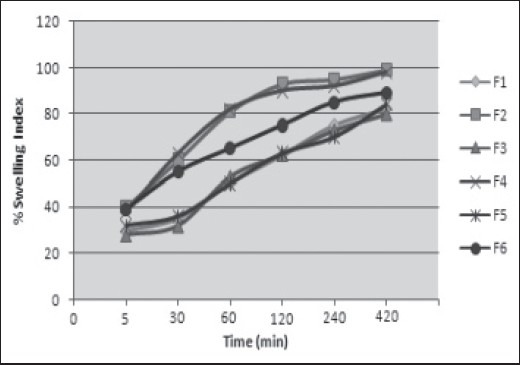
The swelling behavior of formulations F1 to F6
SEM
SEM for F2 formulation was carried out at four different states. At dry state, there is no swelling property and porosity, but it revealed that the surface was smooth up to 4 h after that the swelling and porosity of tablet increased. Maximum swelling and porosity observed after 12 h which indicates the diffusion and swelling mechanism of release shown in Figures 3–6a–d respectively.
Figure 3.
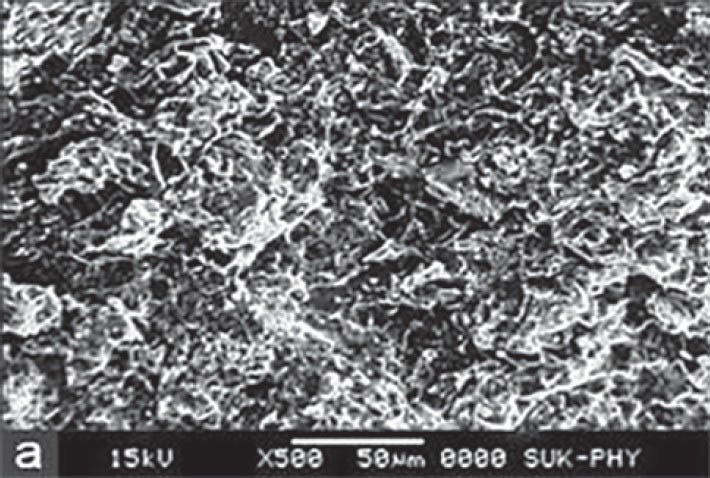
Scanning electron microscopy of optimized formulation F2 at dry state
Figure 6.
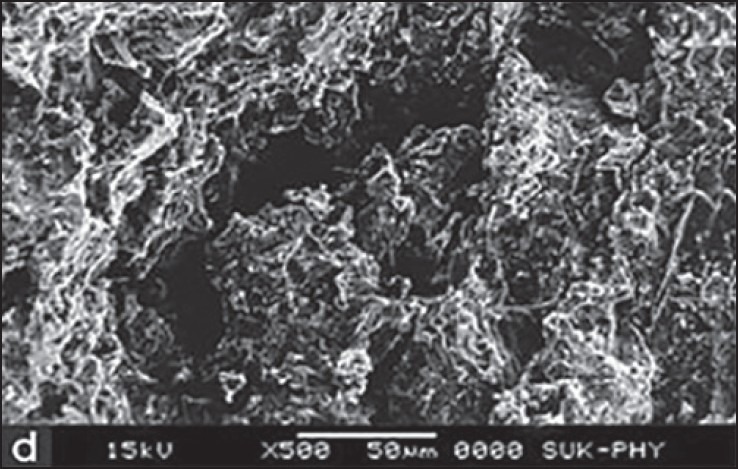
Scanning electron microscopy of optimized formulation F2 after 12 h
Figure 4.
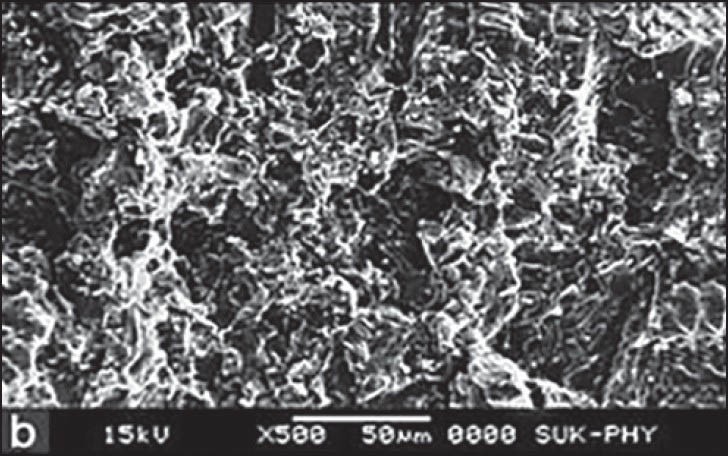
Scanning electron microscopy of optimized formulation F2 after 4 h
Figure 5.
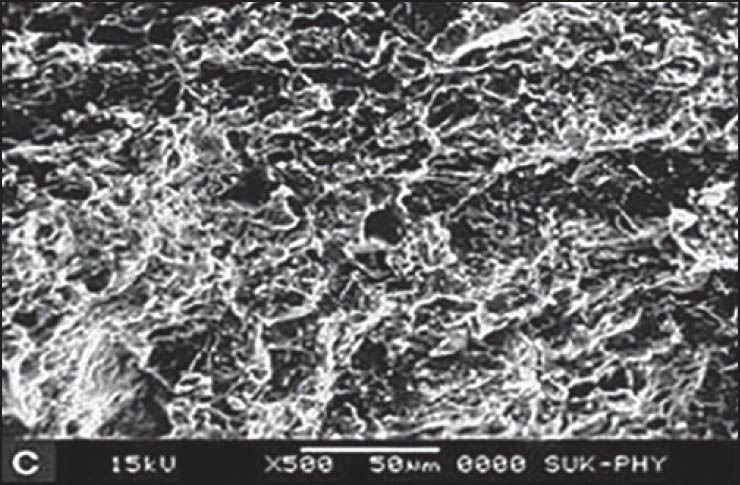
Scanning electron microscopy of optimized formulation F2 after 8 h
CONCLUSION
In the present work, formulations of ciprofloxacin gastroretentive tablets were prepared by direct compression method. Six formulations were formulated and evaluated for both post-compression parameters such as weight variation, hardness, friability, content uniformity, in vitro dissolution studies, floating property and swelling property were evaluated and the results were tabulated.
In vitro release rate studies showed that the maximum drug release was observed in F2, F1 and F5 formulations up to 12 h.
From the above experimental results it can be concluded that SSG and CPD has predominant effect on the buoyancy lag time, while HPMC has predominant effect on total floating time and drug release.
Formulation F2, F4 and F6 containing SSG as super disintegrant shows good swelling nature but the maximum swelling was obtained at latter hours and better dissolution than other formulations. In SEM study of F2 formulation shows maximum swelling and porosity observed after 12 h. Hence, formulation F2 shows the best formulation among the six formulations containing different binders and super disintegrants.
ACKNOWLEDGMENTS
We are thankful to Aurobindo Pharma Ltd., India for providing us gift samples of drug and Torrent pharmaceutical, Ahmedabad, for providing us gift samples of polymers. We are also thankful to department of pharmaceutics, Arvind Gavali College of Pharmacy, Satara.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
REFERENCES
- 1.Shivakumar HG, Gowda DV, Kumar P. Floating controlled drug delivery systems for prolonged gastric residence: A review. Indian J Pharm Educ. 2004;38:172–9. [Google Scholar]
- 2.Gaikwad VD, Yadav VD, Jadhav PD. Formulation and evaluation of floating matrix tablets of diltiazem hydrochloride. Asian J Pharm. 2012;6:245–51. [Google Scholar]
- 3.Jayesh P, Manish R. Tablet formulation design and manufacture: Oral immediate release application. Pharma Times. 2009;41:21–9. [Google Scholar]
- 4.Arora S, Ali J, Ahuja A, Khar RK, Baboota S. Floating drug delivery systems: A review. AAPS PharmSciTech. 2005;6:E372–90. doi: 10.1208/pt060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg R, Gupta GD. Progress in controlled gastroretentive delivery systems. Trop J Pharm Res. 2008;7:1055–66. [Google Scholar]
- 6.Sonar GS, Jain DK, More DM. Preparation and in vitro evaluation of bilayer and floating bioadhesive tablets of rosiglitazone maleate. Asian J Pharm Sci. 2007;2:161–9. [Google Scholar]
- 7.Yadav VD, Gaikwad VD, Gaikwad PV. Development and in vitro evaluation of mucoadhesive gastroretentive matrix tablets of cephalexin. IOSR J Pharm Biol Sci. 2013;7:25–31. [Google Scholar]
- 8.Sunil K Jhain, Jhain NK, Agarwal GP. Gastro retentive floating drug delivery; An overview. Drug Deliv Technol. 2005;5:21–31. [Google Scholar]
- 9.Patel H, Panchal DR, Patel U, Brahmbhatt T. Matrix type drug delivery system: A review. J Pharm Sci Biosci Res. 2011;1:143–51. [Google Scholar]
- 10.Gaikwad VD, Gaikwad MD, Yadav PB. Formulation and in vitro evaluation of gastroretentive drug delivery system containing ofloxacin. IOSR J Pharm Biol Sci. 2013;5:25–30. [Google Scholar]
- 11.Stops F, Fell JT, Collett JH, Martini LG. Floating dosage forms to prolong gastro-retention – The characterisation of calcium alginate beads. Int J Pharm. 2008;350:301–11. doi: 10.1016/j.ijpharm.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Khan F, Razzak SM, Khan ZR, Azam KR, Azad MA. Preparation and in vitro evaluation of theophylline loaded gastroretentive floating tablets of METHOCEL K4M. J Pharm Sci. 2008;7:65–70. [PubMed] [Google Scholar]
- 13.Gaikwad VD, Yadav VD, Jadhav PD. Design and development of sustain release gastro retentive floating tablets for diltiazem hydrochloride. J Pharm Res Opin. 2013;3:9–14. [Google Scholar]
- 14.Deshpande AA, Rhodes CT, Shah NH. Controlled release drug delivery systems for prolonged gastric residence: An overview. Drug Dev Ind Pharm. 1996;22:531–9. [Google Scholar]
- 15.Krishnaiah YS, Satyanarayana V, Dinesh Kumar B, Karthikeyan RS. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur J Pharm Sci. 2002;16:185–92. doi: 10.1016/s0928-0987(02)00081-7. [DOI] [PubMed] [Google Scholar]


