Abstract
Investigations of patients with systemic lupus erythematosus (SLE) have applied insights from studies of the innate immune response to define type I interferon (IFN-I), with IFN-α the dominant mediator, as central to the pathogenesis of this prototype systemic autoimmune disease. Genetic association data identify regulators of nucleic acid degradation and components of TLR-independent, endosomal TLR-dependent, and IFN-I signaling pathways as contributors to lupus disease susceptibility. Together with a gene expression signature characterized by IFNI-induced gene transcripts in lupus blood and tissue, those data support the conclusion that many of the immunologic and pathologic features of this disease are a consequence of a persistent self-directed immune reaction driven by IFN-I and mimicking a sustained anti-virus response. This expanding knowledge of the role of IFN-I and the innate immune response suggests candidate therapeutic targets that are being tested in lupus patients.
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) captures the attention and imagination of medical students and physicians, as well as immunologists and biomedical scientists, due its highly variable clinical presentation; its striking propensity to affect women in their child-bearing years, with nine or ten times more women than men diagnosed with the disease; its protean organ system manifestations and immunologic alterations; and the important lessons that derive from the search for its etiology and study of its pathogenic mechanisms (1). Advances in understanding the molecular basis of innate immunity, accelerated by the elucidation of the TLR system, along with insights gained from analysis of gene transcripts inducible by type I interferon (IFN-I) in blood cells of patients, have led to identification of IFN-I, and particularly IFN-α, as a central mediator in the pathogenesis of SLE. This brief review will describe our current understanding of the role of IFN-I in human lupus. A recent comprehensive review provides an outstanding summary that incorporates insights from studies of murine lupus models (2).
The breadth and systemic nature of the common disease manifestations seen in patients with lupus are consistent with involvement of many immune system players. Rash, photosensitivity, arthritis, and fatigue are features of most patients. Severe and sometimes life-threatening disease manifestations include lupus nephritis, typically characterized by glomerulonephritis along with renal interstitial inflammation and tubular damage; central nervous system disease, ranging from cognitive dysfunction to stroke or seizures; and premature atherosclerosis, conferring increased risk of myocardial infarction (1). Many of those manifestations have been attributed to production and tissue deposition of self-reactive antibodies, often in the form of immune complexes and targeting nucleic acids and nucleic acid-binding proteins. Those autoantibodies are present years before the onset of clinical disease, and their specificities provide important clues, not yet fully unraveled, to the early immunologic events that ultimately lead to organ inflammation and damage (3, 4).
The specific targeting of DNA, cell nuclei and components of ribonucleoprotein particles by lupus autoantibodies, a process that strikes at the core of what we consider “self”, has directed attention of immunologists to the events that account for disruption of T and B cell tolerance mechanisms. The pathogenic properties of class-switched IgG autoantibodies that can access the extravascular space, activate the complement pathway, and initiate Fc receptor-mediated cell activation, leading to recruitment of inflammatory cells and tissue damage, suggested that production of those antibodies is dependent on T cell help (5). Investigation of the role of the adaptive immune system in lupus pathogenesis led to the landmark FDA approval of a therapeutic that inhibits B lymphocyte stimulator (BLyS) (belimumab), as well as anecdotal efficacy (in spite of negative results in randomized controlled clinical trials) of agents that deplete B cells after binding to cell surface CD20 (rituximab) or inhibit T cell activation by blocking signals through CD28 (abatacept) (6-8).
In spite of these important achievements related to the role of the adaptive immune response in lupus pathogenesis, it is recognized that production of autoantibodies is not sufficient for development of clinical lupus, and therapeutic targeting of T and B cells has provided only partial clinical efficacy. In the context of important gaps in our understanding of lupus, the explosion of studies detailing the innate immune response, and particularly the role of endosomal TLRs, has provided an important knowledge base for reconsideration of the early observation of increased IFN-I levels in patients with SLE (9, 10). More recent elucidation of cytoplasmic receptors for RNA and DNA, whether microbial in origin or endogenous, has suggested additional potential mechanisms for induction of IFN-I (11, 12). Investigations by many laboratories have contributed to the current conclusion that IFN-I, particularly IFN-α, is a central pathogenic mediator that accounts for many of the immunologic features and destructive mechanisms that lead to clinical disease in patients (13, 14). Beyond its well-documented role in anti-virus host defense, persistent and excessive production of IFN-α and activation of molecular events downstream of the IFN-I receptor are significant early and sustained events in lupus pathogenesis.
Evidence for increased type I IFN and IFN-inducible gene expression in SLE
Among the multitude of immunologic mediators that are dysregulated and the immune functions that are altered in patients with SLE and in murine lupus models, elevated IFN-I represents a consistent theme over many decades of investigation. In 1969 it was reported that inducing production of IFN-I in vivo in young NZB/NZW F1 mice, genetically susceptible to developing spontaneous lupus-like disease but prior to evident pathology, accelerated production of lupus autoantibodies and tissue damage (15). The stimulus in that system was polyinosinicpolycytidylic acid (poly I:C), mimicking dsRNA. The authors’ interpretation of their data was that in the setting of a genetically susceptible host, nucleic acids can induce autoimmunity and lupus-like disease mediated by IFN-I, a concise view that is now supported by human data. Examples of development of autoimmunity, particularly characterized by autoantibody specificities typical of SLE, along with reports of development of clinical lupus or related systemic autoimmune diseases, in patients who received recombinant IFN-α for treatment of hepatitis C or a hematologic disorder, support the capacity of IFN-I to induce an autoimmune syndrome in some individuals, presumably those endowed with genetic susceptibility supportive of an autoimmune response (16).
Observations of elevated levels of IFN-I in the blood of lupus patients were initially reported in 1979 based on assays that quantified protection of virus-infected cells from death (9). The demonstration of a broad IFN-I-induced gene (IFIG) transcript signature in SLE PBMC emerged from several labs in 2003, providing a compelling visual picture that suggested a dominant role for some components of that cytokine family in the disease (17-21). Recent data from epigenetic analyses of hypomethylated genome sites support activation of many genes related to IFN-I signaling (22, 23). Several hundred gene transcripts define this signature, and while various laboratories have settled on use of panels of IFIG as a biomarker characteristic of SLE, including anywhere from one to twenty-one or more specific transcripts usually quantified by real-time PCR, in fact most are highly correlated, and measurement of a small number of transcripts provides a good measure of the functional effect of IFN-I in vivo based on study of PBMC or whole blood ex vivo (24, 25). Among the classic IFIGs, IFIT1 is particularly representative, but many others are equally informative. While it was possible that a viral mimic of IFN-I's effects, or some other stimulus, was responsible for the striking gene expression pattern, inhibition of IFN-α with neutralizing antibodies eliminated most of the capacity of patient plasma to induce the same IFIGs (26). In adult lupus patients approximately 60-80% will demonstrate the IFIG signature, which is expressed in many cell types (27), and in children with SLE the signature is nearly universal (18). In contrast to many viral infections where IFN-I is produced in the initial stage of infection but is not persistent over time, in patients with SLE the IFN-I gene expression signature, as well as serum or plasma IFN-I activity, persists chronically. In cross-sectional studies the level of IFIG expression is correlated with disease activity, but in longitudinal studies IFIG expression can remain stable or variable over time, with inconsistent relationship to disease flares (28-31).
With continued analysis of large datasets derived from study of lupus peripheral blood, statistical approaches such as K-means cluster analysis have identified subgroups of IFIG transcripts that are differentially expressed in some patients (29, 32). While the clinical significance of these IFIG clusters is not yet clear, it is of interest that a dominant cluster is enriched in those transcripts typically measured in IFN-I biomarker panels and primarily inducible by IFN-α, and a second cluster includes IFIG that are more typically induced by IFN-β. Notably, EIF2AK2, encoding IFN-inducible double-stranded RNA-activated protein kinase (PRKR), is in the second IFIG cluster identified in our dataset and in one of the clusters defined by Chaussabel, is preferentially induced by IFN-β, and is not included among the transcripts measured in most IFN-I biomarker panels (19, 32-35) (Olferiev, M., Crow, M.K., unpublished observations). It should be noted that IFN-γ, typically a product of the adaptive immune response, can contribute to activation of many of the IFIGs, and elevated IFN-γ mRNA is seen in some lupus patients (19, 24, 25, 32, 34, 35). While the collective literature supports IFN-α, comprised of 13 distinct proteins, as the most abundant IFN-I in patients with lupus, the complexity of the IFN signature and the potential for additional IFN-I species, as well as IFN-γ, to contribute to that gene expression signature and lupus pathogenesis is supported by demonstration of increased expression of the IFN-Is IFN-β and IFN-ω in lupus blood compared to that of healthy donors (25). A possible role for IFN-λ, a type III IFN, in SLE remains to be fully explored (36).
The data from patients suggest the view that many of the immunologic and clinical manifestations of SLE might be biologic consequences of IFN-I that is either excessively produced and/or improperly regulated. In that regard, while it has been challenging to accurately measure total IFN-I protein in SLE blood due to the multiple IFN-I family members and the presence of IFN-I inhibitors in some individuals (37), functional assays of IFN-I activity present in plasma or serum of patients support increased production as one important mechanism (26). That interpretation does not discount the important role of genetic factors that impact the cellular response to IFN-I, contributing to a more persistent or exaggerated expression of the cytokine's target genes in some individuals. Overall, elevated circulating IFN-I activity and IFIG expression observed in most SLE patients, and to some extent in those with other systemic autoimmune diseases (38-40), suggest the view that SLE is the prototype disease that behaves immunologically like a poorly-controlled chronic viral infection, albeit in the absence of a documented virus (41-43).
Genetic contributions to activation of the type I IFN pathway
Among the insights that have emerged from genome-wide association studies (GWAS) of lupus patients is the observation that many of the genetic variants associated with SLE encode proteins involved in activation or regulation of the innate immune response (44). Some contribute to availability of endogenous immune stimuli, a number regulate pathways that mediate production of IFN-I and some regulate the cellular response to IFN-I. That increased IFN-I is a genetically conferred risk factor for development of SLE was shown in family studies (45). Other genetic variants associated with SLE encode proteins that are likely to regulate efficiency of antigen presentation, thresholds for activation of T or B lymphocytes or efficiency of intracellular signaling in those cells, and others likely regulate tissue vulnerability to injury or repair mechanisms (44, 46, 47).
Insight into mechanisms that generate inducers of the IFN-I pathway comes from studies of a rare clinical syndrome, Aicardi-Goutieres syndrome (AGS), describing children with distal extremity skin lesions, neurologic dysfunction, anti-DNA antibodies and elevated IFN-I. Genetic studies in several AGS cohorts identified mutations in TREX1, encoding DNase III (48, 49). Rare patients with bona fide SLE have mutations in that gene, and GWAS studies have identified a common variant with significant association with lupus (50). Additional genetic mutations associated with AGS encode enzymes that regulate degradation of RNA or DNA-RNA hybrids; mutations of SAMHD1, ADAR1, and members of the RNASEH2 family are associated with AGS, some cases of SLE and elevated IFN-I (51).
These data complement the longstanding observation of a high risk of SLE among individuals with deficiencies in early components of the complement pathway – C1q, C2 and C4 (52). While those mediators have broad functions in host defense, among their important roles is promoting clearance of debris derived from apoptotic or necrotic cells that might otherwise provide an inappropriate innate immune stimulus. Taken together, these genetic association data point to endogenous nucleic acids as important triggers for IFN-I production, and possibly SLE, and emphasize the significance of effective degradation and removal of potentially stimulatory genomic products.
Additional GWAS data provide strong support for the endosomal TLRs and their downstream signaling components in increased IFN-I production, SLE susceptibility and pathogenesis. Among the genes and genetic loci associated with SLE are TLR7, IFN-regulatory factor 5 (IRF5), IRF7, IRAK1 and TNFAIP3, encoding a regulator of the NFκB pathway activated by endosomal TLRs (44). The relationship of the lupus-associated IRF5 genotype to elevated serum IFN-I activity implicates that downstream mediator of the TLR7 and TLR9 signaling pathways in IFN-I production and might also be relevant to induction of IFN-I by cytoplasmic nucleic acid sensors (53, 54). Of interest, the relationship between the lupus-associated IRF5 genotype and serum IFN-I activity is primarily observed in those lupus patients who demonstrate positive serologic tests for autoantibodies reactive with RNA-associated proteins (Ro, La, Sm or RNP) or DNA (54). The interpretation of those data is that the IRF5 genetic variant, a transcription factor downstream of the endosomal TLRs, is most relevant to lupus pathogenesis and IFN-I production when an appropriate stimulus for those TLRs, nucleic acid-containing immune complexes, is available. The link between such immune complexes and a strong IFN-I signature is a theme that has been supported by many laboratories (55-58). A contribution of the SLE-associated IRF5 genetic haplotype to production of anti-Ro antibody, even in asymptomatic women, and to progression to clinical lupus was demonstrated in a study of mothers of children with neonatal lupus (59). All of the mothers had high titer anti-Ro antibodies, but only some had elevated serum IFN-I. The data suggest that in addition to conferring increased risk for IFN-I production driven by nucleic acid-containing immune complexes, signaling components of the endosomal TLR pathways might contribute to production of lupus autoantibodies. Genetic polymorphisms associated with TLR-independent cytoplasmic sensors of nucleic acids or their downstream signaling mediators are also being identified. Variants in genes encoding the dsRNA sensor IFIH1 (MDA5) and the adaptor MAVS (IPS-1) are associated with SLE (60, 61).
Other genetic associations implicate signaling triggered by the type I IFN receptor (IFNAR) in increased expression of IFIGs. STAT4 and TYK2 encode components of the signaling pathway that induces IFIG transcription (62, 63). In addition, OPN, encoding osteopontin, appears to be important in regulating the IFN-I response (64).
Genetic variants that favor both innate and adaptive immune activation, perhaps in the setting of environmental factors that generate stress or oxidative damage, might be required to achieve a level of immune disruption required for inflammation, tissue damage and clinical signs and symptoms to develop (65).
Mechanisms contributing to type I IFN production in SLE
Plasmacytoid dendritic cells (pDC), which constitutively express IRF7, are the major IFN-α-producing cells, and available data implicate those cells in SLE. Intravenous high dose glucocorticoid therapy, provided as a treatment for lupus clinical flares, or the proteosome inhibitor bortezomib, depletes pDCs from the blood and temporarily ablates the IFN-I gene expression signature (18, 66). Moreover, studies of involved tissue from lupus patients identify pDCs proximate to cells that express high levels of IFIG or their protein products, supporting a direct role for local IFN-I production in the activation of the IFN-I pathway by adjacent cells (67). Other cell types, such as fibroblasts and epithelial cells, are known to produce IFN-β and other IFN-Is and might contribute to the IFN-I that is proposed to be pathogenic in patients. Neutrophils, a cell type that has captured recent interest as an inducer and producer of IFN-I (68-70), likely augment the IFN-I levels detected in patients. In fact, recent data document expression of IFN-α and IFIGs in neutrophils resident in lupus bone marrow, a logical site for TLR-mediated induction of IFN-α, given the presence of autoantibody-producing long-lived plasma cells and cell debris derived from apoptotic cells (71).
Advances in immunology that followed the description of the TLRs and the elucidation of the cytoplasmic nucleic acid sensors coincided with observations of a dominant IFN-I gene expression signature in many lupus patients and together stimulated active investigation of the mechanisms that favor production of IFN-I in SLE. Best developed is literature documenting the capacity and immunopathologic and clinical significance of nucleic acid-containing immune complexes in the induction of IFN-α after those complexes access intracellular endosomal TLRs, particularly TLR7 and TLR9 (55-58). In vitro systems have been used to demonstrate induction of IFN-I-induced gene transcripts, and in some cases IFN-α protein, in unfractionated PBMC or pDCs by serum or plasma from some lupus patients, isolated IgG or immune complexes from patients, or reconstituted immune complexes that contain autoantibodies and either RNA or necrotic or apoptotic cellular material. This effect can be inhibited by blockade of FcγRIIa or degradation of RNA or DNA and is augmented by cell products produced in the setting of inflammation, such as HMGB1 or LL37 (72, 73).
This in vitro demonstration of the potent IFN-inducing capacity of immune complexes containing nucleic acid, particularly those with RNA that can trigger cell activation through TLR7, has provided a view of the pathogenic role for autoantibodies in augmenting and perpetuating immune system activation that goes beyond the more traditional role of autoantibodies and immune complexes as relatively passive inducers of inflammation after deposition in target tissue. The immunopathogenic significance of RNA-containing immune complexes for lupus disease is supported by the clear association between autoantibodies that target the RNA-associated proteins Ro, La, Sm and RNP and the presence of an IFN-I signature in lupus PBMC (74).
Recent studies are defining the contribution of additional signals generated by FcR ligation and intracellular components of the autophagy pathway to the induction of IFN-α triggered through endosomal TLRs (75). Ligation of the FcR on pDCs by a large DNA-containing immune complex regulates recruitment of microtubule-associated protein 1 light chain 3 (LC3) to phagosomes in a PI3K-dependent process that also involves recruitment of autophagy proteins ATG5 and ATG7 to the phagosome. Induction of IFN-α by those complexes via IRF7 requires LC3, a potential point of differential regulation of IFN-I- vs. TNF-dependent clinical syndromes, as DNA-immune complex-mediated TNF production by pDCs does not require LC3. While this pathway has not yet been directly studied in lupus patients, GWAS data have identified lupus-associated polymorphisms in ATG5, supporting the potential immunopathogenic relevance of the coordination of phagosome formation and autophagy proteins in IFN-α production (76).
Less well developed is literature in human SLE supporting a relationship between activation of the TLR7 or TLR9 pathways and induction of those antibody specificities that ultimately target the protein or nucleic acid components of the stimulatory self-particle. The intersection between an endosomal TLR compartment accessed by a U1 RNA-Sm protein particle derived from a spliceosome, for example, or a hY RNA-Ro protein particle, and an antigen processing compartment might provide both self-antigen and adjuvant (the nucleic acid) to induce effective antigen-presenting capacity and, ultimately, T-cell dependent autoantibodies. Extensive data support a relationship between activation of the TLR pathway and production of autoantibodies that target RNA-protein particles (74, 77). Studies in other systems support the capacity of TLR ligand-associated self-proteins to facilitate presentation to T cells (78). As noted, the enrichment of lupus-associated IRF5 polymorphisms in asymptomatic individuals with high titer anti-Ro antibody is consistent with a relationship between the endosomal TLR pathways and autoantibody production (59). Work in the murine system supports the importance of B cell activation through endosomal TLRs in lupus, implicating B cell surface immunoglobulin receptors in capturing self-antigens that co-ligate TLRs and providing a possible mechanism for production of specific autoantibodies (77, 79, 80). Work by Bolland and others in studies of the BXSB murine model, characterized by a translocation and duplication of TLR7, emphasizes the relationship between TLR7, autoantibodies that target RNA-associated proteins, IFN-I induction, and disease pathogenesis (81).
While the role of nucleic acid-containing immune complexes in IFN-I production is well documented, those mechanisms that might account for IFN-I production prior to serologic evidence of autoimmunity are less well developed. The elucidation of the TLR-independent pathways that involve intracellular nucleic acid sensors, along with careful characterization of patients with rare lupus-like syndromes, provides support for a potential role for those pathways early in the pre-clinical stages of systemic autoimmune disease and might involve nonhematopoietic cells (48, 82). As noted, the association of mutations or deficiencies in important cell proteins that control availability of potentially stimulatory nucleic acids, including TREX1, components of the ribonuclease H2 enzyme complex, SAMHD1, and ADAR1, with IFN-I production and lupus-like disease is stimulating active investigation of the nature of nucleic acids that accumulate in the absence of those enzymes (83, 84). An important paper describing increased levels of IFN-β in TREX1-deficient mice drew the conclusion that impaired degradation of intracellular DNA might provide an innate immune stimulus that could drive an IFN-I response and prime the immune system for excessive activation and induction of autoimmunity (85). In that work, Stetson demonstrated DNA fragments enriched in genomic retrotransposon sequences, such as long interspersed nuclear element-1 (LINE-1, L1) and Alu, in DNA cloned from TREX1-deficient mice. In addition, recent data indicate that AGS patients deficient in SAMHD1 function show impaired regulation of L1 transposition (86). Our studies of kidney and salivary gland tissue from patients with SLE or Sjogren's syndrome, respectively, demonstrate expression of L1 RNA transcripts that are highly correlated with IFN-I expression in the same tissue (87) (Sagalovskiy, I., Mavragani, C., Crow, M.K., unpublished observations). Extensive and redundant quality control mechanisms, including those mediated by the noted enzymes along with members of the APOBEC and DNA methyltransferase protein families, contribute to maintenance of genome integrity, and altered function of any one or more of those mechanisms could generate a breech, resulting in virus-like stimuli with the potential to induce a host defense response that mimics a viral infection. However, in contrast to infection by an exogenous virus that induces a rapid burst of IFN-I followed by a well-orchestrated immune response that rapidly brings the infection under control, impaired control of genomic viral-like elements could result in chronic or recurrent activation of innate immune response pathways and alter the threshold for adaptive immune system activation in response to self-antigens, increasing risk for autoimmune disease. Recent data identify the cytoplasmic sensors of endogenous or microbial DNA and RNA as contributors to TLR-independent IFN-I production. DAI/ZBP1 and the cyclic GMP-AMP synthase (cGAS), which binds DNA and induces IFN-β after associating with the adaptor protein STING, and RIG-I and MDA5, recognizing 5’ triphosphate RNA or dsRNA and signaling through the adaptor MAVS, encoded by IFIH1 (with genetic variants associated with SLE), are of interest as mediators of IFN-I production (11, 12, 88-93). Oxidative damage to DNA, as assessed by 8-hydroxyguanosine DNA modifications, might confer resistance of DNA to degradation by TREX1 or other mediators of genome integrity, promoting activation of cytoplasmic DNA sensors (65). Both cytoplasmic DNA and RNA sensing pathways activate IKKε, TBK1 and IRF3, in some cases inducing IFIG, even in the absence of IFN-I, and other cytoplasmic helicases (DDX24) regulate these signaling pathways (94-96). These insights into mechanisms of IFN-pathway activation that do not require autoantibody-containing immune complexes suggest a number of candidate therapeutic targets.
Increased availability of stimulatory nucleic acids would implicate IFN-I production and activation of IFIGs as an underlying and chronic/recurrent mechanism that generates an immune system that is “primed” to respond to additional triggers with further immune activation and inflammation. In that regard, neutrophils are of interest as potential amplifiers of the IFN-I pathway. Long implicated in lupus pathogenesis in studies demonstrating their engulfment of cell nuclei (the so-called “LE cell”), neutrophils also produce and induce IFN-I (68-71). Whether neutrophils induce IFN-I as intact cells or rather as degradation products or subcellular particles remains a question of current interest. The concept of neutrophil extracellular traps (or NETs) was proposed as an organizing mechanism for a collection of stimulatory factors, including DNA, that could promote IFN-I production by pDCs, but the in vivo relevance of this phenomenon has been difficult to demonstrate (97-99). The alternate view that neutrophil-derived organelles containing DNA might serve as delivery vehicles for induction of IFN-I, and potentially for induction of anti-DNA antibodies, is an attractive concept that could be viewed as representing a parallel mechanism to that just described for induction of IFN-I by genomic viral-like elements (100, 101). In settings in which neutrophils become activated, either by exogenous stimuli or by nucleic acid-containing immune complexes, mitochondria enriched in potentially stimulatory DNA might be particularly effective in triggering IFN-I production and might even serve as inducers and targets of anti-DNA autoantibodies. In our longitudinal studies, gene transcripts encoding proteins expressed in neutrophil granules are associated with lupus flares, possibly implicating neutrophil activation in clinical exacerbations (Olferiev, M., Crow, M.K., unpublished observations).
Contribution of IFN-I to immunopathogenesis of SLE
Data from studies of host defense in response to virus infection, along with bioinformatic analysis of IFIG, identify aspects of immune function that might be modified by the high level IFN-I seen in most lupus patients (102, 103). While IFN-I can alter function of myeloid and lymphocyte lineage cells in a manner consistent with many of the immunologic alterations that characterize patients with SLE (104), it is difficult to determine whether those IFN-I effects are directly pathogenic. Recent murine studies examining the role of IFN-I in the context of chronic lymphocytic choriomeningitis virus infection provide an excellent model when considering the pathogenic potential of sustained elevated levels of IFN-I in patients with SLE (42, 43). In those mice, IFN-I blockade reduced pathologic immune activation and restored the architecture of damaged tissue, suggesting that in the lupus scenario, sustained production of IFN-I is also pathogenic.
In addition to data supporting a damaging effect of IFN-I when sustained over time are studies in the human system, including data from patients. An activity in lupus serum that was inhibited by anti-IFN-α antibody augmented T cell stimulatory function in an allogeneic mixed lymphocyte reaction system, suggesting that IFN-α might also promote activation of self-reactive T cells (105). IFN-α induces BLyS, thereby providing support for B cell differentiation, and supports immunoglobulin class switching to generate potentially pathogenic autoantibodies (106). More direct evidence for a pathogenic role for IFN-α in lupus comes from analysis of tissue. Studies of skin biopsies from patients with cutaneous lupus or SLE document pDCs and MX1, a classic IFIG, in the biopsy tissue (67). Synovial tissue from lupus patients with arthritis is rich in IFIG's, including gene transcripts similar to those seen in lupus PBMC. The gene expression pattern in lupus synovial tissue is distinguished from that of patients with rheumatoid arthritis, which shows only modest expression of IFIGs in some patients (107). The functional significance of these distinct patterns has been explored in studies of osteoclast differentiation which suggest that high expression of IFN-β might inhibit osteoclastogenesis and skew myeloid cells toward antigen presenting cell function rather than a bone resorbing phenotype based on induction of CXCL11 by IFN-I (108). Additional data support a pathogenic role for IFN-α in two organ systems that are most associated with morbidity and mortality of lupus patients: the kidney and the cardiovascular system. pDCs are enriched in kidneys of patients with membranoproliferative glomerulonephritis, and IFN-α transcripts are present in biopsy specimens from those patients (109). Data from murine lupus models demonstrate that IFN-α can accelerate disease and contribute to renal damage (110). IFN-α also damages podocytes and induces chemokines that are responsible for recruitment of inflammatory cells, particularly neutrophils, to kidney and other involved tissue (111). In that regard, high level expression of IFN-induced chemokines may be a biomarker of future disease flare (112). Substantial support for IFN-α playing a pathogenic role in premature atherosclerosis in lupus has been provided by several groups, with IFN-α inhibiting production or survival of endothelial progenitor cells that are important for vascular repair and promoting foam cell formation (113, 114). A contribution of IFN-α to the central nervous system manifestations of lupus is suggested by the recent observation that neurotoxic lymphocytes can be activated by IFN-α and mediate CNS damage based on their toxic effects on astrocytes (115).
Taken together, descriptive data from lupus patients, in vitro studies and data from murine models support a variety of pathogenic effects of IFN-α that are likely to contribute to many of the immune system alterations that characterize SLE, and, indirectly, to the tissue manifestations of disease.
Progress in therapeutic targeting of IFN-I in SLE
In view of the abundant data from lupus patients as well as murine lupus models supporting a significant, if not central, role for IFN-α in the pathogenesis of SLE and potentially other systemic autoimmune diseases, therapeutic targeting of the IFN-I pathway has emerged as an important focus of drug development efforts, as recently reviewed (116). Therapeutic strategies addressing mechanisms of induction of IFN-α, direct blockade of IFN-α and its receptor, and modulators of its downstream signaling pathway are being evaluated for their capacity to reduce disease activity, permit reduction of steroid dose or prevent future disease flares.
The most direct approach to therapeutic inhibition of the type I IFN pathway involves blockade of its major stimulus, IFN-α, with a monoclonal antibody (mAb). Three anti-IFN-α mAbs are in development, and safety data indicate that they are generally well tolerated (116, 117). AGS-009 has successfully completed a phase Ia safety trial. Sifalimumab (MEDI-545) partially inhibited expression of IFIGs in whole blood cells and in skin in some patients, but the degree of inhibition of the IFN signature was greater patients with moderate disease (mean SLE Disease Activity Index, SLEDAI, 5.2) than in patients with more active disease (mean SLEDAI of 11.0) (118, 119). Most patients receiving 3 mg/kg or 10 mg/kg IV of rontalizumab (with mean SLEDAI of 3.4) demonstrated >50% reduction of IFIGs in whole blood but did not successfully achieve a level comparable to healthy donors or patients with a low IFN score (120). Of note, all patients receiving single or multiple doses of that mAb recovered their IFN signature by six months after the last dose, suggesting that the stimulus for induction of type I IFN remained operative in vivo. Higher doses of antibody might provide more effective blockade, however a potential role for additional IFN types, including IFN-β or IFN-ω, not targeted by the tested agents, or inadequate blockade of some IFN-α family members, must be considered as an explanation for the incomplete IFIG inhibition noted. In contrast to the inhibition of IFIG in lupus skin by anti-IFN-α mAb, the IFN signature seen in psoriatic skin was not inhibited by sifalimumab, nor was any indication of clinical efficacy seen in psoriasis patients. IFN-κ, a distinct type I IFN, is particularly expressed in skin and might account for the lack of effect of an IFN-α specific mAb in the psoriasis study (121).
A creative approach to generating endogenous polyclonal anti-IFN-α, with the goal of inhibiting pathogenic IFN-α, has been reported in preclinical studies and more recently in a first in human study of a so-called IFN-kinoid, a complex of recombinant IFN-α with KLH (122). Injection of the IFN-kinoid resulted in T cell-dependent production of neutralizing IFN-α antibody that reduced expression of IFIGs in whole blood samples.
The partial amelioration of disease in lupus mice after administration of an antibody specific for IFNAR identifies an alternative approach to reducing the IFN signature (123). MEDI-546 mAb, specific for subunit 1 of IFNAR, prevents association of subunit 1 with subunit 2 of IFNAR, thereby blocking downstream signaling events. This mAb has been administered to patients with diffuse systemic sclerosis and, in contrast to the partial effects on IFIG expression in the sifalimumab and rontalizumab studies, resulted in nearly complete inhibition of IFIG expression in peripheral blood and skin with several dosing regimens (124).
As nucleic acid-containing immune complexes are potent triggers of endosomal TLRs and IFN-I, therapeutic approaches that degrade the nucleic acid components of those complexes or inhibit TLR activation and downstream signaling hold particular promise as they would be predicted to reduce levels of type I IFN, limit the capacity of immune complexes to induce new IFN-I and might also reduce production of autoantibodies (125-127). In that regard, treatment of lupus patients with hydroxychloroquine, documented to inhibit IFN-I production induced by lupus immune complexes in vitro, is now standard of care due to its demonstrated reduction of lupus flares (128). Studies in progress are investigating the safety of several oligonucleotide inhibitors of endosomal TLRs. Additional therapeutic approaches might target kinases and adaptors required for effective induction of IFN-α through the TLR pathway (PI3K) or those activated by cytoplasmic nucleic acids (IKKε, cGAS, STING) (129). While not specific for IFN-I, inhibition of the Jak-STAT signaling pathway with Jak kinase inhibitors might reduce the induction of IFIGs and their downstream effects (130).
Summary and Conclusion
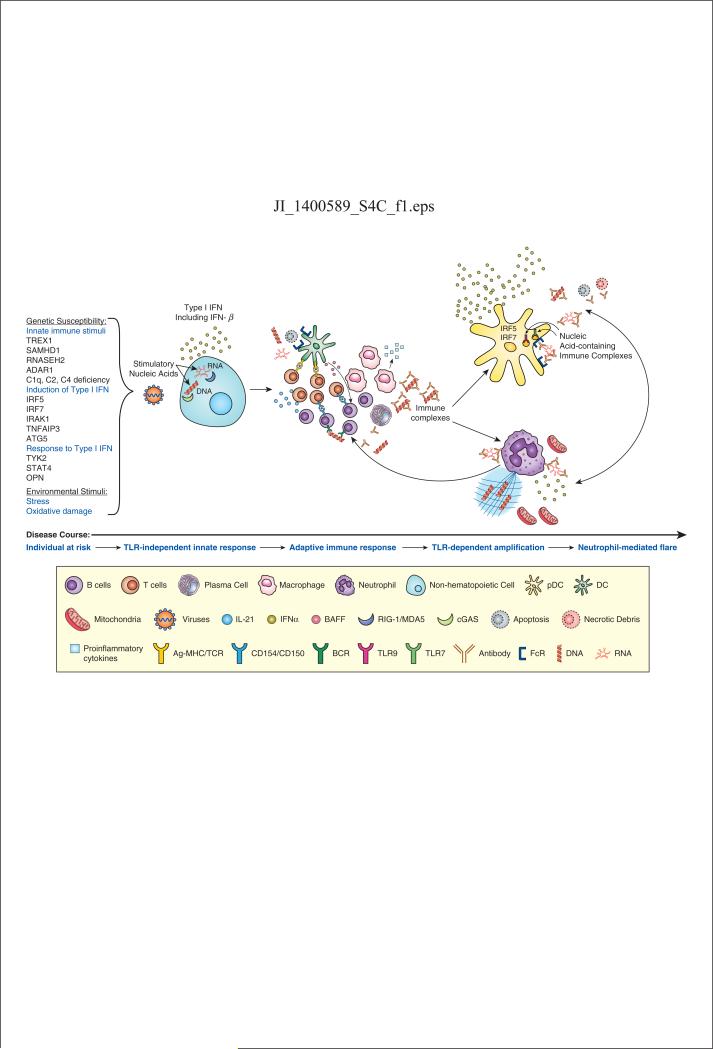
Detailed study of lupus patients and insights from murine models, superimposed on significant advances in the characterization of the mechanisms that trigger and regulate the innate immune system in the setting of viral infection, have identified IFN-I, and particularly IFN-α, as a central pathogenic mediator in SLE. Genetic variants associated with a diagnosis of SLE, along with in vitro and ex vivo studies of patient cells and tissues, point to the significance of effective regulation of endogenous nucleic acids, potential triggers of cytoplasmic receptors, as well as components of TLR-activating immune complexes, in determining lupus susceptibility. A comprehensive view of lupus, with IFN-I a focal point, establishes this prototype disease as one in which endogenous nucleic acids provide a sustained stimulus for a broad immune response that should be reserved for infection with a persistent virus. Our preferred view is that cytoplasmic nucleic acids enriched in virus-like properties might be an early stimulus, while DNA enriched in neutrophil-derived organelles might be particularly associated with lupus flare (Figure 1). Once autoantibodies have been produced, nucleic acid-containing immune complexes provide a particularly potent stimulus for sustained immune dysregulation and disease. Perhaps less important than precisely defining the sequence of events that culminate in clinical lupus is the recognition that multiple points of innate immune regulation, including both TLR-independent and TLR-dependent mechanisms, provide candidate therapeutic targets with potential to lead to therapies that improve outcomes for patients. While important questions remain to be satisfactorily addressed, notably the explanation for the extreme sex skewing of the disease and the contribution of environmental agents to disease pathogenesis, the recent insights into the central role for IFN-I and the innate immune response in this challenging disease hold important potential for advancing care of patients.
Figure 1. Type I IFN-centric view of SLE pathogenesis.
Numerous polymorphisms in genes encoding regulators of nucleic acid degradation or mediators of pathways involved in induction of or response to IFN-I, along with environmental factors that lead to cellular stress or oxidative damage, generate a host endowed with enhanced capacity to activate the IFN-I pathway. A favored view is that stimulatory endogenous nucleic acids activate cytoplasmic nucleic acid sensors and induce IFN-I, possibly enriched in IFN-β, although an exogenous viral trigger remains possible. A recent study suggests that this early event might occur in nonhematopoietic cells (82). The resulting IFN-I will prime an adaptive immune response, and in a host enriched in gene variants that lower the threshold for lymphocyte activation, a self-reactive immune response may be favored. When autoantibodies target nucleic acids or nucleic acid-binding proteins, immune complexes form and strongly amplify IFN-I production, predominantly IFN-α, through Fc receptor-dependent activation of pDCs mediated by endosomal TLRs. The adjuvant activity of the immune complex also amplifies the autoimmune response to the associated nucleic acid-binding proteins. Neutrophils can respond to exposure to IFN-I and immune complexes with extrusion of nucleic acids and associated proteins, in the form of mitochondria or neutrophil extracellular traps (NETs), produce IFN-I and contribute to disease flares. Neutrophils and pDCs can each amplify production of IFN-I by the other cell type, modify function of adaptive immune system cells and amplify autoimmunity. The immunologic consequences of this sustained viral-like response result in broad immune dysregulation, inflammation and tissue damage.
Acknowledgments
The author acknowledges the significant contributions of her colleagues, whose effort and insights have advanced understanding of the role of type I interferon in SLE, particularly Jing Hua, Kyriakos A. Kirou, Clio Mavragani, Timothy Niewold, Mikhail Olferiev and Irina Sagalovskiy.
This work was supported by the Mary Kirkland Center for Lupus Research, National Institutes of Health grant AI059893, and research grants from the Alliance for Lupus Research and the Lupus Research Institute.
Abbreviations used in this article
- SLE
systemic lupus erythematosus
- IFN-I
type I interferon
- IFIG
interferon-induced gene expression
- IFNAR
type I interferon receptor
- AGS
Aicardi-Goutieres syndrome
- IRF5
IFN-regulatory factor 5
- IFNAR
type I interferon receptor
- pDC
plasmacytoid dendritic cells
- SLEDAI
SLE Disease Activity Index
- mAb
monoclonal antibody
References
- 1.Crow MK. Developments in the clinical understanding of lupus. Arthritis Res Ther. 2009;11:245. doi: 10.1186/ar2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kono DH, Baccala R, Theofilopoulos AN. TLRs and interferons: a central paradigm in autoimmunity. Curr Opin Immunol. 2013;25:720–727. doi: 10.1016/j.coi.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 4.Lerner EA, Lerner MR, Hardin JA, Janeway CA, Jr., Steitz JA. Deciphering the mysteries of RNA-containing lupus antigens. Arthritis Rheum. 1982;25:761–766. doi: 10.1002/art.1780250709. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi T, Abe T, Koide J, Hosono O, Morimoto C, Homma M. Cellular mechanism of DNA-specific antibody synthesis by lymphocytes from systemic lupus erythematosus patients. Arthritis Rheum. 1984;27:766–773. doi: 10.1002/art.1780270707. [DOI] [PubMed] [Google Scholar]
- 6.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, van Vollenhoven RF. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witt M, Grunke M, Proft F, Baeuerle M, Aringer M, Burmester G, Chehab G, Fiehn C, Fischer-Betz R, Fleck M, Freivogel K, Haubitz M, Kotter I, Lovric S, Metzler C, Rubberth-Roth A, Schwarting A, Specker C, Tony HP, Unger L, Wassenberg S, Dorner T, Schulze-Koops H. Clinical outcomes and safety of rituximab treatment for patients with systemic lupus erythematosus (SLE) - results from a nationwide cohort in Germany (GRAID). Lupus. 2013;22:1142–1149. doi: 10.1177/0961203313503912. [DOI] [PubMed] [Google Scholar]
- 8.Wofsy D, Hillson JL, Diamond B. Abatacept for lupus nephritis: alternative definitions of complete response support conflicting conclusions. Arthritis Rheum. 2012;64:3660–3665. doi: 10.1002/art.34624. [DOI] [PubMed] [Google Scholar]
- 9.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 10.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 11.Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]
- 12.Unterholzner L. The interferon response to intracellular DNA: why so many receptors? Immunobiology. 2013;218:1312–1321. doi: 10.1016/j.imbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Crow MK, Kirou KA. Interferon-alpha in systemic lupus erythematosus. Curr Opin Rheumatol. 2004;16:541–547. doi: 10.1097/01.bor.0000135453.70424.1b. [DOI] [PubMed] [Google Scholar]
- 14.Ronnblom L, Alm GV, Eloranta ML. The type I interferon system in the development of lupus. Semin Immunol. 2011;23:113–121. doi: 10.1016/j.smim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg AD, Baron S, Talal N. The pathogenesis of autoimmunity in New Zealand mice, I. Induction of antinucleic acid antibodies by polyinosinic-polycytidylic acid. Proc Natl Acad Sci U S A. 1969;63:1102–1107. doi: 10.1073/pnas.63.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity. 2003;36:511–518. doi: 10.1080/08916930310001605873. [DOI] [PubMed] [Google Scholar]
- 17.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- 20.Crow MK, Wohlgemuth J. Microarray analysis of gene expression in lupus. Arthritis Res Ther. 2003;5:279–287. doi: 10.1186/ar1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han GM, Chen SL, Shen N, Ye S, Bao CD, Gu YY. Analysis of gene expression profiles in human systemic lupus erythematosus using oligonucleotide microarray. Genes Immun. 2003;4:177–186. doi: 10.1038/sj.gene.6363966. [DOI] [PubMed] [Google Scholar]
- 22.Absher DM, Li X, Waite LL, Gibson A, Roberts K, Edberg J, Chatham WW, Kimberly RP. Genome-wide DNA methylation analysis of systemic lupus erythematosus reveals persistent hypomethylation of interferon genes and compositional changes to CD4+ T-cell populations. PLoS Genet. 2013;9:e1003678. doi: 10.1371/journal.pgen.1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coit P, Jeffries M, Altorok N, Dozmorov MG, Koelsch KA, Wren JD, Merrill JT, McCune WJ, Sawalha AH. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naive CD4+ T cells from lupus patients. J Autoimmun. 2013;43:78–84. doi: 10.1016/j.jaut.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, Ly N, Woodward RN, Fry KE, Lau AY, Prentice JG, Wohlgemuth JG, Crow MK. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Higgs BW, Morehouse C, de Los Reyes M, Trigona W, Brohawn P, White W, Zhang J, White B, Coyle AJ, Kiener PA, Jallal B. Development of Potential Pharmacodynamic and Diagnostic Markers for Anti-IFN-alpha Monoclonal Antibody Trials in Systemic Lupus Erythematosus. Hum Genomics Proteomics. 20092009 doi: 10.4061/2009/374312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 27.Becker AM, Dao KH, Han BK, Kornu R, Lakhanpal S, Mobley AB, Li QZ, Lian Y, Wu T, Reimold AM, Olsen NJ, Karp DR, Chowdhury FZ, Farrar JD, Satterthwaite AB, Mohan C, Lipsky PE, Wakeland EK, Davis LS. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLoS One. 2013;8:e67003. doi: 10.1371/journal.pone.0067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, Dong X, Chen W, Kim MH, Weng HH, Furst DE, Gorn A, McMahon M, Taylor M, Brahn E, Hahn BH, Tsao BP. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2951–2962. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- 29.Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, Stichweh D, Blankenship D, Li L, Munagala I, Bennett L, Allantaz F, Mejias A, Ardura M, Kaizer E, Monnet L, Allman W, Randall H, Johnson D, Lanier A, Punaro M, Wittkowski KM, White P, Fay J, Klintmalm G, Ramilo O, Palucka AK, Banchereau J, Pascual V. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petri M, Singh S, Tesfasyone H, Dedrick R, Fry K, Lal P, Williams G, Bauer J, Gregersen P, Behrens T, Baechler E. Longitudinal expression of type I interferon responsive genes in systemic lupus erythematosus. Lupus. 2009;18:980–989. doi: 10.1177/0961203309105529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landolt-Marticorena C, Bonventi G, Lubovich A, Ferguson C, Unnithan T, Su J, Gladman DD, Urowitz M, Fortin PR, Wither J. Lack of association between the interferon-alpha signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2009;68:1440–1446. doi: 10.1136/ard.2008.093146. [DOI] [PubMed] [Google Scholar]
- 32.Chiche L, Jourde-Chiche N, Pascual V, Chaussabel D. Current perspectives on systems immunology approaches to rheumatic diseases. Arthritis Rheum. 2013;65:1407–1417. doi: 10.1002/art.37909. [DOI] [PubMed] [Google Scholar]
- 33.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waddell SJ, Popper SJ, Rubins KH, Griffiths MJ, Brown PO, Levin M, Relman DA. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PLoS One. 2010;5:e9753. doi: 10.1371/journal.pone.0009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall JC, Casciola-Rosen L, Berger AE, Kapsogeorgou EK, Cheadle C, Tzioufas AG, Baer AN, Rosen A. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc Natl Acad Sci U S A. 2012;109:17609–17614. doi: 10.1073/pnas.1209724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Q, Yang Q, Lourenco E, Sun H, Zhang Y. Interferon-lambda1 induces peripheral blood mononuclear cell-derived chemokines secretion in patients with systemic lupus erythematosus: its correlation with disease activity. Arthritis Res Ther. 2011;13:R88. doi: 10.1186/ar3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morimoto AM, Flesher DT, Yang J, Wolslegel K, Wang X, Brady A, Abbas AR, Quarmby V, Wakshull E, Richardson B, Townsend MJ, Behrens TW. Association of endogenous anti-interferon-alpha autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:2407–2415. doi: 10.1002/art.30399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg SA, Higgs BW, Morehouse C, Walsh RJ, Kong SW, Brohawn P, Zhu W, Amato A, Salajegheh M, White B, Kiener PA, Jallal B, Yao Y. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun. 2012;13:207–213. doi: 10.1038/gene.2011.61. [DOI] [PubMed] [Google Scholar]
- 39.Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, Segal B, Rhodus NL, Moser KL. Peripheral blood gene expression profiling in Sjogren's syndrome. Genes Immun. 2009;10:285–296. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgs BW, Liu Z, White B, Zhu W, White WI, Morehouse C, Brohawn P, Kiener PA, Richman L, Fiorentino D, Greenberg SA, Jallal B, Yao Y. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis. 2011;70:2029–2036. doi: 10.1136/ard.2011.150326. [DOI] [PubMed] [Google Scholar]
- 41.Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, Mudd J, Schacker T, Funderburg NT, Pilch-Cooper HA, Debernardo R, Rabin RL, Lederman MM, Harding CV. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One. 2013;8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng Y, Tsao BP. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol. 2010;6:683–692. doi: 10.1038/nrrheum.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai X, James RG, Habib T, Singh S, Jackson S, Khim S, Moon RT, Liggitt D, Wolf-Yadlin A, Buckner JH, Rawlings DJ. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest. 2013;123:2024–2036. doi: 10.1172/JCI66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li QZ, Zhou J, Yang R, Yan M, Ye Q, Liu K, Liu S, Shao X, Li L, Zhou XJ, Wakeland EK, Mohan C. The lupus-susceptibility gene kallikrein downmodulates antibody-mediated glomerulonephritis. Genes Immun. 2009;10:503–508. doi: 10.1038/gene.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crow YJ, Rehwinkel J. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum Mol Genet. 2009;18:R130–136. doi: 10.1093/hmg/ddp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee-Kirsch MA, Wolf C, Gunther C. Aicardi-Goutieres syndrome: a model disease for systemic autoimmunity. Clin Exp Immunol. 2014;175:17–24. doi: 10.1111/cei.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Namjou B, Kothari PH, Kelly JA, Glenn SB, Ojwang JO, Adler A, Alarcon-Riquelme ME, Gallant CJ, Boackle SA, Criswell LA, Kimberly RP, Brown E, Edberg J, Stevens AM, Jacob CO, Tsao BP, Gilkeson GS, Kamen DL, Merrill JT, Petri M, Goldman RR, Vila LM, Anaya JM, Niewold TB, Martin J, Pons-Estel BA, Sabio JM, Callejas JL, Vyse TJ, Bae SC, Perrino FW, Freedman BI, Scofield RH, Moser KL, Gaffney PM, James JA, Langefeld CD, Kaufman KM, Harley JB, Atkinson JP. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 2011;12:270–279. doi: 10.1038/gene.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice GI, Forte GM, Szynkiewicz M, Chase DS, Aeby A, Abdel-Hamid MS, Ackroyd S, Allcock R, Bailey KM, Balottin U, Barnerias C, Bernard G, Bodemer C, Botella MP, Cereda C, Chandler KE, Dabydeen L, Dale RC, De Laet C, De Goede CG, Del Toro M, Effat L, Enamorado NN, Fazzi E, Gener B, Haldre M, Lin JP, Livingston JH, Lourenco CM, Marques W, Jr., Oades P, Peterson P, Rasmussen M, Roubertie A, Schmidt JL, Shalev SA, Simon R, Spiegel R, Swoboda KJ, Temtamy SA, Vassallo G, Vilain CN, Vogt J, Wermenbol V, Whitehouse WP, Soler D, Olivieri I, Orcesi S, Aglan MS, Zaki MS, Abdel-Salam GM, Vanderver A, Kisand K, Rozenberg F, Lebon P, Crow YJ. Assessment of interferon-related biomarkers in Aicardi-Goutieres syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol. 2013;12:1159–1169. doi: 10.1016/S1474-4422(13)70258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Troedson C, Wong M, Dalby-Payne J, Wilson M, Dexter M, Rice GI, Crow YJ, Dale RC. Systemic lupus erythematosus due to C1q deficiency with progressive encephalopathy, intracranial calcification and acquired moyamoya cerebral vasculopathy. Lupus. 2013;22:639–643. doi: 10.1177/0961203313486950. [DOI] [PubMed] [Google Scholar]
- 53.Lazear HM, Lancaster A, Wilkins C, Suthar MS, Huang A, Vick SC, Clepper L, Thackray L, Brassil MM, Virgin HW, Nikolich-Zugich J, Moses AV, Gale M, Jr., Fruh K, Diamond MS. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 2013;9:e1003118. doi: 10.1371/journal.ppat.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Ronnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjogren's syndrome autoantigen-associated RNA. Arthritis Rheum. 2006;54:1917–1927. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- 57.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly KM, Zhuang H, Nacionales DC, Scumpia PO, Lyons R, Akaogi J, Lee P, Williams B, Yamamoto M, Akira S, Satoh M, Reeves WH. “Endogenous adjuvant” activity of the RNA components of lupus autoantigens Sm/RNP and Ro 60. Arthritis Rheum. 2006;54:1557–1567. doi: 10.1002/art.21819. [DOI] [PubMed] [Google Scholar]
- 59.Cherian TS, Kariuki SN, Franek BS, Buyon JP, Clancy RM, Niewold TB. Brief Report: IRF5 systemic lupus erythematosus risk haplotype is associated with asymptomatic serologic autoimmunity and progression to clinical autoimmunity in mothers of children with neonatal lupus. Arthritis Rheum. 2012;64:3383–3387. doi: 10.1002/art.34571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson T, Kariuki SN, Franek BS, Kumabe M, Kumar AA, Badaracco M, Mikolaitis RA, Guerrero G, Utset TO, Drevlow BE, Zaacks LS, Grober JS, Cohen LM, Kirou KA, Crow MK, Jolly M, Niewold TB. Autoimmune disease risk variant of IFIH1 is associated with increased sensitivity to IFN-alpha and serologic autoimmunity in lupus patients. J Immunol. 2011;187:1298–1303. doi: 10.4049/jimmunol.1100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pothlichet J, Niewold TB, Vitour D, Solhonne B, Crow MK, Si-Tahar M. A loss-of-function variant of the antiviral molecule MAVS is associated with a subset of systemic lupus patients. EMBO Mol Med. 2011;3:142–152. doi: 10.1002/emmm.201000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cunninghame Graham DS, Morris DL, Bhangale TR, Criswell LA, Syvanen AC, Ronnblom L, Behrens TW, Graham RR, Vyse TJ. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 2011;7:e1002341. doi: 10.1371/journal.pgen.1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol. 2009;182:34–38. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO, Niewold TB. Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun. 2009;10:487–494. doi: 10.1038/gene.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, Tuting T, Hartmann G, Barchet W. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Hirai M, Kadowaki N, Kitawaki T, Fujita H, Takaori-Kondo A, Fukui R, Miyake K, Maeda T, Kamihira S, Miyachi Y, Uchiyama T. Bortezomib suppresses function and survival of plasmacytoid dendritic cells by targeting intracellular trafficking of Toll-like receptors and endoplasmic reticulum homeostasis. Blood. 2011;117:500–509. doi: 10.1182/blood-2010-05-284737. [DOI] [PubMed] [Google Scholar]
- 67.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, McCune WJ, Kaplan MJ. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan MJ. Role of neutrophils in systemic autoimmune diseases. Arthritis Res Ther. 2013;15:219. doi: 10.1186/ar4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindau D, Mussard J, Rabsteyn A, Ribon M, Kotter I, Igney A, Adema GJ, Boissier MC, Rammensee HG, Decker P. TLR9 independent interferon alpha production by neutrophils on NETosis in response to circulating chromatin, a key lupus autoantigen. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-203041. [DOI] [PubMed] [Google Scholar]
- 71.Palanichamy A, Bauer JW, Yalavarthi S, Meednu N, Barnard J, Owen T, Cistrone C, Bird A, Rabinovich A, Nevarez S, Knight JS, Dedrick R, Rosenberg A, Wei C, Rangel-Moreno J, Liesveld J, Sanz I, Baechler E, Kaplan MJ, Anolik JH. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol. 2014;192:906–918. doi: 10.4049/jimmunol.1302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 73.Chamilos G, Gregorio J, Meller S, Lande R, Kontoyiannis DP, Modlin RL, Gilliet M. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood. 2012;120:3699–3707. doi: 10.1182/blood-2012-01-401364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 75.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, Coyle AJ, Kolbeck R, Green DR, Sanjuan MA. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–997. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou XJ, Lu XL, Lv JC, Yang HZ, Qin LX, Zhao MH, Su Y, Li ZG, Zhang H. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis. 2011;70:1330–1337. doi: 10.1136/ard.2010.140111. [DOI] [PubMed] [Google Scholar]
- 77.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 79.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol. 2011;23:106–112. doi: 10.1016/j.smim.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koh YT, Scatizzi JC, Gahan JD, Lawson BR, Baccala R, Pollard KM, Beutler BA, Theofilopoulos AN, Kono DH. Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells. J Immunol. 2013;190:4982–4990. doi: 10.4049/jimmunol.1202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc Natl Acad Sci U S A. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gall A, Treuting P, Elkon KB, Loo YM, Gale M, Jr., Barber GN, Stetson DB. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131:873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 84.Behrendt R, Schumann T, Gerbaulet A, Nguyen LA, Schubert N, Alexopoulou D, Berka U, Lienenklaus S, Peschke K, Gibbert K, Wittmann S, Lindemann D, Weiss S, Dahl A, Naumann R, Dittmer U, Kim B, Mueller W, Gramberg T, Roers A. Mouse SAMHD1 has antiretroviral activity and suppresses a spontaneous cell-intrinsic antiviral response. Cell Rep. 2013;4:689–696. doi: 10.1016/j.celrep.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao K, Du J, Han X, Goodier JL, Li P, Zhou X, Wei W, Evans SL, Li L, Zhang W, Cheung LE, Wang G, Kazazian HH, Jr., Yu XF. Modulation of LINE-1 and Alu/SVA retrotransposition by Aicardi-Goutieres syndrome-related SAMHD1. Cell Rep. 2013;4:1108–1115. doi: 10.1016/j.celrep.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crow MK. Long interspersed nuclear elements (LINE-1): potential triggers of systemic autoimmune disease. Autoimmunity. 2010;43:7–16. doi: 10.3109/08916930903374865. [DOI] [PubMed] [Google Scholar]
- 88.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 89.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 2013 doi: 10.1016/j.it.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 91.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X, Brann TW, Zhou M, Yang J, Oguariri RM, Lidie KB, Imamichi H, Huang DW, Lempicki RA, Baseler MW, Veenstra TD, Young HA, Lane HC, Imamichi T. Cutting edge: Ku70 is a novel cytosolic DNA sensor that induces type III rather than type I IFN. J Immunol. 2011;186:4541–4545. doi: 10.4049/jimmunol.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 95.Hasan M, Koch J, Rakheja D, Pattnaik AK, Brugarolas J, Dozmorov I, Levine B, Wakeland EK, Lee-Kirsch MA, Yan N. Trex1 regulates lysosomal biogenesis and interferon-independent activation of antiviral genes. Nat Immunol. 2013;14:61–71. doi: 10.1038/ni.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma Z, Moore R, Xu X, Barber GN. DDX24 Negatively Regulates Cytosolic RNA-Mediated Innate Immune Signaling. PLoS Pathog. 2013;9:e1003721. doi: 10.1371/journal.ppat.1003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190:1217–1226. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ries M, Schuster P, Thomann S, Donhauser N, Vollmer J, Schmidt B. Identification of novel oligonucleotides from mitochondrial DNA that spontaneously induce plasmacytoid dendritic cell activation. J Leukoc Biol. 2013;94:123–135. doi: 10.1189/jlb.0612278. [DOI] [PubMed] [Google Scholar]
- 102.Feng D, Barnes BJ. Bioinformatics analysis of the factors controlling type I IFN gene expression in autoimmune disease and virus-induced immunity. Front Immunol. 2013;4:291. doi: 10.3389/fimmu.2013.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pace L, Vitale S, Dettori B, Palombi C, La Sorsa V, Belardelli F, Proietti E, Doria G. APC activation by IFN-alpha decreases regulatory T cell and enhances Th cell functions. J Immunol. 2010;184:5969–5979. doi: 10.4049/jimmunol.0900526. [DOI] [PubMed] [Google Scholar]
- 105.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 106.Ittah M, Miceli-Richard C, Eric Gottenberg J, Lavie F, Lazure T, Ba N, Sellam J, Lepajolec C, Mariette X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren's syndrome. Arthritis Res Ther. 2006;8:R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nzeusseu Toukap A, Galant C, Theate I, Maudoux AL, Lories RJ, Houssiau FA, Lauwerys BR. Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 2007;56:1579–1588. doi: 10.1002/art.22578. [DOI] [PubMed] [Google Scholar]
- 108.Coelho LF, Magno de Freitas Almeida G, Mennechet FJ, Blangy A, Uze G. Interferon-alpha and -beta differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression. Proc Natl Acad Sci U S A. 2005;102:11917–11922. doi: 10.1073/pnas.0502188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tucci M, Quatraro C, Lombardi L, Pellegrino C, Dammacco F, Silvestris F. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58:251–262. doi: 10.1002/art.23186. [DOI] [PubMed] [Google Scholar]
- 110.Liu Z, Bethunaickan R, Huang W, Lodhi U, Solano I, Madaio MP, Davidson A. Interferon-alpha accelerates murine systemic lupus erythematosus in a T cell-dependent manner. Arthritis Rheum. 2011;63:219–229. doi: 10.1002/art.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Migliorini A, Angelotti ML, Mulay SR, Kulkarni OO, Demleitner J, Dietrich A, Sagrinati C, Ballerini L, Peired A, Shankland SJ, Liapis H, Romagnani P, Anders HJ. The antiviral cytokines IFN-alpha and IFN-beta modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol. 2013;183:431–440. doi: 10.1016/j.ajpath.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 112.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, Panoskaltsis-Mortari A, Gregersen PK, Behrens TW, Baechler EC. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 2009;60:3098–3107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J, Fu Q, Cui H, Qu B, Pan W, Shen N, Bao C. Interferon-alpha priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-alpha and atherosclerosis in lupus. Arthritis Rheum. 2011;63:492–502. doi: 10.1002/art.30165. [DOI] [PubMed] [Google Scholar]
- 114.Somers EC, Zhao W, Lewis EE, Wang L, Wing JJ, Sundaram B, Kazerooni EA, McCune WJ, Kaplan MJ. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PLoS One. 2012;7:e37000. doi: 10.1371/journal.pone.0037000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pulliero A, Marengo B, Longobardi M, Fazzi E, Orcesi S, Olivieri I, Cereda C, Domenicotti C, Balottin U, Izzotti A. Inhibition of the de-myelinating properties of Aicardi-Goutieres syndrome lymphocytes by cathepsin D silencing. Biochem Biophys Res Commun. 2013;430:957–962. doi: 10.1016/j.bbrc.2012.11.131. [DOI] [PubMed] [Google Scholar]
- 116.Kirou KA, Gkrouzman E. Anti-interferon alpha treatment in SLE. Clin Immunol. 2013;148:303–312. doi: 10.1016/j.clim.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 117.Stohl W. Future prospects in biologic therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2013;9:705–720. doi: 10.1038/nrrheum.2013.136. [DOI] [PubMed] [Google Scholar]
- 118.Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, Brohawn P, Zhang J, White B, Coyle AJ, Kiener PA, Jallal B. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- 119.Merrill JT, Wallace DJ, Petri M, Kirou KA, Yao Y, White WI, Robbie G, Levin R, Berney SM, Chindalore V, Olsen N, Richman L, Le C, Jallal B, White B. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon alpha monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann Rheum Dis. 2011;70:1905–1913. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- 120.McBride JM, Jiang J, Abbas AR, Morimoto A, Li J, Maciuca R, Townsend M, Wallace DJ, Kennedy WP, Drappa J. Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study. Arthritis Rheum. 2012;64:3666–3676. doi: 10.1002/art.34632. [DOI] [PubMed] [Google Scholar]
- 121.Bissonnette R, Papp K, Maari C, Yao Y, Robbie G, White WI, Le C, White B. A randomized, double-blind, placebo-controlled, phase I study of MEDI-545, an anti-interferon-alfa monoclonal antibody, in subjects with chronic psoriasis. J Am Acad Dermatol. 2010;62:427–436. doi: 10.1016/j.jaad.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 122.Lauwerys BR, Hachulla E, Spertini F, Lazaro E, Jorgensen C, Mariette X, Haelterman E, Grouard-Vogel G, Fanget B, Dhellin O, Vandepapeliere P, Houssiau FA. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon alpha-kinoid. Arthritis Rheum. 2013;65:447–456. doi: 10.1002/art.37785. [DOI] [PubMed] [Google Scholar]
- 123.Baccala R, Gonzalez-Quintial R, Schreiber RD, Lawson BR, Kono DH, Theofilopoulos AN. Anti-IFN-alpha/beta receptor antibody treatment ameliorates disease in lupus-predisposed mice. J Immunol. 2012;189:5976–5984. doi: 10.4049/jimmunol.1201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang B, Higgs BW, Chang L, Vainshtein I, Liu Z, Streicher K, Liang M, White WI, Yoo S, Richman L, Jallal B, Roskos L, Yao Y. Pharmacogenomics and translational simulations to bridge indications for an anti-interferon-alpha receptor antibody. Clin Pharmacol Ther. 2013;93:483–492. doi: 10.1038/clpt.2013.35. [DOI] [PubMed] [Google Scholar]
- 125.Sun X, Wiedeman A, Agrawal N, Teal TH, Tanaka L, Hudkins KL, Alpers CE, Bolland S, Buechler MB, Hamerman JA, Ledbetter JA, Liggitt D, Elkon KB. Increased ribonuclease expression reduces inflammation and prolongs survival in TLR7 transgenic mice. J Immunol. 2013;190:2536–2543. doi: 10.4049/jimmunol.1202689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 127.Kandimalla ER, Bhagat L, Wang D, Yu D, Sullivan T, La Monica N, Agrawal S. Design, synthesis and biological evaluation of novel antagonist compounds of Toll-like receptors 7, 8 and 9. Nucleic Acids Res. 2013;41:3947–3961. doi: 10.1093/nar/gkt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R155. doi: 10.1186/ar3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang S, Yang N, Zhang L, Huang B, Tan H, Liang Y, Li Y, Yu X. Jak/STAT signaling is involved in the inflammatory infiltration of the kidneys in MRL/lpr mice. Lupus. 2010;19:1171–1180. doi: 10.1177/0961203310367660. [DOI] [PubMed] [Google Scholar]