Abstract
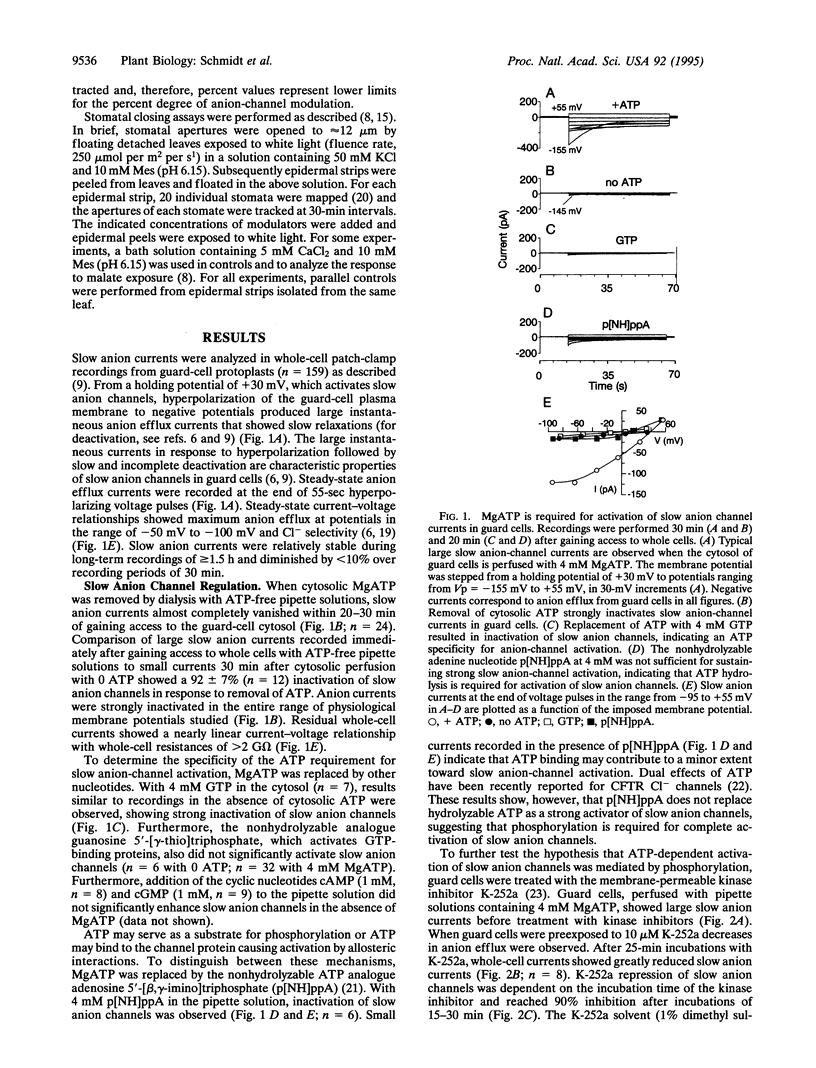
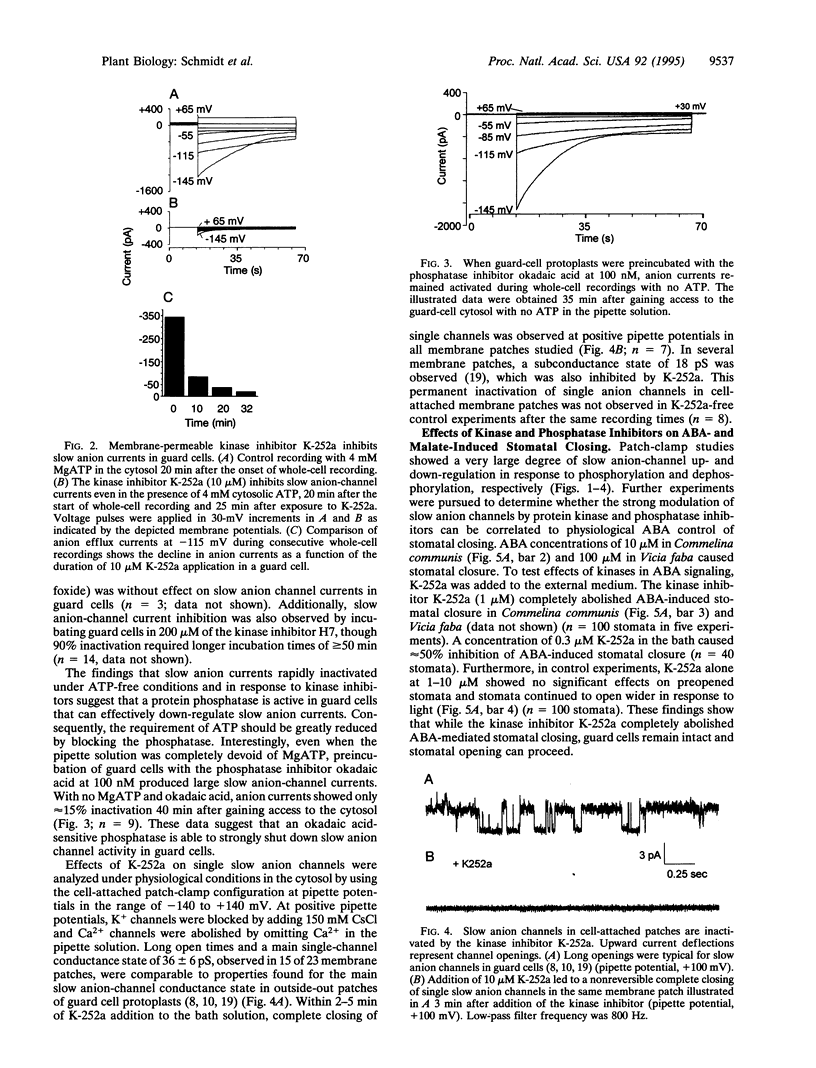
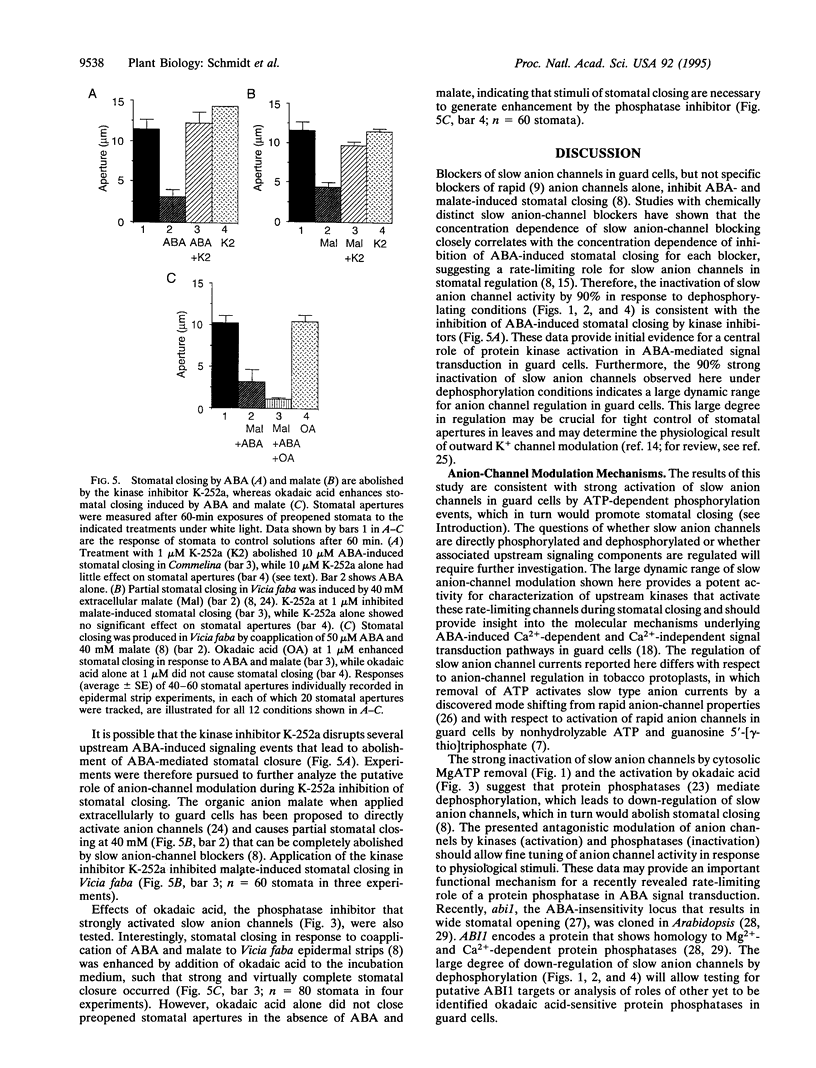
Recent evidence suggests that slow anion channels in guard cells need to be activated to trigger stomatal closing and efficiently inactivated during stomatal opening. The patch-clamp technique was employed here to determine mechanisms that produce strong regulation of slow anion channels in guard cells. MgATP in guard cells, serving as a donor for phosphorylation, leads to strong activation of slow anion channels. Slow anion-channel activity was almost completely abolished by removal of cytosolic ATP or by the kinase inhibitors K-252a and H7. Nonhydrolyzable ATP, GTP, and guanosine 5'-[gamma-thio]triphosphate did not replace the ATP requirement for anion-channel activation. In addition, down-regulation of slow anion channels by ATP removal was inhibited by the phosphatase inhibitor okadaic acid. Stomatal closures in leaves induced by the plant hormone abscisic acid (ABA) and malate were abolished by kinase inhibitors and/or enhanced by okadaic acid. These data suggest that ABA signal transduction may proceed by activation of protein kinases and inhibition of an okadaic acid-sensitive phosphatase. This modulation of ABA-induced stomatal closing correlated to the large dynamic range for up- and down-regulation of slow anion channels by opposing phosphorylation and dephosphorylation events in guard cells. The presented opposing regulation by kinase and phosphatase modulators could provide important mechanisms for signal transduction by ABA and other stimuli during stomatal movements.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A. C., Fricker M. D., Ward J. L., Beale M. H., Trewavas A. J. Two Transduction Pathways Mediate Rapid Effects of Abscisic Acid in Commelina Guard Cells. Plant Cell. 1994 Sep;6(9):1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann S. M. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R., Somerville C. R. Three Classes of Abscisic Acid (ABA)-Insensitive Mutations of Arabidopsis Define Genes that Control Overlapping Subsets of ABA Responses. Plant Physiol. 1990 Nov;94(3):1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton H. L., Williams W. E., Binns M. E. Repeated measurements of aperture for individual stomates. Plant Physiol. 1989 Feb;89(2):387–390. doi: 10.1104/pp.89.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Busch H., Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990 Dec;9(12):3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Marten I. Malate-induced feedback regulation of plasma membrane anion channels could provide a CO2 sensor to guard cells. EMBO J. 1993 Mar;12(3):897–901. doi: 10.1002/j.1460-2075.1993.tb05730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Nagel G., Nairn A. C., Gadsby D. C. Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J., Bouvier-Durand M., Morris P. C., Guerrier D., Chefdor F., Giraudat J. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994 Jun 3;264(5164):1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Linder B., Raschke K. A slow anion channel in guard cells, activating at large hyperpolarization, may be principal for stomatal closing. FEBS Lett. 1992 Nov 16;313(1):27–30. doi: 10.1016/0014-5793(92)81176-m. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., MacKintosh R. W. Inhibitors of protein kinases and phosphatases. Trends Biochem Sci. 1994 Nov;19(11):444–448. doi: 10.1016/0968-0004(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Meyer K., Leube M. P., Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994 Jun 3;264(5164):1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Brown P. H. High Levels of Malic Enzyme Activities in Vicia faba L. Epidermal Tissue. Plant Physiol. 1981 Nov;68(5):1047–1051. doi: 10.1104/pp.68.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Schroeder J. I. Anion Selectivity of Slow Anion Channels in the Plasma Membrane of Guard Cells (Large Nitrate Permeability). Plant Physiol. 1994 Sep;106(1):383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989 May;14(5):187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Keller B. U. Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5025–5029. doi: 10.1073/pnas.89.11.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Schmidt C., Sheaffer J. Identification of High-Affinity Slow Anion Channel Blockers and Evidence for Stomatal Regulation by Slow Anion Channels in Guard Cells. Plant Cell. 1993 Dec;5(12):1831–1841. doi: 10.1105/tpc.5.12.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G., MacRobbie E. A., Blatt M. R. Membrane transport in stomatal guard cells: the importance of voltage control. J Membr Biol. 1992 Feb;126(1):1–18. doi: 10.1007/BF00233456. [DOI] [PubMed] [Google Scholar]



