Angiogenesis depends on specific molecular interactions between vascular cells and components of the extracellular matrix (ECM). This Perspective focuses on the functional role of integrins in angiogenesis and neovascularization. Specifically, we discuss the mechanism by which antagonists of αv integrins disrupt angiogenesis in vivo and how they may impact patients with cancer and inflammatory disease.
Role of ECM and integrins during angiogenesis and vasculogenesis.
Angiogenesis depends not only on growth factors and their receptors but is also influenced by receptors for ECM proteins. In general, cell adhesion to the ECM is mediated by integrins, heterodimeric transmembrane proteins that comprise a diverse family of over 15 α and 8 β subunits. Integrin subunits can heterodimerize in over 20 combinations. Different integrin combinations may recognize a single ECM ligand, while others bind several different ECM proteins. Integrin-mediated adhesion leads to intracellular signaling events that regulate cell survival, proliferation, and migration (1). These signals include elevation in intracellular pH and calcium, inositol lipid synthesis, and the tyrosine phosphorylation of a wide range of nonreceptor tyrosine kinases such as focal adhesion kinase and Src kinases, as well as adaptor proteins such as Shc, p130 CAS, and Crk II. These signaling events trigger a number of downstream signals, including activation of the Ras/mitogen-activated protein (MAP) kinase pathway (1).
During angiogenesis, it is likely that a number of integrins expressed on the surface of activated endothelial cells regulate critical adhesive interactions with a variety of ECM proteins, including fibronectin, vitronectin, laminin, collagen types I and IV, von Willebrand factor, fibrinogen, and denatured collagen. Each of these adhesive interactions may regulate distinct biological events such as cell migration, proliferation, and differentiation. In addition, angiogenesis in different tissues may depend on specific endothelial cell interactions with ECMs that vary considerably in their adhesive protein composition.
Specific role of αv integrins in angiogenesis.
Of the wide spectrum of integrin subunit combinations that are expressed on the surface of cells, integrin αvβ3 has been identified as having an especially interesting expression pattern among vascular cells during angiogenesis and vascular remodeling. Integrin αvβ3 is a receptor for a wide variety of ECM ligands with an exposed RGD sequence, including vitronectin, fibronectin, fibrinogen, thrombospondin, proteolyzed collagen, von Willebrand factor, and osteopontin.
In the adult human, integrin αvβ3 has a rather limited tissue distribution, as it is not typically expressed on epithelial cells and appears at minimal levels on intestinal, vascular, and uterine smooth muscle cells. This receptor is also expressed on a small percentage of activated leukocytes, macrophages, and osteoclasts, where it appears to contribute to immune function and bone resorption. Some invasive tumors, such as metastatic melanoma and late-stage glioblastoma, also express αvβ3, where it contributes to malignant phenotype of the tumor (2). Endothelial cells exposed to growth factors, or those undergoing angiogenesis in tumors, wounds, or inflammatory tissue, express high levels of αvβ3 (3–6). In fact, recent studies suggest that αvβ3 may serve as a useful diagnostic or prognostic indicator of tumors. Specifically, the anti-αvβ3 mAb LM609 coupled to a gadolinium-containing liposome specifically targeted tumor-associated blood vessels in rabbits, as detected by magnetic resonance imaging (7). Recent clinical studies indicate that radiolabeled humanized LM609 (Vitaxin) can be used to detect the vascular bed of human tumors and their metastatic lesions by whole-body imaging with a gamma camera (LoBoglio, A., and co-workers, unpublished observations).
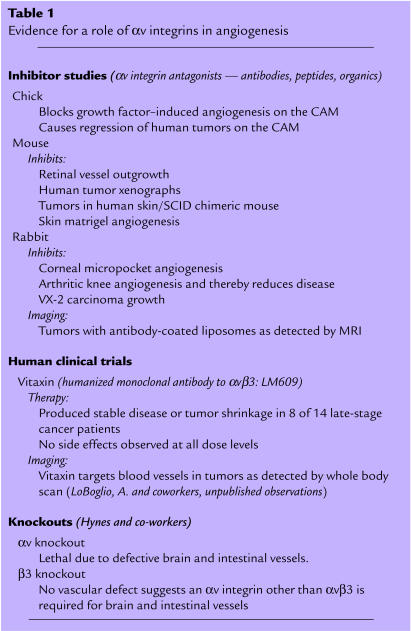
The expression of αvβ3 on activated endothelial cells suggests that this integrin may have an important function during angiogenesis and perhaps developmental neovascularization. In fact, disruption of αvβ3 ligation with antibody (LM609) or peptide antagonists of αvβ3 disrupts blood vessel formation in the chick chorioallantoic membrane (CAM), quail embryo, mouse retina, rabbit cornea, or arthritic knee (4, 6, 8, 9, 10, 11). These antagonists perturb the growth and/or maturation of new blood vessels without detectably influencing the preexisting blood vessels. In tumor models, inhibition of blood vessels by αv integrin antagonists not only blocked tumor-associated angiogenesis but in some cases caused tumor regression (5). Histological examination of tumors treated with the αvβ3 antagonists revealed few, if any, viable tumor cells or detectable blood vessels (5).
Integrin αvβ3 is capable of recognizing a number of ECM molecules in the context of the RGD adhesive sequence. However, recent studies suggest that αvβ3 can bind the matrix metalloproteinase-2 (MMP-2) in a non–RGD-dependent manner where it serves to localize the active form of the enzyme on the surface of angiogenic blood vessels (12). This enables angiogenic endothelial cells to degrade and remodel the ECM during their invasion. Interestingly, native collagen contains RGD sites that are inaccessible to αvβ3. However, after MMP-dependent proteolytic cleavage of collagen, these RGD sites are exposed and become ligated to αvβ3. One might predict that the physical association between MMP-2 and αvβ3 might not only facilitate ECM remodeling but would enable αvβ3-mediated endothelial cell invasion through the proteolyzed matrix by attachment to exposed RGD sites. In recent studies, natural breakdown fragments of MMP-2, termed PEX, were shown to accumulate in tissues that had undergone neovascularization (13). In fact, recombinant PEX was not only able to block MMP-2 binding to αvβ3 but when administered in vivo was able to disrupt tumor-associated angiogenesis (13). Thus, PEX not only prevents endothelial cell–mediated matrix remodeling by MMP-2 but would preclude αvβ3 ligation by elimination of the exposed RGD sites in collagen.
Knockout mice reveal a direct role for αv integrins in vascularization.
Recently, αv integrin knockout mice have been developed to examine the role of the αv integrins in developmental neovascularization (14). Mice deficient in αv integrins express a dramatic vascular pattern. While most animals die in utero, approximately 20% of αv-deficient mice survive to term but die within hours after birth. This lethal mutation is associated with extensive brain and intestinal vessel abnormalities and hemorrhaging, demonstrating that αv integrins are essential during blood vessel formation and/or maturation in these tissues. However, other organs show apparently normal vascularization (14), suggesting that animals lacking αv integrins can compensate to some degree in an organ-specific basis. Interestingly, mice lacking the β3 subunit while having a bleeding disorder contain normal brain and intestinal blood vessels (15). Therefore, an objective comparison of the αv and β3 knockout mice would lead one to conclude that an αv integrin other than αvβ3 was required for brain and gastrointestinal tract neovascularization. The ability of some organs to support neovascularization, even in the absence of the αv integrins, may provide a clue to some of the alternate integrins that may mediate vessel formation in the absence of αvβ3 or αv integrins. For example, integrin αvβ5 can potentiate a pathway of angiogenesis distinct from that mediated by αvβ3 (10). Moreover, mice lacking the fibronectin receptor α5β1 show embryonic lethality with multiple development problems, including severe vascular defects (16). In some cases, knockout mice have provided useful mechanistic information about a given molecule and its biological function. However, the lethality associated with a number of the integrin-deficient mice has made it difficult to decipher how these molecules function in normal animals. In addition, the likelihood for genetic compensation and redundancy provides an additional concern when interpreting the data from these models. Perhaps when conditional knockouts are studied we will gain further insight into the precise role of αv and other integrins in vascular development.
Integrin αvβ3 and vascular cell survival.
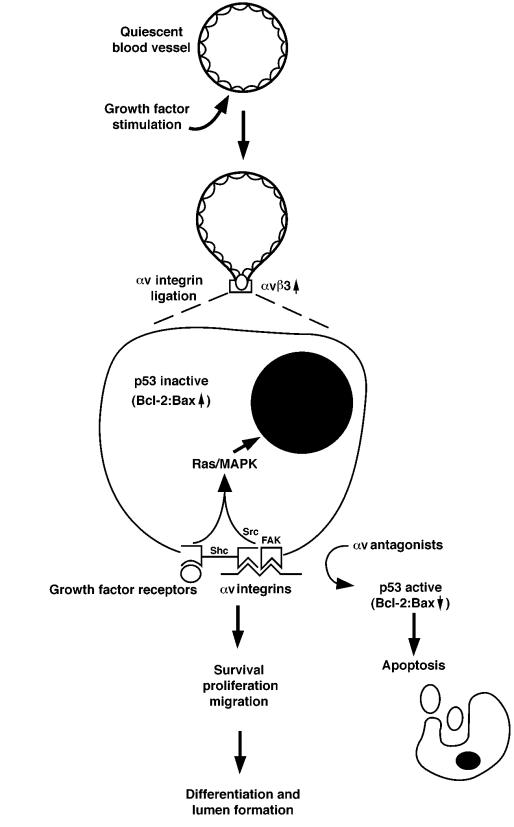
The role of integrin αvβ3 in mediating crucial steps of angiogenic blood vessels has led to the conclusion that this integrin facilitates the survival of stimulated endothelial cells. In fact, systemic administration of αvβ3 antagonists to animals with ongoing angiogenesis shows blood vessels containing high levels of apoptotic endothelial cells. These results suggest that ligation of αvβ3 on vascular cells may mediate a signaling event that is critical for the survival and differentiation of vascular cells undergoing angiogenesis in vivo. In this regard, αvβ3 ligation is essential for the sustained activation of MAP kinase by angiogenic growth factors (17). Ligation of growth factor receptors and integrins by the appropriate ligands is known to potentiate a variety of intracellular signals, including the activation of the Ras/MAP kinase cascade and the activation of focal adhesion kinase (FAK) and the Src tyrosine kinases (Figure 1). In addition, αvβ3 antagonists administered during angiogenesis in the chick CAM induce endothelial cell p53-dependent DNA-binding activity, which in turn leads to an increase in the expression of the cell cycle inhibitor p21WAF1/CIP1 and decreased expression of the death-promoting protein bax (18). Ligand binding by endothelial cell αvβ3 suppresses this apoptotic pathway, thereby facilitating the ultimate differentiation of new blood vessels. It is important to point out that mice clearly survive without p53, indicating that alternative or compensatory apoptotic pathways can allow normal development. The importance of p53 during αv-mediated angiogenesis and endothelial cell survival is underscored by recent preliminary evidence that p53-deficient mice are refractory to the effects of αv antagonists (Stromblad, R., et al., unpublished observations). This provides strong evidence that endothelial cell survival through p53 activity is linked to αv binding to the ECM. In the absence of p53, another compensatory pathway is engaged, facilitating endothelial cell survival in an αv-independent manner. These observations may help explain the compensatory mechanism facilitating blood vessel growth in αv-deficient mice.
Figure 1.
Hypothetical model for the role of αv integrins in angiogenesis. Angiogenic stimulus by growth factors induces expression of αvβ3 and causes cells to invade the surrounding ECM and enter the cell cycle. When ligation of αv integrins is blocked, proliferating vascular cells undergo apoptosis, accompanied by an increase in p53 activity. Angiogenic growth factors induce the activation of Ras/MAP kinase in vascular cells and lead to cell proliferation, differentiation, and migration.
Two angiogenic pathways are characterized by distinct αv integrins.
Two pathways of angiogenesis have recently been identified based on their dependence on the related but distinct integrins αvβ3 and αvβ5 (10). In both the rabbit corneal eye pocket and the chick chorioallantoic membrane angiogenesis assays, anti-αvβ3 mAb blocked bFGF-induced angiogenesis, whereas anti-αvβ5 antagonists blocked VEGF-induced angiogenesis. Furthermore, inhibition of the protein kinase C pathway blocked the VEGF-induced angiogenesis and did not affect bFGF-induced angiogenesis. The biological relevance of these distinct angiogenic pathways is unknown. Perhaps, vascular remodeling in distinct organs depends on the particular growth factors and/or adhesive proteins contained within the specific ECM. As stated above, β3 integrin–deficient mice have normal brain and gut vessel development in the absence of αvβ3, while αv integrin–deficient mice have defective brain and gut blood vessels. This strongly implicates another αv integrin, perhaps αvβ5, in brain and gut developmental neovascularization.
Clinical perspectives.
Based on the strong preclinical data in animal models, the clinical potential of αv-integrin antagonists is currently being evaluated in phase I and phase II clinical trials for patients with late-stage cancer. For example, the humanized form of the anti-αvβ3 mAb LM609 (Vitaxin) has successfully completed phase I trials. Of the 14 cancer patients evaluated, 8 showed disease stabilization and/or some objective tumor reduction (Table 1). Importantly, there was no evidence of toxicity at all doses tested, even when administered for 22 consecutive months. This clinical trial is among the first use of a “targeted” antiangiogenic approach in humans. These clinical data closely mirror the effects observed in tumor-bearing animal models (5). Phase II cancer trials are now under way to further evaluate the efficacy of Vitaxin during long-term treatment. Additional phase I trials are under way to assess the effects of an αvβ3/αvβ5 small-molecule antagonist in late-stage cancer patients.
Table 1.
Evidence for a role of αv integrins in angiogenesis
Conclusions.
Further study of the basic cell biological mechanisms underlying growth factor receptors and integrin function during angiogenesis may continue to provide insight into the evaluation of the clinical benefit of αv-integrin antagonists. The elucidation of the molecular basis of angiogenesis remains a challenge because of the complex interactions between the ECM and vascular cells that must be temporally and spatially coordinated. Examination of the signaling events transduced by cell adhesion molecules to the smooth muscle and endothelial cells may reveal mechanisms in which cells can process cytokine or growth factor stimuli to impact changes in intracellular phosphorylation cascades, gene expression levels, and ECM-associated enzymatic activities. The coordinated response to these inputs will likely impact the processes of vascular cell survival, invasion, migration, proliferation, and differentiation during angiogenesis. Knowledge of these molecular mechanisms will foster the development of novel antiangiogenic therapies to benefit patients with cancer and inflammatory disease.
Acknowledgments
B.P. Eliceiri was supported by a National Research Service Award postdoctoral fellowship(1F32 HL-09435). D.A. Cheresh was supported by grants CA-50286, CA-45726, CA-78045, and HL54444 from the National Institutes of Health.
References
- 1.Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- 2.Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA. Involvement of integrin αv gene expression in human melanoma tumorigenicity. J Clin Invest. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enenstein J, Kramer RH. Confocal microscopic analysis of integrin expression on the microvasculature and its sprouts in the neonatal foreskin. J Invest Dermatol. 1994;103:381–386. doi: 10.1111/1523-1747.ep12395390. [DOI] [PubMed] [Google Scholar]
- 4.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 5.Brooks PC, et al. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 6.Storgard CM, et al. Decreased angiogenesis and arthritic disease in rabbits treated with an αvβ3 antagonist. J Clin Invest. 1999;103:47–54. doi: 10.1172/JCI3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sipkins DA, et al. Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 8.Drake CJ, Cheresh DA, Little CD. An antagonist of integrin αvβ3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- 9.Brooks PC, et al. Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedlander M, et al. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 11.Hammes HP, Brownlee M, Jonczyk A, Sutter A, Preissner KT. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med. 1996;2:529–533. doi: 10.1038/nm0596-529. [DOI] [PubMed] [Google Scholar]
- 12.Brooks PC, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 13.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- 14.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 15.Hodivala-Dilke KM, et al. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 17.Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin αvβ3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stromblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin αvβ3 during angiogenesis. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]