Abstract
Mercury use in small-scale gold mining is ubiquitous across Ghana but little is known about the extent to which such activities have contaminated community residents and miners. Here, occupational exposures to elemental mercury (via urine sampling) and dietary exposures to methylmercury (via hair sampling) were assessed among 120 participants recruited from a mining community located in the Talensi-Nabdam District of Ghana’s Upper East region during summer 2009. More than one-fifth of the participants had moderately high levels of urinary mercury (>10 µg/L) and 5% had urine mercury levels that exceeded the WHO guideline value of 50 µg/L. When participants were stratified according to occupation, those active in the mining industry had the highest mercury levels. Specifically, individuals that burned amalgam had urine mercury levels (median: 43.8 µg/L; mean ± SD: 171.1 ± 296.5 µg/L; n=5) significantly higher than median values measured in mechanical operators (11.6 µg/L, n=4), concession managers/owners (5.6 µg/L, n=11), excavators that blast and chisel ore (4.9 µg/L, n=33), individuals that sift and grind crushed ore (2.2 µg/L, n=47), support workers (0.5 µg/L, n=14), and those with no role in the mining sector (2.5 µg/L, n=6). There was a significant positive spearman correlation between fish consumption and hair mercury levels (r = 0.30) but not with urine mercury (r = 0.18) though further studies are needed to document which types of fish are consumed as well as portion sizes. Given that 200,000 people in Ghana are involved in the small-scale gold mining industry and that the numbers are expected to grow in Ghana and many other regions of the world, elucidating mercury exposure pathways in such communities is important to help shape policies and behaviors that may minimize health risks.
Keywords: exposure assessment, mining, indigenous peoples, risk assessment, fish consumption
INTRODUCTION
Small-scale artisanal gold mining is of global health concern. Between 10 and 15 million people worldwide, mostly poor, are directly involved in small-scale gold mining with another 100 million people estimated to be reliant upon the sector (Hilson and Pardie, 2006; Swain et al., 2007). The practice of small-scale gold mining is increasing worldwide owing to the rising price of gold, the privatization and proliferation of larger mines, and widespread poverty. Gold harvested from small-scale mines may represent 20 to 30% of the world’s output (UNEP, 2002). West Africa, and in particular Ghana, is one of the most important gold mining regions in the world (Spiegel, 2009; Tschakert and Singha, 2007). Though gold has been mined in Ghana for over 1,000 years, its production has increased 700% since 1980 and gold currently accounts for ~40% of the country’s national exports (Hilson, 2002). More than 200,000 Ghanaians are estimated to work in small-scale mining operations (Hilson et al., 2007; Hilson and Pardie, 2006).
Several health concerns exist in small-scale mining communities such as chronic exposure to dust and noise, unsanitary working conditions, and lack of personal protective gear. Exposure to mercury is also of concern since this heavy metal is a potent neurotoxicant (Clarkson and Magos, 2006). After thorough grinding of gold-containing ore or silt, mercury is added to the mixture to create a gold amalgam. Subsequent burning of this amalgam concentrates the gold into a pellet but releases mercury, in its gaseous elemental form (Hg0), into the air. The released elemental mercury vapor poses both an occupational and environmental threat. Occupationally, those involved in small-scale mining (both directly and indirectly) as well as area residents may inhale high levels of elemental mercury. In the atmosphere the elemental mercury may be oxidized and then deposited into nearby waterways. Once in an aquatic system, the mercury will be biomethylated by bacteria into methylmercury (MeHg). Methylmercury biomagnifies through the fish in aquatic ecosystems, and thus poses a risk to those who consume fish. Contamination of human residents and ecological components by mercury (both elemental mercury and methylmercury) has been observed in many small-scale mining regions across the world, such as Brazil (Lebel et al., 1998; Malm et al., 1989; Palheta and Taylor, 1995), Indonesia (Limbong et al., 2003), and Tanzania (Bose-O'Reilly et al., 2010; van Straaten, 2000). Further, exposure to both forms of mercury has been linked with neurotoxicity and other adverse health outcomes effects in several mining sites (Lebel et al., 1998; Swain et al., 2007).
Small-scale gold mining is ubiquitous across Ghana but little is known about the extent to which these mining activities have contaminated local landscapes, area residents and miners with mercury. In the southwest of Ghana, where several small-scale gold mining sites exist, Adimado and Baah (2002) and Donkor et al. (2006) have reported elevated levels of mercury in fish and a small sample of residents but little is known about exposures in other areas of the country.Few studies characterize exposures to both elemental mercury and methylmercury (Ishitobi et al., 2010), though an understanding of both chemicals (their source, fate, exposure, toxicity) is needed to better manage mercury risks in small-scale mining communities (Spiegel and Veiga, 2010). The goal of the current study was to extend upon these aforementioned works and to characterize occupational and environmental exposures to mercury in a Ghanaian small-scale gold mining community in the northern part of the country. To achieve this goal, 120 participants (both area residents and gold miners) were recruited in a mining community located in the Talensi-Nabdam District of Ghana’s Upper East region. Urine and hair were collected to evaluate occupational exposures to elemental mercury and dietary exposures to methylmercury, respectively. Surveys were administered to capture information on occupational, dietary, health and lifestyle factors that may help interpret the mercury biomarker values.
METHODS
Human Subjects Interaction
The study was completed in May and June, 2009. The mining community of focus is located near Bolgatanga in the Upper East region of Ghana in the Talensi-Nabdam District (Figure 1). This district is 912 km2 in area and has a population nearing 100,000 people. Within the mining community, research activities were conducted in three sites: Obuasi, World Bank, and Kejitia. Hilson et al. (2007) conducted field interviews in 2005 with leaders of mining sites in this area and concluded that residents were largely unaware of the health risks of mercury and used little personal protection. From the three sites 120 individuals were recruited into the study. During the monsoon season most of the community members migrate to Bolgatanga. Permission to access the community was obtained in advance from the region’s traditional Chief. Institutional Review Board (IRB) approval was obtained from the University of Michigan (HUM00028444) to interact with adult human research subjects. A locally hired translator facilitated the collection of survey data and interactions with non-English speakers. Personalized and aggregate results were disseminated to stakeholders (participants, government officials) during return visits in 2010 (March, May–June).
Figure 1.
Map of study region in the Upper East region of Ghana is indicated with a solid circle. Source of map: U.S. Central Intelligence Agency [http://www.lib.utexas.edu/maps/africa/ghana_pol_2007.jpg]
Sample and Information Collection
From each participant, 20–50mL of urine was collected in sterile plastic collection cups (BD Vacutainer). Approximately 50 strands of hair from the occipital region were cut close to the scalp, placed cut-side down onto sticky paper and wrapped. Hair was collected from 100 of 120 individuals as 20 did not have sufficient amounts of hair. Urine samples were stored refrigerated (except during transit) until returned to the University of Michigan upon which they were stored frozen at −20°C. Hair was kept at room temperature.
A survey was administered to gather self-reported information on participant demographics (e.g., gender, age, education level, residence), occupation (e.g., type, number of weekly hours, number of years), and diet (weekly servings of key diet components). The survey was also designed to record self-reported measures of health status. Participants were asked polar “yes-no” questions about the health of specific physiological systems (hearing, vision, gastrointestinal neurological, respiratory, renal, dermal). Participants were allowed to elaborate upon their responses in a qualitative manner.
Mercury Analysis
Analysis of total mercury in hair and urine were performed using a Direct Mercury Analyzer 80 (DMA-80, Milestone Inc, CT) according to U.S. EPA Method 7473 as previously described by our group (Nam et al. 2010). Briefly, 500uL of urine was vortexed and placed into a quartz sampling boat. Hair was washed twice with acetone, rinsed with milli-Q water, and dried overnight. About 2 – 5 mg of hair was weighed in a nickel sampling boat. Following placement of sampling boats into the DMA-80, decomposition at 800°C liberated mercury from the sample as a vapor, which was subsequently carried to an absorbance cell (253.65 nm) by oxygen for mercury quantification.
A series of rigorous analytical quality control measures were used. Accuracy and precision were measured by use of several certified reference materials, including NIES CRM #13 (Japanese National Institute for Environment Studies, NIES) and DOLT-3 (Canadian National Research Council). In addition, each batch run contained procedural blanks and replicate runs. The analytical detection limit was calculated as the concentration of mercury which gave a detectable signal above the background noise at greater than the 99% confidence level, so that the detection limit was calculated as 3 times the standard deviation of the mean blank value.
Statistical Analyses
All statistical operations were performed using SPSS (v11.5, Chicago IL). Preliminary data analysis included tabulation of descriptive statistics for all measurements. Hair mercury and urine mercury values were not normally distributed and thus non-parametric statistics (Mann-Whitney U tests, Kruskal-Wallis analysis of variance on ranks, and spearman correlations) were used to analyze the data. The primary relationships of interest were associations between hair (i.e., environmental) and urine (i.e., occupational) mercury levels with gender, mining concession site, occupational role, fish consumption, and self-reported measures of health.
RESULTS
Population Description
Of the 120 participants, 62 were female and the mean age was 39.8 (range: 18–89). Nearly half the participants indicated having no formal education (n=57), about one quarter indicated having primary (n=29) or secondary (n=33) education, and one participant had more than secondary education. The participants were recruited from three gold mining concession sites, Obuasi (n=92), World Bank (n=13), and Kejitia (n=15). Sixty-six participants reported handling mercury on a routine basis, and 100 participants indicated that they were actively involved in small scale mining. Of these 100 individuals involved in small scale mining, 11 were classified as managers or owners, 5 were classified as amalgamators (burn gold), 33 were classified as excavators (blast, remove, chisel ore), 4 were classified as mechanical operators, and 47 were classified as grinders (sift and grind crushed ore). Those in a managerial position were on average older (49.4 years, range: 32–60) than all other groups. For the 20 participants not directly involved in gold mining, 14 provide food and other services to those involved in mining (e.g., assume support roles) and 6 indicated no participation in any mining activity.
Mercury Exposure Biomarkers
Urine was collected from every participant, and hair was collected from each individual that had enough scalp hair (100/120 participants). Mercury was detected in every urine and hair sample analyzed, and the levels spanned more than three orders of magnitude (Table 1). The average theoretical method detection limit (3× SD blanks) for hair was 0.13 ng mercury and for urine was 0.15 ng mercury. The average recovery of mercury from the SRMs for all analysis days was 85.2 ± 8.9% for the NIES SRM and 94.1 ± 9.0 % for the DOLT SRM. Within-day (2.3% for hair, 4.4% for urine) and between day (10.6% for hair, 9.5% for urine) variability of SRMs was calculated, and these values corresponded well to replicate analysis of actual samples provided by participants (data not shown).
TABLE 1.
Descriptive summary of mercury biomarker results with respect to gender and mining site. Significant differences (p<0.05), assessed using Mann-Whitney U tests (gender) or Kruskal Wallis (mining sites), are indicated with letters.
| Urine Mercury (µg/L) | Hair Mercury(µg/g) | |||||
|---|---|---|---|---|---|---|
| Mean (SD) |
Median | Range | Mean (SD) |
Median | Range | |
| All participants | 17.0 (77.3) | 2.5 | 0.2 – 708.0 | 1.1 (3.2) | 0.4 | 0.0 – 22.9 |
| Gender: | ||||||
| Male | 33.1 (116.4)a | 4.4 | 0.2 – 708.0 | 1.5 (3.5)a | 0.6 | 0.2 – 22.9 |
| Female | 4.9 (8.0)b | 2.0 | 0.3 – 43.7 | 0.8 (2.9)b | 0.3 | 0.0 – 21.9 |
| Mining Site: | ||||||
| World Bank | 1.83 (2.3)b | 0.9 | 0.3– 32.9 | 0.3 (0.2)b | 0.3 | 0.0 – 0.6 |
| Obuasi | 15.8 (79.7)b | 2.8 | 0.2 – 708.0 | 1.1 (3.5)a | 0.4 | 0.0 – 22.9 |
| Kejitia | 38.9 (95.7)a | 3.6 | 0.6 – 178.2 | 1.2 (1.4)a | 0.6 | 0.2 – 4.8 |
There was no association of hair or urine mercury levels with a participant’s age or education level. For both hair and urine, mercury levels were significantly higher (more than double) in males compared to females (Table 1). Biomarkers of mercury also varied across the three sampling sites with participants recruited from Kejitia having the highest mercury levels (Table 1).
Fifty-four participants indicated that they handled mercury at some time, and these individuals had urine mercury levels that were significantly (p < 0.05) higher than the 66 participants that indicated “No” to directly handling mercury (median: 4.2 vs 2.3 µg/L; mean ± SD: 25.1 ± 97.2 vs 11.6 ± 42.4 µg/L). When participants were stratified according to occupation, those active in the mining industry had urine and hair mercury levels that were generally higher than individuals not directly involved (i.e., support workers and non-miners; Figure 2). Of the 100 participants actively involved in small scale mining, those that burned amalgam had hair (median: 1.8 µg/g; mean ± SD: 9.7 ± 11.5 µg/g) and urine (median: 43.8 µg/L; mean ± SD: 171.1 ± 296.5 µg/L) mercury levels significantly higher than all other occupational groups. When those actively engaged in mining were queried about the use of personal protection (e.g., masks, gloves), 84 responded “no”, 7 reported “yes”, and the others reported “sometimes”. Urine mercury levels in those reporting “yes” to the use of personal protection were about two-fold lower than individuals that did not use protection (mean values: 4.0 vs 8.0 µg/L).
Figure 2.
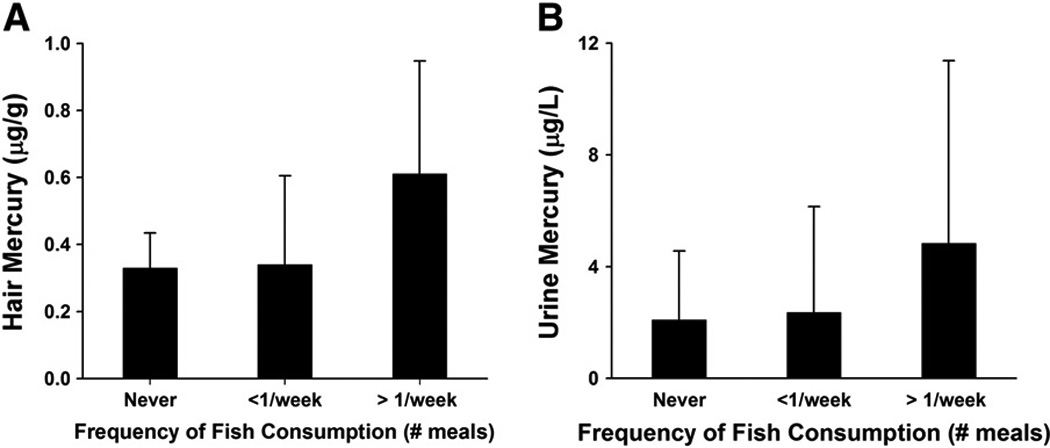
(A) Hair mercury levels and (B) urine mercury levels in relation to frequency of fish consumption. Bars represent median values with upper 75th percentiles indicated as error bars.
Urine and hair mercury levels were compared to established guideline values set by the World Health Organization (Figure 3). For urine, 21% of participants had urine mercury levels that were in excess of 10 µg/L and 5% (n = 6) had urine mercury levels that were in excess of the WHO guideline value of 50 µg/L. When these urinary mercury values were broken down according to gender, mining sites, and occupation, the stratification of values were similar to results reported earlier. For hair, most participants had mercury levels <1 µg/g though six individuals had hair mercury in excess of 3 µg/g and two participants exceeded 20 µg/g.
Figure 3.
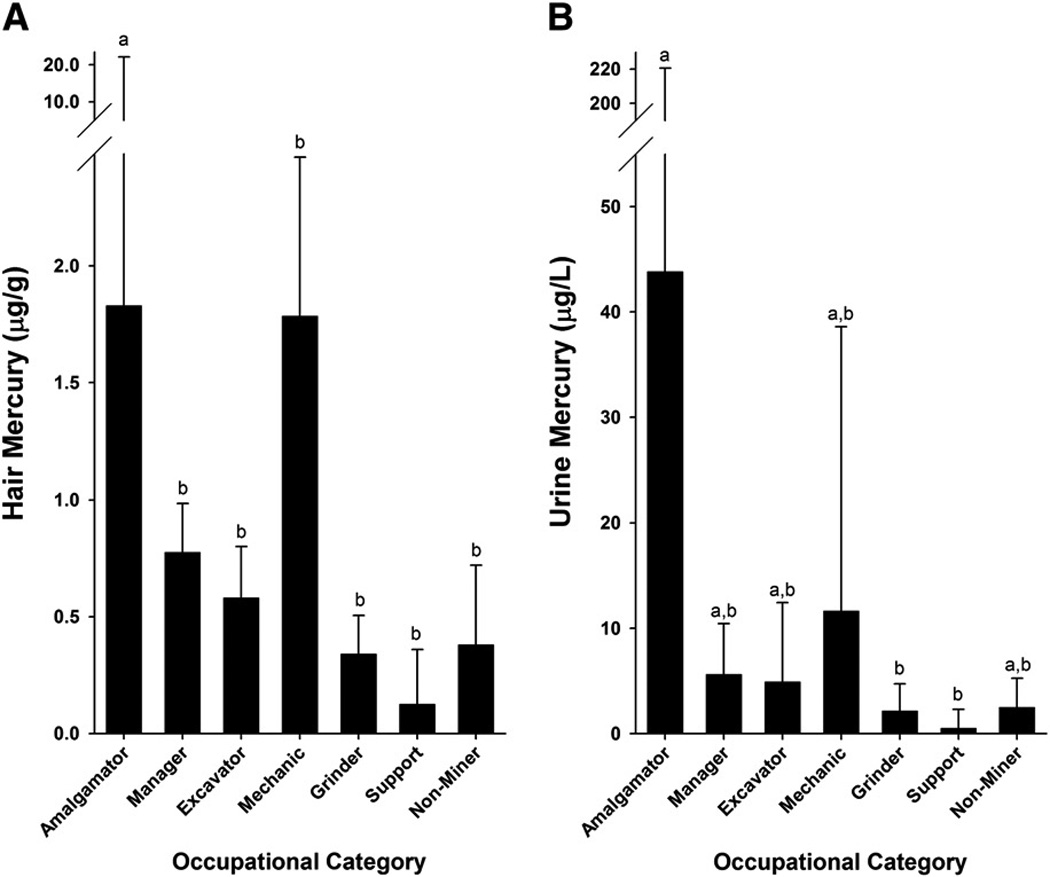
(A) Hair mercury levels and (B) urine mercury levels in relation to a participant’s occupation. Significant differences (p<0.05) are indicated with letters as determined by Kruskal-Wallis analysis of variance on ranks. Bars represent median values with upper 75th percentiles indicated as error bars.
Survey Outcomes
Nearly every participant consumed fish, though it should be noted that this instrument was not designed to gauge intake of specific fish species and portion sizes. Eight participants indicated “No” to eating fish and their hair mercury levels were lower than those that consumed fish (median: 0.3 vs 0.4 µg/g; mean ± SD: 0.5 ± 0.7 vs 1.1 ± 3.3 µg/g). Of those that consumed fish, 37 indicated consumption on at least a weekly basis and the rest consumed fish less than once per week. When frequency of fish consumption was related to mercury biomarkers, there was a significant positive spearman correlation with hair Hg (rs = 0.30, p<0.01) but not with urine mercury (rs = 0.18, p>0.05). When hair and urine mercury were compared against frequency of fish consumption using Kruskal-Wallis analysis of variance, there were no significant differences (Figure 4).
Figure 4.
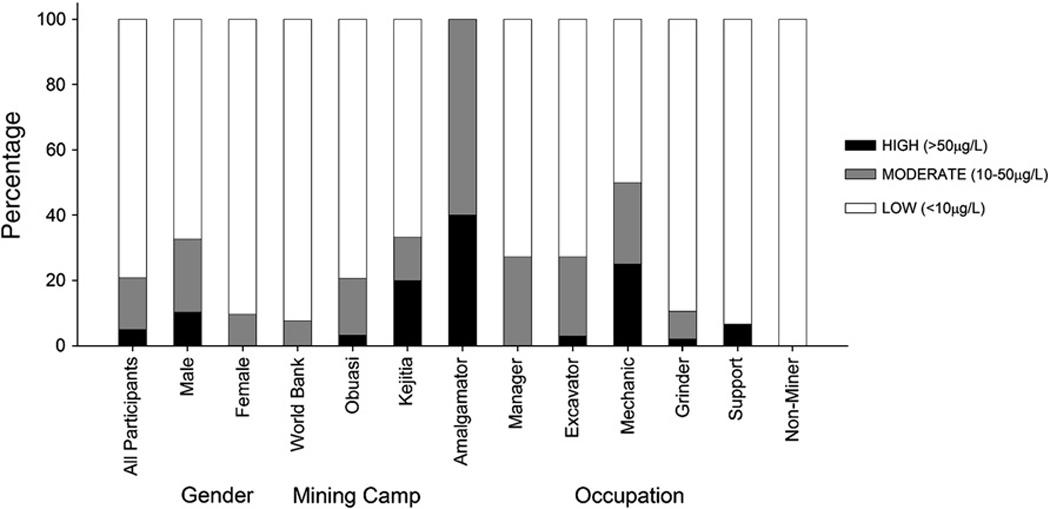
Percentage distribution of participants for a given category (gender, mining site, occupation) with respect to risk thresholds for urine mercury.
To gauge possible health effects in the community, self-reported information on physiologically-specific outcomes were probed using polarized Yes/No questions. There was no significant associations with hair mercury or urine mercury and the following health outcomes (number in brackets indicated “yes”): fatigue (11/120), body ache (45/120), neurological (24/120), infectious diseases (3/120), diarrhea (19/120), breathing difficulty (44/120), vision (10/120) and cardiovascular function (10/120).
DISCUSSION
The key finding of this study was that individuals residing near and working in a small-scale gold mining community in the Talensi-Nabdam District of Ghana are potentially exposed to high levels of mercury via occupational and environmental routes. More than one-fifth of the participants had moderately high levels of urinary mercury (>10 µg/L) and 5% had urine mercury levels that exceeded the WHO guideline value of 50 µg/L, thus reflecting widespread exposure to mercury vapor. While mercury exposures were elevated in several mine workers and area residents, those individuals that handled mercury as part of the amalgamation process experienced the highest exposures. In addition to inhalation exposures, the data suggest that some individuals are exposed to mercury via consumption of contaminated fish. Given that 200,000 people in Ghana are involved in the small-scale gold mining industry and that the numbers are expected to grow in Ghana and many other regions of the world, understanding mercury exposure pathways and health risks in such communities is of importance to help shape policies and behaviors that may minimize health risks.
Mercury levels in urine reflect exposures to inorganic mercury, which is the type of mercury released by amalgam burning. Mercury levels in 79% of the participants were below 10 µg/L which is considered to be a background range (Nordberg et al., 2007). We are not aware of background urine mercury levels across Ghana or West Africa. Though, when considering that the geometric mean urine mercury value in the general U.S. population is 0.5 µg/L with the upper 95% range being 3.3 µg/L (NHANES, 2009), the results presented here suggest that exposures to elemental mercury are widespread and elevated in the small-scale mining areas investigated here.
Urine mercury levels were significantly higher in participants that actively burned amalgams. Twenty-one % of participants had urine mercury levels that were moderately elevated (i.e., > 10 µg/L) and every individual that actively burned amalgams had urine mercury levels that were either moderately elevated or greater than the WHO guideline value of 50 µg/L. In fact, several of the miners had urine mercury levels exceeding 100 µg/L with the greatest value being 708 µg/L. Such high exposures are not uncommon in small-scale gold mining sites. For example, in Brazil (Palheta and Taylor, 1995) and Tanzania (van Straaten, 2000), urine mercury levels in small-scale gold miners often exceed 50 µg/L. Further, in a study of people living near small-scale gold mining sites in southwestern Ghana 68% of the participants had urine mercury levels that ranged between 50 and 200 µg/L (Adimado and Baah, 2002). At this moment it is not clear why mercury exposures are much higher in this particular site in southwest Ghana when compared to our study in the Upper East region.
There is ample evidence that exposure of miners to mercury vapor results in several neurological effects, namely peripheral nervous system and neuropsychological disturbances. While a threshold value of 50 µg/L mercury in urine is used to gauge risk, health effects such as tremors and incoordination have been documented in studies of occupationally-exposed individuals with urine levels less than 50 µg/L (Clarkson and Magos, 2006). In the current study there was no association between urinary mercury levels and self-reported health measures, though owing to the basic nature of the present inquiry future studies should employ objective and rigorous measures to assess possible health impacts.
Mercury levels in hair generally reflect environmental exposures to methylmercury, though on average about 20% of mercury in hair may be derived from inorganic sources (Clarkson and Magos, 2006) and thus some of the hair mercury measured here may be due to inhalation of elemental mercury, and the exact contribution in such small-scale gold mining communities needs further investigation. The main route of methylmercury exposure is via fish consumption. Though our questions regarding fish consumption did not account for specific fish species or portion sizes, a positive association was observed between fish consumption and hair mercury levels. Such an association has been observed in several other small-scale mining regions. Notably are findings from mining areas in the Brazilian Amazon in which heightened exposures to both methylmercury (through fish consumption) and elemental mercury (through inhalation) (Lebel et al., 1998; Malm et al., 1989; Palheta and Taylor, 1995) have been documented. Mercury levels in fish have been reported from other parts of Ghana (Adimado and Baah, 2002; Donkor et al., 2006), but to our knowledge such a study has not been completed in the Upper East region. During our field research we were informed about a nearby dam from which some residents obtained fish, and future studies should follow-up on this as a possible source of methylmercury exposure in the community. In any future study concerning fish consumption in the region, mercury risks need to be balanced against nutritional, economical, and cultural benefits.
The hair mercury results obtained here were similar to other values reported across Ghana, in both urban and rural centers. A recent study on 123 individuals spanning 10 major cities in Ghana revealed that the mean value of hair mercury was 0.8 µg/g (range: 0.1 – 4.1) (Voegborlo et al. 2010). In southwest Ghana, where several small-scale gold mining sites exist, the reported hair mercury levels were similar to what we found here in the Upper East region. For example, in the Ankobra river basin the mean hair mercury value was 2.7 (range: 0.2–5.9, n=100) and in the Tano river basin the mean value was 3.5 (rage: 0.1–3.5, n=117) (Adimado and Baah, 2002).
An earlier study by Hilson et al. (2007) in the same communities studied here noted that residents were largely unaware of the health risks of mercury and that they did not use personal protection. During our conversations with area residents, most have now recognized that mercury is of health concern. However, owing to the widespread poverty in this region (as well as other sites across Ghana and the world) and the rising price of gold, the practice of small-scale gold mining is expected to continue and grow. In this community, technological and behavioral changes to minimize mercury exposures have been considered and are still under investigation by local mining officials, concession owners, academic researchers, and the workers themselves. Here, only seven individuals (out of 120) indicated the use of any form of protection device (e.g., masks) and perhaps not surprisingly their urine mercury levels were lower than the rest of the participants.
In conclusion this study provides science-based exposure evidence to support the notion by others (e.g., Hilson et al., 2007) that individuals residing in small-scale gold mining communities in the Upper East region of Ghana are exposed to potentially high levels of mercury. Though individuals directly involved in gold amalgamation experienced the highest exposures to mercury, our data suggest that most community members (including women) are exposed to elevated levels of mercury. Additional studies are needed to determine if such exposures are related to adverse health outcomes. We learned that community residents have gained some awareness of the health risks associated with mercury use, though protective schemes such as retorts and mercury-alternates to amalgamation are not employed and further studies are needed to understand why such barriers exist. Further, like other small-scale gold mining communities across the world we found some evidence of environmental exposure to methylmercury likely via the diet and studies are required to better understand this mercury exposure pathway.
ACKNOWLEDGEMENTS
This study was funded by several internal sources at the University of Michigan, namely the Office of Vice President of Research (OVPR), the Undergraduate Research Opportunities Program (UROP), the Center for Afroamerican and African Studies (CAAS), the International Center, the African Social Research Initiative (ASRI), the George and Jennifer Stone Scholarhsip program, and the School of Public Health. We acknowledge the support of Chief NaabPurbotaaba as well as Ephraim SowahKomey for translational services. The support of Professors Kenneth Pelig-Ba (University of Development Studies, Navrongo), Osman Al-Hassan (University of Ghana, Legon), and officials with the Minerals Commission of Ghana (Simon Attebiya, Wilson W. Zooghah) is acknowledged.
REFERENCES
- Adimado AA, Baah DA. Mercury in human blood, urine, hair, nail, and fish from the Ankobra and Tano River Basins in southwestern Ghana. Bull Environ Contam Toxicol. 2002;68:339–346. doi: 10.1007/s001280259. [DOI] [PubMed] [Google Scholar]
- Bose-O'Reilly S, Drasch G, Beinhoff C, Tesha A, Drasch K, Roider G, Taylor H, Appleton D, Siebert U. Health assessment of artisanal gold miners in Tanzania. Sci Total Environ. 2010;408:796–805. doi: 10.1016/j.scitotenv.2009.10.051. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Donkor AK, Bonzongo JC, Nartey VK, Adotey DK. Mercury in different environmental compartments of the Pra River Basin, Ghana. Sci Total Environ. 2006;368:164–176. doi: 10.1016/j.scitotenv.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Hilson G. Harvesting mineral riches: 1000 years of gold mining in Ghana. Resources Policy. 2002;28:13–26. [Google Scholar]
- Hilson G, Hilson CJ, Pardie S. Improving awareness of mercury pollution in small-scale gold mining communities: challenges and ways forward in rural Ghana. Environ Res. 2007;103:275–287. doi: 10.1016/j.envres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hilson G, Pardie S. Mercury: An agent of poverty in Ghana's small-scale gold mining industry? Resources Policy. 2006;31:106–116. [Google Scholar]
- Ishitobi H, Stern S, Thurston SW, Zareba G, Langdon M, Gelein R, Weiss B. Organic and inorganic mercury in neonatal rat brain after prenatal exposure to methylmercury and mercury vapor. Environ Health Perspect. 2010;118:242–248. doi: 10.1289/ehp.0900956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, Dolbec J. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- Limbong D, Kumampung J, Rimper J, Arai T, Miyazaki N. Emissions and environmental implications of mercury from artisanal gold mining in North Sulawesi, Indonesia. Sci Total Environ. 2003;302:227–236. doi: 10.1016/s0048-9697(02)00397-2. [DOI] [PubMed] [Google Scholar]
- Malm O, Pfeiffer WC, Souza CMM, Reuther R. Mercury pollution due to gold mining in the Madeira River Basin, Brazil. Ambio. 1989;19:11–15. [Google Scholar]
- Nam DH, Adams DH, Flewelling LJ, Basu N. Neurochemical alterations in lemon shark (Negaprionbrevirostris) brains in association with brevetoxin exposure. Aquat Toxicol. 2010 doi: 10.1016/j.aquatox.2010.05.014. In Press. [DOI] [PubMed] [Google Scholar]
- NHANES. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta: U.S. Centers for Disease Control and Prevention; 2009. [Google Scholar]
- Nordberg G, Fowler B, Nordberg M, Friberg L, editors. Handbook on the Toxicology of Metals. 3rd edition. Elsevier; 2007. [Google Scholar]
- Palheta D, Taylor A. Mercury in environmental and biological samples from a gold mining area in the Amazon region of Brazil. Sci Total Environ. 1995;168:63–69. doi: 10.1016/0048-9697(95)04533-7. [DOI] [PubMed] [Google Scholar]
- Spiegel SJ. Occupational health, mercury exposure and environmental justice: Learning from experiences in Tanzania. Am J Public Health. 2009;99:S550–S558. doi: 10.2105/AJPH.2008.148940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel SJ, Veiga MM. International guidelines on mercury management in small-scale gold mining. J Clean Prod. 2010;18:375–385. [Google Scholar]
- Swain EB, Jakus PM, Rice G, Lupi F, Maxson PA, Pacyna JM, Penn A, Spiegel SJ, Veiga MM. Socioeconomic consequences of mercury use and pollution. Ambio. 2007;36:45–61. doi: 10.1579/0044-7447(2007)36[45:scomua]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tschakert P, Singha K. Contaminated identities: mercury and marginalization in Ghana's artisanal mining sector. Geoforum. 2007;38:1304–1321. [Google Scholar]
- UNEP. Global Mercury Assessment. Inter-organizational Programme for the Sound Management of Chemicals. Geneva, Switzerland: A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and OECD; 2002. [Google Scholar]
- vanStraaten P. Human exposure to mercury due to small scale gold mining in northern Tanzania. Sci Total Environ. 2000;259:45–53. doi: 10.1016/s0048-9697(00)00548-9. [DOI] [PubMed] [Google Scholar]
- Voegborlo RB, Matsuyama A, Adimado AA, Akagi H. Head hair total mercury and methylmercury levels in some Ghanaian individuals for the estimation of their exposure to mercury: preliminary studies. Bull Environ Contam Toxicol. 2010;84:34–38. doi: 10.1007/s00128-009-9901-7. [DOI] [PubMed] [Google Scholar]


