Abstract
Clostridium botulinum has been classified into four groupings (groups I to IV) based on physiological characteristics and 16S rRNA sequencing. We have examined the lipid compositions of 11 representative strains of C. botulinum and a strain of Clostridium sporogenes by 2D-TLC and by MS. All strains contained phosphatidylglycerol (PG), cardiolipin (CL) and phosphatidylethanolamine (PE) in both the all-acyl and the alk-1′-enyl (plasmalogen) forms. Five strains in proteolytic group I, which are related to C. sporogenes, contained varying amounts of an ethanolamine-phosphate derivative of N-acetylglucosaminyl-diradylglycerol, which is also present in C. sporogenes. Three strains in group II, which are related to Clostridium butyricum, Clostridium beijerinckii and Clostridium acetobutylicum, contained lipids characteristic of these saccharolytic species: a glycerol acetal and a PG acetal of the plasmalogen form of PE. Two group III strains, which are related to Clostridium novyi, contained amino-acyl derivatives of PG, which are also found in C. novyi. A strain in group IV had PE, PG and CL, but none of the distinguishing lipids. This work shows that the lipidome of C. botulinum is consistent with its classification by other methods.
Introduction
Clostridium botulinum has been known as the cause of botulism, a potentially fatal foodborne toxinosis, since the late 19th century. Botulinum neurotoxins (BoNTs) are the most poisonous substances known and are classified into seven serologically distinct groups (serotypes A to G) (Hatheway, 1995). Most strains produce only one serotype of BoNT, but some produce more than one toxin serotype, including Ab, Ba, Af and Bf (Barash & Arnon, 2004; Gimenez & Gimenez, 1993; Santos-Buelga et al., 1998), whereby the highest quantity of BoNT produced is designated by the upper-case letter. Many strains also contain unexpressed (silent) BoNT genes, where the silent gene is shown in parentheses, e.g. A(B) (Franciosa et al., 1994). Human botulism is commonly caused by groups I and II C. botulinum (Sebaihia et al., 2007). Other species of clostridia have been isolated that produce BoNTs, including Clostridium argentinense, and rare strains of Clostridium baratii and Clostridium butyricum (Hatheway & Johnson, 1998). Neurotoxin-producing clostridia are paraphyletic, and are designated solely by their ability to produce their characteristic neurotoxin.
Early biochemical and physiological studies of C. botulinum indicated that they were distinct physiologically and could be divided into four groups, which were designated groups I, II, III and IV (Hatheway, 1990). These physiological groups were based on proteolytic and saccharolytic characteristics, acid and alcohol fermentation end products, ability to grow at low temperatures, heat resistance of spores, and ability to grow in the presence of acids, alcohols and salts (Hatheway & Johnson, 1998).
The diversity of the neurotoxin-producing clostridia has been supported by molecular taxonomic studies (Collins & East, 1998; Collins et al., 1994; Hill et al., 2007). Johnson and Francis divided the genus Clostridium into four major groups, I to IV, based on the G+C content of their DNA and rRNA gene homologies. C. botulinum strains were all in the low %G+C group I, and were separated into groups I-A, I-F and I-H based on rRNA homologies (Johnson & Francis, 1975). Further 16S rRNA analyses of the genus Clostridium confirmed the classification of C. botulinum into four groupings which corresponded to the four physiological groups, I to IV (Collins & East, 1998; Hutson et al., 1993a, 1993b). An analysis of 174 C. botulinum strains by amplified fragment length polymorphism (AFLP) and by sequencing of 16S rRNA and BoNT genes confirmed the existence of at least four distinct genomic backgrounds, each of which has likely independently acquired BoNT genes through horizontal gene transfer (Hill et al., 2007).
The proteolytic group I strains of C. botulinum expressing toxin types A, B and F are closely related to Clostridium sporogenes. The saccharolytic group II strains expressing toxin types B, E and F are related to Clostridium butyricum, Clostridium beijerinckii and Clostridium acetobutylicum. Group III strains expressing toxin types C and D cluster with Clostridium novyi and Clostridium haemolyticum, and group IV strains expressing toxin type G cluster with Clostridium subterminale, Clostridium proteolyticus, C. argentinense and Clostridium schirmacherense (Collins et al., 1994; Hill et al., 2007). The lipids of C. butyricum, C. beijerinckii and C. acetobutylicum have been studied intensively (Baumann et al., 1965; Johnston & Goldfine, 1983, 1992; Oulevey et al., 1986; Matsumoto et al., 1971) and the polar lipids of C. novyi were recently elucidated (Guan et al., 2011). We have also studied the polar lipids of C. sporogenes. Thus it became possible to compare the lipid compositions of a number of strains of C. botulinum with previously completed analyses of clostridial lipids. The results of a detailed study of the lipids of 11 strains of C. botulinum by 2D-TLC and by MS are here presented. These lipidomic studies demonstrate clear relationships of C. botulinum strains with those that have been found to be related by DNA sequence and physiological analyses.
Methods
Strains.
The C. botulinum strains examined in this study listed in Table 1 are from our (E. A. Johnson) culture collection. They were stored in 50 % glycerol/50 % TPGY (trypticase-peptone-glucose-yeast extract) medium at −80 °C.
Table 1. C. botulinum strains analysed.
| Strain | Serotype | Group | Source |
| Loch Maree | A-3 | I | CDC |
| Hall A Hyper | A | I | Our laboratory |
| Okra B | B | I | Our laboratory |
| ATCC 3502 | A | I | Our laboratory |
| 62A | A | I | Our laboratory |
| Eklund 17B | B | II | Mel Eklund |
| Alaska E | E | II | Mel Eklund |
| Beluga E | E | II | Our laboratory |
| ATCC 1873 | D | III | Our laboratory |
| Africa C | C/D | III | Our laboratory |
| G89 | G | IV | CDC |
| C. sporogenes ATCC 3584 | na | na | ATCC |
Lipid isolation.
Frozen stocks were thawed and grown anaerobically overnight in 10 ml TPGY medium at 30 °C (strains 17B, Alaska E, Beluga E and type G) or 37 °C (strains Loch Maree, Hall A Hyper, Okra B, ATCC 3502, 62A and C. sporogenes). These cultures were inoculated at 1 %, v/v, into 500 ml anaerobic TPGY, and grown statically for 18 h at 30 or 37 °C, and the cells were harvested by centrifugation at 4 °C. The wet cell pellets were extracted with chloroform/methanol/water by the method of Bligh & Dyer (1959), with modifications. The lipid extracts were dried under a stream of nitrogen, while being warmed in a heating block. They were dissolved in chloroform and stored at −20 °C.
TLC.
2D-TLC was performed on silica gel 60, 10×10 cm thin-layer plates. The solvents used were chloroform/methanol/concentrated ammonia/water, 65 : 30 : 2.5 : 2.5 (by vol.), in the first dimension and chloroform/methanol/acetic acid/water, 80 : 18 : 12 : 5 (by vol.), in the second dimension. Acid hydrolysis of lipids on TLC plates using HCl fumes for detection of plasmalogens has been described previously (Johnston et al., 2010). Amine-containing lipids were detected using 0.3 % ninhydrin in ethanol, followed by heating at 120 °C for 5–10 min. Phosphorus-containing lipids were detected with 0.3 % (w/v) molybdenum blue (Sigma). Standards of phosphatidylethanolamine (PE), phosphatidylglycerol (PG), cardiolipin (CL) from Sigma-Aldrich, and of glycerol acetal of plasmenylethanolamine (GAPlaE) isolated from C. butyricum, were run alongside the lipid samples in both the first and second dimensions. The standards were spotted in two lanes at the left side of the plate for the first dimension run and at the right side of the top of the plate for the second dimension. HCl hydrolysis of plasmalogens results in the formation of the corresponding lyso lipid, i.e. with the remaining sn-2 acyl chain and long-chain aldehydes. HCl hydrolysis of the GAPlaE results in the formation of lyso-PE, and hydrolysis of phosphatidylglycerol acetal of plasmenylethanolamine (PGAPlaE) results in the formation of lyso-PE and PG in addition to long-chain aldehydes (Johnston & Goldfine, 1988; MacDonald & Goldfine, 1990).
Quantification of C. sporogenes lipids.
The relative amounts of the polar lipids of C. sporogenes were determined as previously described for the lipids of Clostridium tetani (Johnston et al., 2010). Briefly, triplicate 10 ml cultures were prelabelled with 10 µCi (370 kBq) [1-14C]acetate and grown overnight. The cells were harvested by centrifugation and the lipids extracted with chloroform/methanol. They were subjected to 2D-TLC as described above and quantified with a phosphorimager.
Liquid chromatography/MS (LC/MS).
Normal-phase LC-electrospray ionization (ESI)/MS of lipids was performed using an Agilent 1200 Quaternary LC system, coupled to a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems). LC was performed on an Ascentis Si HPLC column (5 µm, 25 cm×2.1 mm). The elution program has been described previously (Guan et al., 2011). The post-column splitter diverted ~10 % of the LC flow to the ESI source of the QSTAR XL mass spectrometer, with MS settings as follows: IS = −4500 V, CUR = 20 p.s.i., GS1 = 20 p.s.i., DP = −55 V and FP = −150 V. Nitrogen was used as the collision gas for MS/MS experiments. Data acquisition and analysis were performed using the Analyst QS software (Applied Biosystems). Polar lipids emerged from this column as follows: PG, 12–14 min; CL, 14–15 min; PE and phosphatidylmonomethylethanolamine (PMME), 16.5–17.5 min; PGAPlaE, 17–18 min; GAPlaE, 18–20 min; lysyl-PG, 20–22 min; EtnP-GlcNAcDAG, 22–24 min. In some runs there was partial overlap between PG and CL.
Results
The C. botulinum strains used in this study are a representative sample of the four physiological groupings. At least one strain from each major group is represented. Although the sample size is small, the results so far indicate that lipid composition mirrors taxonomy based on other physiological characteristics and DNA sequencing.
Group I
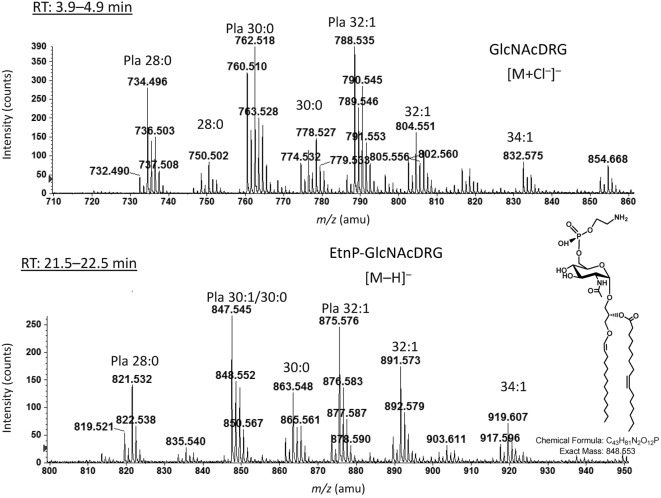
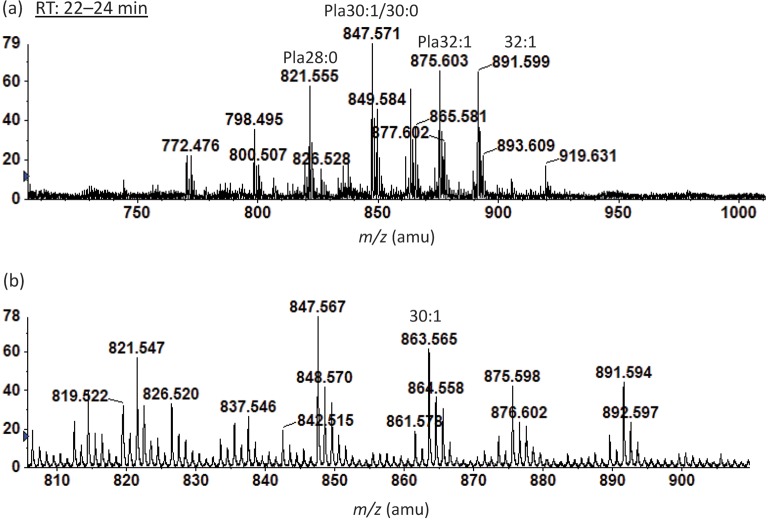
This group, which secretes BoNT types A, B or F and is proteolytic, is closely related to C. sporogenes and Clostridium putrificum, as revealed by 16S rRNA sequencing (Hill et al. 2007; Collins et al., 1994; Collins & East, 1998). Analysis of the polar lipids of C. sporogenes revealed the presence of PE, PG and CL in both the all-acyl forms and as plasmalogens. The presence of the acyl forms of these lipids is predicted based on the presence of genes annotated as pss, psd, pgpA or -B and cls. All three phospholipids are rich in plasmalogens (1-O-alk-1′-enyl ether lipids), which have been found in many anaerobic, but not in aerobic or facultative bacteria (Goldfine & Johnston, 2005; Goldfine, 2010b). In addition, 5.4 % of the polar lipids of C. sporogenes were N-acetylglucosylaminyl-diradylglycerol (GlcNAcDRG) and 3.0 % were a recently discovered phosphoethanolamine-modified GlcNAcDRG (EtnP-GlcNAcDRG; Johnston et al., 2010), both of which contained substantial amounts of the plasmalogen form (Fig. 1). EtnP-GlcNAcDRG was originally identified in C. tetani (Johnston et al., 2010), which is moderately related to C. sporogenes (Collins et al., 1994). We found EtnP-GlcNAcDRG in five group I C. botulinum strains: Hall A Hyper (Fig. 2a), 62A (Fig. 2b), Loch Maree, Okra B and ATCC 3502 (not shown) (Table 2). Upon MS/MS, EtnP-GlcNAcDRG produced a fragment (EtnP) ion at m/z 140.0, which was seen in the MS/MS spectra of these lipids from C. sporogenes and the group I C. botulinum strains.
Fig. 1.
Negative ion ESI/MS spectra of GlcNAcDRG and EtnP-GlcNAcDRG species from C. sporogenes strain ATCC 3584. Pla, plasmalogen species. The structure of the plasmalogen form of EtnP-GlcNAcDAG (30 : 1) is shown. The number before the colon is the sum of the chain lengths and the number after the colon is the number of double bonds.
Fig. 2.
Negative ion ESI/MS spectra of EtnP-GlcNAcDRG species from (a) C. botulinum strain Hall A Hyper and (b) strain 62A. Pla, plasmalogen species. The number before the colon is the sum of the chain lengths and the number after the colon is the number of double bonds.
Table 2. Polar lipids of C. botulinum strains.
The ‘+’ symbols indicate the relative amounts of these lipids as judged by molybdate staining and/or charring. Abbreviation: Pla, plasmalogen form.
| Strain | Group | PE/PlaE | PG/PlaG | CL/PlaCL | GAPlaE | PGAPlaE | Ala- or Lys-PG | EtnP-GlcNAc-DRG | GlcNAcDRG |
| HAH* | I | ++/+++ | ++/+++ | +/+++† | + (Mostly Pla) | ||||
| LM A-3‡ | I | ++/+++ | +/++ | ++/+++ | Trace | ||||
| Okra B | I | +/+++ | +/+++ | +/+++ | Trace | ||||
| 62A | I | +++/++ | ++/+++ | ++/+++ | Trace | ||||
| ATCC 3502 (type A) | I | ++/++ | +/+++ | ++/+++† | + (Mostly Pla) | ||||
| C. sporogenes | ++/+++ | ++/+++ | ++/+++ | + | |||||
| Eklund 17B | II | ++/+++ | +/++ | ++/++ +† | ++ | + | + (Diacyl) | ||
| BE§ | II | +/+++ | +/++ | ++/+++ | ++ | ++|| | + (Diacyl) | ||
| Alaska E | II | +/++ | +++/+ | ++/++ | ++ | ++ | Lys-PG | ||
| D1873 | III | +++/+++ | ++/++ | ++/++ | Lys-PG/Pla Ala-PG/Pla | ||||
| Africa C | III | +++/+ | +++/+ | +++/+ | Lys-PG | ||||
| G89 | IV | +/+++ | ++/+++ | ++/+++ |
Hall A Hyper.
Di-alkenyl species found.
Loch Maree A3.
Beluga E.
Both PG and PlaG acetals of PlaE were found.
Group II
Members of this group, which secrete BoNT types B, E or F, are typically saccharolytic and non-proteolytic. According to 16S rRNA sequencing, they cluster with C. acetobutylicum, C. beijerinckii and C. butyricum (Collins et al., 1994; Hill et al., 2007; Collins & East, 1998). In terms of lipid composition this is the best-studied group of the genus. Like most clostridia and indeed many bacteria, these species contain PE, PG and CL. C. acetobutylicum differs from the other two in having large amounts of glycosyl-diradylglycerols in addition to phospholipids (Johnston & Goldfine, 1992; Oulevey et al., 1986). A distinguishing feature of these species is the presence of significant levels of glycerol-acetals of plasmenylethanolamine (PlaE) (Matsumoto et al., 1971; Khuller & Goldfine, 1974) and smaller amounts of PG-acetals of PlaE (Johnston & Goldfine, 1988; MacDonald & Goldfine, 1990). Glycerol and PG acetals of PlaE were seen by 2D-TLC (Fig. 3) and MS (Fig. 4) in strain Beluga E, and in Eklund 17B and Alaska E (data not shown) (Table 2). Interestingly, strain Beluga E has both PG and plasmenylglycerol (PlaG) acetals of PlaE (Fig. 4a). This represents the first identification of PlaG acetals of PlaE.
Fig. 3.
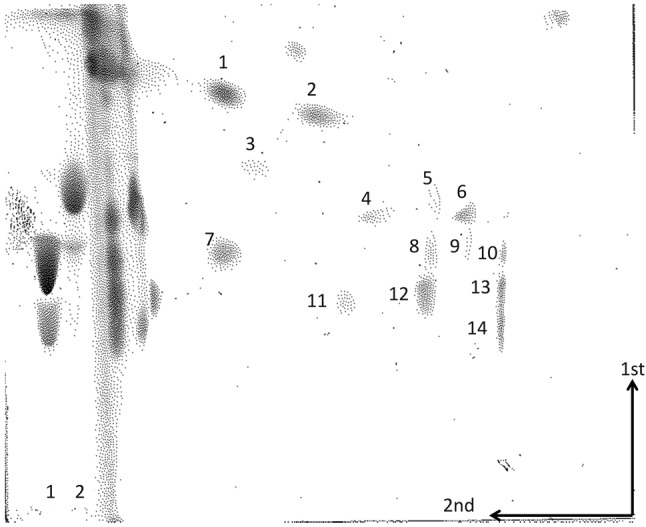
Analysis by 2D-TLC of 14C-labelled lipids from C. botulinum strain Beluga E. The lipids were subjected to hydrolysis with HCl fumes between the first and second chromatographies. Based on their positions and staining, the major lipids were tentatively identified as: 1, monoglycosyldiacylglycerol; 2, unknown phospholipid; 3, unknown phospholipid; 4, CL; 5, PG; 6. lyso-CL derived from PlaCL; 7, unknown (phosphorus-negative, ninhydrin-negative); 8, PE; 9, unknown; 10, lyso-PE derived from PlaE; 11, unknown (phosphorus-negative, ninhydrin-negative); 12, PG derived from PGAPlaE; 13, lyso-PE derived from PGAPlaE and lyso-PG derived from PlaGAPlaE; 14, lyso-PE derived from GAPlaE. Spots at the solvent front of the second dimension are aldehydes and their condensation products derived from the plasmalogens. A slightly slower-moving product is seen in the lane containing PGAPlaE and PlaGAPlaE. Standards on the left side were: 1, PE and GA; 2, PG. The arrows indicate the 1st and 2nd dimensions.
Fig. 4.
(a) Negative ion ESI/MS spectra of PG and PlaG acetals of PlaE. PlaGAPlaE species are underlined. The structure of PlaGAPlaE (64 : 2) is shown. (b) Glycerol acetals of PlaE (GAPlaE) from C. botulinum strain Beluga E. The structure of GAPlaE (30 : 1) is shown. The number before the colon is the sum of the chain lengths and the number after the colon is the number of double bonds.
Group III
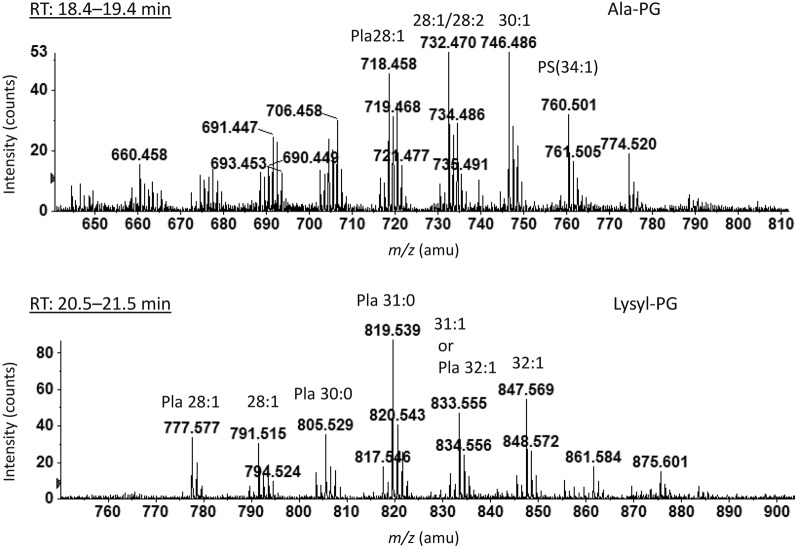
C. botulinum strains in this group produce type C and D toxins and are genetically related to C. novyi (Collins et al., 1994; Collins & East, 1998; Hill et al., 2007). Members of this group are non-proteolytic and have higher optimal growth temperatures than members of groups I and II (Collins & East, 1998). A distinguishing feature of the lipidome of C. novyi is the presence of the aminoacyl-PGs lysyl- and alanyl-PG (Guan et al., 2011). Like C. novyi, strain D1873 contains both lysyl-PG and alanyl-PG (Fig. 5), and strain C. Africa CP contains only lysyl-PG. Lysyl-PG was also observed in C. botulinum Alaska E (Fig. S1).
Fig. 5.
Negative ion ESI/MS spectra of Ala-PG and Lys-PG from C. botulinum strain D1873. Pla, plasmalogen species. The number before the colon is the sum of the chain lengths and the number after the colon is the number of double bonds.
Group IV
There are relatively few C. botulinum strains in group IV, which is characterized by secretion of the G type toxin. These strains do not ferment sugars and differ from the other groups by lack of lipase production. By 16S rRNA sequence analysis they cluster most closely with C. subterminale and Clostridium estertheticum (Collins & East, 1998) and also with C. proteolyticus, C. argentinense and C. schirmacherense (Hill et al., 2007). The strain G that we examined contained PE, PG and CL with abundant plasmalogen forms (Table 2).
Discussion
Polar lipid compositions clearly distinguish groups I, II and III strains of C. botulinum. Group I strains are closely related to C. sporogenes and more distantly related to C. tetani (Collins & East, 1998; Hill et al., 2007; Sebaihia et al., 2007). In addition to PG, CL and PE, phospholipids that are found in all strains of C. botulinum that we have examined, group I strains contain low amounts of EtnP-GlcNAcDRG, which was detected by MS of the total lipids. This phosphoethanolamine-modified diradylglycerol glycolipid was initially characterized in C. tetani (Johnston et al., 2010). It is also found in C. sporogenes (Fig. 1). Since there is no rapid method to date for detecting this lipid, it would not serve as a useful diagnostic tool for this group at this time.
The saccharolytic group II strains cluster with C. acetobutylicum, C. beijerinckii and C. butyricum (Collins et al., 1994; Hill et al., 2007; Collins & East, 1998). The lipids that are unique to this group of organisms are glycerol acetals and PG acetals of PlaE (Goldfine & Johnston, 2005), and these lipids were found in the three group II strains of C. botulinum that we examined. These lipids are readily detectable on 2D-TLC of the total lipids, as they have distinct mobilities and produce specific products of HCl hydrolysis when plates are exposed to HCl fumes prior to chromatography in the second dimension (Fig. 3). C. acetobutylicum and C. beijerinckii are noted for their ability to produce acetone and butanol, and have been used for industrial production of these compounds (Jones & Woods, 1986; Keis et al., 2001). In these solventogenic bacteria the conversion of PlaE to GAPlaE, possibly by way of the intermediate PGAPlaE, serves to stabilize the bilayer arrangement of the cell membrane in response to the formation of solvents (MacDonald & Goldfine, 1990, 1991; Goldfine, 2010a; Goldfine et al., 1987). The retention of these lipids in C. botulinum group II strains suggests that they meet similar challenges during and after growth in sugar-containing substrates.
The two group III strains, D1873 and Africa C, like C. novyi to which they are related, contain aminoacyl-PGs. The gene products responsible for the biosynthesis of aminoacyl-PG have been designated MprF (multiple peptide resistance factors), and are considered to be virulence factors that control cellular permeability to cationic antibiotics by transferring an amino acid from tRNA to the free distal hydroxyl group of the glycerol moiety of PG. Roy and Ibba found 117 sequences of MprF-related proteins in 22 genera of Gram-positive bacteria (Roy & Ibba, 2008). MprF is also widely distributed in Gram-negative species. In Gram-positive bacteria MprF is mostly found in Firmicutes (bacilli and clostridia), but not in Mollicutes. It is also found in Actinobacteria (Roy & Ibba, 2008).
MprF of C. novyi NT is 93 % identical to that of C. botulinum type C, 80 % identical to that of strain BKT015925, and 78 % identical to that of D strain 1873. We should also note that lysyl-PG is also present in the group II strain Alaska E.
There are only a few known strains of C. botulinum in group IV which produce BoNT/G (Suen et al., 1988). They are related to C. subterminale, C. proteolyticus, C. argentinense and C. schirmacherense (Hill et al., 2007). We have analysed strain G89, and found that it contains PE, PG and CL, some minor lipids, but none of the distinguishing polar lipids. None of these genomes has been sequenced, making it difficult to predict their phospholipid-synthesizing capabilities.
Notably, all strains of C. botulinum that we have examined have varying amounts of plasmalogen, as demonstrated both by 2D-TLC with HCl treatment and by MS. The only earlier report concerning the polar lipids of C. botulinum was on strain NCIB 4270 grown at 30 °C. This non-proteolytic strain, which was isolated by the Torry Research Station from sea-bottom deposits off the Scandinavian coast prior to 1967 (Hodgkiss et al., 1967), contained PE, PG, CL, phosphatidylserine and a phosphorus-containing glycolipid of unknown structure (Evans et al., 1998). None of these lipid classes had plasmalogen equivalents. A similar situation has been observed in C. tetani, in which one strain was found to be negative for plasmalogens, but four other strains were positive. The loss of plasmalogen-synthesizing capabilities has been noted in laboratory-passaged strains (Johnston et al., 2010). Another possibility is that the cool marine environment permitted the loss of plasmalogens. It would be interesting to examine other clostridia isolated from this environment. Clearly, other C. botulinum strains with various histories have retained this capacity.
It has been acknowledged for several decades that 16S rRNA sequencing and more recently total genome sequencing provide the best evidence for taxonomic relationships among bacteria and Archaea. However, lipid analysis can provide significant additional information about these relationships (Goldfine, 1982). Indeed, before the distinct evolutionary position of Archaea was shown by 16S rRNA sequencing (Woese & Fox, 1977), it was known that halophiles, later classified among Archaea, contain unique 2,3-diphytanyl-sn-glycerol ether lipids, characteristic of this group (Kates, 1972). The distinct nature of the lipids was one of the first clues that this group of organisms was very different from bacteria, which are largely characterized by lipids containing fatty acids, and in the case of anaerobes, by vinyl-ethers (Goldfine, 1982). Since most bacteria contain PG, CL and PE, taxonomic relationships are better shown with lipids that are found in relatively small clusters of organisms. In this paper we have shown that for C. botulinum, EtnP-GlcNacDRG, glycerol- and PG-acetals of PlaE, and aminoacyl-PGs represent polar lipids that are of diagnostic value in bacterial taxonomy.
Acknowledgements
We wish to acknowledge Mark J. Jacobson for growth of C. botulinum strains and lipid extractions. The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z. G. are supported by the NIH LIPID MAPS Large Scale Collaborative Grant number GM-069338. This work was supported by a research grant from the University of Pennsylvania Research Foundation. This project was partially supported in E. A. J.’s laboratory by the Pacific Southwest Regional Center of Excellence grant U54 AI065359 and the Region V ‘Great Lakes’ RCE (NIH award 1-U54-AI-057153).
Abbreviations:
- BoNT
botulinum neurotoxin
- CL
cardiolipin
- EtnP-GlcNAcDRG
phosphoethanolamine GlcNAc-diradylglycerol
- GAPlaE
glycerol acetal of plasmenylethanolamine
- GlcNAcDRG
GlcNAc-diradylglycerol
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PGAPlaE
phosphatidylglycerol acetal of plasmenylethanolamine
- PlaE
plasmenylethanolamine
- PlaG
plasmenylglycerol
Footnotes
A supplementary figure is available with the online version of this paper.
References
- Barash J. R., Arnon S. S. (2004). Dual toxin-producing strain of Clostridium botulinum type Bf isolated from a California patient with infant botulism. J Clin Microbiol 42, 1713–1715. 10.1128/JCM.42.4.1713-1715.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N. A., Hagen P.-O., Goldfine H. (1965). Phospholipids of Clostridium butyricum: studies on plasmalogen composition and biosynthesis. J Biol Chem 240, 1559–1567. [PubMed] [Google Scholar]
- Bligh E. G., Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37, 911–917. 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- Collins M. D., East A. K. (1998). Phylogeny and taxonomy of the food-borne pathogen Clostridium botulinum and its neurotoxins. J Appl Microbiol 84, 5–17. 10.1046/j.1365-2672.1997.00313.x [DOI] [PubMed] [Google Scholar]
- Collins M. D., Lawson P. A., Willems A., Cordoba J. J., Fernandez-Garayzabal J., Garcia P., Cai J., Hippe H., Farrow J. A. E. (1994). The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol 44, 812–826. 10.1099/00207713-44-4-812 [DOI] [PubMed] [Google Scholar]
- Evans R. I., McClure P. J., Gould G. W., Russell N. J. (1998). The effect of growth temperature on the phospholipid and fatty acyl compositions of non-proteolytic Clostridium botulinum. Int J Food Microbiol 40, 159–167. 10.1016/S0168-1605(98)00029-4 [DOI] [PubMed] [Google Scholar]
- Franciosa G., Ferreira J. L., Hatheway C. L. (1994). Detection of type A, B, and E botulism neurotoxin genes in Clostridium botulinum and other Clostridium species by PCR: evidence of unexpressed type B toxin genes in type A toxigenic organisms. J Clin Microbiol 32, 1911–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez D. F., Gimenez J. A. (1993). Serological subtypes of botulinal neurotoxins. In Botulinum and Tetanus Neurotoxins, pp. 421–431. Edited by DasGupta B. R. New York: Plenum Press. [Google Scholar]
- Goldfine H. (1982). Lipids of procaryotes – structure and distribution. Current Topics Membr Transp 17, 1–43. 10.1016/S0070-2161(08)60307-X [DOI] [Google Scholar]
- Goldfine H. (2010a). Membrane biogenesis. In Hydrocarbons, Oils and Lipids: Diversity, Properties and Formation, pp. 417–424. Edited by Timmis K. N. Berlin: Springer. [Google Scholar]
- Goldfine H. (2010b). The appearance, disappearance and reappearance of plasmalogens in evolution. Prog Lipid Res 49, 498. [DOI] [PubMed] [Google Scholar]
- Goldfine H., Johnston N. C. (2005). Membrane lipids of clostridia. In Handbook on Clostridia, pp. 297–310. Edited by Dürre P. Boca Raton, FL: Taylor & Francis; 10.1201/9780203489819.pt3 [DOI] [Google Scholar]
- Goldfine H., Johnston N. C., Mattai J., Shipley G. G. (1987). Regulation of bilayer stability in Clostridium butyricum: studies on the polymorphic phase behavior of the ether lipids. Biochemistry 26, 2814–2822. 10.1021/bi00384a024 [DOI] [PubMed] [Google Scholar]
- Guan Z., Johnston N. C., Aygun-Sunar S., Daldal F., Raetz C. R., Goldfine H. (2011). Structural characterization of the polar lipids of Clostridium novyi NT. Further evidence for a novel anaerobic biosynthetic pathway to plasmalogens. Biochim Biophys Acta 1811, 186–193. 10.1016/j.bbalip.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatheway C. L. (1990). Toxigenic clostridia. Clin Microbiol Rev 3, 66–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatheway C. L. (1995). Botulism: the present status of the disease. Curr Top Microbiol Immunol 195, 55–75. 10.1007/978-3-642-85173-5_3 [DOI] [PubMed] [Google Scholar]
- Hatheway C. L., Johnson E. A. 1998. Clostridium: the spore-bearing anaerobes. In: Topley and Wilson’s Microbiology and Microbial Infections, 9th Edn, pp. 731–782. Edited by Coller L., Balows A., Sussman M. London: Arnold. [Google Scholar]
- Hill K. K., Smith T. J., Helma C. H., Ticknor L. O., Foley B. T., Svensson R. T., Brown J. L., Johnson E. A., Smith L. A., Okinaka R. T., Jackson P. J., Marks J. D., 2007. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J Bacteriol 189, 818–832. 10.1128/JB.01180-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkiss W., Ordal Z. J., Cann D. C. (1967). The morphology and ultrastructure of the spore and exosporium of some Clostridium species. J Gen Microbiol 47, 213–225. 10.1099/00221287-47-2-213 [DOI] [PubMed] [Google Scholar]
- Hutson R. A., Thompson D. E., Collins M. D. (1993a). Genetic interrelationships of saccharolytic Clostridium botulinum types B, E and F and related clostridia as revealed by small-subunit rRNA gene sequences. FEMS Microbiol Lett 108, 103–110. 10.1111/j.1574-6968.1993.tb06081.x [DOI] [PubMed] [Google Scholar]
- Hutson R. A., Thompson D. E., Lawson P. A., Schockenitturino R. P., Bottger E. C., Collins M. D. (1993b). Genetic interrelationships of proteolytic Clostridium botulinum types A, B, and F and other members of the Clostridium botulinum complex as revealed by small-subunit rRNA gene sequences. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology 64, 273–283. 10.1007/BF00873087 [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Francis B. S. (1975). Taxonomy of the clostridia: ribosomal ribonucleic acid homologies among the species. J Gen Microbiol 88, 229–244. 10.1099/00221287-88-2-229 [DOI] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. (1983). Lipid composition in the classification of the butyric acid-producing clostridia. J Gen Microbiol 129, 1075–1081. [DOI] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. (1988). Isolation and characterization of a novel four chain phospholipid, the phosphatidylglycerol acetal of plasmenylethanolamine from Clostridium butyricum. Biochim Biophys Acta 961, 1–12. 10.1016/0005-2760(88)90124-5 [DOI] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. (1992). Replacement of the aliphatic chains of Clostridium acetobutylicum by exogenous fatty acids: regulation of phospholipid and glycolipid composition. J Bacteriol 174, 1848–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston N. C., Aygun-Sunar S., Guan Z., Ribeiro A. A., Daldal F., Raetz C. R., Goldfine H. (2010). A phosphoethanolamine-modified glycosyl diradylglycerol in the polar lipids of Clostridium tetani. J Lipid Res 51, 1953–1961. 10.1194/jlr.M004788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. (1986). Acetone-butanol fermentation revisited. Microbiol Rev 50, 484–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. (1972). Ether-linked lipids in extemely halophilic bacteria. In Ether Lipids: Chemistry and Biology, pp. 351–398. Edited by Snyder F. New York: Academic Press. [Google Scholar]
- Kates M. (1986). Techniques of Lipidology. Isolation, Analysis and Identification of Lipids, 2nd Edn Amsterdam: North-Holland Publishing Company. [Google Scholar]
- Keis S., Shaheen R., Jones D. T. (2001). Emended descriptions of Clostridium acetobutylicum and Clostridium beijerinckii, and descriptions of Clostridium saccharoperbutylacetonicum sp. nov. and Clostridium saccharobutylicum sp. nov. Int J Syst Evol Microbiol 51, 2095–2103. 10.1099/00207713-51-6-2095 [DOI] [PubMed] [Google Scholar]
- Khuller G. K., Goldfine H. (1974). Phospholipids of Clostridium butyricum. V. Effects of growth temperature on fatty acid, alk-1-enyl ether group, and phospholipid composition. J Lipid Res 15, 500–507. [PubMed] [Google Scholar]
- MacDonald D. L., Goldfine H. (1990). Phosphatidylglycerol acetal of plasmenylethanolamine as an intermediate in ether lipid formation in Clostridium butyricum. Biochem Cell Biol 68, 225–230. 10.1139/o90-030 [DOI] [PubMed] [Google Scholar]
- MacDonald D. L., Goldfine H. (1991). Effects of solvents and alcohols on the polar lipid composition of Clostridium butyricum under conditions of controlled lipid chain composition. Appl Environ Microbiol 57, 3517–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Tamiya K., Koizumi K. (1971). Studies on neutral lipids and a new type of aldehydogenic ethanolamine phospholipid in Clostridium butyricum. J Biochem 69, 617–620. [PubMed] [Google Scholar]
- Oulevey J., Bahl H., Thiele O. W. (1986). Novel alk-1-enyl ether lipids isolated from Clostridium acetobutylicum. Arch Microbiol 144, 166–168. 10.1007/BF00414729 [DOI] [Google Scholar]
- Roy H., Ibba M. (2008). RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc Natl Acad Sci U S A 105, 4667–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Buelga J. A., Collins M. D., East A. K. (1998). Characterization of the genes encoding the botulinum neurotoxin complex in a strain of Clostridium botulinum producing type B and F neurotoxins. Curr Microbiol 37, 312–318. [DOI] [PubMed] [Google Scholar]
- Sebaihia M., Peck M. W., Minton N. P., Thomson N. R., Holden M. T. G., Mitchell W. J., Carter A. T., Bentley S. D., Mason D. R. & other authors (2007). Genome sequence of a proteolytic (Group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res 17, 1082–1092. 10.1101/gr.6282807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen J. C., Hatheway C. L., Steigerwalt A. G., Brenner D. J. (1988). Genetic confirmation of identities of neurotoxigenic Clostridium baratii and Clostridium butyricum implicated as agents of infant botulism. J Clin Microbiol 26, 2191–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. (1977). Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A 74, 5088–5090. 10.1073/pnas.74.11.5088 [DOI] [PMC free article] [PubMed] [Google Scholar]