Abstract
Background
Patient overall perception of health may provide an effective early warning for risk of hospitalization and death among heart failure patients.
Objective
Determine whether overall perceived health predicts all-cause hospitalization or death in heart failure patients after adjusting for confounding factors in a sample of adults with heart failure.
Design
Prospective, longitudinal, observational study.
Settings
Three outpatient urban settings in the northeast United States between 2007 and 2010.
Participants
Adults with chronic Stage C heart failure confirmed by echocardiographic and clinical evidence.
Methods
A secondary analysis was conducted using data collected on 273 Stage C patients with heart failure. Participants in the parent study were followed for 6 months. Overall perceived health was measured by self-report. Hospitalization and death were assessed from electronic hospital records and confirmed with county death records as needed. Cox proportional hazards models were used to examine the association between perceptions of health and rates of hospitalization and death.
Results
Patients with poor or fair perceived health had over 5.5 times the rate of death or hospitalization over the 6-month period (hazard ratio; 95% confidence interval: 2.0-15.6; p=0.001) after controlling for model covariates. The predictive ability of perceived health attenuated over time such that at 30-days patients who reported poor or fair perceived health had only 1.2 times the rate of an event and virtually no difference in event rate by 60-days.
Conclusions
Overall perceived health is a powerful indicator of impending events and can be a quick tool for prioritizing heart failure patients who are at highest risk of imminent death and hospitalization. Questions about perceived health need to be asked of patients regularly in order to have clinical utility.
Introduction
Heart failure is estimated to affect about 5.7 million adults in the United States (Roger et al., 2012). The prevalence of heart failure is rising as treatment approaches improve and survival among elderly patients with heart failure increases (Roger et al., 2012). At this time, the age-adjusted 5-year mortality estimate for heart failure is approximately 50% (Roger et al., 2004). Heart failure causes frequent hospitalization; (Hunt et al., 2009, Kosiborod et al., 2006) among Medicare beneficiaries it is the most frequent cause of hospitalization (Cheng and Nayar, 2009). In 2010, the estimated direct and indirect costs of heart failure were over $39.2 billion (Lloyd-Jones et al., 2010), most of which were attributed to hospitalizations.
One of the present challenges of heart failure management is identifying which patients are at highest risk of hospitalization in order to intervene early or prevent hospitalization entirely with better self-care and disease management. Preventing hospitalizations can reduce costs and improve patients’ quality of life. There are multiple ways to determine which patients are at highest risk of hospitalization. Two commonly used prognostic indicators include New York Heart Association (NYHA) functional class and exercise capacity (Havranek et al., 2001). These outcomes are limited by being determined exclusively by clinicians, rather than by patients. A simple question about overall perceived health consistently predicts mortality, (Farkas et al., 2009, Havranek et al., 2001, Idler and Benyamini, 1997, Johansson et al., 2008, Rosen et al., 1997) physical disability, (Idler and Kasl, 1995, Mossey et al., 1989) worsening health, (Havranek et al., 2001) and hospital utilization (Wolinsky et al., 1994) better than objective clinical information or physician rating (Winter et al., 2007). Therefore, the aim of this study was to determine the contribution of overall perceived health to the risk of all-cause hospitalization or death after adjusting for confounding factors in a sample of adults with heart failure.
Overall perceived health (or perceived health as it is subsequently termed in this paper) is synonymous with the term self-rated health, is a self-assessed, subjective measure of general health status measured by a single question, “In general would you say your health is excellent, very good, good, fair or poor?” This question is sensitive to changes in social, physical and psychological state (Bailis et al., 2003, Winter et al., 2007). Besides predicting outcomes, perceived health influences health behaviors such as engagement in physical activity and adherence to medical therapy (DiMatteo et al., 2007, Zimmermann et al., 2008).
Patients’ reports on the status of their health are valuable because many patients are sensitive and aware of the subtle changes in their health status, which may not be quantitatively assessed by their health provider. Perceived health incorporates both the current level of health as well as changes in health status (Idler and Benyamini, 1997). In a sample of patients with heart failure, Rosen et al. reported that perceived health was influenced primarily by levels of physical function, emotional distress, and socioeconomic status measured by income (Rosen et al., 1997). In their conceptual model, Wilson and Cleary proposed perceived health as one of the four domains of a patient’s health related quality of life, along with biological/physiological factors, symptom and functional status (Wilson and Cleary, 1995). The existing literature on perceived health suggests that this concept may be an important indicator of heart failure outcomes, so in this study we tested the contribution of perceived health to the risk of all-cause hospitalization or death after adjusting for confounding factors.
Methods
This study was a secondary analysis of data collected in a prospective cohort study of 280 community-dwelling adults with heart failure. The parent study was conducted to investigate the relationship between excessive daytime sleepiness and self-care. The detailed methodology for this study has been reported elsewhere and is briefly summarized here (Riegel et al., 2011).
Patient Characteristics
Inclusion criteria were specified by the parent study as enrolment of adults with chronic Stage C heart failure, defined by the American College of Cardiology as those with previous or current heart failure symptoms in the context of an underlying structural heart problem causing the heart failure, in spite of medical treatment (Jessup et al., 2009). Patients were screened for visual acuity and hearing sufficient to engage in dialogue and English literacy. Exclusion criteria included residence in a long-term care setting, working nights or rotating shifts, renal failure requiring dialysis, imminently terminal illness, plans to move out of the area, history of serious drug or alcohol abuse within the past year, or major depressive illness.
Illness and Treatment Characteristics
Clinical information, including the etiology of heart failure and left-ventricular ejection fraction, was abstracted from the medical record by registered nurses. Research assistants collected data during home visits between 2007 and 2010. NYHA functional class was obtained using a standardized interview (Kubo et al., 2004), and then scored by a single board certified cardiologist. Patients were followed up in person at three and six months following enrolment.
Questionnaires
At baseline, participants were asked to complete a number of questionnaires and a question about perceived health; “In general would you say your health is, excellent, very good, good, fair or poor?” The Charlson Comorbidity Index was used to measure comorbid conditions. The Charlson Comorbidity Index is a well-accepted measure of 17 comorbid conditions with possible scores ranging 0 to 30. Raw scores can be categorized as low (1 or 2) versus medium (3 or 4) or high risk (5 or more) of death within 10 years (Charlson et al., 1987).
Statistical Analysis
The aim of the analysis was to assess the prognostic value of perceived health as a determinant of heart failure events, defined as all-cause hospitalization or death over 6 months or 180 days of follow-up. The categories were amalgamated to facilitate statistical analysis, as other authors have done (Johansson et al., 2008), and clinical interpretation. Perceived health was recoded into two categories: excellent, very good, or good versus fair or poor because few participants rated their health as excellent or very good.
A Cox proportional hazards survival analysis model was used to examine the association between perceived health and event rates. Time was censored as the date of last follow-up for patients who were still alive and had not been hospitalized. Covariates included in the multivariate analysis included age, highest year of education, race, gender, income, NYHA class, type and etiology of heart failure, site of enrolment, and Charlson Comorbidity Index score. Race, gender, site, and NYHA class have been used as covariates in all analyses associated with the parent study. In addition, we included additional covariates following preliminary regression analyses, including all variables significantly associated with the outcome (events). Select variables were also retained in the model based on their theoretical relevance to the outcome; including education, heart failure etiology and type of heart failure.
The proportional hazards assumption was tested with a global test and coefficient-specific Schoenfeld residuals. The proportionality assumption was relaxed and interaction terms with time were included for covariates with statistically significant Schoenfeld residuals (p <0.05). The Kaplan-Meier event-free survival graphs controlled for all covariates in the regression model. The sample size for the current analysis was determined by the requirements of the parent study. All analyses were performed using STATA version 11 (StataCorp, 2009).
Results
A total of 280 patients with heart failure were included in the parent study. The final Cox regression analysis included 273 patients because six patients were lost to follow-up prior to study completion and one patient was excluded by listwise deletion due to missing data on two independent variables, heart failure etiology and type of heart failure. There were a total of 97 events in this analysis (6 deaths and 91 hospitalizations) over the 180-days of follow-up. The majority of subjects (65%) were event-free at the end of the six-month follow-up period.
At baseline the sample had a mean age of 62 years. The majority was male (64%) and functionally limited in NYHA class III/IV (76%). Most had systolic heart failure (69%) with an average ejection fraction of 36% and non-ischemic etiology (64%). More than half of the sample (54%) had completed at least 2 years of college education. Income was measured by its impact on household needs; 16% had less than needed and 49% had enough to meet their needs. Few patients (13%) rated their health as poor, although among patients with events, 65% perceived their health as poor and 34% perceived their health as fair. Clinical characteristics are shown in Table 1. Charlson Comorbidity Index raw scores ranged from 1 to 11; 90% of the sample had a low or moderate level of comorbid illness with raw scores between 1 and 4. Overall, 53% were in the low risk category and 47% had either moderate or high risk of ten-year mortality based on their Charlson Comorbidity Index score.
Table 1.
Baseline characteristics of the sample by overall perceived health
| Variables | Categories of overall perceived health (mean +/− SD or %) |
||
|---|---|---|---|
| All (n=280) |
Excellent/very good/good (n=128) |
Fair/Poor (n=152) | |
| Age | 61.9 (12.5) | 63.3 (12.7) | 60.9 (12.2) |
| Ejection fraction | 35.4 (17.0) | 34.7 (16.7) | 35.9 (17.4) |
| Dutch Knowledge of Heart | |||
| Failure Score (n=270) | 11.7 (0.1) | 11.7 (1.6) (n=122) | 11.7 (1.7) (n=148) |
| Gender | |||
| Male | 180 (64.3) | 75 (41.7) | 105 (58.3) |
| Female | 100 (35.7) | 53 (53.0) | 47 (47.0) |
| Race | |||
| Black/Other | 105 (37.5) | 25 (25.7) | 78 (74.3) |
| White | 175 (62.5) | 101 (57.7) | 74 (42.3) |
| Highest Level of Education | |||
| < High school | 27 (9.6) | 11 (40.7) | 16 (59.3) |
| High school | 102 (36.4) | 39 (38.2) | 63 (61.7) |
| > 2 years of college | 151 (53.9) | 78 (51.7) | 73 (48.3) |
| Income | |||
| More than needed | 98 (35.0) | 70 (71.4) | 28 (28.6) |
| Enough to meet needs | 137 (48.9) | 49 (35.8) | 88 (64.2) |
| Less than needed | 45 (16.1) | 9 (20.0) | 36 (80.0) |
| HF type | |||
| Systolic | 194 (69.3) | 95 (49.0) | 99 (51.0) |
| Diastolic | 53 (18.9) | 21 (39.6) | 32 (60.4) |
| Mixed | 32 (11.4) | 12 (37.5) | 20 (62.5) |
| Unspecified | 1 (0.4) | 0 | 1 (100) |
| HF etiology | |||
| Ischemic | 102 (36.6) | 43 (42.2) | 59 (57.8) |
| Nonischemic | 177 (63.4) | 85 (48.0) | 92 (52.0) |
| NYHA Functional Class | |||
| Class I/II | 66 (23.6) | 44 (66.7) | 22 (33.3) |
| Class III | 164 (58.6) | 76 (46.3) | 88 (53.7) |
| Class IV | 50 (17.9) | 8 (16.0) | 42 (84.0) |
| Charlson Comorbidity Index | |||
| Low (1–2) | 149 (53.2) | 79 (53.0) | 70 (47.0) |
| Moderate (3–4) and | |||
| High (5–11) | 131 (46.8) | 49 (37.4) | 82 (62.6) |
| Diuretic medical therapy | |||
| No | 54 (19.3) | 39 (72.2) | 15 (27.8) |
| Yes | 226 (80.7) | 89 (39.4) | 137 (60.6) |
HF: heart failure; NYHA: New York Heart Association
At baseline we examined for differences in medical therapies between participants who classified themselves as having excellent/good versus fair/poor self-perceived health. There were no differences between the two groups (p>0.05) for the following medical therapies: angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, or treatment for sleep-disordered breathing such as treatment with continuous positive airway pressure. There was a difference in the proportions of participants on diuretics between the two groups (p<0.001) so it was included as a covariate in the final model. Most of the participants in this cohort study were followed in specialty heart failure clinics and the vast majority was followed up consistency with standard medical therapy. There were some differences among the three sites in the intensity with which patients were followed but these differences were controlled for by including a variable for site as a covariate in our final model.
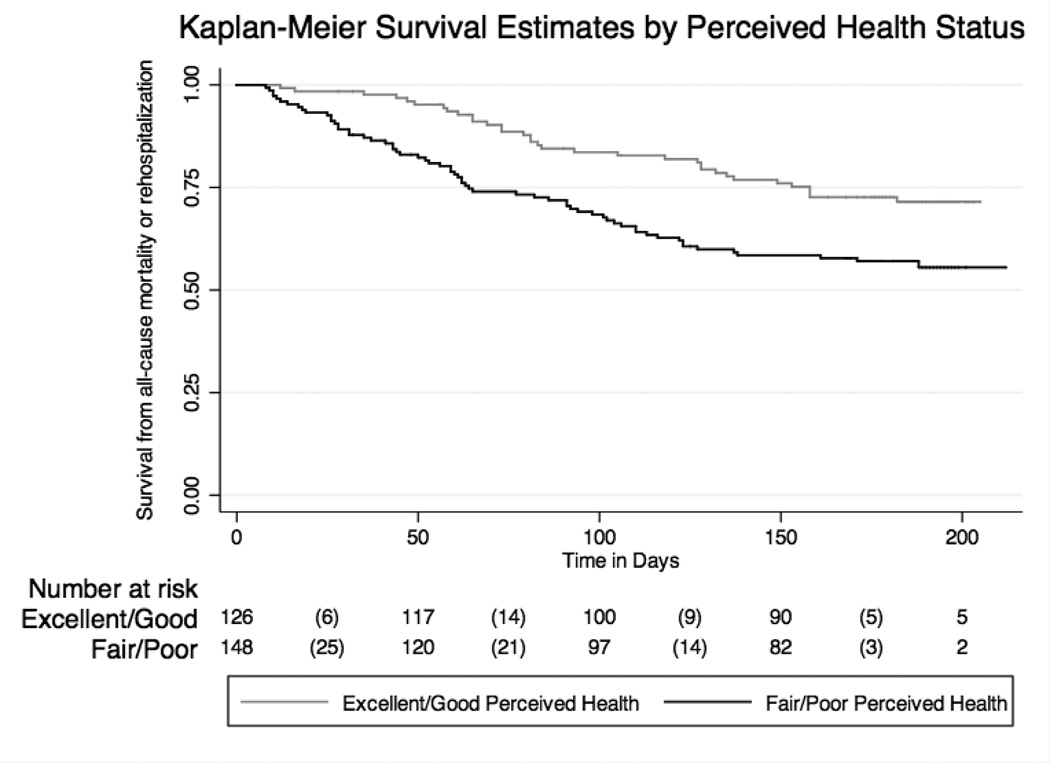
Of all covariates included in the model, patient perception of health had the largest impact on the event rate (Table 2). Controlling for eleven covariates, patients with fair or poor perceived health 5.5 times the rate of dying or being hospitalized compared to patients with good perceived health (adjusted hazard ratio (HR): 5.7; 95% confidence interval (CI): 2.0 to 15.6; p=0.001). A Kaplan Meier survival graph illustrates the survival functions for the two categories of perceived health (excellent/very good/good and fair/poor). The Kaplan Meier graph was adjusted for the covariates included in the final model, including enrolment site, age, highest year of education, race, gender, income, NYHA class, heart failure type, heart failure etiology, diuretic therapy and comorbid conditions (Figure 1).
Table 2.
Cox proportional hazard model: overall perceived health and event risk
| Variable | Adjusted HR |
95% CI | p-value |
|---|---|---|---|
| Perceived Health | |||
| Excellent/very good/good | 1.0 | -- | -- |
| Fair/poor | 5.61 | 2.04 – 15.41 | 0.001 |
| Income | |||
| More than needed | 1.0 | -- | -- |
| Enough to meet needs | 0.39 | 0.19–0.79 | 0.010 |
| Less than needed | 0.12 | 0.03–0.45 | 0.002 |
| NYHA | |||
| I/II | 1.0 | -- | -- |
| III | 1.45 | 0.77–2.71 | 0.249 |
| IV | 3.22 | 1.57–6.58 | 0.001 |
| Site of Enrollment | |||
| A | 1.0 | -- | -- |
| B | 2.46 | 1.18–5.13 | 0.017 |
| C | 2.81 | 0.93–8.49 | 0.066 |
NYHA: New York Heart Association functional class; HR: hazard ratio; CI: confidence interval. Results are based on models controlling for age, gender, race, education, income, comorbid conditions, heart failure type, diuretic therapy, heart failure etiologies, NYHA and enrolment site.
Figure 1. Event-free survival: Cox proportional hazard model.
Results indicate the difference in composite event risk for patients who had excellent or good versus fair or poor overall perceived health. Results control for the following covariates: age, gender, highest year of education, race, income, HF etiologies, HF type, New York Heart Association functional class, diuretic therapy, comorbid conditions and enrolment site. The risk table shows the number of participants who are at risk of having an event at each time point. The number of failures are reported in parentheses between the displayed at-risk times.
There were statistically significant differences in the rate of events between participants who reported different levels of income. The event rate was lowest in the income group that reported having less than enough income to meet needs compared to those reporting more than enough income (hazard ratio (HR): 0.12, 95% CI: 0.03–0.45, 95% confidence interval (CI): 0.002). People with the most severe NYHA class IV had 3 times the rate of an event compared to patients in NYHA class I or II (HR: 3.0, 95% CI: 1.50–6.16, p=0.002). There was no difference in the event rate between people with different levels of comorbid conditions.
The Cox regression assumption of non-proportionality was tested with Schoenfeld residuals, which computes tests for both individual covariates and the model as a whole. The results of the global test were significant for a violation of the proportional hazards assumption (p=0.002). More specifically, there was evidence (p<0.05) of non-proportional hazards for the following covariates: income, enrolment site, perceived health and comorbid conditions, indicating that interaction terms and time needed to be included for each of these covariates in the final model (Table 3). Most importantly, the effect size of the interactions between perceived health and time weakened over the follow-up period, such that after 30 days the hazard of having an event after reporting fair or poor health on day one decreased from 5.67 to 1.20. The hazard rate attenuated further to 0.67 at 60 days, reaching 0.14 by 90 days (Table 3).
Table 3.
Covariates with an interaction with time
| Variable | Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Income | 0.011 | 0.003 to 0.018 | 0.004 | |
| Site | −0.007 | −0.013 to −0.001 | 0.020 | |
| Perceived health | −0.018 | −0.028 to −0.007 | 0.001 | |
| Comorbidity | 0.016 | 0.006 to 0.026 | 0.002 | |
| Attenuation of perceived health over time | ||||
| HR 1 day | HR 30 days | HR 60 days | HR 90 days | |
| Poor vs. good* | 5.67 | 1.20 | 0.67 | 0.14 |
CI: Confidence interval; HR: adjusted hazard ratio; NYHA: New York Heart Association functional class
Discussion
The major finding of this study is that patients with heart failure who reported poor or fair perceived health had over five and a half times the rate of having an event within six months compared to patients with heart failure who reported excellent or good perceived health. What is interesting and novel about these findings is that the predictive ability of this question to predict event rates declined remarkably within 30 days and was virtually null by 60 days. In addition, as expected, patients with more severe heart failure, measured with NYHA functional class, had over three times the risk of having an event compared to people with less severe heart failure. Patients with lower income levels had lower event rates compared to people who self-reported higher incomes.
The major finding on the increased risk of death and hospitalization for people who reported fair and poor perceived health is consistent with the literature (Farkas et al., 2009, Havranek et al., 2001, Johansson et al., 2008). The primary difference between this study and prior research is that prior studies do not report the results of interactions between perceived health and time. In a post hoc analysis of the IntraMural infusion of low molecular weight heparin to Prevent REStenosis after Stent implantation (IMPRESS) trial, Havranek et al report a protective effect of higher health perception, measured using a Visual Analog Scale, on the risk of death or hospitalization (adjusted HR of 0.74, 95% CI: 0.61–88, p=0.001) (Havranek et al., 2001). These results are from a clinical trial population, which in general tends to be younger, healthier and male. The outcome was also measured with a visual analog scale rather than the global question used in most studies, as it was in the present study. In a patient population similar to ours, Johannson et al reported a four times higher event rate of cardiovascular mortality in patients with heart failure who reported poor versus very good perceived health status (adjusted HR 4.1, CI 95% 1.8–9.4) (Johansson et al., 2008) over a ten-year follow-up period. One of the limitations of the Johannson et al study was that they did not account for the potential interaction between perceived health and time. Perceived health is a dynamic measure that is likely to change over the course of 10 years of follow-up. Another study by Farkas et al. also reported similar results in a Slovenian patient population. Heart failure patients with poor or worse health had almost double the risk of dying over 48 months compared to people who rated their health as average or better (adjusted HR: 1.92, 95% CI 1.06–3.48) (Farkas et al., 2009). The outcome in the Farkas et al study was exclusively mortality (hospitalizations were not included as an event) and the sample size was limited with only 100 patients. This present study adds to the literature by confirming the value of measuring perceived health status and adds that the relationship changes over time. This result suggests that the question about perceived health should be repeated at follow-up appointments in clinical practice because the effect of perceived health on outcomes attenuates over time.
After perceived health, NYHA functional class was the best predictor of having an event in this study. NYHA functional class is an indirect indicator of heart failure severity, so we would expect patients with more severe heart failure to have a higher rate of having an event. In this analysis, there was also a curious protective effect of lower socioeconomic status on the risk of an event. This may be explained by the fact that 73% of the people in the lowest income bracket had medical insurance through Medicare or Medicaid, compared to people reporting higher baseline income, 44% of whom had coverage through a preferred provider organization and 13% through a health maintenance organization. Perhaps government-funded coverage provides better incentives for follow-up than private insurers; however, this is only speculation and the association with low socioeconomic status may not hold clinical significance.
Strengths and Limitations of the study
The strengths of the study were that it has a relatively large sample size, the data were collected from three study sites, and there was good representation of women and minorities in the study. The primary limitations of the study are the number of events and generalizability of the results. The rule of thumb for Cox Regression analysis is that there should be 10 outcome events per predictor variable (Concato et al., 1995, Peduzzi et al., 1995, Peduzzi et al., 1996). In this study, there are 97 events and 11 predictor variables. The event per predictor variable ratio is 8.8. This rule of thumb has been relaxed based on simulation studies done by Vittinghoff and McCulloch in which they demonstrated that errors were uncommon with 5–9 event per predictor variable and really no different with 10–16 event per predictor variable based on the assumption that we regard confidence interval coverage less than 93 percent, type 1 error greater than 7 percent as problematic (Vittinghoff and McCulloch, 2007). Given the aforementioned findings, the likelihood of our results being due to chance is unlikely.
Like many randomized clinical trials, the mean age for this heart failure sample was relatively young (62 years) and thus can be criticized for not being representative of community heart failure patients. While it is true that this sample is younger than other community-based samples, because most came from a university-based referral center, they were comparable in terms of heart failure etiology and severity. Another limitation is that there might be some selection bias due to the cohort design of the study and the exclusion criteria. As individual cohorts of the parent study were saturated, patient eligibility focused on criteria related to excessive daytime sleepiness and cognitive status, thus eliminating over 300 individuals who would have otherwise been eligible (Riegel et al., 2011). Overall, the generalizability of these results is limited to community dwelling, English speaking heart failure patients, without moderate or major cognitive decline, major depression or severe concomitant renal failure requiring dialysis.
In this study, as with others, (Farkas et al., 2009, Havranek et al., 2001, Johansson et al., 2008) perceived health was based on a single assessment at baseline. It would be optimal to measure the association between perceived health and events at baseline and at all follow-up time periods.
Conclusions and Clinical Implications
Overall, health perception is a stronger predictor than NYHA functional class, income, education, or comorbid conditions for predicting events in patients with heart failure, at least in the short-term. This is not a surprise in light of previous work that demonstrates health perception is able to integrate emotional, socioeconomic factors with functional status (Rosen et al., 1997). The implications are that in an elderly population with heart failure, a single global question on perceived health at one time point may be a helpful predictor of a patient’s event rate over the next month, but it needs to be repeated in follow-up visits to capture changes in health. This makes sense given the dynamic nature of health, especially in an older adult population with a syndrome as serious as heart failure. With further support from future research, the assessment of patients’ perceived health in routine clinical visits may be an efficient and cost-effective triage tool, as other authors have suggested as well (Carlson, 2012, Farkas et al., 2009, Johansson et al., 2008). Future observational studies and clinical trials with patients with heart failure should consider including repeated measures of perceived health so that comparisons across time can be made to determine the consistency of this finding in other samples.
What is already known about the topic?
In heart failure patients, overall perceived health is a predictor of physical disability, worsening health and hospital utilization.
Overall perceived health influences health behaviors such as engagement in physical activity and adherence to medical therapy.
What this paper adds?
A single question about overall perceived health is a short-term predictor (0–30 days) of heart failure rehospitalization and death.
The predictive ability of a single question about overall perceived health attenuates after 30 days.
This is a comparison between poor/fair versus excellent/very good and good perceived health. The hazard ratios are adjusted for the following covariates: age, gender, race, education, income, comorbid conditions, diuretic therapy, heart failure type, heart failure etiologies, NYHA and enrollment site.
Acknowledgements
The authors would like to acknowledge that this work was funded by a grant from the National Heart, Lung & Blood Institute (RO1 HL084394-01A1) and by the Philadelphia Veterans Affairs Medical Center, VISN 4 Mental Illness Research, Education, and Clinical Center (MIREC). The authors also gratefully acknowledge Thomas A. Gillespie, MD, FACC for scoring the New York Heart Association (NYHA) interviews.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ruth Masterson Creber, Email: rumaster@nursing.upenn.edu, University of Pennsylvania School of Nursing, Philadelphia, PA 19104.
Paul D. Allison, Email: Allison@soc.upenn.edu, University of Pennsylvania School of Arts and Sciences, Philadelphia, PA 19104.
Barbara Riegel, Email: briegel@nursing.upenn.edu, University of Pennsylvania School of Nursing, Philadelphia, PA 19104.
References
- Bailis DS, Segall A, Chipperfield JG. Two views of self-rated general health status. Soc Sci Med. 2003;56(2):203–217. doi: 10.1016/s0277-9536(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Carlson B, Pozehl B, Hertzog M, Zimmerman L, Riegel B. Predictors of Overall Perceived Health in Patients with Heart Failure. Journal of Cardiovascular Nursing. 2012 doi: 10.1097/JCN.0b013e31824987a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cheng JW, Nayar M. A review of heart failure management in the elderly population. Am J Geriatr Pharmacother. 2009;7(5):233–249. doi: 10.1016/j.amjopharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Concato J, Peduzzi P, Holford TR, Feinstein AR. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol. 1995;48(12):1495–1501. doi: 10.1016/0895-4356(95)00510-2. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care. 2007;45(6):521–528. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- Farkas J, Nabb S, Zaletel-Kragelj L, Cleland JG, Lainscak M. Self-rated health and mortality in patients with chronic heart failure. Eur J Heart Fail. 2009;11(5):518–524. doi: 10.1093/eurjhf/hfp038. [DOI] [PubMed] [Google Scholar]
- Havranek EP, Lapuerta P, Simon TA, L'Italien G, Block AJ, Rouleau JL. A health perception score predicts cardiac events in patients with heart failure: results from the IMPRESS trial. J Card Fail. 2001;7(2):153–157. doi: 10.1054/jcaf.2001.24121. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37. [PubMed] [Google Scholar]
- Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344–S353. doi: 10.1093/geronb/50b.6.s344. [DOI] [PubMed] [Google Scholar]
- Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- Johansson P, Brostrom A, Dahlstrom U, Alehagen U. Global perceived health and ten-year cardiovascular mortality in elderly primary care patients with possible heart failure. Eur J Heart Fail. 2008;10(10):1040–1047. doi: 10.1016/j.ejheart.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Kosiborod M, Lichtman JH, Heidenreich PA, Normand SL, Wang Y, Brass LM, Krumholz HM. National trends in outcomes among elderly patients with heart failure. Am J Med. 2006;119(7):616 e611–617 e611. doi: 10.1016/j.amjmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Kubo SH, Schulman S, Starling RC, Jessup M, Wentworth D, Burkhoff D. Development and validation of a patient questionnaire to determine New York Heart Association classification. J Card Fail. 2004;10(3):228–235. doi: 10.1016/j.cardfail.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Mossey JM, Mutran E, Knott K, Craik R. Determinants of recovery 12 months after hip fracture: the importance of psychosocial factors. Am J Public Health. 1989;79(3):279–286. doi: 10.2105/ajph.79.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Riegel B, Moelter ST, Ratcliffe SJ, Pressler SJ, De Geest S, Potashnik S, Fleck D, Sha D, Sayers SL, Weintraub WS, Weaver TE, Goldberg LR. Excessive Daytime Sleepiness is Associated With Poor Medication Adherence in Adults With Heart Failure. J Card Fail. 2011;17(4):340–348. doi: 10.1016/j.cardfail.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the american heart association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Contrada RJ, Gorkin L, Kostis JB. Determinants of perceived health in patients with left ventricular dysfunction: a structural modeling analysis. Psychosom Med. 1997;59(2):193–200. doi: 10.1097/00006842-199703000-00012. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 11.2. College Station, TX: StataCorp, LP; 2009. [Google Scholar]
- Vittinghoff E, McCulloch CE. Relaxing the Rule of Ten Events per Variable in Logistic and Cox Regression. American Journal of Epidemiology. 2007;165(6):710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- Winter L, Lawton MP, Langston CA, Ruckdeschel K, Sando R. Symptoms, affects, and self-rated health: evidence for a subjective trajectory of health. J Aging Health. 2007;19(3):453–469. doi: 10.1177/0898264307300167. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Culler SD, Callahan CM, Johnson RJ. Hospital resource consumption among older adults: a prospective analysis of episodes, length of stay, and charges over a seven-year period. J Gerontol. 1994;49(5):S240–S252. doi: 10.1093/geronj/49.5.s240. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Ekholm O, Gronbaek M, Curtis T. Predictors of changes in physical activity in a prospective cohort study of the Danish adult population. Scand J Public Health. 2008;36(3):235–241. doi: 10.1177/1403494808086982. [DOI] [PubMed] [Google Scholar]