Abstract
Background and Purpose
Despite the reported functional recovery in transplanted stroke models and patients, the mechanism of action underlying stem cell therapy remains not well understood. Here, we examined the role of stem cell-mediated vascular repair in stroke.
Methods
Adult rats were exposed to transient occlusion of the middle cerebral artery and 3 hours later randomly stereotaxically transplantated with 100K, 200K, or 400K human cerebral endothelial cell 6 viable cells or vehicle. Animals underwent neurological examination and motor test up to day 7 after transplantation then euthanized for immunostaining against neuronal, vascular, and specific human antigens. A parallel in vitro study cocultured rat primary neuronal cells with human cerebral endothelial cell 6 under oxygen-glucose deprivation and treated with vascular endothelial growth factor (VEGF) and anti-VEGF.
Results
Stroke animals that received vehicle infusion displayed typical occlusion of the middle cerebral artery-induced behavioral impairments that were dose-dependently reduced in transplanted stroke animals at days 3 and 7 after transplantation and accompanied by increased expression of host neuronal and vascular markers adjacent to the transplanted cells. Some transplanted cells showed a microvascular phenotype and juxtaposed to the host vasculature. Infarct volume in transplanted stroke animals was significantly smaller than vehicle-infused stroke animals. Moreover, rat neurons cocultured with human cerebral endothelial cell 6 or treated with VEGF exhibited significantly less oxygen-glucose deprivation-induced cell death that was blocked by anti-VEGF treatment.
Conclusions
We found attenuation of behavioral and histological deficits coupled with robust vasculogenesis and neurogenesis in endothelial cell-transplanted stroke animals, suggesting that targeting vascular repair sets in motion a regenerative process in experimental stroke possibly via the VEGF pathway.
Keywords: endothelial cells, neurogenesis, stem cells, stroke
Stem cell therapy has reached limited clinical trials in patients with stroke on the basis of safety and efficacy data from preclinical studies. 1–4 However, a major gap in our knowledge is the mechanism of action underlying stem cell therapy. Cell transplantation studies in stroke have mostly focused on neuronal stem or progenitor cells as donor cell type,5–15 primarily to replace the dead or injured neuronal cells of the stroke brain. Indeed, such transplantation of neuronal stem or progenitor cells has resulted in neurogenesis with reported functional recovery in transplanted stroke animals.5–15 Despite these positive observations, varying levels of behavioral and histological improvement accompany transplanted stroke animals. We posit that while new neurons have been generated from the either exogenously transplanted stem cells or transplant-mediated solicitation of endogenous stem cells, a key component of the neurovascular unit that has been largely neglected is the repair of the vasculature. To this end, we tested the hypothesis that stem cell transplantation in stroke using endothelial cells, which are the main cell type of the vasculature, should promote vasculogenesis and then likely serve as substrate for enhanced endogenous neurogenesis.
During the neurodevelopmental period, vasculogenesis and neurogenesis seem to occur at the same time,16–18 suggesting that these 2 cell lineage processes are critical. Recently, the important role of endothelial cells in regulating both vasculogenesis and neurogenesis has been recognized. 19–22 Cerebral endothelial cells serve many cerebrovascular maintenance functions, including structural element, blood brain barrier formation, regulation of neurotransmitter and ion, and controlling of blood flow.23,24 Interestingly, these same cerebrovascular functions are compromised after ischemic stroke. If therapies could be designed to protect the brain vasculature from stroke-induced alterations, then we should be able to reduce the pathophysiological outcomes of stroke. Hence, the present study sought to engage the endothelial cell component of the neurovascular unit in an effort to abrogate stroke.
The potential therapeutic benefits of transplanting exogenous or mobilizing endogenous endothelial progenitor cells in stroke animal models have been demonstrated,25,26 but the mechanisms remain not well understood. In this study, we transplanted the immortalized cell line of human cerebral endothelial cells (HEN6) into the striatum of experimentally ischemic stroke rats and assessed endogenous and exogenous vasculogenesis and neurogenesis to demonstrate the role of vascular repair in stroke. We hypothesized that transplantation of endothelial cells would afford behavioral and histological benefits against stroke via the vasculogenic reparative pathway in facilitating the neurogenic regenerative process.
Material and Methods
In Vivo Study
Subjects
We used adult Sprague-Dawley rats (weighing, 200–250 g at the beginning of experiments; Harlan Sprague Dawley, Indianapolis, IN) according to the approved guidelines of the University of South Florida System Institutional Animal Care and Use Committee. They were housed singly in a temperature and humidity controlled room that was maintained on 12-hour light/dark cycles with free access to food and water. All surgical procedures were conducted under aseptic conditions, and every effort was made to minimize animal suffering and to reduce the number of animals used.
Human Cerebral Endothelial Cells
HEN6 were kindly provided by Dr Kim (University of British Columbia, Vancouver, BC, Canada). Briefly, primary dissociated cell cultures from the periventricular region of human telencephalic tissues of 14-week gestation were prepared and grown for 10 days. Thereafter, cells were infected with an amphotropic, replication-incompetent retroviral vector containing v-myc. Finally, HEN6 were subcultured at ≈90% confluence and subjected to further experiments. Next, we phenotypically characterized HEN6 in vitro and found that these cells were immunocytochemically positive against a human mitochondrial marker (Mito), von Willebrand factor (vWF), and CD31 that the positivity was >99% for each phenotypic marker (Figure 1).
Figure 1.
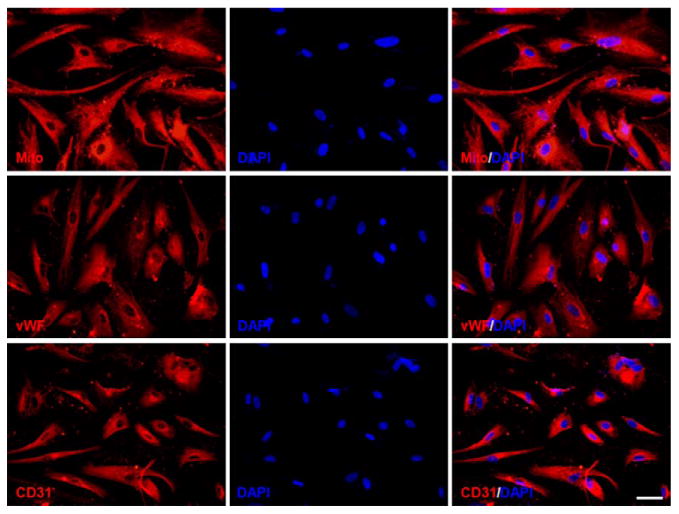
Immunocytochemical analysis of human cerebral endothelial cells (HEN6), immortalized HEN6, immunocytochemically expressed human-specific mitochondrial marker (Mito), von Willebrand factor (vWF), and CD31. DAPI indicates 4′,6-diamidino-2-phenylindole.
Stroke Surgery
The present ischemic stroke model used the middle cerebral artery occlusion (MCAo) technique as described in our previous studies.27–30 We used 46 rats for the surgery, and 40 rats reaching our criterion of successful MCAo, as described elsewhere, were enrolled in the study. Briefly, animals were anesthetized by a mixture of 1% to 2% isofluran in NO/oxygen (69%/30%) via a face mask. Body temperature was maintained at 37±0.3°C during the surgical procedures. The midline skin incision was made in the neck with subsequent exploration of the right common carotid artery, the external carotid artery, and internal carotid artery. A 4-0 monofilament nylon suture (27.0–28.0 mm) was advanced from the common carotid artery bifurcation until it blocked the origin of the MCA. Animals were allowed to recover from anesthesia during MCAo. After 60 minutes of transient MCAo, animals were reanesthetized and reperfused by withdrawal of the nylon thread. Animals receiving the sham operation were anesthetized with the same gas via a face mask. A midline incision was made in the neck and the right common carotid artery was isolated. The animals were then closed and allowed to recover from anesthesia. We have standardized the MCAo model, with stroke animals showing ≥80% reduction in regional cerebral blood flow during the occlusion period as determined by laser Doppler (Perimed). To ensure further similar degree of stroke insults, physiological parameters, including Pao2, PaCO2, and plasma PH measurements, were monitored, and we found no significant differences in our stroke animals.
HEN6 Cells Transplantation
Forty rats (n=10 for each group) were randomly assigned to receive stereotaxic transplantation of PBS as a control, 100K, 200K, or 400K HEN6/9 μL, 3 hours after MCAo. Transplantation targeted the striatum via a single Hamilton (25 gauge) needle pass with 3 dorsoventral deposits (bregma, +1.2 mm; medial-lateral, +2.5 mm; dorsal-ventral, −5.0/−4.5/−4.0 mm).31
Motor and Neurological Tests
All investigators testing the animals were blinded to the treatment condition. Animals were subjected to elevated body swing test and neurological examination before stroke surgery (baseline) then at days 3, 5, and 7 after surgery. Elevated body swing test involved handling the animal by its tail and recording the direction of the swings. The test apparatus consisted of a clear Plexiglas box (40×40×35.5 cm). The animal was gently picked up at the base of the tail and elevated by the tail until the animal's nose is at a height of 2 inches (5 cm) above the surface. The direction of the swing, either left or right, was counted once the animals head moves sideways ≈10° from the midline position of the body. These steps are repeated 20× for each animal. Intact rats displayed a 50% swing bias (ie, the same number of swings to the left and to the right). A 75% swing bias was used as a criterion of stroke-induced motor asymmetry. Animals were also tested in a modified neurological examination. Neurological score for each rat was obtained using 3 tests that included (1) forelimb akinesia that measured the ability of the animal to replace the forelimb after it was displaced laterally by 2 to 3 cm, graded from 0 (immediate replacement) to 3 (replacement after several seconds or no replacement); (2) beam walking ability, graded 0 for a rat that readily traversed a 2.4-cm-wide, 80-cm-long beam to 3 for a rat unable to stay on the beam for 10 seconds; and (3) paw grasp that measured the ability to hold onto a 2-mm-diameter steel rod, graded 0 for a rat with normal forepaw grasping behavior to 3 for a rat unable to grasp with the forepaws. The scores from this battery of 3 neurological tests were pooled to obtain the mean neurological score for each treatment group. A ≥2.5 mean neurological score was used as a criterion of stroke-induced neurological impairment.
Histology and Immunohistochemistry
Twenty-four rats (n=6 for each group) were deeply anesthetized and perfused transcardially with 4% paraformaldehyde 7 days after HEN6 transplantation. Brains were harvested and postfixed in the same fixative for 24 hours followed by 30% sucrose in PBS for 1 week. Frozen sections were then cut at 30 μm in a cryostat and stored at −20°C. To demonstrate graft survival, neuronal and vascular phenotype expression and immunohistochemical investigations were performed. Free-floating sections throughout the transplanted striatum and continuing cortex involving the stroke area were incubated overnight at 4°C with an anti-nestin antibody (mouse; anti-nestin antibody [ab6142]; abcam), glial fibrillary acidic protein (GFAP) (mouse, anti-GFAP antibody [MAB360]; Millipore), collagen IV (rabbit, anti-collagen IV antibody [ab19808]; abcam), and HuNu (mouse, anti-human nuclei antibody [MAB1281]; Millipore) for engrafted HEN6, with 5% serum and 0.2% triton X-100 (Fischer Scientific, Pittsburgh, PA). After rinsing 3× in PBS, sections were incubated for 2 hours at room temperature in goat antimouse IgG Alexa Fluor 488 conjugate (Invitrogen) and goat antirabbit IgG Alexa Fluor 594 conjugate (Invitrogen) with Hoechst33342 (Sigma). Next, the sections were washed again 3× in PBS and mounted on glass slides using mounting medium. Control studies included exclusion of primary antibody substituted with 10% goat serum in PBS. No immunoreactivity was observed in these controls.
HEN6 Graft Survival Assessment
For evaluation of graft survival, HuNu-positive cells were counted every fifth 30-μm-thick coronal tissue section throughout the transplanted striatum by an observer blinded to the experimental group assignment. Abercrombie formula was used to eliminate bias of counting the same cell in 2 consecutive sections.
Infarct Measurement
The remaining 16 rats (n=4 for each group) were euthanized at 7 days PBS or HEN6 transplantation. Coronal sections of the brains were sliced at 2 mm, immersed in 2% 2,3,5-triphenyltetrazolium chloride (TTC; T8877; Sigma), and then fixed with 4% paraformaldehyde. The size of infarct area, which was devoid of red staining, was determined on the digital images using ImageJ software and calculated the ratio of infarct area toward whole brain by an observer blinded to the experimental group assignment as described in our reports.7,29,30
Analysis of Glial Formation and Blood Vessels
As described above, cryosections were processed for immunohistochemical staining against GFAP for glial formation and collagen IV for blood vessels. Glial formation is defined as the proportion of the area occupied by GFAP-positive cells, which was calculated every fifth 30-μm-thick coronal tissue section throughout the stroke striatal penumbra. In a similar fashion, we measured vessel density defined as the proportion of the area occupied by collagen IV–positive area in the striatum. They were measured using ImageJ software by an observer blinded to the experimental group assignment.
In Vitro Study
Vascular Endothelial Growth Factor Role in Oxygen-Glucose Deprivation
Primary rat neonatal neuronal cells (PRNCs) were obtained from BrainBit. According to the supplier protocol, cells (4×104 cells/well) were suspended in 200-μL supplemented neurobasal medium containing 2-mmol/L L-glutamine and 2% B27 in the absence of antibiotics and grown in poly-L-Lysine–coated 96-well plate (BD Biosciences) at 37°C in humidified atmosphere containing 5% carbon dioxide in 40% of the neuron and 60% astrocytes cell population and validated immunocytochemically using vesicular glutamate transporter-1. PRNCs were grown until reaching cell confluence of ≈70% then subjected to 90-minute oxygen-glucose deprivation (OGD) as described previously.32 Briefly, the PRNCs were initially exposed to OGD medium (116 mmol/L NaCl, 5.4 mmol/L KCl, 0.8 mmol/L MgSO4, 1 mmol/L NaH2PO4, 26.2 mmol/L NaHCO3, 0.01 mmol/L glycine, 1.8 mmol/L CaCl2; pH 7.4) and placed in an anaerobic chamber (PlasLabs) containing 95% nitrogen and 5% carbon dioxide for 15 minutes at 37°C, and finally the chamber was sealed and incubated for 90 minutes at 37°C (hypoxic-ischemic condition). After the hypoxic-ischemic exposure, the cell culture was reintroduced to reperfusion-like condition containing 5 mmol/L glucose under normoxia for 2 hours.
To reveal the involvement of vascular endothelial growth factor (VEGF) in HEN6 neuroprotection, we initially examined the therapeutic benefits of exogenous VEGF by manipulating the basal medium when the reperfusion started (Figure I in the online-only Data Supplement) as follows: group A: basal medium as a control; group B: low concentration (0.5 ng/mL) of VEGF; group C: high concentration (5.0 ng/mL) of VEGF; group D: group A with anti-VEGF; group E: group B with anti-VEGF; and group F: group C with anti-VEGF. Two hours after the reperfusion, the supernatant was collected from the culture and the PRNCs were subjected to the mitochondrial activity assay. For the mitochondrial activity assay, reduction of 3-(4, 5-dimethyl-2-thiazoyl)-2,5-diphenyltetrazolium bromide (MTT) by cellular dehydrogenases was used as described in our previous report.33
Coculture of Neuronal Cells After the OGD Condition With HEN6 or Fibroblasts
HEN6 and fibroblasts, as control cells, were separately grown to a subconfluent monolayer in 10-cm dishes, and then they were rinsed twice with PBS before being plated in culture plate inserts (3-μm membrane pore size; BD Biosciences). In parallel, the 4×104 of PRNCs were plated in 96-well companion plates (BD Biosciences) and subjected to 90-minute OGD condition. After the OGD condition for PRNCs, the inserts containing HEN6 and fibroblasts were transferred to these wells, and the coculture was introduced to one of the conditions (Figure I in the online-only Data Supplement) in the normoxic incubator for 2 hours (these conditions corresponded to reperfusion with or without VEGF and anti-VEGF). The coculture was assigned to further experiments. Before cell viability analyses, the culture plate inserts containing HEN6 or fibroblast were removed, and then we used the bottom of the plate cultured with only neuronal cells for analyses.
For the neuronal cell viability, we used the fluorescent live/dead cell assay.34 The green fluorescence of the live cells was measured by the Gemini EX fluorescence plate reader (Molecular Device). To evaluate the VEGF levels in the supernatants collected from pre and post reperfusion process, we used ELISA VEGF detection kits (R&D systems). To reveal the localization of damaged cells, we examined immunocytochemical against caspase 3.
Statistical Analysis
We used repeated ANOVA followed by Fisher protected least significant difference post hoc tests to reveal any statistical significance between treatments (P<0.05). All data were presented as mean±SEM. In addition, Pearson R coefficient of correlation was performed to show interactions between neuroprotective mechanisms and functional recovery. In the statistical analyses of in vitro data, because of the differences in the baseline of treatment conditions, basal media data were normalized among single culture, coculture with basal media, and coculture with HEN6.
Results
Transplanted HEN6 Cells Survive Dose Dependently in Stroke Brain
To reveal transplanted HEN6 survival, we used immunohistochemical detection of the specific human antigen HuNu. The number of HuNu-positive cells per visual field in 400K group (106.9±43.3) was significantly higher than that in 200K (70.4±31.7) and 100K (34.8±23.6) transplanted groups (P<0.05; Figure II in the online-only Data Supplement), thus the number of surviving transplanted HEN6 was dose dependent. However, the percentage of survival was comparable and not significantly different across all transplanted animals: 400K (0.23±0.08%), 200K (0.26±0.12%), 100K (0.26±0.18%).
HEN6 Transplantation Dose Dependently Ameliorates Stroke-Induced Behavioral Deficits
All animals included in this study did not display any detectable behavioral deficits at baseline (Figure 2). After MCAo stroke surgery, animals exhibited significant impairments in both motor and neurological performance, which were evident during the 1-hour MCAo (data not shown), and was maintained throughout the 7-day study period in those stroke animals that received vehicle infusion. In contrast, stroke animals that received HEN6 exhibited a dose-dependent improvement in behavioral outcomes (pairwise comparisons between groups, P<0.05), with the highest dose of 400K displaying the most pronounced functional recovery (F4,26=83.26; P<0.01). This dose-dependent behavioral recovery was consistent for both motor and neurological assays and across all times points examined (ie, days 3, 5, and 7). Sham-operated animals (normal) did not show any detectable deficits throughout the study period. The HEN6 transplanted stroke animals, while demonstrating 20% to 45% improvement versus the vehicle-infused stroke animals, were still significantly impaired compared with this normal group (P<0.05).
Figure 2.
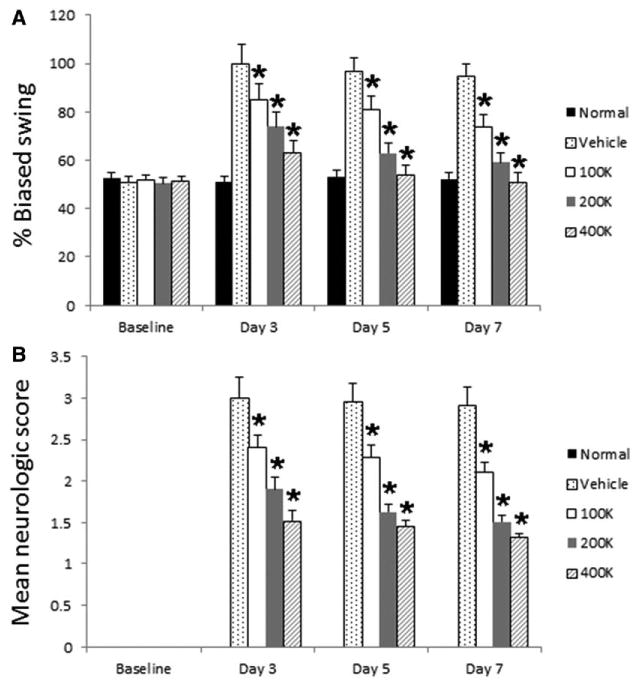
Human cerebral endothelial cells (HEN6) ameliorates stroke-induced behavioral deficits. All animals enrolled in this study displayed no detectable behavioral deficits at baseline, with sham-operated animals (normal) exhibiting normal behaviors throughout the study period. After middle cerebral artery occlusion stroke surgery throughout the 7-day study period, stroke animals that received vehicle infusion displayed significant motor (A) and neurological impairments (B). In contrast, dose-dependent improvements across all times points in both behavioral outcomes were displayed by stroke animals transplanted with HEN6, with the highest dose of 400K most improved. The HEN6 transplanted stroke animals, although significantly improved compared with the vehicle-infused stroke animals, were still significantly impaired compared with the normal group (*P<0.05).
HEN6 Transplantation Reduces Infarct Volume, Suppresses Reactive Gliosis, and Induces Vasculogenesis
We used TTC to determine the therapeutic effect of HEN6 on the brain infarct. The infarct volumes (total, in cortex, and in transplanted striatum) were 87.7±17.4 (27.1±5.4%), 57.4±11.1, and 30.2±9.6 mm3 in control; 73.2±14.1 (23.9±3.1%), 52.8±8.0, and 20.4±10.0 mm3 in 100K group; 40.4±15.8 (13.1±4.5%), 32.4±7.7, and 8.0±9.2 mm3 in 200K group; and 30.8±14.3 (10.4±4.7%), 27.1±11.0, and 3.7±3.4 mm3 in 400K group (Figure 3). The infarct volume was significantly reduced in 400K and 200K HEN6 transplanted groups compared with control group, which received PBS injection (P<0.05). Next, we used GFAP immunostaining to reveal reactive gliosis on peri-infarct area. The intensity ratio of GFAP-positive area in control, 100K, 200K, and 400K was 29.5±10.0, 26.7±9.2, 12.7±2.5, and 13.3±4.6% (Figure 3). Glial formation was significantly reduced in 400K and 200K HEN6 transplanted groups compared with 100K and control groups (P<0.05). In addition, we used collagen IV immunostaining to reveal vascularization within the area of the transplanted striatum. The intensities of collagen IV–positive area in control, 100K, 200K, and 400K were 0.2±0.3%, 6.3±6.9%, 18.2±10.7%, and 25.4±9.3% (Figure 3). Vasculogenesis within the striatum was significantly increased in 400K and 200K HEN6 transplanted groups compared with 100K and control groups (P<0.01).
Figure 3.
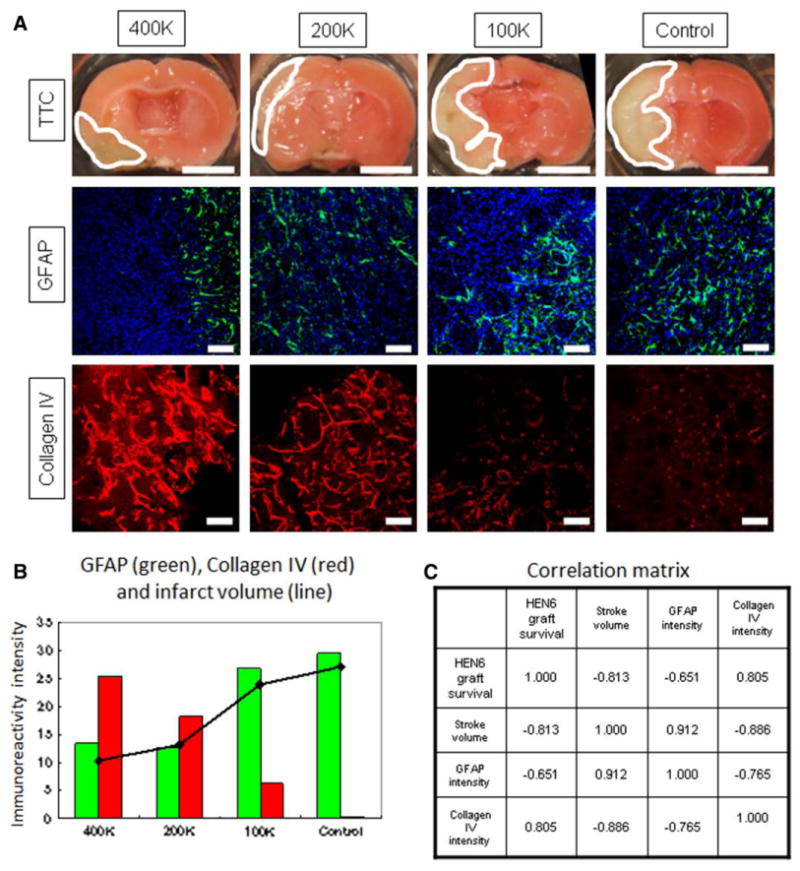
Human cerebral endothelial cells (HEN6) attenuates stroke-induced histological deficits. Transplantation of HEN6 reduced infarct volume (2,3,5-triphenyltetrazolium chloride [TTC]), suppressed reactive gliosis (GFAP), and induced vasculogenesis (collagen IV; A). Graphical rendition of correlations among GFAP (green), collagen IV (red), and infarct volume (line) are presented (B), indicating that with HEN6 reducing the infarct volumes in 400K and 200K transplanted stroke animals, there was a corresponding suppression of reactive gliosis (GFAP) and elevation of collagen IV. Numeric coefficients of correlation are shown (C). Scale bar in TTC-stained brains is 5 mm. Scale bar in GFAP and collagen IV equals 50 μm. GFAP indicates glial fibrillary acidic protein.
HEN6-Mediated Neuroprotective Cellular Processes Correlate With Infarct Volume and Reactive Gliosis
All brain repair parameters, including survival of transplanted cells, reduction in infarct volume, suppression of gliosis change, and enhanced vasculogenesis, showed good correlations. The dose-dependent survival of transplanted cells correlated with infarct volume, gliosis (GFAP immunoreactivity), and vasculogenesis (collagen IV immunoreactivity; Figure 3B). The correlation matrix of these brain repair parameters showed positive correlations between infarct volume and gliosis and a negative correlation between the infarct volume and the vasculogenesis. In addition, the correlation matrix showed that HEN6 graft survival was positively correlated with vasculogenesis but negatively correlated with gliosis, and gliosis was negatively correlated with vasculogenesis (Figure 3C).
HEN6 Induces Vasculogenesis in Endogenous and Exogenous Cells
To answer the question whether transplanted HEN6 afford neovascularization, we used double-immunolabeling of HuNu and collagen IV in the stroke brain. Surviving HuNu-positive transplanted HEN6 in ischemic striatum formed a new microvascular-like structure that was positive against collagen IV and juxtaposed to the host vasculature that was also positively stained against collagen IV (Figure 4). The formation of this microvascular-like structure was dose dependent, reflecting the dose-dependent HEN6 graft survival.
Figure 4.

Human cerebral endothelial cells (HEN6) induces endogenous and exogenous vasculogenesis and neurogenesis. HEN6 grafts were labeled with human-specific antigen HuNu (A–C; green). The vascular marker collagen IV (A; red) revealed labeling of the exogenous transplanted HEN6 (asterisk in A) and the endogenous vasculature (arrowhead in A). Double positive cells, using the other vascular marker von Willebrand factor (vWF; B; red) colabeled with HuNu, correspond to exogenous vasculature (arrow in B), whereas vWF-positive cells but negative for HuNu represent endogenous vasculature (arrowhead in B). In addition, cells positive for the immature neural marker nestin (C; red) and colabeled with the HuNu-positive transplanted HEN6 indicate exogenous neurogenesis, whereas the nestin-positive cells but negative for HuNu represent endogenous neurogenesis. Abundant nestin-positive cells (D; green) surrounding the transplants suggest that endogenous neurogenesis within the striatum was enhanced by the HEN6 grafts. Scale bar, 50 μm.
HEN6 Promotes Endogenous Neurogenesis
To reveal whether the HEN6 histological benefits extend to neurogenesis, we used double-immunolabeling of HuNu and nestin in the stroke brain. Nestin-positive cells were detected migrating from centrally located HuNu-positive-transplanted cells. In addition, colocalization of nestin-positive cells around HuNu-positive cells was observed (Figure 4).
VEGF Protects PRNCs Against OGD Condition
We examined cultured PRNCs with VEGF treatment under OGD condition as a prelude to assessing a VEGF role in HEN6 neuroprotection in stroke. The number of apoptotic caspase 3-positive cells in both VEGF treatment group (groups B [41.47±4.39%] and C [44.85±7.85%]) was significantly reduced than no VEGF treatment group [group A [59.15±8.62%]). Treatment with anti-VEGF in combination with VEGF in groups E (66.28±3.89%) and F (61.97±9.84%) abolished the VEGF neuroprotective effect (P<0.05). Moreover, anti-VEGF treatments alone (without VEGF; group D [89.75±3.92%]) significantly exacerbated the OGD-induced neurotoxicity compared with other groups (P<0.05; Figures I and V in the online-only Data Supplement). Relative mitochondrial reductase activity in VEGF treatment group (groups B [32.72±2.47%] and C [35.3±1.13%]) was significantly higher than in the basal medium (group A [25.77±0.57%]) and anti-VEGF treatment groups (groups D [13.35±2.21], E [16.02±1.45], and F [18.52±2.62%]; P<0.05; Figure 5).
Figure 5.
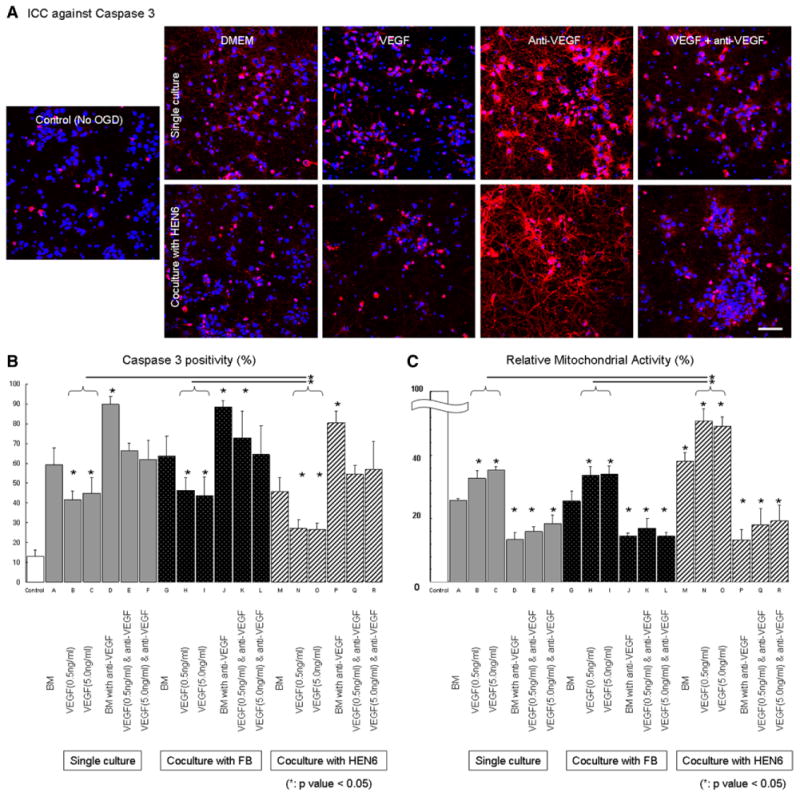
Human cerebral endothelial cells (HEN6) reduces oxygen-glucose deprivation (OGD)–induced cell death in cocultured primary neuronal cells (PRNSCs). Representative caspase 3 immunocytochemical (ICC) images of control (no OGD) vs OGD under single culture of PRNCs or cocultured with HEN6 in the routine DMEM culture condition or supplemented with vascular endothelial growth factor (VEGF), anti-VEGF or combination of both (A). In addition, as appropriate coculture control condition, PRNCs were cocultured with fibroblasts (FB; ICC images not shown but comparable with single culture condition). Quantifications of all treatment conditions revealed that OGD produced significant apoptotic cell death (caspase 3; B) and impaired the oxidative metabolism (relative mitochondrial activity; C), which were blocked by VEGF treatment, and such neuroprotective effects were further enhanced by coculture with HEN6 but not with FB. *P<0.05. Scale bar, 50 μm. BM indicates basal medium.
Coculture of PRNCs and HEN6 Combined With VEGF Treatment Optimally Attenuates OGD-Induced Neuronal Cell Death
PRNCs exposed to the OGD condition were then cocultured with HEN6 or fibroblasts in the medium containing VEGF and anti-VEGF. Additional 12 treatment groups (groups G-R) are provided in Figure I in the online-only Data Supplement. On the basis of the apoptosis caspase 3 immunohistochemistry and the MTT assay, we found that PRNCs cocultured with fibroblasts (groups G–L) displayed significant cell death when compared with the singly cultured PRNCs subjected to OGD as described above. However, PRNCs cocultured with HEN6 (groups M–R) exhibited significantly reduced OGD-induced cell death. In addition, VEGF treatments boosted the HEN6 neuroprotective effects that not only blocked the anti-VEGF treatment, but also exacerbated the OGD-induced neurotoxicity. These results were summarized in Figures I and V in the online-only Data Supplement. In the ELISA analysis, VEGF concentrations across all VEGF treatment groups alone and HEN6 alone were comparable and did not differ significantly, except for the combination of HEN6 and VEGF that showed a trend of much higher VEGF upregulation (data not shown). These VEGF-treated groups, as well as the HEN6 alone groups, showed significantly increased levels of VEGF in comparison with no VEGF/no HEN6 coculture and anti–VEGF-treated groups (data not shown).
Discussion
We demonstrated in the present study that transplantation of the human endothelial cell line, HEN6, reduced infarct volume and behavioral deficits accompanied by enhanced endogenous vasculogenesis and neurogenesis and with some grafted cells exhibiting a new microvasculature indicating exogenous vasculogenesis. The mechanism of HEN6 neuroprotective effects was likely mediated by the VEGF signaling pathway. Altogether, repairing the endothelial component of the neurovascular unit was shown here as a key neuroprotective process for stroke therapy.
Despite the reported functional recovery in transplanted stroke animals and limited clinical trials in patients with stroke,1–4 a major gap in our knowledge is the mechanism of action underlying cell therapy. We speculated that vascular repair is a vital neuroprotective process in stroke as documented previously.24,26,35–38 However, it is not clear whether the cell graft-induced neovascularization has endogenous and exogenous components. Furthermore, the correlation between vasculogenesis and neurogenesis in stroke after cell transplantation remains uncertain. Here, we demonstrated that HEN6 transplantation exerted both endogenous and exogenous neovascularization. To this end, we show both collagen IV and vWF as markers of vasculogenesis. In support of delineating endogenous from exogenous vasculogenesis, we observed blood vessels stained with the human-specific antigen marker, HuNu, that colabeled with collagen IV or vWF corresponding to exogenous vasculogenesis (Figure 4). In addition, we detected that juxtaposed to this exogenous vasculogenesis is collagen IV or vWF-stained vessels but without HuNu labeling indicating endogenous vasculogenesis. However, our present data do not distinguish between new vessels and preserved cells after stroke and transplantation. An equally important finding here is that graft-induced vascularization is accompanied by enhanced neurogenesis.21,38 Similar to our observed exogenous and endogenous vasculogenesis, we found nestin-labeled cells positively or negatively stained with HuNu, suggesting exogenous and endogenous neurogenesis, respectively. Of note, the robust nestin expression enveloping the HuNu-positive cells implies enhanced endogenous neurogenesis. Further characterization of HEN6 graft effects on both angiogenesis and neurogenesis is warranted.
That HEN6 showed VEGF upregulation, and that a trend of improved VEGF elevation detected in the combination group of VEGF and HEN6, both of which blocked by anti-VEGF, suggest that the neuroprotection afforded by HEN6 is regulated by the VEGF pathway. Alternatively, other trophic factors secreted by HEN6 (not examined here) might be contributing additive effects to the exogenous VEGF in producing the neuroprotection against OGD-induced cell death. These therapeutic substances seem to be specific to HEN6 because the fibroblasts coculture did not afford protection against OGD. In addition, the HEN6 cell-to-cell contact with PRNCs, as opposed to the substrate produced by fibroblast-PRNC coculture, could have also mitigated the observed therapeutic benefits. The detection of microvascular morphology in HEN6-grafted stroke animals might have been similarly achieved in the in vitro condition, although likely masked by the short period and incomplete neurovascular unit in the cell culture system. The host microenvironment, in particular the notion of a vasculome in the brain,39 may also contribute to the fate and function of transplanted cells. These speculative secretory and cell substrate mechanisms warrant further investigations.
HEN6 may display multiple endothelial cell functions, such as enhancing vasculogenesis and fostering the integrity of the blood brain barrier. An important molecule that interacts with endothelial cells is VEGF that could induce vasculogenesis by promoting proliferation and migration of endothelial cells to the site of injury.40 Furthermore, VEGF is a key factor stimulated within a few hours after cerebral ischemia.41,42 The present study suggests that a combination of increased VEGF level (as detected in vitro) and enhanced HEN6/host endothelial cell proliferation, migration, and microvasculature-like structure formation within the ischemic brain contributed to the observed therapeutic benefits in this stroke model.
As we translate these findings to the clinic, basic questions on safety and efficacy of the transplant regimen arise. The present intracerebral transplantation targeting the striatal penumbra in the supra-acute stroke stage while effective may not be practical in the clinic. Extending such neurosurgical procedure to a few days after stroke may be more feasible in the clinic. For cell dose, we demonstrated the minimum effective dose of 200K for purified HEN6 transplantation that is comparable with other kinds of cell type. 10,43–47 In view of our findings that VEGF contributed to the therapeutic benefits, combination therapy of VEGF and cell therapy is indicated as described previously.35,48
This proof-of-concept study demonstrated efficacy and mechanism of action mediating immortalized cerebral endothelial cell transplantation in stroke. The present 7-day graft maturation period requires long-term assessment of benefit and safety. The present study is focused on the acute therapeutic effects of HEN6 transplantation, warranting the need for determining the chronic effects of cell therapy on angiogenesis and neurogenesis. Although stroke has been widely considered as an acute neurological disease, an equally deleterious secondary cell death ensues after the initial ischemic insult that will require aggressive therapeutic intervention with stable neurostructural and functional benefits over time. Highly regulated immortalized6,49 or nonimmortalized endothelial progenitor cells23,24,36,38 have potential cell-based transplant applications in stroke.
Supplementary Material
Supplemental Figure 1. Culture conditions for Primary rat neuronal cells (PRNCs). PRNCs were either singly cultured or co-cultured with fibroblasts or HEN6. Additionally, PRNCs were grown under the routine DMEM culture condition or supplemented with VEGF, anti-VEGF, or combination of both.
Supplemental Figure 2. HEN6 graft survival. Dose-dependent graft survival of transplanted HEN6 was detected. However, the percent graft survival was comparable and not significantly different across all transplanted animals.
Acknowledgments
We express their gratitude to Meaghan Staples, Cyrus Tamboli, Travis Dailey, Chris Metcalf, Diana Hernandez-Ontiveros, Mibel Pabon, and Sandra Acosta for technical assistance during the conduct of the study.
Sources of Funding: Dr Borlongan is supported by James and Esther King Foundation for Biomedical Research Program 1KG01-33966 and National Institutes of Health National Institute of Neurological Disorders and Stroke RO1 1R01NS071956-01.
Footnotes
Disclosures: None.
References
- 1.Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem cell therapies as an emerging paradigm in stroke (steps): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 2.Borlongan CV, Chopp M, Steinberg GK, Bliss TM, Li Y, Lu M, et al. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3:249–250. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chopp M, Steinberg GK, Kondziolka D, Lu M, Bliss TM, Li Y, et al. Who's in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant. 2009;18:691–693. doi: 10.3727/096368909X470883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 5.Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–2263. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- 6.Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 7.Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. CNS grafts of rat choroid plexus protect against cerebral ischemia in adult rats. Neuroreport. 2004;15:1543–1547. doi: 10.1097/01.wnr.0000133298.84901.cf. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, et al. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- 10.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimura T, Dezawa M, Kanno H, Yamamoto I. Behavioral and histological evaluation of a focal cerebral infarction rat model transplanted with neurons induced from bone marrow stromal cells. J Neuropathol Exp Neurol. 2005;64:1108–1117. doi: 10.1097/01.jnen.0000190068.03009.b5. [DOI] [PubMed] [Google Scholar]
- 12.Hicks AU, Hewlett K, Windle V, Chernenko G, Ploughman M, Jolkkonen J, et al. Enriched environment enhances transplanted subventricular zone stem cell migration and functional recovery after stroke. Neuroscience. 2007;146:31–40. doi: 10.1016/j.neuroscience.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, et al. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- 14.Minnerup J, Kim JB, Schmidt A, Diederich K, Bauer H, Schilling M, et al. Effects of neural progenitor cells on sensorimotor recovery and endogenous repair mechanisms after photothrombotic stroke. Stroke. 2011;42:1757–1763. doi: 10.1161/STROKEAHA.110.599282. [DOI] [PubMed] [Google Scholar]
- 15.Sakata H, Niizuma K, Wakai T, Narasimhan P, Maier CM, Chan PH. Neural stem cells genetically modified to overexpress Cu/Zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke. 2012;43:2423–2429. doi: 10.1161/STROKEAHA.112.656900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 17.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 18.Strong LH. The early embryonic pattern of internal vascularization of the mammalian cerebral cortex. J Comp Neurol. 1964;123:121–138. doi: 10.1002/cne.901230111. [DOI] [PubMed] [Google Scholar]
- 19.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 21.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 24.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 25.Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. 2011;7:208–220. doi: 10.1007/s12015-010-9157-y. [DOI] [PubMed] [Google Scholar]
- 26.Oyamada N, Itoh H, Sone M, Yamahara K, Miyashita K, Park K, et al. Transplantation of vascular cells derived from human embryonic stem cells contributes to vascular regeneration after stroke in mice. J Transl Med. 2008;6:54. doi: 10.1186/1479-5876-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 28.Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010:108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 29.Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, et al. Intracerebral xenografts of mouse bone marrow cells in adult rats facilitate restoration of cerebral blood flow and blood-brain barrier. Brain Res. 2004;1009:26–33. doi: 10.1016/j.brainres.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 30.Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke. 2004;35:2206–2210. doi: 10.1161/01.STR.0000138954.25825.0b. [DOI] [PubMed] [Google Scholar]
- 31.Parxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 2005. [Google Scholar]
- 32.Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, et al. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;10:126. doi: 10.1186/1471-2202-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, et al. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19:439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell E, Cao X, Moibi JA, Greene SR, Young R, Trucco M, et al. Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes. 2003;52:2731–2739. doi: 10.2337/diabetes.52.11.2731. [DOI] [PubMed] [Google Scholar]
- 35.Harms KM, Li L, Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS One. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, et al. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185–2195. doi: 10.1002/stem.161. [DOI] [PubMed] [Google Scholar]
- 37.Shimotake J, Derugin N, Wendland M, Vexler ZS, Ferriero DM. Vascular endothelial growth factor receptor-2 inhibition promotes cell death and limits endothelial cell proliferation in a neonatal rodent model of stroke. Stroke. 2010;41:343–349. doi: 10.1161/STROKEAHA.109.564229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo S, Zhou Y, Xing C, Lok J, Som AT, Ning M, et al. The vasculome of the mouse brain. PLoS One. 2012;7:e52665. doi: 10.1371/journal.pone.0052665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 41.Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- 42.Plate KH, Beck H, Danner S, Allegrini PR, Wiessner C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654–666. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 44.Saporta S, Borlongan CV, Sanberg PR. Neural transplantation of human neuroteratocarcinoma (hNT) neurons into ischemic rats. A quantitative dose-response analysis of cell survival and behavioral recovery. Neuroscience. 1999;91:519–525. doi: 10.1016/s0306-4522(98)00610-1. [DOI] [PubMed] [Google Scholar]
- 45.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. Neuroreport. 1998;9:3703–3709. doi: 10.1097/00001756-199811160-00025. [DOI] [PubMed] [Google Scholar]
- 46.Grabowski M, Johansson BB, Brundin P. Survival of fetal neocortical grafts implanted in brain infarcts of adult rats: the influence of postlesion time and age of donor tissue. Exp Neurol. 1994;127:126–136. doi: 10.1006/exnr.1994.1086. [DOI] [PubMed] [Google Scholar]
- 47.Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, et al. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31:235–242. doi: 10.1038/jcbfm.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maurer MH, Thomas C, Bürgers HF, Kuschinsky W. Transplantation of adult neural progenitor cells transfected with vascular endothelial growth factor rescues grafted cells in the rat brain. Int J Biol Sci. 2008;4:1–7. doi: 10.7150/ijbs.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS One. 2006;1:e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Culture conditions for Primary rat neuronal cells (PRNCs). PRNCs were either singly cultured or co-cultured with fibroblasts or HEN6. Additionally, PRNCs were grown under the routine DMEM culture condition or supplemented with VEGF, anti-VEGF, or combination of both.
Supplemental Figure 2. HEN6 graft survival. Dose-dependent graft survival of transplanted HEN6 was detected. However, the percent graft survival was comparable and not significantly different across all transplanted animals.


