SUMMARY
Psychological studies in humans and behavioral studies of model organisms suggest that forgetting is a common and biologically regulated process, but the molecular, cellular, and circuit mechanisms underlying forgetting are poorly understood. Here we show that the bidirectional modulation of a small subset of dopamine neurons (DANs) after olfactory learning regulates the rate of forgetting of both punishing (aversive) and rewarding (appetitive) memories. Two of these DANs, MP1 and MV1, exhibit synchronized ongoing activity in the mushroom body neuropil in alive and awake flies before and after learning, as revealed by functional cellular imaging. Furthermore, while the mushroom-body-expressed dDA1 dopamine receptor is essential for the acquisition of memory, we show that the dopamine receptor DAMB, also highly expressed in mushroom body neurons, is required for forgetting. We propose a dual role for dopamine: memory acquisition through dDA1 signaling and forgetting through DAMB signaling in the mushroom body neurons.
INTRODUCTION
Optimum cognitive fitness is predicted to occur with a robust ability to form new memories along with a strong capacity to forget irrelevant or harmful memories. Presently, there exists controversy as to whether memories are forgotten through passive decay or through active mechanisms, such as retroactive interference caused by subsequent learning events and mental activity (Wixted, 2004). Recently, molecular genetic studies using Drosophila pointed toward the involvement of the small GTPase Rac1 for the forgetting of early and labile olfactory memories within the mushroom body (MB) intrinsic neurons (Shuai et al., 2010), neurons known to be critical for forming and retrieving olfactory memories in insects (Berry et al., 2008; Menzel, 2001). Thus, emerging evidence supports the hypothesis that forgetting is a biologically regulated process. However, it remains unclear what other molecular pathways might regulate forgetting. Furthermore, it is unknown whether forgetting is internally regulated within the MB intrinsic neurons or whether forgetting is a circuit-based phenomenon involving MB extrinsic neurons.
The neurotransmitter dopamine has been implicated in behavioral control and its disorders across species to include motor control (Joshua et al., 2009), motivation (Wise, 2004; Krashes et al., 2009), decision making (Doya, 2008; Zhang et al., 2007), arousal (Andretic et al., 2005), addiction (Lüscher and Malenka, 2011), and learning (Schwaerzel et al., 2003; Claridge-Chang et al., 2009; Wise, 2004). The vast array of behavioral processes influenced by dopamine can be accounted for, in part, by the multiplicity of dopamine receptors, distinct intracellular signaling pathways enabled by receptor activation and inactivation (Beaulieu and Gainetdinov, 2011), different time courses for behaviors influenced by dopamine signaling (Schultz, 2007), the complex innervation of many brain areas by discrete clusters of dopamine neurons (DANs) (Mao and Davis, 2009; Björklund and Dunnett, 2007), and the innervation of subcellular domains of individual neurons by different DANs (Mao and Davis, 2009). Untangling this complexity to understand singular dopamine functions requires temporally precise manipulation of the activity of individual or small groups of DANs innervating defined neuronal targets that mediate discrete behaviors.
The cell bodies of DANs are organized as 15 clusters distributed throughout the adult fly brain (Mao and Davis, 2009; Nässel and Elekes, 1992). The PPL1 cluster contains five distinct DAN types with stereotyped innervation zones within the MB lobes, the neuropil housing the axon fibers of MB intrinsic neurons (Mao and Davis, 2009). DAN output has been shown to be necessary for the acquisition of aversive olfactory memories (Schwaerzel et al., 2003), and artificial stimulation of the PPL1 DANs in the presence of an odor is sufficient to form aversive olfactory memory (Claridge-Chang et al., 2009). These studies provide evidence that the PPL1 DANs convey the unconditioned stimulus (US) to the MBs, where it converges with the olfactory conditioned stimulus (CS) for the acquisition of aversive olfactory memories. Two distinct dopamine receptors, dDA1 and DAMB, are highly expressed within the MB intrinsic neurons and are coupled to the cAMP signaling pathway, and thus are likely mediators of dopaminergic effects on olfactory memory (Sugamori et al., 1995; Han et al., 1996; Kim et al., 2003). Indeed, the dDA1 receptor is required for both aversive and appetitive olfactory memory formation in adult flies (Kim et al., 2007). While the DAMB receptor mutant was reported to produce aversive olfactory memory defects in larvae (Selcho et al., 2009), these results were confounded by odor preference defects and leave the role of DAMB in adult olfactory learning and memory largely unknown.
Here we utilize bidirectional modulation of DAN activity with temporal precision, in vivo functional imaging of DAN activity, and dopamine receptor mutant analysis to address the role that dopamine plays in memory. Our results indicate that dopamine has a dual role in both the acquisition of olfactory memories and the forgetting of these memories.
RESULTS
Bidirectional Modulation of Dopamine Neuron Activity after Learning Regulates Forgetting
We used the GAL4 > UAS system (Brand and Perrimon, 1993) to acutely modulate the activity of Drosophila’s DANs during the period of memory retention after olfactory classical conditioning. Our initial studies employed a tyrosine-hydroxylase (TH) gal4 line (TH-gal4) to drive UAS-transgene expression in the DANs in the fly brain (Mao and Davis, 2009; Friggi-Grelin et al., 2003). We drove expression of a UAS-shits1 transgene encoding a temperature-sensitive Dynamin protein that blocks synaptic output at restrictive temperatures (Kitamoto, 2001) or a UAS-trpA1 transgene encoding a temperature-sensitive cation channel to stimulate DANs at elevated temperatures (Hamada et al., 2008). Both of these transgenes provide for normal neuronal function below 25°C but modulate activity at temperatures above 29 °C. Thus, these two tools allow for the control of neuronal activity in a bidirectional way.
Remarkably, we discovered that blocking synaptic output from DANs with UAS-shits1 for 40 min or more after learning significantly enhanced memory measured at 3 hr (Figure 1A), whereas there was no significant increase in memory with control +/UAS-shits1 flies. The maximum enhancement of memory occurred with 80 min of elevated temperature at any period between acquisition and testing (Figure 1B). Furthermore, blocking synaptic output from DANs did not affect subsequent odor avoidance behavior (see Figure S1A available online). Therefore, it is unlikely that the enhanced memory expression is due to altering odor perception or locomotor function required at retrieval for choosing between the trained and the control odors. These data indicate that DANs in wild-type flies exhibit continued synaptic activity after learning that erodes the expression of memory by either inhibiting memory consolidation or promoting forgetting.
Figure 1. Bidirectional Modulation of Dopamine Neuron Activity after Learning Regulates Forgetting.
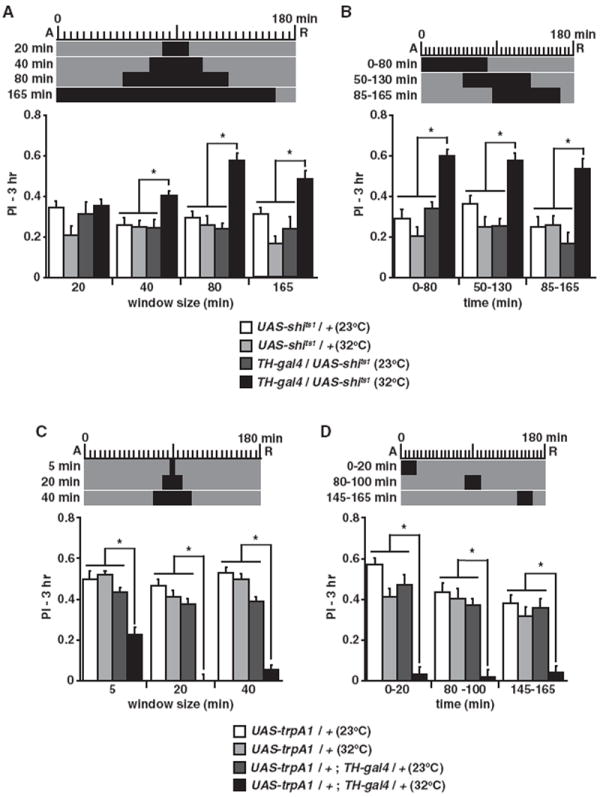
The experimental design above each graph shows the time of acquisition (A) and retrieval (R) relative to the time of elevated temperature at 32°C (black) from the 23°C (gray) baseline.
(A) Three hour performance index (PI) was significantly enhanced after a 40 min or longer elevated temperature window for TH-gal4/UAS-shits1 versus all other groups (*p < 0.02, n ≥ 7).
(B) An 80 min treatment window that varied in the time of onset significantly enhanced PIs for TH-gal4/UAS-shits1 versus all other groups at all time windows (*p < 0.002, n ≥ 8). The enhanced PIs for all time windows were not statistically different (p > 0.4).
(C) Three hour PI was significantly reduced after a 5 min or longer elevated temperature window for UAS-trpA1/+; TH-gal4/+ versus all other groups (*p < 0.0001, n ≥ 8). PIs for UAS-trpA1/+;TH-gal4/+ after a 20 min treatment were reduced to a level not significantly different from zero (p = 0.8).
(D) A 20 min treatment window significantly reduced PIs for UAS-trpA1/+;TH-gal4/+ versus all other groups at all time windows (*p < 0.0001, n ≥ 8). Scores for the treated UAS-trpA1/+;TH-gal4/+ flies were not significantly different from each other (p > 0.8) or from zero (p ≥ 0.178). Results are presented as means ± SEM.
We reasoned that potentiating DAN activity after learning should inhibit consolidation or accelerate forgetting. Stimulation of DANs by activation of TrpA1 for a minimum of 5 min after learning significantly decreased memory at 3 hr (Figure 1C). Remarkably, stimulation for 20 min or longer at any time window after training completely abolished memory expression (Figure 1D). Moreover, the abolished memory expression was not due to altered odor perception or avoidance (Figure S1B). These results support the conclusion that DAN activity after learning inhibits memory consolidation and/or promotes forgetting.
The process of consolidation is known to occur within distinct time windows after acquisition. In Drosophila, a portion of memory that is initially labile and sensitive to cold shock is consolidated into a stable and resistant form within 60 min after training (Tully et al., 1994). If DAN activity after training normally functions to inhibit consolidation, then the synaptic blockade and DAN stimulation experiments should only produce effects during this time window but not thereafter. Our results show an equally potent effect on performance of modulating DAN activity during or after this consolidation window (Figures 1B and 1D). These results, along with those described below (Figures 3A-3B), indicate that DAN activity after training must be for modulating forgetting rather than for modulating consolidation into a cold-resistant form of memory.
Figure 3. Activity of c150-gal4 DA Neurons after Training Is Necessary for Forgetting Early Labile Memories.
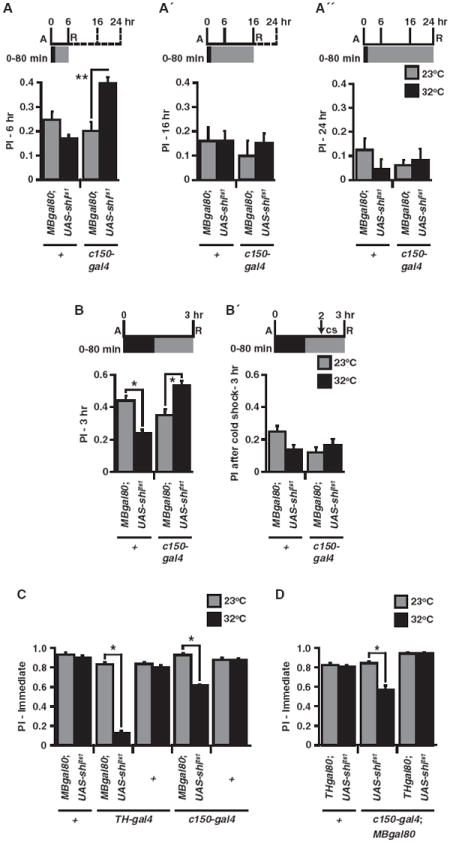
(A–A″) PI was significantly enhanced after a 0–80 min elevated temperature exposure for MBgal80/+; c150-gal4/UAS-shits1 flies at 6 hr (A) but not at 16 (A′) or 24 hr (A″) compared to normal temperature treatment (**p < 0.0005, n ≥ 8).
(B and B′) As in Figure 2B, 3 hr PIs were significantly enhanced when blocking c150 neurons for 0–80 min, while there was a significant decrease for control MBgal80/+; UAS-shits1/+ flies in this experiment compared to normal temperature (*p < 0.003, n ≥ 7). However, there was no significant enhancement after blocking c150 neurons for 0–80 min with an additional cold shock (cs) at 2 hr (p = 0.777, n = 12).
(C) Acquisition and immediate retrieval of aversive memory at 23°C or 32°C. PIs immediately after conditioning were significantly reduced at elevated temperature for MBgal80/+; TH-gal4/UAS-shits1 and MBgal80/+; c150-gal4/UAS-shits1 flies compared to normal temperature (*p < 0.0001, n = 8). A significantly lower PI was observed with TH-gal4 compared to c150-gal4 at 32°C (*p < 0.0001, n = 8).
(D) PI immediately after conditioning was significantly reduced, as in (C), at elevated temperature for flies carrying c150-gal4, MBgal80, and UAS-shits1 (*p < 0.0001, n = 8), but not for those that also contained THgal80 (p = 1.00) when compared to normal temperature. Results are presented as means ± SEM.
Activity from the c150-gal4 Subset of DANs Regulates Forgetting
Given the central role of the MBs in olfactory learning and memory in insects (Davis, 2005; Heisenberg, 2003; Menzel, 2001), we reasoned that the PPL1 DANs that innervate this brain neuropil would be the most likely candidates for those involved in forgetting. Utilizing a panel of PPL1-gal4 lines (Figures 2A and S2A) that drive expression in distinct subsets of PPL1 DANs, we screened for PPL1 DANs involved in the forgetting process by using both UAS-shits1 and UAS-trpA1. We included in the genotypes a gal80 transgene expressed in the MB intrinsic neurons (MBgal80) to suppress the GAL4 activity that is present there in most of the PPL1-gal4 lines. Interestingly, a synaptic blockade of the PPL1 DAN neurons included in the c150-gal4 (also Krasavietz; Dubnau et al., 2003) expression pattern produced a memory enhancement similar to that observed with TH-gal4 (Figure 2B). Although we observed an occasional effect of heat stress on memory performance in control lines like +/MBGAL80; +/UAS-shits1, an effect that has been observed in many prior temperature-shift experiments (Zhang et al., 2008; Waddell et al., 2000; McGuire et al., 2001; Dubnau et al., 2001) and is potentially due to heat-induced dopaminergic activity (Zhang et al., 2008), the effect was to decrease rather than enhance memory performance. The c150-gal4 driver is expressed in three DAN classes innervating the heel/peduncle (MP1), lower stalk/junction (MV1), and upper stalk (V1). Blocking synaptic output with c061-gal4, MZ604-gal4, or NP7135 that are expressed in DANs innervating only the heel/peduncle, lower stalk/junction/alpha tip, or upper stalk, respectively, did not enhance memory retention. This suggests that the activity of at least two of the three classes of PPL1 neurons must be blocked for memory enhancement. The lack of gal4 lines that are expressed specifically in the α or α′ tip DAN neurons prohibited us from determining whether these neurons are also involved in forgetting. However, because the quantitative effect of blocking the c150-gal4 neurons was the same as blocking all PPL1 DANs with TH-gal4, it is likely that the α or α′ tip DAN neurons play, at most, a minor role in memory decay.
Figure 2. Blocking Synaptic Release from a Subset of DANs Reduces Forgetting and Stimulation Accelerates Forgetting.
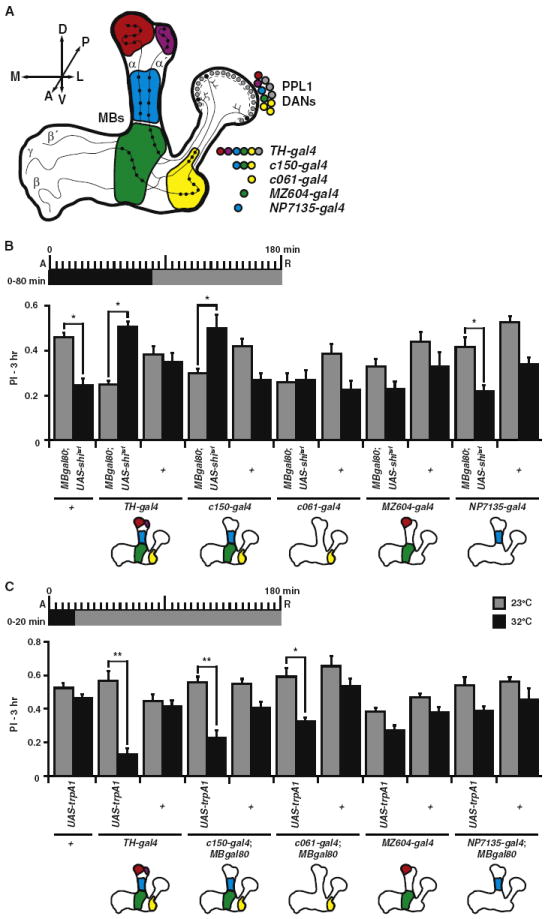
(A) Schematic diagram illustrating putative connectivity (black dots) between the mushroom body (MB) neuron classes (α′/β′, α/α, and γ) and the PPL1 DAN class (Tanaka et al., 2008; Mao and Davis, 2009). The cell bodies, corresponding innervation of the MB lobes, and representative gal4 lines used in this study for each DAN class are color coded (yellow, heel/peduncle; green, lower stalk/junction; blue, upper stalk; red, tip of α lobe; purple, tip of α′ lobe). Axis labels: A, anterior; P, posterior; D, dorsal; V, ventral; M, medial; L, lateral.
(B) Three hour PIs were significantly enhanced after a 0–80 min elevated temperature window only for flies that carried TH-gal4 or c150-gal4 along with MBgal80/+; UAS-shits1/+ when compared to normal temperature treatment (*p < 0.05, n ≥ 7). The 80 min elevated temperature treatment caused a significant decrease in PI for MBgal80; UAS-shits1 and NP7135-gal4/+; MBgal80/+; UAS-shits1/+ in this experiment compared to normal temperature (*p < 0.05, n ≥ 7).
(C) Three hour PIs were significantly reduced after a 20 min elevated temperature exposure immediately after training for flies carrying UAS-trpA1 with TH-gal4, c150-gal4, or c061-gal4 compared to normal temperature treatment (**p < 0.0001, *p < 0.0005, n ≥ 8). Results are presented as means ± SEM.
In a reciprocal experiment, we stimulated the c150-gal4 PPL1 DANs by using UAS-trpA1 (Figure 2C). Stimulation of MBgal80/+; c150-gal4/+ neurons produced a significant decrease in memory retention similar to stimulation with MBgal80/+; TH-gal4/+. Interestingly, stimulation of c061-gal4/+; MBgal80/+ neurons also led to a significant decrease in memory retention. Therefore, stimulated activity of c150-gal4 or c061-gal4/+; MBgal80/+ neurons is sufficient to accelerate forgetting. These two gal4 drivers share expression in the DAN class of neurons that uniquely innervates the MB heel/peduncle and intersects with the processes of both α/β and γ MB neurons, neurons in which Rac-mediated forgetting has been suggested to occur (Shuai et al., 2010). This suggests that concurrent dopamine input into these two types of MB intrinsic neurons is sufficient to accelerate forgetting.
To confirm that the DANs within c150-gal4 expression pattern are responsible for the phenotypes observed when blocking synaptic transmission and stimulating activity, we introduced a THgal80 transgene to repress GAL4 activity within the DANs. We compared memory retention of MBgal80/+; c150-gal4/+ flies with or without THgal80 (Figure S2B). As before, blocking MBgal80/+; c150-gal4/+ neurons led to a significant increase in memory retention, whereas blocking in the presence of THgal80 did not. Stimulating the MBgal80/+; c150-gal4/+ neurons with trpA1 led to pronounced forgetting compared to stimulating the MBgal80/THgal80; c150-gal4/+ subset of neurons (Figure S2C). Thus, excluding the DANs from the MBgal80/+; c150-gal4/+ expression pattern with TH-gal80 expression largely rescued the effect of stimulating MBgal80/+; c150-gal4/+ neurons.
Blocking c150-gal4 DAN Output after Training Preserves Labile Memory
To further confirm that the enhanced memory expression observed with synaptic blockade of DAN is due to protecting memories from forgetting rather than increasing consolidation, and to delimit the time window for enhanced expression, we conducted two different experiments. First, we assayed the lifetime of the enhanced memory after the synaptic blockade of MBgal80/+; c150-gal4/+ neurons (Figures 3A–3A″). Memory was significantly enhanced at 6 hr after conditioning, like at 3 hr (Figure 2B), but not at 16 or 24 hr. This observation indicates that the enhanced performance is due to preserving early memories and that the additional memory is forgotten sometime between 6–16 hr after conditioning. The alternative hypothesis, that synaptic blockade increases consolidation, predicts that any additional consolidated memory gained during the blockade would be stable and still be present at later time points. Second, we blocked the synaptic activity of MBgal80/+; c150-gal4/+ neurons for 80 min after conditioning (as in Figure 2B), but with a parallel group we additionally disrupted all labile memory existing at 2 hr with a 0°C cold shock and measured 3 hr memory (Figures 3B and 3B′). Interestingly, while we reproduced an enhancement of 3 hr memory, we found that the cold-resistant, consolidated memory was not significantly altered after blocking c150 DANs, indicating that the memory preserved by synaptic blockade was labile because it was sensitive to cold shock. Together, these data support the conclusion that ongoing activity from c150-gal4 DANs after training induces the forgetting of early labile memories without affecting cold-resistant, consolidated memories or the consolidation process itself.
To determine whether the activity of c150-gal4 DANs is restricted to the process of forgetting after memory is acquired, we imposed a synaptic blockade on both TH-gal4/+ and MBgal80/+; c150-gal4/+ neurons during acquisition and immediate retrieval (Figure 3C). As observed previously (Schwaerzel et al., 2003) and confirmed here, blocking the majority of DANs with TH-gal4 led to a robust reduction in memory performance. By comparison, blocking MBgal80/+; c150-gal4/+ DANs led to a lesser, but still significant, decrement in immediate memory performance. To ensure that the DANs within c150-gal4 expression pattern were responsible for this decrement in immediate memory, we measured memory in flies with or without the THgal80 transgene (Figure 3D). Removing DANs from the c150-gal4 expression pattern via THgal80 expression produced a complete rescue of immediate memory. Because DAN output is not required for retrieval of aversive olfactory memories (Schwaerzel et al., 2003), these data indicate that the activity of c150-gal4 DANs during training is required for optimal acquisition in addition to a later requirement in the process of forgetting.
Stimulation of c150-gal4 DANs Induces Forgetting of Consolidated Aversive and Appetitive Memories
To determine whether consolidated memories can be influenced by DAN-mediated forgetting, as suggested by the data in Figure 1D, we stimulated MBgal80/+; c150-gal4/+ neurons for 20 min immediately prior to a 6 hr memory test; a time at which memories are fully consolidated into a cold-resistant form (Figure 4A). This stimulation caused a robust and significant decrease in memory. To further ensure that this decrement was due to a loss of consolidated memory rather than any remaining labile memory, we imposed a cold shock at 2 hr to eliminate labile memory, followed by a 20 min stimulation with trpA1 just prior to a 3 hr memory test (Figure 4B). Remarkably, we found that this stimulation of MBgal80/+; c150-gal4/+ neurons led to a complete loss of consolidated memory. These data, along with Figure 1D, indicate that, while early labile memories are more sensitive than consolidated memories to endogenous dopamine activity after learning (Figures 3A–3B), excessive stimulation of these neurons with TrpA1 is sufficient to weaken both forms of memory.
Figure 4. Stimulation of c150 DANs Causes Forgetting of Consolidated Aversive and Appetitive Odor Memories.
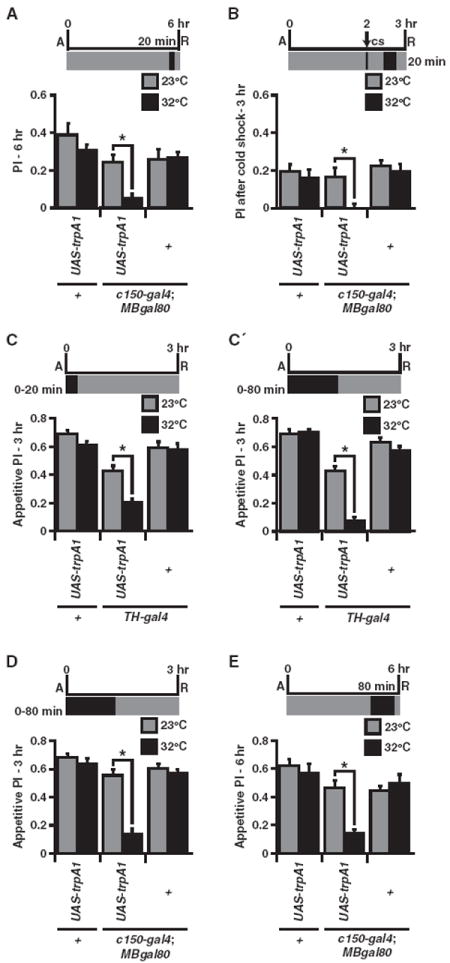
(A) Six hour aversive PIs were significantly reduced after a 20 min elevated temperature window 15 min prior to retrieval for MBgal80/UAS-trpA1; c150-gal4/+ flies compared to normal temperature treatment (*p < 0.05, n = 8).
(B) Three hour aversive PI was significantly reduced after a cold shock at 2 hr followed by a 20 min elevated temperature window 15 min prior to retrieval for MBgal80/UAS-trpA1; c150-gal4/+ flies compared to normal temperature (*p < 0.05, n = 8). The reduced PI was not significantly different from zero (p = 0.751).
(C and C′) Appetitive 3 hr PI was significantly reduced after a 20 or 80 min elevated temperature window for UAS-trpA1/+; TH-gal4/+ flies compared to normal temperature (*p < 0.05, n = 8).
(D) Appetitive 3 hr PI was significantly reduced after a 0–80 min elevated temperature window for MBgal80/UAS-trpA1; c150-gal4/+ flies compared to normal temperature (*p < 0.05, n = 8).
(E) Appetitive 6 hr PI was significantly reduced after an 80 min heat exposure presented 15 min prior to retrieval for MBgal80/UAS-trpA1; c150-gal4/+ flies when compared to normal temperatures (*p < 0.05, n = 8). Results are presented as means ± SEM.
Appetitive olfactory memories are consolidated within the first few hours after training to form a stable memory that lasts for days (Tempel et al., 1983; Krashes and Waddell, 2008). Although the formation of appetitive memory has been shown to be independent of synaptic activity of DANs during acquisition (Schwaerzel et al., 2003), we wondered whether this form of memory is vulnerable to DAN-mediated forgetting. Interestingly, stimulating TH-gal4 neurons for 20 or 80 min after appetitive memory training led to a robust and significant decrease in memory expression measured at 3 hr (Figures 4C and 4C′), an effect we mapped to the MBgal80/+; c150-gal4/+ neurons (Figure 4D). To eliminate the possibility that stimulation of c150-gal4 DANs was interfering with the consolidation of appetitive memory, we performed an 80 min stimulation of MBgal80/+; c150-gal4/+ neurons just prior to a 6 hr retrieval test (Figure 4E). Once again, we observed a significant decrease in memory performance when stimulating just prior to testing at 6 hr. Together, these data indicate that stimulated activity of c150-gal4 DANs can also induce the forgetting of consolidated appetitive memories.
MP1 and MV1 DANs Display Synchronized Ongoing Activity before and after Learning
Our blocking experiments of synaptic activity strongly indicate that some of the c150-gal4 PPL1 DANs (MP1, heel/peduncle; MV1, junction/lower stalk; V1, upper stalk; Figure 5A) that innervate the mushroom bodies have continued synaptic activity after conditioning. To verify and measure this activity, we expressed UAS-GCaMP3.0 (Tian et al., 2009), which encodes a Ca2+-sensitive enhanced green fluorescent protein (GFP), within the DANs via TH-gal4. In order to isolate the Ca2+-based increases in fluorescence from motion-based changes in fluorescence, we included a UAS-RFP (Pramatarova et al., 2003), which encodes a Ca2+-insensitive red fluorescent protein (RFP) with an emission spectrum largely separate and distinct from the GCaMP3.0. We performed in vivo functional imaging experiments on naive-, forward-, or backward-conditioned awake UAS-GCaMP3.0/UAS-RFP; TH-gal4 flies. Remarkably, and in agreement with our behavioral results, we found robust, ongoing Ca2+-based activity within the MP1 and MV1 DAN processes that innervate the MBs, while the V1 innervation of the MBs was silent (Figures 5A-5C). Additionally, we observed that the DAN innervations of the anterior inferior medial protocerebrum (aimpr) (innervated extensively by MP1 and MV1 DANs; Tanaka et al., 2008) also displayed robust activity. Importantly, learning did not alter DAN activity in any of these regions as neither forward nor backward conditioning caused significant alterations to the overall activity per second. Interestingly, while simultaneously recording multiple regions, we observed that the ongoing activity appeared highly synchronized between MP1, MV1, and the aimpr (Figure 5B). We calculated a normalized cross-correlation between simultaneously recorded signals between these regions (Figure 5D) and found that the MP1, MV1, and aimpr activities were highly correlated, while V1 was not. Behavioral conditioning did not significantly alter the activity correlations. These data, along with our blocking experiments (Figures 1A, 1B, and 2B), demonstrate that the MP1 and MV1 DANs have ongoing activity and that the MBs receive continued dopaminergic input after memory acquisition. Furthermore, this forgetting signal is synchronized between these two DANs.
Figure 5. MV1 and MP1 DANs Exhibit Ongoing Synchronized Ca2+-Based Activity before and after Training in Awake, Living Animals.
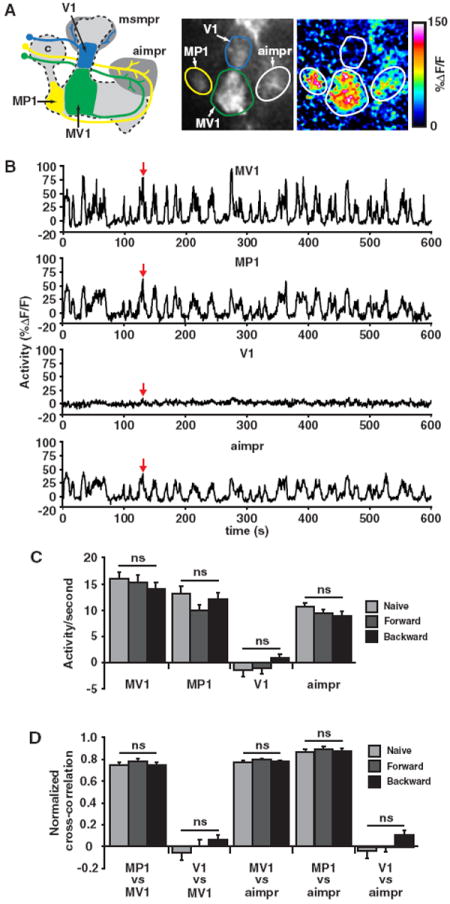
(A) The left panel illustrates the projections of the V1, MV1, and MP1 DANs to both the MB neuropil (blue, green, and yellow areas) and protocerebrum (dark gray areas) imaged in this study. The PPL1 DAN cell bodies, located adjacent to the calyx (“c”), project a neurite anteriormedially toward the MB lobes. The V1 DAN (blue) neurite branches to innervate both the middle superior medial protocerebrum (msmpr) just posterior to the vertical tips of the MBs and the middle segments of α′ and α MB lobes. The MV1 and MP1 neurites innervate the anterior inferior medial protocerebrum (aimpr) located dorsal and anterior to the horizontal MB lobes. These neurites then travel around the tip of the horizontal lobes to finally innervate distinct regions of the MBs. The MV1 terminates in the anterior face of the γ lobe and the lower-stalk region of the α′ lobe, while MP1 terminates in the heel region of the γ lobe and the α/β layers of the peduncle. The MV1 and MP1 neurons project to other areas of the protocerebrum, which are not shown for graphical simplicity. The middle and right panels show GCaMP3.0 expression and the corresponding pseudocolored activity (%ΔDF/F, see Experimental Procedures) from an activity event (red arrow in B) occurring in a representative recording. The regions of interest used to measure activity are identified by outlining.
(B) Activity traces (%ΔDF/F) from a 600 s recording from the representative animal in (A) are shown for the MV1, MP1, and V1 innervations of the MBs along with the DAN innervations within the aimpr. The red arrows indicate the time point used for collecting the static images in the middle and right panels of (A).
(C) Group data of activity per second (see Experimental Procedures for calculation) for recordings from the MV1, MP1, and V1 innervations of the MBs and the DAN innervations of the aimpr as assayed in naive animals and in animals 15 min after forward and backward conditioning. MV1, MP1, and the aimpr displayed robust ongoing activity in naive animals and after both conditioning paradigms (significantly different from zero, p < 0.0001, n ≥ 20), while V1 showed no significant activity (not significantly different from zero, p ≥ 0.245, n ≥ 17). Learning did not significantly alter the activity in any of the regions measured (ns, p ≥ 0.185). The scores for animals tested behaviorally in parallel with optical imaging were PI = 0.586 ± 0.015 (forward conditioning) and PI = −0.052 ± 0.015 (backward conditioning); p < 0.0001, n ≥ 35.
(D) Group data of normalized cross-correlations of activity (see Experimental Procedures) between simultaneously recorded regions of interest. In naive animals, simultaneously recorded activities of MV1, MP1, and the aimpr were highly correlated to each other (significantly different from zero, p < 0.0001, n ≥ 9), while V1 was not significantly correlated with MV1 or the aimpr (not significantly different from zero, p ≥ 0.384, n = 18). Forward and backward conditioning did not significantly alter activity correlations in any of the regions measured (ns, p ≥ 0.194, n ≥ 9). Results are presented as means ± SEM.
The Dopamine Receptor DAMB Is Specifically Required for Forgetting
We reasoned that if dopamine is mediating forgetting of memory stored within the MBs, then loss of dopamine receptors expressed in the MBs would block this forgetting pathway. Both the dDA1 and DAMB dopamine receptors are highly expressed within the MBs (Han et al., 1996; Kim et al., 2003). However, because dDA1 mutants do not form aversive olfactory memories due to the dDA1 role in acquisition (Kim et al., 2007), we chose to look at the potential role for DAMB in forgetting. Remarkably, we found that despite a slightly decreased immediate memory, damb mutants exhibited significantly enhanced memory retention at time points up to 24 hr, a time at which memory in control Canton-S flies was completely forgotten (Figure 6A). This more persistent increase in memory expression with the complete loss of DAMB compared to that observed after transiently blocking synaptic activity of DANs (Figures 3A–3A″) is probably due to the constitutive disruption of the dopamine signaling pathway across the entire retention window. We wondered also whether, in addition to gradual forgetting during memory retention, DAMB also played a role in the acute forgetting that occurs during reversal learning (Shuai et al., 2010). It has been shown that if flies are trained to one odor pair, then immediately trained to the reverse CS+/CS− contingency, they exhibit a stronger preference for the most recent and reversed contingency when subsequently tested. Indeed, we found that while control Canton-S flies robustly learned and remembered the reversed odor contingency, damb mutants displayed reduced memory expression for the reversal odor conditioning. This suggests that damb flies are defective in their ability to forget the first contingency, and this interferes with expressing memory of the reversal contingency. To better assess the nature of the immediate memory defect in damb mutant flies (Figure 6A), we performed a memory acquisition curve by varying the number of electric-shock pulses given during the training (Figure 6C). We found that damb mutants acquired memory at a similar rate as control flies up to six shocks, but their memory plateaued at a slight but significantly lower level at 12 shocks. To determine whether damb mutants exhibit behaviors consistent with having normal sensorimotor systems that underlie olfactory classical conditioning, we performed shock and odor avoidance controls. We found that at higher voltages, including the 90V standardly used in training, damb mutants were impaired in shock avoidance (Figure 6D), while their odor avoidance was not significantly different from the control (Figure 6E). Thus, DAMB appears to be required for effective perception of the electric shock US, which may explain the slight deficiency in immediate learning in damb mutants (Figures 6A and 6C). All together, these data indicate that, while the dDA1 receptor is important for forming aversive memories, the DAMB receptor is important for forgetting them.
Figure 6. Dopamine Receptor DAMB Is Required for Forgetting.
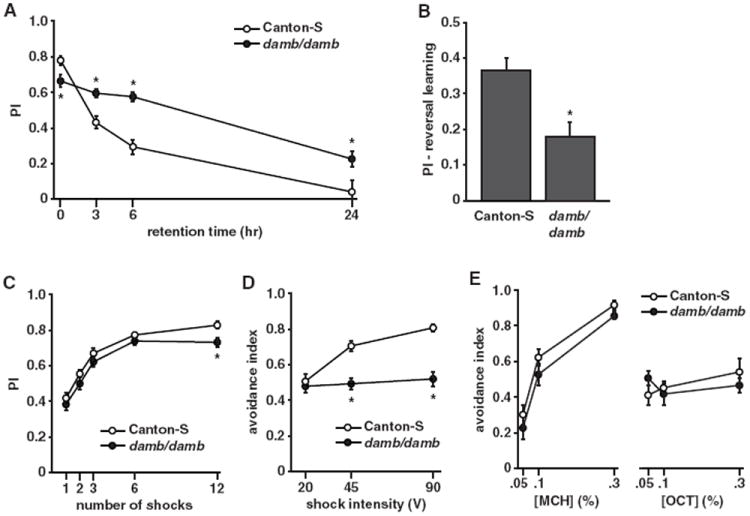
(A) Memory retention was significantly enhanced at 3 hr (p = 0.0046, n = 8), 6 hr (p = 0.0003, n = 8), and 24 hr (p = 0.0083, n = 8) after training in animals lacking the DAMB receptor despite a slight but significantly reduced immediate memory (p = 0.0453, n = 8).
(B) damb mutants showed a significant reduction in a reversal learning paradigm. Performance index is measured with respect to the second “reversal” odor contingency (p = 0.0166, n = 8).
(C) Animals lacking the DAMB receptor acquired memory at a similar rate for one (p = 0.3738, n = 8), two (p = 0.2473, n = 8), three (p = 0.1835, n = 8), and six (p = 0.2673, n = 8) shocks but plateaued at a significantly lower level than Canton-S for 12 shocks (p = 0.0355, n = 8).
(D) damb mutants displayed significantly reduced shock avoidance at the higher shock intensities of 45V (p = 0.0004, n = 16) and 90V (p < 0.0001, n = 16) relative to the control.
(E) damb mutants exhibit odor avoidance indistinguishable from the control to both MCH and OCT at several odor concentrations (*p < 0.05, n = 8). Results are presented as means ± SEM.
DISCUSSION
By modulating the activity of DANs in an acute and reversible way, visualizing Ca2+-based DAN synaptic activity, and conducting behavioral analyses of a dopamine receptor mutant, we have established that dopamine (DA) plays a dual role in learning and forgetting. We propose that after DANs fulfill their role in the acquisition of memory by providing a US signal to the MBs predominantly through the dopamine receptor dDA1, they continue to release dopamine onto the MBs that signals through the DAMB receptor to cause forgetting of recently acquired labile memories (Figure 7). We hypothesize that consolidation works to shield important memories from this ongoing dopamine-MB forgetting mechanism.
Figure 7. Model of Dopamine-Mediated Learning and Forgetting.
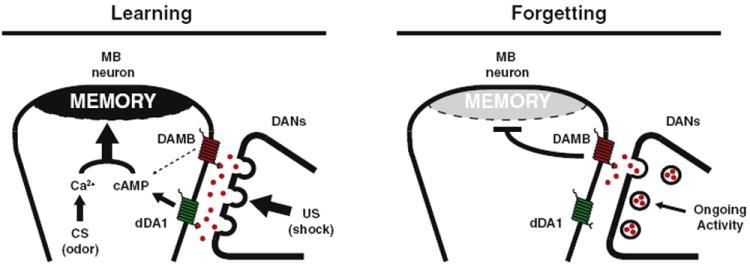
The left panel illustrates dopamine-mediated learning. Upon electric shock, a US signal is delivered when DANs release large amounts of dopamine (red spheres) onto the MBs. Binding of dopamine to the dDA1 receptor (green) leads to increased cAMP, while the CS odor stimuli results in increased Ca2+ signaling. Coincident Ca2+ and cAMP signaling leads to strong memory formation within the MBs (Tomchik and Davis, 2009). The right panel illustrates dopamine-mediated forgetting. After learning, a low level of ongoing dopamine release is sensed by the DAMB receptor (red) and weakens the memory over time. Differences in affinity of the two receptors for ligand, compartmentalization, coupling to downstream signaling pathways, or signaling kinetics may account for the dominant action of dDA1 in acquisition and DAMB in forgetting. Results are presented as means ± SEM.
This model is based on several specific lines of evidence: we discovered that blocking the output from DANs after learning enhances memory expression (Figures 1A and 1B), while stimulating DANs accelerated memory decay (Figures 1C and 1D). These effects were delimited to the c150-gal4 subset of DANs (Figures 2B and 2C), which includes the PPL1 DANs that project to the heel/peduncle (MP1), junction/lower-stalk (MV1), and upper-stalk regions of the MB neuropil (V1). We confirmed that the MP1 and MV1 DANs exhibit activity in naive animals through G-CaMP functional imaging as predicted by the synaptic blocking experiments, and this activity is synchronized between the two DANs and persists after learning (Figure 5). Finally, we confirmed and extended prior studies showing that blocking dopamine signaling during training impairs acquisition (Figures 3C and 3D), consistent with the role for the dDA1 receptor in acquisition, and showed that mutation of DAMB increases memory expression and impairs reversal learning (Figure 6). Our model predicts as one of several future epistasis experiments that DAMB mutation should block the increased forgetting caused by DAN activation. Overall, our observations are consistent with separate roles for the two receptors in the MBs for acquisition and forgetting.
The dopamine-based forgetting mechanism described here appears to preferentially remove labile memories, because a blockade of DAN synaptic activity enhances labile but not cold-resistant, consolidated memories (Figures 3A-3B′). Nevertheless, excessive stimulation of the mechanism with TrpA1 can induce the forgetting of consolidated memories (Figures 1C, 1D, and 4). Presumably, the TrpA1-mediated stimulation leads to overall higher levels of dopamine signaling, recruits additional DANs into the signaling network, or creates a different temporal pattern of activity that renders consolidated memory, formed for either aversive or appetitive conditioning, susceptible to forgetting.
Recently, Plaçais et al. (2012) presented data that is inconsistent with ours in support of the overriding conclusion that normal DAN activity specifically inhibits consolidated (cold-resistant) as opposed to labile memories. This conclusion was based largely on claims that blocking DAN activity specifically enhanced cold-resistant memory and that activation of DANs specifically inhibited cold-resistant memory. Our results indicate that blocking DAN activity specifically enhances labile memories and that activation of DANs can diminish both cold-resistant and labile aversive memories (Figures 1B, 1D, 3A, 3B, 3B′, 4A, and 4B) and appetitive memories (Figures 4C-4E). We offer several explanations for the discrepancies. First, in their cold-shock experiments, Plaçais et al. (2012) used the TH-gal4 driver and Shibirets to block the majority of DANs including those that innervate the α and α′ tips of the mushroom bodies, while we blocked only a subset of the DANs (c150-gal4). It is conceivable that the broader block of DAN activity partially underlies the differences in results. Second, the DAN activity block was applied across the entire 3 hr window between acquisition and retrieval with the cold shock overlaid on top of the activity block, whereas we applied the cold shock well after a shorter 80 min activity block. It is possible that the simultaneous cold shock and activity block somehow interact to confound the results. Most interestingly, Plaçais et al. (2012) found that blocking DAN activity in radish mutant flies that form only labile memories (Folkers et al., 1993) produced enhanced memory retention. This observation is consistent with our interpretation that blocking DAN activity preserves labile memories. Finally, the DAN stimulation experiments performed by Plaçais et al. (2012) with trpA1 utilized only a 1 min heat stimulation. Our results indicate that 20 min of heat is necessary to observe the complete effect of this stimulation (Figure 1C). The elimination of essentially all memory performance in multiple experiments (Figures 1C and 1D) strongly indicates that DAN stimulation can induce the forgetting of both labile and consolidated memories.
How can a single neurotransmitter, dopamine, have two seemingly opposite roles in both forming and weakening olfactory memories? And how can two different dopamine receptors, expressed broadly in the MBs as revealed by light microscopic analysis, serve acquisition on the one hand and forgetting on the other? One important consideration is the context and timing for the signaling that occurs during learning or afterwards. Prior studies have shown that dopamine delivery (the US) coupled with acetylcholine stimulation (the CS) leads to synergistic cAMP elevation within the MB intrinsic neurons, and this physiological response, as well as behavioral learning, is dependent upon the adenylyl cyclase encoded by the rutabaga gene (Tomchik and Davis, 2009). However, dopamine in isolation elevates cAMP levels independently of rutabaga, possibly due to the actions of other adenylyl cyclases. Thus, ongoing dopamine activity after learning should induce cAMP signaling in the absence of the calcium elevation due to the CS of odor stimulation. Therefore, the cellular context and timing of the dopamine-based acquisition signal is different from the dopamine-based forgetting signal. It is also possible that the receptors induce distinct intracellular signaling. Moreover, although the two receptors, dDA1 and DAMB, appear to be colocalized within the MB neuropil at the light microscope level, there may exist differences in subcellular localization between the two that help dictate their individual roles in learning and forgetting.
We propose that when a new memory is formed, there exists an active and dopamine-based forgetting mechanism, represented by ongoing DAN activity, that begins erasure unless some importance is assigned to the memory, perhaps through a consolidation mechanism. In other words, consolidation processes may counter the active forgetting mechanism. Whether the ongoing DAN activity is chronic or whether it is modulated by environmental factors remains unknown. The DAN forgetting mechanism does not preclude some passive loss of memory through stochastic breakdown of memory substrates within the MB intrinsic neurons. However, we speculate that active forgetting is the dominant force, because most if not all mechanisms in biology have both forward and reverse pathways (i.e., kinases versus phosphatases and protein synthesis versus protein degradative pathways). In addition, it may be that other mechanisms implicated in forgetting, such as proactive interference, retroactive interference, mental exertion, and stress (Jonides et al., 2008; Wixted, 2004), function by modulating the activity of the dopamine-based forgetting mechanism described here.
EXPERIMENTAL PROCEDURES
Fly Stocks and Genetics
Fly stocks were cultured on standard medium at room temperature. Crosses were raised at 25°C with 70% relative humidity with a 12 hr light-dark cycle. To obtain the data in Figure 1 and Figure S1, we crossed virgin female UAS-shits1 or UAS-trpA1 flies to males from either wCS10(UAS-/+) or TH-gal4 (TH-gal4/+). For all other data, we crossed virgin females from gal4 lines (with or without MBgal80) to males of either wCS10 (+) or UAS transgene stocks. Gal4 drivers used in this study include TH-gal4 (Friggi-Grelin et al., 2003), c061-gal4 (Krashes et al., 2009), MZ604-gal4 (Ito et al., 1998; Tanaka et al., 2008), and NP7135-gal4 (Tanaka et al., 2008). The THgal80 transgene was described in Sitaraman et al. (2008). The MBgal80 transgene was constructed by Hiromu Tanimoto. damb mutant flies were generated by Kyung-An Han using P element imprecise excision, which created a deletion of the damb locus (Selcho et al., 2009). The damb mutant flies were backcrossed with Canton-S.
Behavior
We used 2- to 6-day-old flies for all behavioral experiments except for imaging experiments (see below), in which flies were at least 5 days old to achieve adequate basal fluorescence. Flies were first equilibrated for ~15 min in a fresh food vial to the environment of a behavioral room dimly lit with red light at 23°C (or 32°C for Figures 3C and 3D) and 70% humidity. Standard aversive olfactory conditioning experiments were performed as described (Beck et al., 2000). Briefly, a group of 60–70 flies were loaded into a training tube where they received the following sequence of stimuli: 30 s of air, 1 min of an odor paired with 12 pulses of 90V electric shock (CS+), 30 s of air, 1 min of a second odor with no electric shock (CS−), and finally 30 s of air. For conditioning odors, we bubbled fresh air through 3-octanol (OCT) and 4-methylcyclohexonal (MCH) at concentrations of 0.055% and 0.05% in mineral oil, respectively. To measure early memory (Figures 3C and 3D), we immediately transferred the flies into a T maze where they were allowed 2 min to choose between an arm with the CS+ odor and an arm with the CS− odor. To test memory retention, we tapped the flies after conditioning back into a food vial to be tested at a later time point (3, 6, 16, or 24 hr). For all experiments, two groups were trained and tested simultaneously. One group was trained with OCT as the conditioned stimulus paired with reinforcer (CS+) and MCH unpaired with reinforcer (CS−), while the other group was trained with MCH as CS+ and OCT as CS−. Each group (60–70 flies) tested provides a half performance index (half PI): half PI = ([number of flies in CS− arm] – [number of flies in CS+ arm]) / (number of flies in both arms). A final PI was calculated by averaging the two half PIs. Because the two groups were trained to opposite CS+/CS− odor pairs, this method balances out naive odor biases.
During temperature-shift experiments, flies were transferred into preheated food vials at 32°C for a rapid temperature shift. The flies remained in a 32°C incubator at 70% humidity for a predefined period. In all cases, flies were returned to 23°C at least 15 min prior to a retrieval test.
Cold-shock experiments were performed by transferring trained flies to a precooled glass vial in an ice-water bath (~0°C). The flies were anesthetized almost immediately and remained in the bath for 2 min and then returned to a food vial at 23°C.
Appetitive olfactory memory experiments were performed as described (Krashes and Waddell, 2008). Briefly, flies were first starved for 16–24 hr prior to appetitive training on 0.8% nonnutritive agar. The CS+ and CS− odors and their concentrations were as described above for aversive conditioning. Flies were first exposed to the CS− odor for 2 min in a tube containing a dry filter paper previously saturated with water followed by 30 s of air. The flies were then transferred to a second tube containing a dry filter paper previously saturated with a 2 M sucrose solution and exposed for 2 min to a second odor (CS+). After conditioning, flies were maintained in nonnutritive agar vials at either 23°C or 32°C. Memory testing was performed as described above following aversive conditioning.
Acquisition curves for Canton-S and damb mutants were conducted as follows. Flies were exposed to 1, 2, 3, 6, or 12 shock pulses evenly distributed over 1 min of CS+ exposure such that the last shock pulse (or the only shock pulse) was always given at the last 1.25 s of odor exposure. After 30 s of air and the 1 min CS− exposure, flies were immediately tested for memory recall.
Reversal-learning experiments were conducted by training with an odor-pair contingency (for example, CS+ = OCT, CS− = MCH), waiting 1 min, training to the reverse odor-pair contingency (for example, CS+ = MCH, CS− = OCT), and immediately testing memory performance. If flies remember both contingencies equally, then one expects a PI of zero, while a positive PI would suggest a stronger memory performance with respect to the reversal contingency.
Odor avoidance tests were conducted by allowing naive flies to choose for 2 min in a T maze between an odor on one side and fresh air on the other. An avoidance index is calculated as the ([number of flies in fresh air arm] – [number of flies in odor arm]) / (number of flies in both arms).
Shock avoidance tests were conducted by allowing naive flies to choose for 2 min in a T maze between one arm containing an electrified copper grid (same as used for training above) and the other arm containing a nonelectrified copper grid. The side that is electrified is alternated to account for any side-to-side T maze bias.
In Vivo Functional Imaging Experiments
TH-gal4 virgin females were crossed to male UAS-GCaMP3.0, UAS-RFP flies. Progeny from this cross were collected and separated into three groups: (1) a “naive” group that received no odor or shock stimuli, (2) a “forward” group that received the standard olfactory conditioning (see above), and (3) a “backward” group that received the same odor and shock stimulation as the forward group but with the shock preceding the odor stimuli by 45 s. Approximately 15 min after behavioral conditioning (or directly from the vial in the case of the naive group), a 5- to 6-day-old male fly was aspirated, without anesthesia, into a narrow slot the width of a male fly in a custom-designed imaging chamber, while the remaining forward- and backward-conditioned flies were tested for behavioral memory. The fly head was oriented in one of two ways. One set of flies were oriented in a manner that presented a frontal view of the MB lobes, allowing us to obtain simultaneous recordings of the aimpr and the V1 and MV1 innervations of the MBs, with a second deeper recording of the MP1 innervation of the MB heel. We alternated which plane was recorded first for every animal. A second set of flies was oriented with the head rotated slightly, allowing the simultaneous recording of the aimpr and the MP1 and MV1 innervation of the MBs. The eyes, proboscis, and front two legs of the fly were restrained by using myristic acid. After placing a small piece of clear tape on the top surface of the slot containing the fly, a small square hole was cut out allowing access for dissection of the head cuticle. A small piece of the dorsal head cuticle was then removed along with the air sacs and fat bodies so that a clear optical path was created to the MBs. Fresh saline (103 mM NaCl, 3 mM KCl, 5 mM HEPES, 1.5 mM CaCl2, 4 mM MgCl2, 26 mM NaHCO3, 1 mM NaH2PO4, 10 mM trehalose, 7 mM sucrose, and 10 mM glucose [pH 7.2]) was perfused immediately across the brain to prevent desiccation and ensure the health of the fly. The remaining four legs and abdomen were free to move and flies frequently kicked their legs and moved their abdomens. All animals included in the data showed vigorous leg and abdomen movements after the procedures, indicating that they maintained health across the recording time. Using a 20× water-immersion objective and a Leica TCS SP5 II confocal microscope with a 488 nm argon laser, we imaged the DAN innervation of the MBs at 2 Hz. We used one PMT channel (510–550 nm) to detect GCaMP3.0 fluorescence and a second PMT channel (610–700 nm) to detect RFP fluorescence.
In Vivo Functional Imaging Analysis
A region of interest (ROI) was drawn around the DAN innervation of the mushroom bodies or the aimpr, and the average F for that ROI was calculated across time for both the GCaMP3.0 and RFP channels in Image J, G(t) and R(t), respectively. All further analysis was done in MATLAB using custom-written algorithms. To correct for photobleaching, we first calculated baseline fluorescence for GCaMP3.0 (Gb(t)) and RFP (Rb(t)) by fitting a line to the minimums of 10 s bins of the recording. We then calculated the normalized GCaMP3.0 and RFP signals as:
Finally, in order to correct for motion, we calculated the “activity” (Figure 5B) as:
The “activity per second” (Figure 5C) was calculated for each recording by taking the integral of the activity function and dividing it by the total time in seconds. To pseudocolor an “event” (right panel of Figure 5A), we first performed a Gaussian blur (sigma = 2.0) on all image frames by using ImageJ. We then calculated on a pixel-by-pixel basis an average baseline (Gb and Rb) from five consecutive time points in a trough just prior to the activity event marked with a red arrow in Figure 5B. Then we calculated the normalized GCaMP3.0 and RFP (GN and RN) signal and the resulting activity for the event on a pixel-by-pixel basis by using the calculations shown above. In order to measure synchronization of DAN activity within distinct MB lobes innervations and the aimpr, we first computed a normalized cross-correlation (Ryx) function between simultaneously recorded signals as follows:
where y and x are the two simultaneous recording activity signals across discrete time t, m is the lag, and N is the total sample length of the recordings. If two signals were synchronized in phase, then Ryx would be maximum with zero lag (m = 1). Therefore, we calculated a zero-lag normalized cross-correlation (Figure 5D) as
If two signals are perfectly identical, Ryx(zero lag) = 1.
Immunostaining and Microscopy
Whole brains were isolated in ice-cold PBS and maintained at 4°C during all steps until mounting them on microscope slides. Brains were fixed in a solution of 4% paraformaldehyde and PBS+T (0.3% Triton X-100 in PBS). After 6 × 10 min washes with PBS+T, the brains were blocked overnight with 5% normal goat serum in PBS+T solution. Brains were then incubated with rabbit anti-GFP (1:200, Molecular Probes) and mouse anti-FasII (1:10, DSHB) primary antibodies overnight. After washing for 6 × 10 min in PBS+T, we incubated the brains overnight in a solution containing goat anti-rabbit IgG conjugated with Alexa Fluor 488 and goat anti-mouse IgG conjugated with Alexa Fluor 633 (1:1,000, Molecular Probes) secondary antibodies. After an additional washing for 6 × 10 min with PBS+T, we mounted the brains on slides in Vectashield (Vector Laboratories). Images were collected by using a 10× dry objective and a Leica TCS SP5 II confocal microscope. The step size for z stacks was 1 μm with images collected at 512 × 512 pixel resolution.
Statistical Methods
Excel Stat and Prism were used for statistical analyses. Because PI values obtained from the classical olfactory assay are normally distributed (Tully et al., 1994), we used ANOVAs to make comparisons among different groups. For all comparisons of the effect of temperature across different genotypes, we performed a two-way ANOVA with both temperature and genotype as factors. We followed the two-way ANOVA with a Tukey post hoc comparison among the relevant groups. We performed a one-way ANOVA followed by a Tukey post hoc test for making comparisons within a genotype (TH-gal4/UAS-shits1 or UAS-trpA1/+; TH-gal4/+) treated at 32°C across time windows of different lengths (Figures 1A and 1C) or times (Figures 1B and 1D), for comparing among naive, forward, and backward groups of “activity per second” Figure 5C) and “normalized cross-correlation” (Figure 5D) and all damb statistics. A one-sample t test was used to make a comparison to zero. All tests were two tailed and confidence levels were set at α = 0.05.
Supplementary Material
Acknowledgments
We would like to acknowledge expert technical support from Daniel A. Richter. These research studies were supported by grant NS19904 from the National Institutes of Health to R.L.D.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes two figures and can be found with this article online at doi:10.1016/j.neuron.2012.04.007.
References
- Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beck CDO, Schroeder B, Davis RL. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci. 2000;20:2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J, Krause WC, Davis RL. Olfactory memory traces in Drosophila. Prog Brain Res. 2008;169:293–304. doi: 10.1016/S0079-6123(07)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenböck G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nat Neurosci. 2008;11:410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neuro-transmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, et al. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Folkers E, Drain P, Quinn WG. Radish, a Drosophila mutant deficient in consolidated memory. Proc Natl Acad Sci USA. 1993;90:8123–8127. doi: 10.1073/pnas.90.17.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, Strausfeld NJ. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn Mem. 1998;5:52–77. [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Adler A, Bergman H. The dynamics of dopamine in control of motor behavior. Curr Opin Neurobiol. 2009;19:615–620. doi: 10.1016/j.conb.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Seong CS, Han KA. Expression of a D1 dopamine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr Patterns. 2003;3:237–245. doi: 10.1016/s1567-133x(02)00098-4. [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- Menzel R. Searching for the memory trace in a mini-brain, the honeybee. Learn Mem. 2001;8:53–62. doi: 10.1101/lm.38801. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Elekes K. Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 1992;267:147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- Plaçais P-Y, Trannoy S, Isabel G, Aso Y, Siwanowicz I, Belliart-Guérin G, Vernier P, Birman S, Tanimoto H, Preat T. Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat Neurosci. 2012;15:592–599. doi: 10.1038/nn.3055. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Ochalski PG, Chen K, Gropman A, Myers S, Min KT, Howell BW. Nck beta interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol Cell Biol. 2003;23:7210–7221. doi: 10.1128/MCB.23.20.7210-7221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcho M, Pauls D, Han KA, Stocker RF, Thum AS. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS ONE. 2009;4:e5897. doi: 10.1371/journal.pone.0005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai Y, Lu B, Hu Y, Wang L, Sun K, Zhong Y. Forgetting is regulated through Rac activity in Drosophila. Cell. 2010;140:579–589. doi: 10.1016/j.cell.2009.12.044. [DOI] [PubMed] [Google Scholar]
- Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci USA. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamori KS, Demchyshyn LL, McConkey F, Forte MA, Niznik HB. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci USA. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Davis RL. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron. 2009;64:510–521. doi: 10.1016/j.neuron.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- Zhang K, Guo JZ, Peng Y, Xi W, Guo A. Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science. 2007;316:1901–1904. doi: 10.1126/science.1137357. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yin Y, Lu H, Guo A. Increased dopaminergic signaling impairs aversive olfactory memory retention in Drosophila. Biochem Biophys Res Commun. 2008;370:82–86. doi: 10.1016/j.bbrc.2008.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


