Abstract
Oxidized LDL is implicated in atherosclerosis; however, the pathways that convert LDL into an atherogenic form in vivo are not established. Production of reactive nitrogen species may be one important pathway, since LDL recovered from human atherosclerotic aorta is enriched in nitrotyrosine. We now report that reactive nitrogen species generated by the MPO-H2O2-NO2– system of monocytes convert LDL into a form (NO2-LDL) that is avidly taken up and degraded by macrophages, leading to massive cholesterol deposition and foam cell formation, essential steps in lesion development. Incubation of LDL with isolated MPO, an H2O2-generating system, and nitrite (NO2–)— a major end-product of NO metabolism—resulted in nitration of apolipoprotein B 100 tyrosyl residues and initiation of LDL lipid peroxidation. The time course of LDL protein nitration and lipid peroxidation paralleled the acquisition of high-affinity, concentration-dependent, and saturable binding of NO2-LDL to human monocyte–derived macrophages and mouse peritoneal macrophages. LDL modification and conversion into a high-uptake form occurred in the absence of free metal ions, required NO2–, occurred at physiological levels of Cl–, and was inhibited by heme poisons, catalase, and BHT. Macrophage binding of NO2-LDL was specific and mediated by neither the LDL receptor nor the scavenger receptor class A type I. Exposure of macrophages to NO2-LDL promoted cholesteryl ester synthesis, intracellular cholesterol and cholesteryl ester accumulation, and foam cell formation. Collectively, these results identify MPO-generated reactive nitrogen species as a physiologically plausible pathway for converting LDL into an atherogenic form.
Introduction
Oxidative modification of LDL is implicated in atherogenesis (1–5). A key early event is believed to be the conversion of LDL into a form capable of being recognized by “scavenger” or “oxidized LDL” receptors, whose surface expression is not diminished upon exposure to excess cholesterol (1–5). Direct demonstration of the oxidation pathways responsible for rendering LDL atherogenic in vivo, however, has proved difficult because of the evanescent nature of reactive oxidant species and the noninformative products they typically produce (6).
One potential pathway for LDL modification and conversion into a high-uptake form in vivo may involve reactive nitrogen species derived from nitric oxide (nitrogen monoxide, NO), a long-lived free radical that is formed by multiple vascular wall cells (7). NO is relatively unreactive toward most biomolecules and does not promote protein and lipid oxidation at neutral pH. However, a variety of reactive nitrogen species derived from NO are capable of damaging biologic targets (8–14). Antibodies specific for nitrotyrosine, a marker for protein damage by reactive nitrogen species (9, 14, 15), recognize epitopes in human atheroma but not in adjacent normal aortic intima (16). Mass spectrometric studies demonstrated nearly 100-fold–higher levels of nitrotyrosine in LDL recovered from human atherosclerotic aortae compared with LDL isolated from plasma of healthy donors (17). Thus, multiple lines of evidence suggest that nitrating intermediates promote LDL oxidation in the human artery wall during atherosclerosis; however, the precise nitrating intermediates formed, the pathways responsible for their generation in vivo, and the biologic consequences of LDL nitration are still unknown.
The most well-studied pathway for generating nitrating intermediates from NO involves formation of peroxynitrite (ONOO–), a product of NO and superoxide (O2–) (12). Bolus addition of reagent ONOO– to biologic targets promotes nitration of protein tyrosyl residues (14), depletes lipid-soluble antioxidants (18, 19), and initiates lipid peroxidation (11, 13). Multiple cellular components of human atherosclerotic lesions produce both O2– and NO (7), suggesting that ONOO– may contribute to LDL oxidation and nitrotyrosine formation in human atherosclerotic lesions. Recent studies have questioned the significance of ONOO– in mediating protein nitration in vivo, however, because of the inefficient tyrosine nitration observed when this oxidizing agent is formed in situ at physiological pH and low equivalent fluxes of NO and O2– (20). Moreover, enhanced NO production by macrophages has been shown to have a protective effect on the extent of cell-mediated oxidative damage of LDL (8).
Another potential pathway for generating reactive nitrogen species in vivo has recently been described that involves myeloperoxidase (MPO), an enzyme implicated in atherosclerosis. MPO is an abundant heme protein that is secreted from activated phagocytes (21). It amplifies the oxidizing potential of H2O2 by using it as cosubstrate to generate a variety of cytotoxic oxidants (22–27). Antibodies specific for MPO demonstrate that the enzyme is localized in human atherosclerotic lesions (28). One major and unique oxidant formed by MPO in vivo is hypochlorous acid (HOCl), a potent chlorinating agent generated by the oxidation of chloride (23, 29, 30). Both mass spectrometric and immunohistochemical studies demonstrate that HOCl-generated protein oxidation products are enriched in human atherosclerotic aortic intima and LDL recovered from human atherosclerotic lesions (29, 31). Thus, MPO-generated oxidants are formed in diseased human vascular tissues.
Several recent studies demonstrate that MPO also utilizes H2O2 and nitrite (NO2–), the autoxidation product of NO, as cosubstrates to generate a microbicidal oxidant (32) capable of nitrating phenolic compounds and proteins in vitro (25, 26, 33, 34). NO2– is ubiquitous in biologic tissues and fluids (25). Plasma and extracellular levels of NO2– are markedly increased in inflammatory and infectious processes in which NO production is enhanced (35–37). Studies with isolated MPO and human neutrophils demonstrate that the enzyme catalyzes aromatic nitration in media containing concentrations of NO2– approximating those found in biologic fluids, confirming that this major NO metabolite can serve as a physiological substrate for MPO in the presence of plasma levels of chloride (26, 38).
Here, we explore the role of MPO-generated reactive nitrogen species in converting LDL into a novel proatherogenic form. We now report that exposure of LDL to the MPO-H2O2-NO2– system of phagocytes results in concomitant apo B-100 protein nitration, induction of LDL lipid peroxidation, and conversion of the lipoprotein into a form capable of promoting macrophage cholesteryl ester accumulation and foam cell formation, essential steps in the atherosclerotic process.
Methods
Materials.
L-[13C6]tyrosine was purchased from Cambridge Isotopes Inc. (Andover, Massachusetts, USA). Tissue culture media and additives were purchased from Life Technologies Inc. (Gaithersburg, Maryland, USA). [125I]NaI and [14C]oleate were supplied by ICN Pharmaceuticals Inc. (Costa Mesa, California, USA). C57BL/6 mice (16–20 weeks of age) were purchased from the Trudeau Institute (Saranac Lake, New York, USA). THP-1 cells were obtained from American Type Culture Collection (Rockville, Maryland, USA). All other reagents were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA) unless otherwise specified.
General procedures.
All buffers were passed over a column of Chelex-100 resin (Bio-Rad Laboratories Inc., Hercules, California, USA) and supplemented with diethylenetriamine pentaacetic acid (DTPA) to remove any potential transition metal ions that might catalyze LDL oxidation during incubations. Peroxynitrite was synthesized and quantified as described (14). L-3-[13C6]nitrotyrosine was made from L-[13C6]tyrosine and peroxynitrite and was isolated by reverse-phase HPLC to remove residual NO2– before use (17). Protein content was determined by the Markwell-modified Lowry protein assay (39), with BSA as standard. Production of H2O2 by glucose/glucose oxidase (GGOx) was quantified by oxidation of Fe(II) and formation of an Fe(III)-thiocyanate complex (25). The concentration of reagent H2O2 was determined spectrophotometrically (ε240 = 39.4 M-1/cm-1; ref. 40). LDL was acetylated with acetic acid anhydride (41). Acetylated lipoproteins were dialyzed against 0.15 M NaCl, 0.3 mM Na2EDTA, and 100 μM DTPA (pH 7.4). LDL labeling with [125I]NaI and agarose gel electrophoresis were performed as described previously (42). The specific activity of labeled LDL preparations was between 100 and 250 dpm/ng protein. Cholesterol and cholesteryl ester content, and cholesteryl [14C]oleate synthesis by cells incubated with the indicated lipoproteins (50 μg/mL), were determined by published methods (41). α-tocopherol levels were determined as described (43). LDL concentrations are expressed as milligrams of protein per milliliter.
MPO and lipoprotein isolation.
MPO (donor: hydrogen peroxide, oxidoreductase, EC 1.11.1.7) was initially purified from detergent extracts of human leukocytes by sequential lectin affinity and gel filtration chromatography as described by Rakita et al. (44). Trace levels of contaminating eosinophil peroxidase were then removed by passage over a sulfopropyl Sephadex column (45). Purity of isolated MPO was established by demonstrating an RZ of 0.87 (A430/A280), SDS-PAGE analysis with Coomassie blue staining, and in-gel tetramethylbenzidine peroxidase staining (46). Enzyme concentration was determined spectrophotometrically utilizing an extinction coefficient of 89,000 M-1/cm-1/heme of MPO (22). The concentration of the MPO dimer was calculated as half the indicated concentration of heme-like chromophore. LDL was isolated from fresh plasma by sequential ultracentrifugation as a 1.019 < d < 1.063 g/mL fraction (47). Final preparations were placed in 50 mM sodium phosphate (pH 7.0), 200 μM DTPA (by passage over 10-DG desalting columns; Bio-Rad Laboratories Inc.) and stored under N2 until use.
Lipoprotein oxidation.
NO2-LDL was prepared by incubating LDL (0.2 mg protein/mL) at 37°C in 50 mM sodium phosphate (pH 7.0), 100 μM DTPA in the presence of 30 nM MPO, 100 μg/mL glucose, 20 ng/mL glucose oxidase (grade II; Boehringer Mannheim Biochemicals, Indianapolis, Indiana, USA), and 0.5 mM NaNO2 for 8 hours unless otherwise specified. Preliminary studies demonstrated that under these conditions, a constant flux of H2O2 (0.18 μM/min) is generated by the GGOx system. Unless otherwise stated, oxidation reactions were terminated by addition of 40 μM butylated hydroxytoluene (BHT; from a 100 mM ethanolic stock) and 300 nM catalase to the reaction mixture. Preliminary studies demonstrated that monocytes isolated by methods that use adhesion/washing steps contained less MPO per cell (presumably because of some premature degranulation during the isolation procedure). Human peripheral blood monocytes used for LDL oxidation were therefore isolated by elutriation (48). Monocytes (1 × 106/mL) were incubated with LDL (0.2 mg/mL) in HBSS (magnesium-, calcium-, phenol-, and bicarbonate-free; pH 7.20) supplemented with DTPA (100 μM) for 8 hours at 37°C in the presence of the additions or deletions indicated. Reactions were terminated by addition of BHT (20 μM) and pelleting of cells. Oxidation of LDL (0.2 mg/mL) by copper was performed by dialysis vs. 5 μM CuSO4 in PBS for 24 hours. Oxidation was terminated by addition of BHT (40 μM) and dialysis against PBS containing DTPA (100 μM). Aggregated LDL for cytochalasin D experiments was produced by vortexing solutions of LDL (500 μg/mL in 0.15 M NaCl, 0.3 mM Na2EDTA, 40 μM BHT).
Analysis of lipid peroxidation products.
Lipids were extracted by a modified Dole procedure as described by Savenkova et al. (49). Lipids were dried under nitrogen and resuspended in 1% sodium dodecylsulfate, and total lipid hydroperoxides were then measured as described (50). Total (free and esterified) 9-hydroxy-10,12-octadecadienoic acid + 9-hydroperoxy-10,12-octadecadienoic acid [9-H(P)ODE] formed was determined by reverse-phase HPLC of triphenylphosphine-reduced lipid extracts after base hydrolysis as described previously (49). Authentic (E,Z)-9-hydroxy-10,12-octadecadienoic acid (9-HODE; Cayman Chemical Co., Ann Arbor, Michigan, USA) was used as standard. Cholesteryl-9-hydroxy-10,12-octadecadienoate + cholesteryl-9-hydroperoxy-10,12-octadecadienoate [9-H(P)ODE] was quantified by reverse-phase HPLC of triphenylphosphine-reduced lipid extracts (not subjected to base hydrolysis) as described (49). (E,Z)-cholest-5-en-ol(3β)-9-hydroxy-10,12-octadecadienoate (cholesteryl-9-HODE; Cayman Chemical Co.) was used as standard. The content of thiobarbituric acid (TBA) reactive products in LDL preparations was determined by fluorescence analysis (51) in the presence of 0.05% (wt/vol) BHT. Fluorescence measurements (2-nm slit width, excitation 515 nm, emission 553 nm) were made on an LS-3 Fluorescence Spectrometer (Perkin-Elmer Corp., Norwalk, Connecticut, USA) using an external calibration curve constructed with known amounts of malondialdehyde (MDA) obtained from hydrolysis of 1,1,3,3-tetramethoxypropane in 20% acetic acid (pH 3.5). Total lipid hydroperoxides, total 9-H(P)ODE, cholesteryl-9-H(P)ODE, and TBA reactive products are expressed as nanomole of oxidation product per milligram of LDL protein.
Analysis of LDL protein oxidation products and amino acids.
Nitrotyrosine content was determined by stable isotope dilution gas chromatography-mass spectrometry (GC/MS) on a Turbomass Spectrometer (Perkin-Elmer Corp.) equipped with chemical ionization probe. Briefly, reactions were terminated by addition of BHT and catalase as already described here; lipids and salts were removed by extraction with a single-phase solvent mixture of H2O/water-washed diethyl ether/methanol (1:3:7, vol/vol/vol) (52); and the nitrotyrosine content in amino acid hydrolysates was determined after reduction to amino tyrosine as an n-propyl, per heptafluorobutyryl derivative (53). Nitrotyrosine content is normalized to the content of tyrosine determined by stable isotope dilution GC/MS (24).
Samples from time-course experiments quantifying remaining lysine and tryptophan residues were quenched with addition of 200 μM BHT and immediately analyzed. Unmodified lysine residues in LDL were quantified by fluorescamine fluorescence (54). Nα-acetyl lysine was used as standard to construct an external calibration curve. The fluorescence of treated samples was expressed relative to that of native LDL, assuming that the lipoprotein contains 341 lysine residues per particle, the number of lysines present in mature apo B-100 (55). Tryptophan fluorescence of LDL was measured using excitation and emission wavelengths of 280 nm and 335 nm, respectively (54). A linear relationship between loss of fluorescence and number of tryptophan residues oxidized was assumed. Results are expressed relative to those of native LDL; it was assumed that apo B-100 of native LDL contains 37 tryptophan residues (55).
Cells.
Elicited mouse peritoneal macrophages (MPMs) were harvested by peritoneal lavage with ice-cold PBS 2–3 days after thioglycollate stimulation of female C57BL/6 mice (42). Resident MPMs were similarly harvested from mice not subjected to thioglycollate stimulation. Primary cultures were prepared at a density of 106 cells per 16-mm-diameter well in RPMI-1640 containing 10% FBS and were used 48 hours after plating. Chinese hamster ovary (CHO) cells expressing mouse scavenger receptor class A type I (CHO-mSR-AI) and control vector-transfected parental CHO cells were a generous gift of M. Krieger (Massachusetts Institute of Technology, Boston, Massachusetts, USA) and were cultured as described elsewhere (56). Experiments were performed on confluent cell monolayers in Ham’s F-12 medium containing 3% lipoprotein-deficient FCS, BHT (20 μM), DTPA (100 μM), and catalase (300 nM). THP-1 cells were plated at a density of 0.5 × 106 cells per well and incubated with 64 nM PMA in RPMI-1640 with 10% FBS and 50 μM β-mercaptoethanol for 3 days before use. hMDMs were prepared from isolated human peripheral blood monocytes cultured 10–14 days in macrophage serum-free media (Gibco BRL, Grand Island, New York, USA) supplemented with GM-CSF (20 ng/mL) (48). Experiments with hMDMs were performed on confluent cell monolayers in the same medium containing 200 μg/mL LDL (to block LDL receptor–mediated recognition of LDL preparations), BHT (20 μM), DTPA (100 μM), and catalase (300 nM).
Lipoprotein uptake, degradation, and binding by cultured cells.
BHT (20 μM), catalase (300 nM), and DTPA (100 μM) were included in the media of all uptake, degradation, and binding experiments to inhibit lipoprotein oxidation during incubation with cells. Cells were washed with serum-free medium, and the indicated amounts of 125I-labeled native or modified LDL were added in 250 μL of the appropriate media. After a 5-hour incubation at 37°C, lipoprotein degradation was assessed by removal of media and quantifying TCA-soluble, noniodide degradation products (41, 42). Cells were also washed and assayed for cell-associated label and protein content (41, 42). Cell-free control experiments were performed in parallel and subtracted from counts in cell-containing reactions to calculate cell-dependent lipoprotein degradation (cell-free controls were <10% of the observed TCA-soluble counts in cell-containing reactions). Total uptake was calculated from the sum of degradation and cell-associated label. In competition experiments, 125I-labeled lipoprotein (5 μg/mL) was incubated with cells in the presence of 200 μg/mL of unlabeled competitor. Lipoprotein binding to macrophages at 4°C was determined after 4-hour incubations of the indicated amounts of modified forms of [125I]LDL, as described (41).
Size exclusion chromatography.
[125I]LDL (2 mg in 0.5 mL of 50 mM sodium phosphate buffer [pH 7.0] containing 200 μM DTPA, 5 mg/mL BSA) was applied to a Sephacryl S400-HR column (1.0 × 45 cm) pre-equilibrated in 50 mM sodium phosphate buffer (pH 7.0) containing 200 μM DTPA. Lipoproteins were eluted at a flow rate 10 mL/h, and radioactivity in 1.1-mL fractions was determined.
Statistics.
Data represent the mean ± SD of the indicated number of samples. Statistical analyses were made using a paired Student’s t test. For all hypotheses, the significance level was 0.05. When multiple comparisons were made, a Bonferroni correction to the significance criterion for each test was made.
Results
Human monocytes use the MPO-H2O2-NO2– system to convert LDL into a high-uptake form.
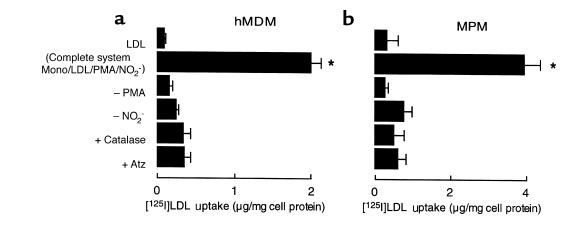
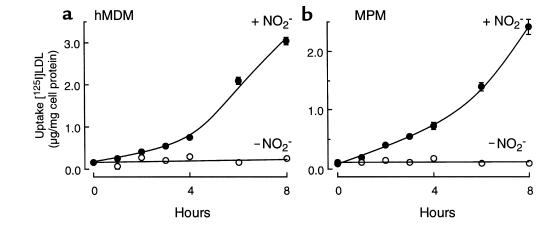
We hypothesized that phagocytes might use the MPO-H2O2-NO2– system to generate a nitrating intermediate that can oxidatively modify LDL, rendering it a ligand for macrophage recognition. To test this hypothesis, we incubated LDL with activated human monocytes in the absence and presence of NO2– and then determined whether the lipoprotein was converted into a high-uptake form for macrophages. LDL modified by activated human monocytes in the absence of NO2– demonstrated only a modest increase in the extent of LDL uptake by macrophages (Figure 1). In contrast, human monocytes activated in the presence of NO2– converted the lipoprotein into a form that was readily taken up and degraded by both hMDMs (Figure 1a) and thioglycollate-elicited MPMs (Figure 1b). Conversion of LDL into a high-uptake form by monocytes required cell activation and was inhibited by either the H2O2 scavenger catalase or peroxidase inhibitors, e.g., 3-aminotriazole). These results suggest that monocytes may use the MPO-H2O2-NO2– system for oxidative conversion of LDL into an atherogenic form. They also suggest that the MPO-H2O2 system of monocytes may use NO2– as substrate even in the presence of plasma levels of Cl–. Finally, macrophage recognition of monocyte-modified LDL appears to involve a mechanism(s) distinct from the LDL receptor, as excess native LDL that was included in the media during exposure of hMDMs to monocyte-modified [125I]LDL preparations (see Methods) did not inhibit’s uptake of monocyte-modified LDL.
Figure 1.
Degradation of [125I]LDL by hMDMs and MPMs after modification by activated human monocytes. Isolated human monocytes (106/mL) were incubated at 37°C in HBSS supplemented with DTPA (100 μM), [125I]LDL (0.2 mg/mL), and NO2– (500 μM). Monocytes were activated with phorbol ester (200 nM) and maintained in suspensions by intermittent inversion (complete system) for 8 hours. Additions or deletions to the complete system were as indicated. Reactions were stopped by addition of BHT (40 μM) and catalase (300 nM); the cells were pelleted; and then 125I-labeled lipoproteins (5 μg/mL) were incubated with either hMDMs (a) or thioglycollate-elicited MPMs (b) at 37°C for 5 hours in the appropriate media containing additional catalase (300 nM) and BHT (20 μM). Cellular uptake of lipoproteins was subsequently determined as described in Methods. The final concentrations of additions to the complete system were catalase (300 nM) and 3-aminotriazole (1 mM). Data represent the mean ± SD of 3 separate experiments. *P < 0.001 for complete system vs. complete system – NO2–. Atz, 3-aminotriazole; Mono, human peripheral blood monocyte.
LDL oxidized by MPO, H2O2, and physiological levels of NO2– is converted into a form (NO2-LDL) that is avidly taken up and degraded by macrophages.
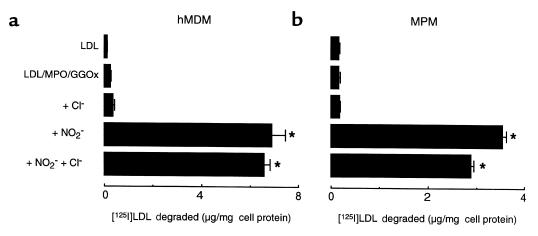
Activated monocytes can generate a host of reactive oxidant species, which could have contributed to LDL modification and conversion into a high-uptake form. For example, direct exposure of LDL to additions of either HOCl (54, 57) or HOCl in the presence of NO2– (58) has been reported to result in lipoprotein aggregation and conversion into a high-uptake form for macrophages. Thus, a role for HOCl production by the MPO-H2O2 system of the cells could not be excluded as a contributor to the cell-dependent conversion of LDL into a form with enhanced macrophage recognition. To explore more fully the mechanism(s) through which the MPO-H2O2 system of monocytes might render LDL atherogenic, we used model systems to evaluate the ability of MPO-generated chlorinating and nitrating intermediates to convert LDL into a form that is recognized by macrophages. [125I]LDL was incubated with MPO isolated from human leukocytes, an H2O2-generating system (GGOx) that produced a low flux of peroxide similar to that which might occur in vivo, and substrate (either Cl– or NO2–, or both). The ability of the modified lipoprotein to be taken up and degraded by either hMDMs or thioglycollate-elicited MPMs was then determined. Incubation of LDL in the presence of an HOCl-generating system (MPO, GGOx, and Cl–) failed to convert the lipoprotein into a stable high-uptake form (Figure 2). In contrast, exposure of LDL to MPO and the H2O2-generating system, but with NO2– added, transformed the particle into a form readily taken up and degraded by macrophages (Figure 2). Moreover, addition of plasma levels of Cl– to NO2–-containing reaction mixtures only modestly inhibited conversion of LDL into a high-uptake form. These results suggest that under conditions in which MPO-generated oxidants are formed by a physiological flux of H2O2, the reactive nitrogen species formed by the MPO-H2O2-NO2– system (presumably NO2; ref. 25) is more effective than either HOCl (product of the MPO-H2O2-Cl– system) or NO2Cl (the presumed nitrating intermediate formed by the reaction of NO2– and HOCl; ref. 33) in converting LDL into a stable high-uptake form for macrophages.
Figure 2.
Degradation of [125I]LDL by hMDMs and MPMs after modification by MPO-generated chlorinating and nitrating intermediates. [125I]LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM), glucose (100 μM), and glucose oxidase (20 ng/mL) in the presence of the indicated additions in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) overnight at 37°C, as described in Methods. Under these conditions, a constant flux of H2O2 (0.18 μM/min) is generated by the GGOx system. Reactions were stopped by addition of BHT (40 μM) and catalase (300 nM), and then 125I-labeled lipoproteins (5 μg/mL) were incubated with either hMDMs (a) or thioglycollate-elicited MPMs (b) at 37°C for 5 hours in the appropriate media containing additional catalase (300 nM) and BHT (20 μM). Cellular uptake of lipoproteins was subsequently determined as described in Methods. When indicated, Cl– (100 mM) or NO2– (500 μM) were added during LDL modification by MPO. Data represent the mean ± SD of triplicate determinations. Similar results were observed in 3 independent experiments. *P < 0.001 for comparison vs. LDL modified in the presence of MPO and an H2O2-generating system (LDL/MPO/GGOx).
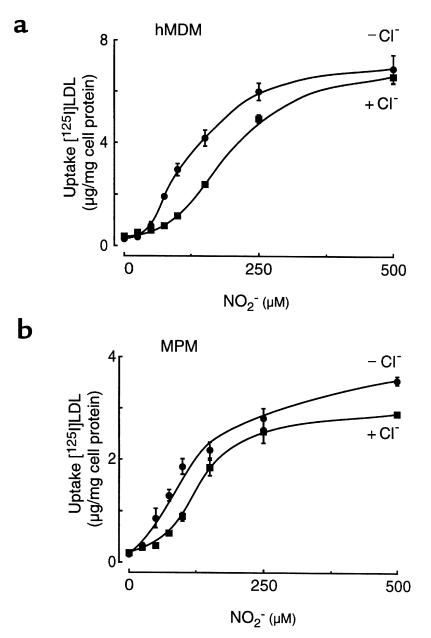
The concentration of NO2– in aortic intima is unknown. Plasma levels of NO2– are normally 1–5 μM but can increase more than 10-fold during inflammation. Because the major pathway for NO2– removal is through reaction with oxyhemoglobin-forming nitrate (NO3–), levels of NO2– in inflamed extracellular fluids and tissues are likely higher (25, 35–37). We therefore evaluated the NO2– concentration dependence of LDL modification and conversion into a high-uptake form by MPO in the absence and presence of plasma levels (100 mM) of Cl–. Incubation of LDL with MPO and an H2O2-generating system in the presence of increasing concentrations of NO2– resulted in progressively enhanced association, uptake, and degradation of the modified lipoprotein by all macrophages examined, i.e., both resident and elicited MPMs, THP-1 cells (a human acute monocytic leukemia cell line), and hMDMs. Data for lipoprotein uptake after modification by the MPO-H2O2-NO2– system in the absence and presence of Cl– by hMDMs (Figure 3a) and thioglycollate-elicited MPMs (Figure 3b) are shown. The presence of plasma levels of Cl– (100 mM) during MPO-dependent modification of the lipoprotein only modestly inhibited the ability of the peroxidase to utilize NO2– as substrate and convert the lipoprotein into a high-uptake form. Half maximal uptake by macrophages was observed at ∼100 μM NO2– for both hMDMs and MPMs (Figure 3), and significant increases in cell recognition were noticeable even when LDL was modified by MPO in the presence of 25 μM NO2–, the lowest concentration examined (P < 0.05 when comparing LDL modified with 0 vs. 25 μM NO2– in both the absence and presence of Cl– for both cell types). Collectively, these results indicate that concentrations of NO2– comparable to those found in inflammatory tissues and fluids are sufficient for MPO-dependent conversion of LDL into a high-uptake form. They also demonstrate that HOCl formation is not obligatory for conversion of LDL into a high-uptake form and that MPO (either isolated or derived from activated monocytes) utilizes NO2– as substrate to modify LDL into a form that binds and is taken up and degraded by macrophages, even in the presence of plasma levels of Cl–. Because MPO-generated reactive chlorinating species can modify protein and lipid components of lipoproteins (29, 31, 52) but are not required for conversion of LDL into a stable high-uptake form by the MPO-H2O2-NO2– system of monocytes, subsequent studies characterizing the biochemical and biologic properties of LDL modified by MPO-generated nitrating intermediates were performed under Cl-free conditions.
Figure 3.
NO2– concentration dependence of MPO-mediated modification of LDL and conversion into a high-uptake form for macrophages. [125I]LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM), glucose (100 μM), glucose oxidase (20 ng/mL), and the indicated concentrations of NO2–, in the absence (filled circles) and presence (filled squares) of Cl– (100 mM) in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) overnight at 37°C, as described in Methods. Uptake of the modified lipoproteins was then assessed using hMDMs (a) and thioglycollate-elicited MPMs (b) as described in Methods. Data represent the mean ± SD of triplicate determinations. Similar results were observed in 3 independent experiments.
The MPO-H2O2-NO2– system promotes LDL protein nitration and induces LDL lipid peroxidation.
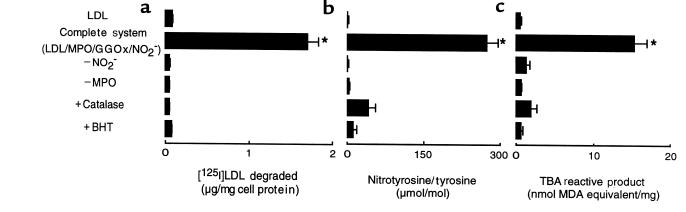
The nitrating intermediate formed by the MPO-H2O2-NO2– system is known to promote nitration of aromatic compounds and soluble proteins (25, 26); however, the products formed by this reactive intermediate after incubation with lipids and lipoproteins have not yet been reported. This is of particular interest because nitrogen dioxide (NO2), the one electron oxidation product of NO2–, has been suggested to be the reactive nitrogen species formed during peroxidase-catalyzed oxidation of NO2– (25). NO2 is known to initiate lipid peroxidation both in vitro and after inhalation (59, 60); thus, exposure of LDL to the MPO-H2O2-NO2– system might also represent a mechanism to promote lipid peroxidation. To test this hypothesis, we determined whether markers of protein and lipid oxidation, such as LDL nitrotyrosine content and TBA reactive products, were increased in LDL preparations after exposure to MPO, NO2–, and an H2O2-generating system, using conditions that convert the lipoprotein into a high-uptake form for macrophages. Incubation of LDL with MPO-generated reactive nitrogen species resulted in concomitant conversion of the lipoprotein into a high-uptake form for elicited MPM, nitration of apo B-100 tyrosine residues, and induction of lipid peroxidation (Figure 4). Similar results (enhanced macrophage uptake) were also obtained using cultured hMDMs, resident MPMs, or THP-1 cells (data not shown). Acquisition of macrophage recognition, LDL protein nitration, and lipid peroxidation all demonstrated absolute requirements for MPO and NO2–, were inhibited by catalase and BHT, and occurred in the absence of metal ions (Figure 4). These results are consistent with a role for MPO, H2O2, and NO2– in producing a free radical intermediate that promotes nitration of apo B-100 tyrosyl residues, initiation of lipid peroxidation, and acquisition of macrophage recognition.
Figure 4.
LDL protein nitration, lipid peroxidation, and conversion into a high-uptake form for macrophages mediated by the MPO-H2O2-NO2– system. [125I]LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM), glucose (100 μM), glucose oxidase (20 ng/mL), and NO2– (500 μM) in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) (complete system) or the indicated additions or deletions. The degradation of lipoproteins by thioglycollate-elicited MPMs (a), the content of nitrotyrosine (b), and TBA reactive products (c) generated were then determined as described in Methods. Data represent the mean ± SD for 3 independent experiments. *P < 0.001 for complete system vs. complete system – NO2–.
Multiple protein and lipid components of LDL are modified by the MPO-H2O2-NO2– system during the acquisition of macrophage recognition.
In the next set of experiments, we studied the time course of LDL conversion into a high-uptake form after exposure to MPO, NO2–, and a low steady flux of H2O2 (Figure 5). In parallel, we also characterized the extent of lipoprotein modification by a variety of independent indices of protein oxidative damage (Figure 6) and lipid peroxidation (Figure 7). Statistically significant (P < 0.01) increases in LDL uptake and degradation were observed with hMDMs and MPMs after a brief (2 hour) incubation of the lipoprotein with the complete MPO-H2O2-NO2– system (Figure 5). Cell recognition of the modified lipoprotein progressively increased in a nonlinear manner without indications of plateau after up to 8 hours of incubation. In stark contrast, no increase in macrophage uptake or degradation was observed in LDL incubated with MPO and H2O2 in the absence of NO2– over the entire time interval examined (Figure 5).
Figure 5.
Time course of LDL conversion into a high-uptake form after exposure to the MPO-H2O2-NO2– system. [125I]LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM), glucose (100 μM), and glucose oxidase (20 ng/mL) in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) for the indicated times in either the presence (+ NO2–; filled circles) or absence (– NO2–; open circles) of NO2– (500 μM) as described in Methods. Uptake of the modified lipoprotein by hMDMs (a) and thioglycollate-elicited MPMs (b) was then determined as described in Methods. Data represent the mean ± SD for triplicate determinations. Similar results were observed in 3 independent experiments.
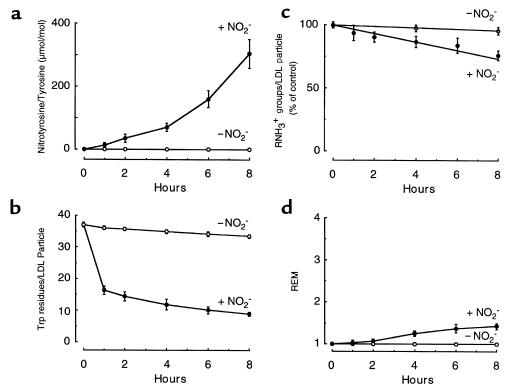
Figure 6.
Characterization of multiple protein components of LDL exposed to the MPO-H2O2-NO2– system. LDL samples prepared for Figure 5 were analyzed for nitrotyrosine content (a) tryptophan fluorescence (b), unmodified Nε-lysine groups (c), and lipoprotein REM (d), as described in Methods. Under the conditions used, the REM for exhaustively acetylated LDL is 3.8. Data represent the mean ± SD of 3 independent experiments (1 using 125I-labeled LDL as starting material, and 2 using nonlabeled LDL as starting material). + NO2– (filled circles), LDL modified by MPO, an H2O2-generating system (GGOx), and NO2– as described in Figure 5; – NO2– (open circles), LDL modified by MPO and an H2O2-generating system (GGOx) as described in Figure 5.
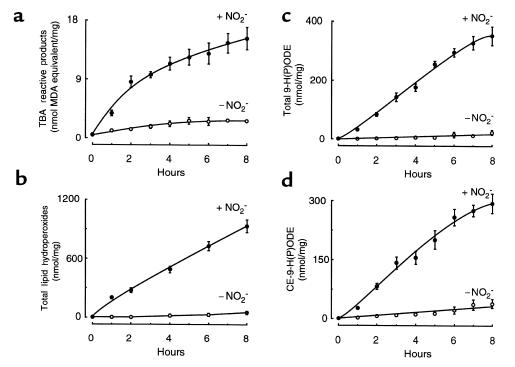
Figure 7.
Quantification of multiple lipid oxidation products formed during LDL modification by the MPO-H2O2-NO2– system. LDL samples prepared for Figure 5 were analyzed for TBA reactive products (a), total lipid hydroperoxides (b), total 9-H(P)ODE (c), and cholesteryl-9-H(P)ODE (d) as described in Methods. Data represent the mean ± SD of 3 independent experiments (1 using [125I]LDL as starting material and 2 using nonlabeled LDL as starting material). + NO2– (filled circles), LDL modified by MPO, an H2O2-generating system (GGOx), and NO2– as described in Figure 5; – NO2– (open circles), LDL modified by MPO and an H2O2-generating system (GGOx) as described in Figure 5.
To characterize LDL preparations after exposure to MPO-generated reactive nitrogen species, we first examined several markers of protein modification during the same time interval over which macrophage recognition of the nitrated lipoprotein occurred (Figure 5). Nitrotyrosine was not observed during GC/MS analysis of native LDL; however, after brief exposure to the MPO-H2O2-NO2– system, the nitrated amino acid was detected (Figure 6a). The nitrotyrosine content of NO2-LDL rose progressively with longer periods of incubation. The curvilinear nature of the plot suggests that alternative targets on LDL may be preferentially oxidized initially by the MPO-generated nitrating intermediate. One such protein target appears to be tryptophan residues, as incubation of LDL with the MPO-H2O2-NO2– system resulted in their rapid initial loss (Figure 6b). It has been demonstrated that marked increases in electrophoretic mobility and loss of Nε-amino groups of lysine are required for macrophage recognition of other modified forms of LDL, such as acetylated LDL, copper-oxidized LDL, and MDA-modified LDL (61, 62). However, after exposure to the MPO-H2O2-NO2– system, the content of free amine groups on LDL apo B-100 demonstrated only gradual progressive decreases with time (Figure 6c). After 2 hours of modification in the presence of NO2–, <10% of lysine residues were modified (Figure 6c), yet statistically significant increases in LDL uptake and degradation were detected (Figure 5) (P < 0.01 and P < 0.001 for 0 vs. 2 hours for hMDMs and MPMs, respectively). Consistent with these findings, only a modest progressive increase in the relative electrophoretic mobility (REM) of LDL was observed (Figure 6d), suggesting that only a slight increase in anionic charge of LDL occurred, despite significant macrophage recognition.
Exposure of LDL to MPO, NO2–, and an H2O2-generating system resulted in rapid initiation of LDL lipid peroxidation (Figure 7). Even at the earliest time points examined, multiple lipid peroxidation products [TBA reactive products, total lipid hydroperoxides, total 9-H(P)ODE] — including those of core lipids [cholesteryl-9-H(P)ODE] — were formed, suggesting that the oxidant generated by the MPO-H2O2-NO2– system readily promotes hydrogen atom abstraction in unsaturated lipids (Figure 7). As with the protein markers of oxidation, production of lipid oxidation products was minimal in the absence of NO2–. In a separate set of experiments, we examined α-tocopherol levels vs. time in LDL preparations exposed to the MPO-H2O2-NO2– system, as depicted in Figures 5–7. At 2 hours and 8 hours, 68 ± 6.3% and 93 ± 6.4%, respectively, was consumed (mean ± SD; n = 3 independent experiments). Thus, modification of LDL by the MPO-H2O2-NO2– system is accompanied by oxidation of multiple protein and lipid components of the lipoprotein and by significant depletion of lipid-soluble antioxidant species.
LDL modified by the MPO-H2O2-NO2– system is not aggregated.
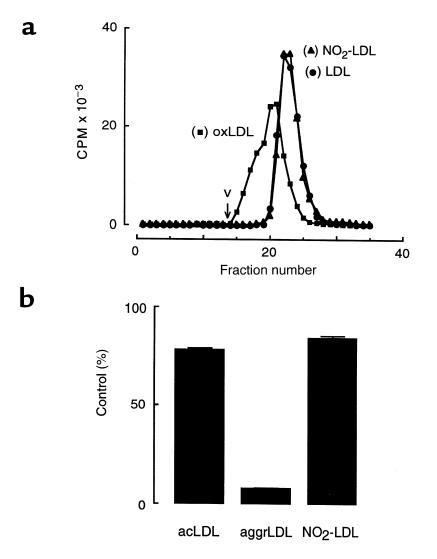
Aggregation of LDL represents one mechanism for conversion of the lipoprotein into a high-uptake form (63, 64). A number of modifications, including exposure to reagent HOCl and other types of oxidation, result in LDL aggregation (54, 57, 65, 66). Preliminary studies suggested that NO2-LDL was not aggregated, because only negligible amounts (<0.1%) of the lipoprotein failed to enter 0.5% agarose gels during electrophoresis and because no significant light scattering in NO2-LDL preparations was observed. To exclude more fully the possibility that small LDL aggregates formed during modification by the MPO-H2O2-NO2– system might contribute to the observed enhanced uptake and degradation by macrophages, additional experiments were performed. No evidence of particle aggregation in NO2-LDL preparations was observed during analysis by size exclusion chromatography (Figure 8a). The elution profile of NO2-LDL was identical to that of native LDL, whereas copper-oxidized LDL clearly demonstrated formation of particles of increased size (Figure 8a). We also examined the effect of cytochalasin D, an inhibitor of microfilament formation, on uptake and degradation of modified forms of lipoprotein by MPMs, as cytochalasin D has been effectively used as an inhibitor of phagocytosis of aggregated forms of LDL (66). Macrophage degradation of NO2-LDL was only modestly affected by cytochalasin D treatment (Figure 8b). Comparable results were seen with acetylated LDL, a prototypic ligand for scavenger receptor–mediated endocytosis (41). In contrast, degradation of vortex-aggregated LDL, which is internalized through a phagocytic mechanism (63), was inhibited by >90% by cytochalasin D treatment (Figure 8b). Collectively, these results suggest that oxidation of LDL by the MPO-H2O2-NO2– system does not promote aggregation of the lipoprotein; moreover, they also suggest that the observed enhanced uptake of NO2-LDL is not the result of phagocytosis of the modified lipoprotein.
Figure 8.
(a) Size exclusion chromatography. (b) The effect of cytochalasin D treatment on macrophage degradation of modified forms of LDL. (a) Native [125I]LDL (LDL; filled circles), copper oxidized [125I]LDL (oxLDL; filled squares), and [125I]LDL modified by the complete MPO-H2O2-NO2– system (NO2-LDL; filled triangles) were prepared and individually fractionated on a Sephacryl S400-HR column as described in Methods. The elution profiles of native and modified lipoproteins are shown. The void volume (v) of the column is indicated. (b) Acetylated [125I]LDL (acLDL), vortex-aggregated [125I]LDL (aggrLDL), and [125I]LDL modified by the complete MPO-H2O2-NO2– system (NO2-LDL) were prepared as described in Methods. The 125I-labeled lipoprotein preparations were then individually incubated (5 μg/mL) with thioglycollate-elicited MPMs at 37°C for 5 hours in the presence or absence of cytochalasin D (1 μg/mL) in media supplemented with catalase (300 nM) and BHT (20 μM). Cellular degradation of lipoprotein was subsequently determined as described in Methods. Results are expressed as the percentage of lipoprotein degradation observed in the presence vs. absence of cytochalasin D treatment. Lipoprotein degradation by non-cytochalasin D–treated macrophages (control) exposed to acLDL, aggrLDL, and NO2-LDL preparations was 3.69 ± 0.14, 1.78 ± 0.03, and 1.49 ± 0.08 μg LDL per milligram of cell protein, respectively. Data represent the mean ± SD of triplicate determinations from a representative experiment performed in duplicate.
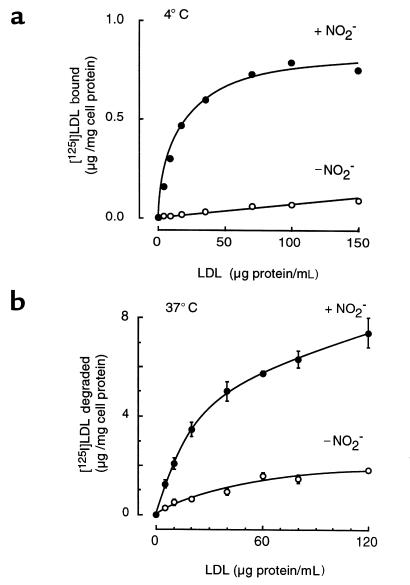
Macrophage binding of LDL modified by the MPO-H2O2-NO2– system is saturable and specific.
The lack of lipoprotein aggregation after modification by the MPO-H2O2-NO2– system, and the inability of cytochalasin D to significantly inhibit macrophage uptake and degradation of NO2-LDL, suggested that cellular recognition was likely mediated through a receptor-mediated process. To test this hypothesis, we examined whether [125I]NO2-LDL binding and degradation by MPMs were saturable and specific. Macrophage binding of LDL that was nitrated by the complete MPO-H2O2-NO2– system was saturable (Figure 9a). Under the conditions used, the half-maximal concentration for [125I]NO2-LDL binding to macrophages was ∼10 μg/mL (20 nM lipoprotein), confirming that the cells recognize NO2-LDL with high affinity. Macrophage binding of NO2-LDL was also specific because binding of LDL exposed to MPO and H2O2 in the absence of NO2– was negligible (Figure 9a). Moreover, coincubation of [125I]NO2-LDL either with a 40-fold excess of nonlabeled native LDL or with LDL modified in the presence of MPO and H2O2 without NO2– failed to significantly block (≤10% inhibition) macrophage recognition of the modified lipoprotein. In contrast, competition studies examining [125I]NO2-LDL binding, uptake, and degradation to macrophages demonstrated that excess nonlabeled NO2-LDL was an effective competitor (>70% inhibition). The concentration dependence of NO2-LDL degradation (Figure 9b) by MPMs (at 37°C) was also examined and demonstrated comparable hyperbolic plots, consistent with uptake and degradation of the modified lipoprotein through a saturable process. In contrast, cell-mediated uptake and degradation of LDL modified by MPO and H2O2 in the absence of NO2– were minimal (Figure 9b). Collectively, these results are consistent with macrophage recognition of NO2-LDL through a specific high-affinity receptor–mediated process.
Figure 9.
Concentration dependence of macrophage binding (a) and degradation (b) of LDL modified by the MPO-H2O2-NO2– system. (a) [125I]LDL was modified in the presence of MPO and an H2O2-generating system (GGOx) in either the presence (+ NO2–; filled circles) or absence (– NO2–; open circles) of NO2– in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) as described in Methods. Thioglycollate-elicited MPM binding (a) and degradation (b) of the indicated concentrations of lipoproteins were then assessed as described in Methods. Data represent the mean of duplicate determinations (binding studies) or the mean ± SD of triplicate determinations (degradation studies). Similar results were observed in 3 independent experiments.
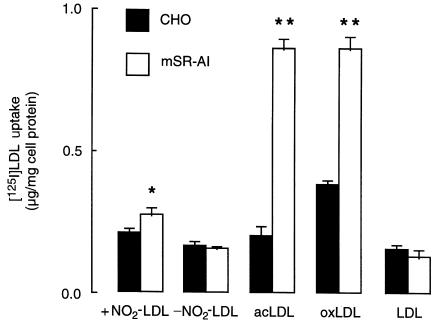
Macrophage recognition of LDL modified by the MPO-H2O2-NO2– system occurs through a pathway independent of the class A type I scavenger receptor.
Increased macrophage binding, uptake, and degradation of [125I]LDL modified by the MPO-H2O2-NO2– system, which is saturable and not inhibited by excess native LDL, suggests that the nitrated lipoprotein is being bound and internalized by a receptor that is not the LDL receptor. To characterize the nature of the macrophage receptor mediating recognition of NO2-LDL, we initially performed competition studies using known inhibitors of scavenger receptor class A. Excess polyanionic inhibitors such as polyinosinic acid, polyguanylate, dextran sulfate, and fucoidan all failed to inhibit MPM uptake of NO2-LDL by >50% (data not shown). These results suggested that scavenger receptor class A, if involved in NO2-LDL recognition, could only partially account for the recognition of this modified form of LDL. To assess further whether the classic scavenger receptor participates in the recognition of NO2-LDL, we examined the uptake and degradation of the nitrated lipoprotein using CHO cells expressing mSR-AI. CHO-mSR-AI demonstrated significant uptake and degradation of both acetylated LDL and copper-oxidized LDL when compared with control vector-transfected CHO cells, as reported previously (56). In contrast, there was only a negligible increase in the uptake and degradation of NO2-LDL by CHO-mSR-AI cells compared with control vector-transfected cells (Figure 10). Although the difference was significant (P < 0.05), expression of mSR-AI only augmented cell uptake of NO2-LDL by ∼20% over basal levels. Thus, the scavenger receptor SR-AI does not appear to play an important role in macrophage recognition of LDL modified by MPO-generated reactive nitrogen species.
Figure 10.
Uptake of 125I-labeled lipoproteins by CHO cells transfected with the murine scavenger receptor class A type I. Confluent CHO cells expressing the murine scavenger receptor class A type I (white bars) or control vector-transfected parental CHO cells that lack the LDL receptor (black bars) were incubated for 5 hours at 37°C with 5 μg/mL of the indicated 125I-labeled lipoproteins prepared as described in Methods. Cellular uptake of modified lipoproteins was then determined as described in Methods. Data represent the mean ± SD of triplicate determinations. Similar results were observed in 3 independent experiments. *P < 0.05 vs. control vector-transfected parental CHO cells; **P < 0.001 vs. control vector-transfected parental CHO cells. acLDL, acetylated LDL; LDL, native LDL; mSR-AI, CHO cells expressing the murine scavenger receptor class A type I; + NO2-LDL, LDL modified by the complete MPO-H2O2-NO2- system; – NO2-LDL, LDL modified by the complete system in the absence of NO2–; oxLDL, copper oxidized (5 hours) LDL.
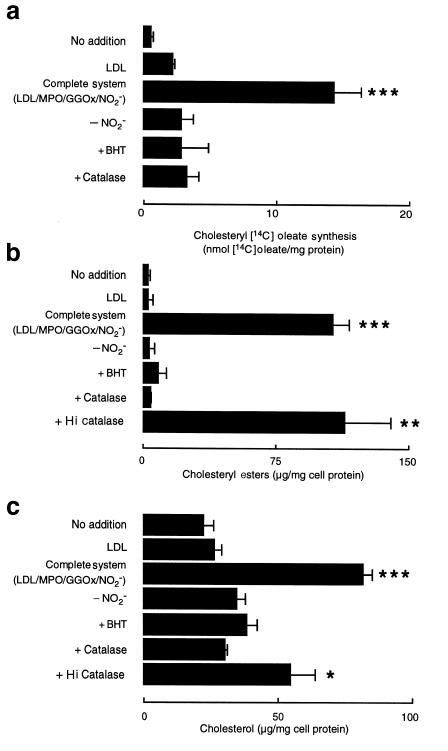
LDL modified by the MPO-H2O2-NO2– system promotes macrophage cholesteryl ester synthesis, lipid loading, and foam cell formation.
Protein nitration, induction of lipid peroxidation, and conversion of the lipoprotein into a high-uptake form are all properties consistent with conversion of LDL into a proatherogenic form. To determine whether LDL modification by MPO-generated reactive nitrogen species might contribute to cholesterol accumulation in macrophages, a critical process in lesion development, we first evaluated the ability of NO2-LDL to stimulate cholesteryl ester biosynthesis by MPMs. NO2-LDL markedly stimulated [14C]oleate incorporation into cellular cholesteryl ester pools in MPMs (Figure 11a). In contrast, additions or deletions to the complete system that prevented LDL protein nitration, lipid peroxidation, and macrophage recognition all resulted in ablation of macrophage cholesteryl ester formation from exogenous modified lipoprotein (compare Figures 4 and 11). In a parallel set of experiments, the ability of NO2-LDL to promote cellular accumulation of intracellular sterol pools was examined. Again, only LDL modified in the presence of each component of the complete MPO-H2O2-NO2- system, but not in the presence of catalase or BHT, promoted significant increases in free and esterified cholesterol content of macrophages (Figure 11).
Figure 11.
Cholesteryl oleate synthesis (a), cholesteryl ester mass (b), and cholesterol mass (c) of cells exposed to LDL modified by the MPO-H2O2-NO2– system. [125I]LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM), glucose (100 μM), glucose oxidase (20 ng/mL), and NO2– (500 μM) in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) (complete system) or the indicated additions or deletions. Lipoproteins were then incubated with thioglycollate-elicited MPMs, and the extent of cholesteryl [14C]oleate formation (a), cellular cholesteryl ester mass (b), and free cholesterol mass (c) was determined as described in Methods. Data represent the mean ± SD of triplicate determinations. Similar results were observed in 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 for comparison vs. LDL modified by MPO and an H2O2-generating system only (– NO2–). No significant cholesteryl [14C]oleate formation or increase in cholesteryl ester or free cholesterol mass above basal values was observed in LDL preparations modified by the complete system in the absence of either MPO or GGOx. Hi catalase, heat-inactivated catalase.
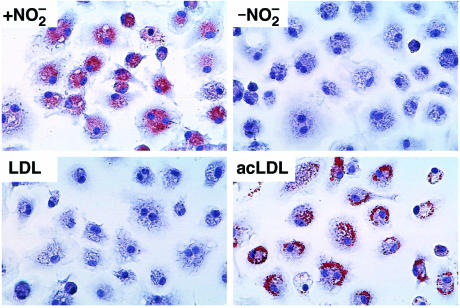
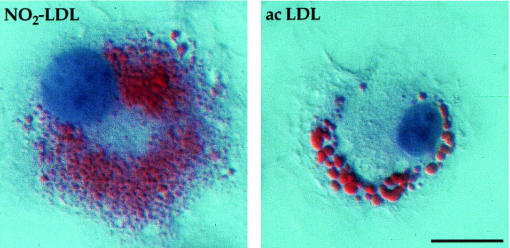
In a final set of experiments, we examined whether stimulation of cholesteryl ester biosynthesis and cellular lipid accumulation in macrophages incubated with NO2-LDL resulted in the formation of lipid-laden foam cells, an early cellular event in the atherosclerotic process. Thioglycollate-elicited MPMs were incubated for 72 hours with either native LDL (negative control), acetylated LDL (positive control), NO2-LDL, or LDL exposed to MPO and H2O2 in the absence of NO2–, and then microscopically examined after neutral lipid staining with oil red O. LDL nitrated by MPO promoted massive cholesterol deposition in macrophages (Figure 12). Moreover, the oil red O–positive staining of NO2-LDL demonstrated comparable intensity to that observed in macrophages exposed to an equivalent level of exhaustively acetylated LDL (Figure 12). The total cholesterol content (cholesteryl ester + free cholesterol) of macrophages exposed to equivalent levels of NO2-LDL and exhaustively acetylated LDL was 189 ± 13 μg/mg cell protein and 333 ± 100 μg/mg cell protein, respectively (mean ± SD; n = 3). Interestingly, the lipid droplets within the cytoplasm of the NO2-LDL–treated cells were more punctate and diffusely spread than those observed with acetylated LDL (Figure 13), suggesting that the intracellular processing of nitrated and acetylated lipoproteins differs. In contrast, cells incubated with either native LDL or with LDL exposed to MPO and H2O2 in the absence of NO2– were devoid of oil red O–positive droplets (Figure 12). Collectively, these results demonstrate that LDL modification by MPO-generated reactive nitrogen species converts the lipoprotein into a form that promotes extensive lipid loading of macrophages and foam cell formation.
Figure 12.
Macrophage foam cell formation by exposure to LDL modified by the MPO-H2O2-NO2– system. Thioglycollate-elicited MPMs were grown for 48 hours in RPMI-1640 containing 10% FBS. Cells were then incubated for 72 hours in the same media containing catalase (100 nM), BHT (20 μM), and vitamin E (20 μM) in the presence of the following lipoprotein preparations (50 μg/mL): LDL (0.2 mg/mL) previously incubated with isolated human MPO (30 nM), glucose (100 μM), and glucose oxidase (20 ng/mL) in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) at 37°C for 8 hours in the presence (+ NO2–) and absence (– NO2–) of NO2– (500 μM), native LDL (LDL), and acetylated LDL (acLDL). Media containing the appropriate modified lipoproteins were exchanged after 24-hour incubation. Cells were fixed with 4% formaldehyde and stained with hematoxylin and oil red O. Original magnification: ×300.
Figure 13.
High-power magnification view of macrophage foam cells formed by exposure to LDL modified by the MPO-H2O2-NO2– system and acetylated LDL (acLDL). Differential interference contrast microscopy of hematoxylin and oil red O–stained thioglycollate-elicited MPMs exposed to NO2-LDL and acLDL as in Figure 12. Scale bar: 10 μm.
Discussion
Reactive nitrogen species have been implicated in promoting oxidative damage of LDL and tissues in atherosclerosis (16, 17). However, neither the pathways for nitrating LDL in vivo nor the biologic consequences of these reactions are established. The results of the current study demonstrate that nitrating intermediates formed by the MPO-H2O2-NO2– system of monocytes mediate LDL protein nitration, initiate LDL lipid peroxidation, and convert the lipoprotein into a form that promotes cholesterol deposition in macrophages and foam cell formation.
Despite intense interest in the role of reactive nitrogen species in inflammatory injury and vascular disease, few studies have examined the effects of nitrating intermediates on LDL recognition by macrophages. LDL incubated with SIN-1, a compound that spontaneously decomposes to generate comparable rates of O2– and NO production, results in increased levels of lipid peroxidation products but does not generate a high-uptake form of LDL (8, 13). Studies examining the effects of modulators of NO synthesis on phorbol ester–stimulated MPMs are consistent with NO playing a protective role, as increased NO production inhibited, and decreased NO production promoted, LDL oxidation and conversion into a high-uptake form (8). LDL exposed to reagent ONOO– has been shown to promote macrophage cholesteryl ester biosynthesis (13). However, the mechanism(s) of cell recognition of ONOO–-modified lipoproteins has not yet been examined. Several observations suggest that macrophage recognition of ONOO–-modified LDL occurs through mechanisms distinct from those involved with LDL modified by the MPO-H2O2-NO2– system. Stimulation of macrophage cholesteryl oleate synthesis by ONOO–-treated LDL requires millimolar quantities of oxidant and extensive LDL modification (∼60% lysine modification, REM > 2) (13). In contrast, modification into a high-uptake form by MPO-generated nitrating intermediates requires only low levels of H2O2, results in minimal depletion of free lysine residues, and only modestly enhances lipoprotein REM (Figure 6). Moreover, modification of LDL with high levels of ONOO– results in lipoprotein aggregation, as judged by light scattering and size exclusion chromatography (D. Schmitt and S.L. Hazen, unpublished study); thus, cellular uptake mechanisms of ONOO–-modified LDL may be via phagocytosis of aggregates. Similarly, LDL aggregation (and hence, phagocytosis by macrophages) has been demonstrated after exposure of LDL to an atmosphere containing NO2 gas (67), vortexed mixtures of HOCl and NO2– (58), and millimolar levels of NO2– for prolonged periods (68).
In contrast, multiple lines of evidence suggest that macrophage uptake of LDL modified by MPO, H2O2, and NO2– occurs via a pathway involving receptor-mediated endocytosis rather than phagocytosis. Binding of NO2-LDL to macrophages occurred through a high-affinity, saturable, and specific interaction — characteristics of a receptor-mediated process. Moreover, uptake and degradation of the modified lipoprotein were saturable, unlike bulk-phase phagocytosis of lipoprotein aggregates (63, 64). Competition studies examining [125I]NO2-LDL binding, uptake, and degradation by macrophages further confirm the specificity of the interaction because excess nonlabeled NO2-LDL was an effective competitor, but native LDL and LDL modified in the presence of MPO and H2O2 without NO2– were not. Finally, lipoprotein aggregation during oxidative modification by the MPO-H2O2-NO2– system was excluded by a variety of methods including size exclusion chromatography, light scattering, gel electrophoresis, and cytochalasin D treatment of macrophages.
The distinction between macrophage uptake and degradation of an aggregated lipoprotein compared with a monomeric/soluble oxidized lipoprotein also underscores the unexpected findings observed when examining the potential role of MPO-generated chlorinating and nitrating intermediates in conversion of LDL into a high-uptake form. Previous studies reported by Hazell et al. (57) noted that LDL treatment with reagent HOCl becomes aggregated and, like other aggregated forms of lipoproteins, promotes intracellular lipid accumulation in macrophages (54). However, in the present study, LDL incubated with either activated human monocytes in Cl–-containing media (Figure 1) or an HOCl-generating system (MPO, GGOx, and Cl–; Figure 2) was not converted into a stable high-uptake form. These results suggest that direct addition of reagent HOCl and in situ formation of HOCl by the MPO-H2O2-Cl– system of phagocytes modify LDL in different ways. A similar conclusion was reached in a recent mass spectrometric study examining peptide oxidation products in tryptic digests of apo B-100 from LDL exposed to either reagent HOCl or the MPO-H2O2-Cl– system (69). Such a difference might be observed if MPO binds directly to LDL and locally delivers oxidant to select sites on the lipoprotein particle. Alternatively, certain targets for oxidation on LDL may only be modified in the presence of high local concentrations of oxidants produced during bolus addition in vitro, but that otherwise might not be observed under more physiological conditions. Indeed, under the conditions of a low steady flux of H2O2 used in the present study, no significant LDL aggregation was observed during exposure of the lipoprotein to the MPO-H2O2-Cl– system. In contrast, LDL aggregation and conversion into a particle ingested by phagocytosis was observed after addition of reagent HOCl or after bolus addition of H2O2 to LDL incubated in the presence of MPO and Cl– (E. Podrez et al., unpublished study). The results of the present study do not rule out formation of a labile oxidation product from the MPO-H2O2-Cl– system (e.g., N-monochloramines) that is subsequently repaired (57) during incubations with cells. Whether such a labile modified form of LDL is a ligand for macrophages, and might thus play a role in LDL uptake in vivo in circumstances in which repair processes might become depleted, cannot be excluded. Taken together, the results of the present study suggest that under conditions in which only a low flux of H2O2 is formed, MPO-generated nitrating intermediates are more effective than chlorinating intermediates in promoting oxidative conversion of LDL into a stable high-uptake form.
Because of the high concentrations of Cl– in biologic matrices, reactive chlorinating species have routinely been thought to be the predominant oxidant formed by the MPO-H2O2 system of phagocytes in vivo (21–24). However, the results of the present study clearly demonstrate that MPO (either isolated or derived from activated monocytes) utilizes NO2– to convert LDL into a form that binds to, and is taken up and degraded by, macrophages, even in the presence of plasma levels of Cl–. Furthermore, these results also demonstrate that HOCl formation is not obligatory for MPO- and NO2–-dependent conversion of LDL into a high-uptake form. Consequently, many of the experiments described in the present study were performed with no Cl– present during the oxidation reaction because from a mechanistic standpoint, it is then possible to demonstrate that both the alterations in the biologic end points examined (e.g., binding, cell association, uptake, degradation, sterol accumulation) and the oxidation products formed (e.g., nitrotyrosine, various lipid peroxidation products) are the result of an oxidant species derived from MPO-dependent oxidation of NO2–. Although the nitrating intermediate(s) formed by the reaction of NO2– with the MPO-H2O2 system has not been identified, NO2 (the one electron oxidation product of NO2–) and NO2Cl (formed by reaction of HOCl with NO2–) have been implicated (25, 26, 33). Thus, under physiological conditions in which both Cl– and NO2– are present, a variety of halogenating and nitrating intermediates may be formed by MPO (24, 25, 30, 33, 52). The product(s) of the MPO-H2O2-NO2– system is anticipated to be particularly effective at converting LDL into a high-uptake form for intimal macrophages.
Despite evidence suggesting that a receptor-mediated pathway is involved in macrophage recognition of NO2-LDL, the identity of the receptor and ligand mediating the interaction is unknown. Experiments using CHO cells expressing mSR-AI demonstrated no difference in NO2-LDL binding, uptake, or degradation when compared with control vector-transfected CHO cells, strongly suggesting that class A type I scavenger receptor does not participate in recognition of the nitrated lipoprotein. This lack of recognition is consistent with the relatively low level of apo B-100 lysine blockage and REM observed (Figure 6) compared with that required for recognition of other modified forms of LDL (61, 62). Future studies identifying the nature of the ligand(s) and receptor(s) mediating macrophage recognition of NO2-LDL are warranted.
The ability of modified forms of LDL to promote accumulation of cellular cholesteryl esters has been widely accepted as an index of the atherogenic potential of a lipoprotein. While our studies show that incubation of LDL with activated monocytes in the presence NO2– transforms the lipoprotein into a high-uptake form, the biologic significance of this type of modification in vivo is not yet established. All of the in vitro cell experiments in the present study were performed under serum-free conditions. Whether or not the MPO-H2O2-NO2– system of monocytes effectively modifies LDL in a more physiological, serum/plasma- containing milieu remains to be explored. However, considerable evidence suggests that MPO may contribute to nitration of LDL in human atheroma because all of the components of the MPO-H2O2-NO2– system are present and available for lipoprotein and tissue oxidative damage in human lesions, and products of this pathway — e.g., nitrotyrosine (16, 17), dityrosine (70), and lipid hydroperoxides (43, 71) — are enriched in human lesions. Indeed, immunohistochemical staining demonstrates that MPO colocalizes with lipid-laden macrophages (28), and our recent mass spectrometric studies demonstrate that chlorotyrosine, a specific marker for MPO-dependent protein damage by chlorinating intermediates (24, 72, 73), is significantly enriched in LDL recovered from human aortic lesions and atherosclerotic intima (29). Thus, catalytically active MPO and sufficient levels of H2O2 to generate reactive oxidants with the enzyme are present in human vascular tissues. Moreover, the low rate of H2O2 flux used for lipoprotein modification in this study is less than that generated by circulating levels of leukocytes activated in suspension (74) or on biologic surfaces (75). Furthermore, although the actual concentration of NO2– in arterial intima is not known, the concentration of NO2– required for MPO-dependent conversion of LDL into a high-uptake form is well within the range of that observed in other inflammatory fluids and tissues (35–37). Finally, the levels of nitrotyrosine observed in apo B–containing LDL-like particles recovered from human atherosclerotic lesions (17) are several-fold greater than those observed in NO2-LDL preparations, which are avidly taken up and degraded by macrophages. Thus, MPO-generated nitrating intermediates may represent a physiologically plausible mechanism for promoting protein nitration, lipid peroxidation, and cholesterol deposition in macrophage-rich human atherosclerotic tissues.
Acknowledgments
We thank D.M. Driscoll and G.M. Chisolm for thoughtful comments and criticisms, H.L. Copeland for advice on statistical analyses, and P.A. Cohen and M.K. Cathcart for generously providing human monocytes used for the preparation of hMDMs in this study. We also thank Monty Krieger for generously providing CHO cells transfected with mSRA-1. This work was supported in part by a Scientist Development Grant from the American Heart Association and by National Institutes of Health grants HL-61878 and HL-62526 (to S.L. Hazen) and HL-53315 (to H.F. Hoff).
References
- 1.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinbrecher UP, Zhang HF, Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9:155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- 3.Chisolm, G.M., and Penn, M.S. 1998. Oxidized lipoproteins and atherosclerosis. In Atherosclerosis and coronary artery disease. V. Fuster, R. Ross, and E. J. Topol, editors. Lippincott-Raven Publishers. Philadelphia, PA. 129–149.
- 4.Berliner JA, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg D. Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 6.Heinecke JW. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 1998;141:1–15. doi: 10.1016/s0021-9150(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 8.Jessup W, Mohr D, Gieseg SP, Dean RT, Stocker R. The participation of nitric oxide in cell free- and its restriction of macrophage-mediated oxidation of low-density lipoprotein. Biochem Biophys Acta. 1992;1180:73–82. doi: 10.1016/0925-4439(92)90029-m. [DOI] [PubMed] [Google Scholar]
- 9.van der Vliet A, Eiserich JP, Kaur H, Cross CE, Halliwell B. Nitrotyrosine as biomarker for reactive nitrogen species. Methods Enzymol. 1996;269:175–184. doi: 10.1016/s0076-6879(96)69019-3. [DOI] [PubMed] [Google Scholar]
- 10.Lymar SV, Jiang Q, Hurst JK. Mechanism of carbon dioxide–catalyzed oxidation of tyrosine by peroxynitrite. Biochemistry. 1996;35:7855–7861. doi: 10.1021/bi960331h. [DOI] [PubMed] [Google Scholar]
- 11.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham A, et al. Peroxynitrite modification of low-density lipoprotein leads to recognition by the macrophage scavenger receptor. FEBS Lett. 1993;330:181–185. doi: 10.1016/0014-5793(93)80269-z. [DOI] [PubMed] [Google Scholar]
- 14.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- 16.Beckman JS, et al. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1993;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 17.Leeuwenburgh C, et al. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem. 1997;272:1433–1436. doi: 10.1074/jbc.272.3.1433. [DOI] [PubMed] [Google Scholar]
- 18.Christen S, et al. Gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci USA. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogg N, Darley-Usmar VM, Wilson MT, Moncada S. The oxidation of alpha-tocopherol in human low-density lipoprotein by the simultaneous generation of superoxide and nitric oxide. FEBS Lett. 1993;326:199–203. doi: 10.1016/0014-5793(93)81790-7. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer S, Mayer B. Lack of tyrosine nitration by peroxynitrite generated at physiological pH. J Biol Chem. 1998;273:27280–27285. doi: 10.1074/jbc.273.42.27280. [DOI] [PubMed] [Google Scholar]
- 21.Klebanoff, S.J., and Clark, R.A. 1978. The neutrophil: function and clinical disorders. Elsevier/North Holland Biomedical Press. Amsterdam, the Netherlands. 447–451.
- 22.Agner, K. 1972. Structure and function of oxidation-reduction enzymes. A. Akeson and A. Ehrenberg, editors. Pergamon Press. Tarrytown, NY. 329–335.
- 23.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 24.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Vleit A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 26.Eiserich JP, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 27.Krinsky NI. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974;186:363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- 28.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazell LJ, et al. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klebanoff SJ. Reactive nitrogen intermediates and antimicrobial activity: role of nitrite. Free Radic Biol Med. 1993;14:351–360. doi: 10.1016/0891-5849(93)90084-8. [DOI] [PubMed] [Google Scholar]
- 33.Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vleit A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid: a novel mechanism for nitric oxide-mediated protein modification. J Biol Chem. 1996;271:19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- 34.Sampson JB, Ye Y, Rosen H, Beckman JS. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch Biochem Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- 35.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51:1219–1222. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torre D, et al. Serum concentrations of nitrite in patients with HIV-1 infection. J Clin Pathol. 1996;49:574–576. doi: 10.1136/jcp.49.7.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaston B, et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci USA. 1993;90:10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Q, Hurst JK. Relative chlorinating, nitrating and oxidizing capabilities of neutrophil determined with phagocytosable probes. J Biol Chem. 1997;272:32767–32772. doi: 10.1074/jbc.272.52.32767. [DOI] [PubMed] [Google Scholar]
- 39.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 40.Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H2O2 solutions in the UV) Anal Biochem. 1972;49:474–478. doi: 10.1016/0003-2697(72)90451-4. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoppe G, O’Neil J, Hoff HF. Inactivation of lysosomal proteases by oxidized low density lipoprotein is partially responsible for its poor degradation by mouse peritoneal macrophages. J Clin Invest. 1994;94:1506–1512. doi: 10.1172/JCI117490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suarna C, Dean RT, May J, Stocker R. Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of alpha-tocopherol and ascorbate. Arterioscler Thromb Vasc Biol. 1995;15:1616–1624. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- 44.Rakita RM, Michel BR, Rosen H. Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry. 1990;29:1075–1080. doi: 10.1021/bi00456a033. [DOI] [PubMed] [Google Scholar]
- 45.Wever R, Plat H, Hamers MN. Human eosinophil peroxidase: a novel isolation procedure, spectral properties and chlorinating activity. FEBS Lett. 1981;123:327–331. doi: 10.1016/0014-5793(81)80320-1. [DOI] [PubMed] [Google Scholar]
- 46.van Dalen CJ, Whitehouse MW, Winterbourn CC, Kettle AJ. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem J. 1997;327:487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatch FT. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- 48.Czerniecki BJ, et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J Immunol. 1997;159:3823–3837. [PubMed] [Google Scholar]
- 49.Savenkova ML, Mueller DM, Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994;269:20394–20400. [PubMed] [Google Scholar]
- 50.el-Saadani M, et al. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res. 1989;30:627–630. [PubMed] [Google Scholar]
- 51.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 52.Hazen SL, Hsu FF, Gaut JP, Crowley JR, Heinecke JW. Modification of proteins and lipids by myeloperoxidase-derived oxidants. Methods Enzymol. 1999;300:88–105. doi: 10.1016/s0076-6879(99)00117-2. [DOI] [PubMed] [Google Scholar]
- 53.Crowley JR, Yarasheski K, Leeuwenburgh C, Turk J, Heinecke JW. Isotope dilution mass spectrometric quantification of 3-nitrotyrosine in proteins and tissues is facilitated by reduction to 3-aminotyrosine. Anal Biochem. 1998;259:127–135. doi: 10.1006/abio.1998.2635. [DOI] [PubMed] [Google Scholar]
- 54.Hazell LJ, Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J. 1993;290:165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen SH, et al. The complete cDNA and amino acid sequence of human apolipoprotein B-100. J Biol Chem. 1986;261:12918–12921. [PubMed] [Google Scholar]
- 56.Ashkenas J, et al. Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J Lipid Res. 1993;34:983–1000. [PubMed] [Google Scholar]
- 57.Hazell LJ, van den Berg JJ, Stocker R. Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation. Biochem J. 1994;302:297–304. doi: 10.1042/bj3020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panasenko OM, Briviba K, Klotz LO, Sies H. Oxidative modification and nitration of human low-density lipoproteins by the reaction of hypochlorous acid with nitrite. Arch Biochem Biophys. 1997;343:254–259. doi: 10.1006/abbi.1997.0171. [DOI] [PubMed] [Google Scholar]
- 59.Pryor WA, Lightsey JW. Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous acid. Science. 1981;214:435–437. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- 60.Thomas HV, Mueller PK, Lyman RL. Lipoperoxidation of lung lipids in rats exposed to nitrogen dioxide. Science. 1968;159:532–534. doi: 10.1126/science.159.3814.532. [DOI] [PubMed] [Google Scholar]
- 61.Steinbrecher UP, Witztum JL, Parthasarathy S, Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis. 1987;7:135–143. doi: 10.1161/01.atv.7.2.135. [DOI] [PubMed] [Google Scholar]
- 62.Haberland ME, Olch CL, Folgelman AM. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984;259:11305–11311. [PubMed] [Google Scholar]
- 63.Khoo JC, Miller E, McLoughlin P, Steinberg D. Enhanced macrophage uptake of low density lipoprotein after self-aggregation. Arteriosclerosis. 1988;8:348–358. doi: 10.1161/01.atv.8.4.348. [DOI] [PubMed] [Google Scholar]
- 64.Suits AG, Chait A, Aviram M, Heinecke JW. Phagocytosis of aggregated lipoprotein by macrophages: low density lipoprotein receptor–dependent foam-cell formation. Proc Natl Acad Sci USA. 1989;86:2713–2717. doi: 10.1073/pnas.86.8.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schissel SL, et al. Rabbit aorta and human atherosclerotic lesions hydrolyze sphingomyelin of retained low-density lipoprotein: proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest. 1996;98:1455–1464. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoff HF, Whitaker TE, O’Neil J. Oxidation of low density lipoprotein leads to particle aggregation and altered macrophage recognition. J Biol Chem. 1992;267:602–609. [PubMed] [Google Scholar]
- 67.Kikugawa K, Beppu M, Okamoto Y. Uptake by macrophages of low-density lipoprotein damaged by nitrogen dioxide in air. Lipids. 1995;30:313–320. doi: 10.1007/BF02536038. [DOI] [PubMed] [Google Scholar]
- 68.Chang GJ, et al. Oxidation of LDL to a biologically active form by derivatives of nitric oxide and nitrite in the absence of superoxide. Dependence on pH and oxygen. Arterioscler Thromb. 1994;14:1808–1814. doi: 10.1161/01.atv.14.11.1808. [DOI] [PubMed] [Google Scholar]
- 69.Cy Y, et al. Selective modification of apo B-100 in the oxidation of low density lipoproteins by myeloperoxidase in vitro. J Lipid Res. 1999;40:686–698. [PubMed] [Google Scholar]
- 70.Leeuwenburgh C, et al. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem. 1997;272:3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 71.Folcik VA, Aristy-Nivar RA, Krajewski LP, Cathcart MK. Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. J Clin Invest. 1995;96:504–510. doi: 10.1172/JCI118062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kettle AJ. Neutrophils convert tyrosyl residues in albumin to chlorotyrosine. FEBS Lett. 1996;379:103–106. doi: 10.1016/0014-5793(95)01494-2. [DOI] [PubMed] [Google Scholar]
- 73.Hazen SL, Crowley JR, Mueller DM, Heinecke JW. Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: a sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation. Free Radic Biol Med. 1997;23:909–916. doi: 10.1016/s0891-5849(97)00084-1. [DOI] [PubMed] [Google Scholar]
- 74.Weiss SJ, Lampert MB, Test ST. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science. 1983;222:625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]
- 75.Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]