Abstract
Tumor lysis syndrome (TLS) is a potentially deadly complication of tumors or their treatment. This syndrome consists of a constellation of laboratory findings such as hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, known as laboratory TLS. When clinical complications such as seizures, acute renal failure, and cardiac dysrhythmias occur in patients with laboratory TLS, the syndrome is called clinical TLS. TLS is especially common in patients with hematological malignancies with rapid cellular turnover rates such as acute lymphocytic leukemia and Burkitt lymphoma, but is very rare in patients with solid tumors. Nevertheless, there are multiple reports in the literature on the occurrence of TLS in patients with solid tumors. In this review article, we summarize the current data on the occurrence of TLS in patients with solid tumors. We propose an algorithm of risk stratification and prevention of TLS in patients with solid cancers.
Key words: tumor lysis syndrome, solid cancers
Introduction
Malignant neoplasms comprise a heterogeneous group of disorders in which aberrant or erratic cellular proliferation leads to profound derangements in organism well-being. Malignant tumors vary in pathophysiology and genetics, clinical presentation, diagnosis, treatment and prognosis. More importantly, cancer was found to be the second leading cause of death among United States residents in 2011 by Centers for Disease Control and Prevention.1 Therefore, given the high prevalence of malignant neoplasms it is essential that health care providers are aware of the major complications of cancer itself and/or its treatment.
Tumor lysis syndrome (TLS) is one of the major oncological emergencies. TLS comprises a clinico-laboratory derangement of cellular metabolism which can lead to acute renal impairment, cardiac rhythm disturbances, seizures and death.2 Cellular death mediated by cancer therapy (chemotherapy or other pharmacological antitumor intervention or radiation therapy) or spontaneous cellular death in rapidly dividing tumors (which is known as spontaneous TLS) leads to efflux of cellular material that is rich in potassium, phosphorus, and uric acid. On the other hand, serum calcium is typically decreased in patients with TLS because of binding to phosphorus. This in turn can lead to acute impairment of renal function, cardiac arrhythmogenicity, central nervous system toxicity, and eventually death. The most widely used diagnostic criteria were proposed by Cairo and Bishop in 2004.2 According to their classification, TLS can be defined as laboratory TLS, when TLS is clinically silent, as well as clinical TLS, when laboratory evidence of TLS is complicated by clinical manifestations such as arrhythmias, renal insult, seizures and ultimately death. The diagnostic criteria proposed by Cairo and Bishop are presented in Tables 1 and 2. It is important to mention that laboratory TLS is defined as the presence of at least two or more biochemical variables within 3 days before chemotherapy or 7 days after chemotherapy in the face of adequate hydration and use of uric acid lowering agent. Clinical TLS is defined as the presence of at least one clinical criterion that is not believed to be attributable to chemotherapy agent.2 However, this definition is not perfect since radiation therapy may lead to TLS and TLS can occur spontaneously in rapidly proliferating and bulky malignancies.
Table 1.
Cairo-Bishop definition of laboratory tumor lysis syndrome for adults.
| Variable | Value | Change from baseline value |
|---|---|---|
| Uric acid | ≥8 mg/dL (476 mmol/L) | 25% increase |
| Potassium | ≥6.0 mEq/L (or 6 mmol/L) | 25% increase |
| Phosphorus | ≥4.5 mg/dL (1.45 mmol/L) for adults and >2.1 mmol/L (6.5 mg/dL) for children | 25% increase |
Table 2.
Cairo-Bishop grading of clinical tumor lysis syndrome for adults.
| Variable | Grade 0 | Grade I | Grade II | Grade III | Grade IV | Grade V |
|---|---|---|---|---|---|---|
| Creatinine | None | 1.5 times ULN | >1.5-3.0 times ULN | >3.0-6.0 times ULN | >6.0 times ULN | Death |
| Cardiac arrhythmia | None | Intervention not indicated | Non-urgent medical intervention indicated | Symptomatic and incompletely controlled medically or controlled with device (e.g., defibrillator) | Life-threatening (e.g., arrhythmia associated with heart failure, hypotension, syncope, shock) | Death |
| Seizures | None | - | One brief, generalized seizure; seizure(s) well controlled by anticonvulsants or focal motor seizures not interfering with ADL | Seizure in which consciousness is altered; poorly controlled seizure disorder; with breakthrough generalized seizures despite medical intervention | Seizure of any kind which are prolonged, repetitive or difficult to control (e.g., status epilepticus, intractable epilepsy) | Death |
ULN, upper limits of normal; ADL, activities of daily living.
Certain tumor and patient characteristics can be used to predict the risk of future TLS. Examples, of predisposing factors include highly proliferating and bulky malignancies, sensitivity to chemotherapy, baseline renal dysfunction (like the presence of diabetes mellitus, hypertension, congestive heart failure etc.), exposure to nephrotoxic substances (like non-steroidal anti-inflammatory agents, certain antihypertensive medications, etc.), decreased oral intake and baseline elevations in uric acid and phosphorus.2,3
It is well known that rapidly proliferating hematological malignancies comprise the vast majority of TLS cases. For example, tumors such as Burkitt leukemia, advanced Burkitt lymphoma, advanced diffuse large B cell lymphoma, acute myelogenous leukemia and acute lymphocytic leukemia carry a high risk for the development of TLS.2-4 The common medical principle that prevention is always better than treatment is also relevant in TLS. Stratifying cancer patients based on the risk of TLS is essential in TLS prevention. Vigorous hydration and use of uric acid lowering agent are the mainstay of management. Patients at low risk for TLS can be managed with allopurinol, but patients at high risk for TLS should be managed with rasburicase (after excluding deficiency glucose-6 phosphate dehydrogenase since this medication can lead to severe hemolysis in such patients). Patients at intermediate risk for TLS can be managed with either allopurinol or rasburicase. Patients with clinically established TLS should be managed with aggressive hydration, rasburicase, and hemodialysis in advanced cases.2-6 Comprehensive discussion of TLS pathophysiology, clinical presentation and management is outside the scope of this manuscript. The interested reader is referred to well written review articles on this topic.2-6
As noted above hematological malignancies comprise the vast majority of TLS which is believed to be secondary to sensitivity to treatment and rapid proliferative rates. Nevertheless, TLS can occur in patients with solid cancers as a result of therapy or even spontaneously. We will review the current literature on TLS in patients with solid tumors. The search strategy is presented below. First, we will review reported cases of TLS in patients with pulmonary malignancies. Second, TLS in patients with malignant breast lesions will be discussed. Third, the literature on TLS in patients with gynecological cancers will be reviewed. Fourth, we will review the data on TLS in patients with gastrointestinal cancers. Fifth, we will discuss the literature on TLS in patients with neurological malignancies. Sixth, cases of TLS in patients with sarcomas will be discussed. In a final section we will review the literature on miscellaneous solid tumors.
Search strategy
We searched PubMed/Medline, Scopus, Embase and Web of Science for articles focused on TLS in patients with solid tumors published from 1950 to February 2014. The search terms were: tumor lysis syndrome, tumor lysis in solid malignancies, tumor lysis syndrome in solid cancers, tumor lysis syndrome in solid tumors, tumor lysis syndrome in organ specific malignancies (e.g. lung cancer, breast cancer etc.) and their combination. The reference lists of the identified articles were further screened for potentially relevant articles that could be overlooked by an electronic search. The search methodology was adapted from the scientific search guidelines published in 2011.7
Tumor lysis syndrome in lung cancers
Lung cancer is among the most common cancers worldwide. More specifically, according to the American Cancer Society lung cancer holds the second place in terms of prevalence in both sexes and first place in terms of mortality.8 It is estimated that 116,000 new cases of lung cancer will be diagnosed in the USA and there will be 108,210 deaths due to lung cancer in 2014.8 Histological diagnosis is of essential importance, since treatment and prognosis vary tremendously.9 In particular, lung carcinomas comprise the vast majority of cases and are typically divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC).9 NSCLC is further subdivided into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Among carcinomas, adenocarcinoma has the highest prevalence, followed by squamous cell carcinoma, SCLC, and large cell carcinoma, respectively.9 Other types of lung cancer have a much lower prevalence and include neuroendocrine tumors other than SCLC (such as carcinoid tumor), mesothelioma, sarcomatoid lung carcinoma, pulmonary lymphoma and others.9 Issues pertinent to disease manifestations, diagnosis and treatment are beyond the scope of this manuscript and can be easily found elsewhere. We will discuss the occurrence of TLS in patients with NSCLC and SCLC in separate sections.
Tumor lysis syndrome in non-small cell lung cancer
Eight cases were found in the literature search described above.10-17 The age of the patients ranged from 38 to 74 years with the mean age being 58.6 years. Seven of the reported patients were males. All of the reported patients had some form of smoking history: one patient had a history of pipe smoking,11 while others had a history of cigarette smoking. It is important to note that all of the reported patients had evidence of metastatic disease. Five patients had adenocarcinoma,10,11,13,14,17 two patients had squamous cell carcinoma,15,16 and one patient had evidence of mixed NSCLC and SCLC.12 Five patients developed TLS secondary to chemotherapy.10,12-14,17 It is interesting to note that one case report attributed TLS to zoledronic acid, which was used to manage hypercalcemia of malignancy.13 The authors attributed this association to possible antitumor properties of zoledronic acid.13 Two patients developed spontaneous TLS (adenocarcinoma and squamous cell carcinoma respectively),11,16 and one patient developed TLS secondary to radiation therapy (squamous cell carcinoma).15
It was noted that some patients had pretreatment elevation of lactate dehydrogenase (LDH).10,11 However, other reports did not provide data on baseline LDH and other laboratory parameters. One case report had baseline pretreatment elevation of potassium and uric acid.17 However, potassium elevation in that case was mild (5.5 mEq/L) and did not meet the diagnostic criterion for TLS.
Nevertheless, it is reasonable to conclude that patients with NSCLC at high risk for TLS should have evidence of advanced metastatic disease. Most of the reports had evidence of liver involvement. However, whether this has something to do with TLS pathogenesis or the liver is simply a common site for metastatic spread is unclear. LDH as well as other laboratory variables such as potassium, uric acid, and phosphorus may be of use in assessing the risk of TLS in patients with advanced lung cancer. In conclusion, it is reasonable to consider the possibility of impending TLS in patients with advanced metastatic NSCLC and the elevated laboratory variables mentioned above. Whether TLS prevention in such cases will be beneficial is unknown and should be decided on a case by case basis. A summary of TLS cases in patients with NSCLC is presented in Supplementary Table 1.
Tumor lysis syndrome in patients with small cell lung cancer
Thirteen cases of TLS were found in the literature search described above.18-30 One case was in the form of an abstract.23 In addition, one case of mixed SCLC and NSCLC has already been described above.12 It is important to keep in mind that SCLC lies behind adenocarcinoma and squamous cell carcinoma in terms of prevalence, but has a higher number of reported cases of TLS. This can be attributed to random reporting of cases (some cases of NSCLC might not be reported) or it can be explained by the fact that SCLC is known to be a chemosensitive malignancy, with resultant massive cell death after chemotherapy commencement.8
The age of the patients ranged from 52 to 78 years with the mean age being 67.3 years, which is similar to the mean age for SCLC.9 Thus, patients with TLS secondary to SCLC tend to be older than patients with NSCLC (please see above). Seven of the reported cases were females.18,22,26-30 Most of the patients had evidence of metastatic disease, except one case of a 70 year old female who had limited stage SCLC.30 Most of the cases were attributable to chemotherapy, except two cases of spontaneous TLS.28,29 Padhi and Singh incorrectly mentioned that their case was the first reported case of spontaneous TLS among patients with SCLC.29 Spontaneous TLS was first officially reported by a group from our institution in 2011.28 It is important to mention that most of the patients with SCLC who developed TLS had baseline elevation of LDH and other biochemical variables such as uric acid, potassium, creatinine and phosphorus.
Therefore, it is reasonable to consider the possibility of TLS in patients with SCLC (particularly metastatic). Clinicians might consider assessing baseline levels of LDH, uric acid and phosphorus in addition to common biochemical laboratory tests to stratify the risk of TLS. Clinicians should assess each case individually and if deemed necessary administer prophylactic treatment against TLS. A summary of TLS cases in patients with SCLC is presented in Supplementary Table 2.
Tumor lysis syndrome in breast cancers
Breast cancer is the most common cancer in women and the second leading cancer related cause of death among females.8 Various histological types of breast adenocarcinoma exist.31 Despite its rarity it is important to remember that breast cancer can occur in men.32 A detailed discussion of breast cancer is outside the scope of this manuscript and can be easily found elsewhere. Below we will review the scientific literature on TLS in patients with breast cancer.
Twelve published reports consisting of 13 cases of TLS in patients with breast cancer were identified using the search methodology described above.27,33-43 All patients had metastatic breast adenocarcinomas. One case of TLS was associated with prior radiation therapy and treatment with hydrocortisone,39 while the others were clearly associated with chemotherapy or hormonal therapy. However, one patient had evidence of laboratory TLS prior to initiation of chemotherapy.37
The age of the patients ranged from 31 years to 94 years with the mean age being 54.1 years.
Most of the patients had baseline elevation in LDH and some had baseline increase of uric acid. A summary of reported cases of TLS in patients with breast cancer is presented in Supplementary Table 3.
Tumor lysis syndrome in gynecological cancers
Gynecological cancers can be divided into vulvar and vaginal cancers, cervical cancer, uterine cancer, gestational trophoblastic neoplasia, ovarian cancer, fallopian and peritoneal carcinomas.44 A more detailed discussion on clinical aspects pertaining to gynecological cancers is outside the scope of this manuscript and can be found elsewhere.
Ten published reports of TLS cases in patients with gynecological cancer were identified using the search methodology described above.45-55 Six cases of ovarian cancer complicated with TLS were found.45-50 However, one case was excluded because it was found to be ovarian Burkitt lymphoma,49 which is known to pose a very high intrinsic risk for TLS.4 All the included ovarian cancer represented epithelial adenocarcinomas. The age at diagnosis varied from 47 years to 63 years, with the mean age being 56.25 years. All of the cases of TLS were associated with chemotherapy. TLS developed as early as 2 days 46 to as long as 2 weeks after initiation of chemotherapy.46 Two published reports did not have baseline laboratory values,46,49 one case had baseline mild elevation of LDH,45 and baseline laboratory values in one case were within normal limits.47
Two cases of vulvar carcinoma were found.50,51 Two cases of uterine cancer were identified with one being recurrent endometrial adenocarcinoma and the second being uterine leiomyosarcoma.53 We decided to include uterine leiomyosarcoma in this section because of its location. It is important to note that most reports did not include baseline laboratory parameters for their patient. Some reports did mention elevation of baseline creatinine and uric acid were elevated,54 as well as baseline phosphorus 52 and LDH.45,54 A case of uterine leiomyosarcoma was complicated by the presence of obstructive uropathy, which might increase the risk for TLS.53 One case of gestational trophoblastic neoplasia was noted.54 All of the TLS cases were chemotherapy induced. Baseline elevation of LDH and likely potassium, phosphorus, creatinine, and uric acid is suggestive of increased risk for TLS. It is important to consider preventive measures such as hydration and urate lowering therapy in patients with advanced gynecological cancers. A summary of reported cases of TLS in patients with gynecological cancers is presented in Supplementary Table 4.
Tumor lysis syndrome in genitourinary and urological cancers
This section includes cancers originating from the genitourinary system including testicular tumors in men. For greater coverage on general topics related to these cancers, the reader is referred to a good review source.44
Thirteen published manuscripts were identified using the search methodology described above.35,56-69 One report described two patients and another three patients,62,63 making the total number of patients sixteen. Among those found, there were five cases of prostate adenocarcinoma.56-60 The age of the affected patients ranged from 60 to 80 years old with the mean age being 66.1 years. All of patients with the prostate cancer had evidence of metastatic disease and four of them had hormone refractory cancer.57-59 One published case did not include baseline laboratory values,59 whereas the others reported baseline elevated levels of LDH,56,57,60 uric acid,56-58 and creatinine.58,60 Four of the TLS cases in patients with prostate cancer occurred after the administration either a chemotherapy or hormonal therapy. One patient developed TLS after the initiation of radiation therapy for bone metastasis.60
TLS in testicular cancers and extratesticular cancers are reviewed together. We identified five published reports,35,61-64 with one describing three patients and another two patients,62,63 making the total number of patients eight. There were five cases of seminoma,35,61-63 with one being of extratesticular origin.61 All but one of the seminoma cases were metastatic.35 The other tumors reported comprised metastatic extratesticular choriocarcinoma,61 metastatic testicular germ cell tumor and metastatic testicular non seminomatous tumor.63 There were three cases of spontaneous TLS all of which were seminoma.62,64 The others, had evidence of elevated LDH,35,61,63 creatinine,35,61 and uric acid.35 The age ranged from 22 to 58 years with the mean age being 39.75 years. Nevertheless, it is important to note that Kattan et al. failed to demonstrate the occurrence of TLS among 46 patients with germ cell tumors.65
There were four cases of renal cancers associated with the development of TLS.66-69 All of the renal cancers were metastatic. Two out of four patients were females.66,67 On a histological level there were two cases of clear cell carcinoma,67,68 one case of transitional cell carcinoma and one case of chromophobe cancer.66,69 It is important to note that some reports did not include the whole spectrum of baseline laboratory values pertinent to TLS. Nevertheless, two patients had elevated baseline levels of creatinine,66,69 one patient had baseline increase in uric acid and one had elevated baseline levels of LDH.66,67 All of the TLS cases occurred after the administration of chemotherapy. A summary of reported cases of TLS in patients with genitourinary cancers is presented in Supplementary Table 5.
Tumor lysis syndrome in patients with gastrointestinal cancers
Gastrointestinal malignancies include cancers originating from the esophagus, stomach, intestines, liver, gallbladder and biliary tree and pancreatic cancer. We will separately review the association between TLS and hepatic cancers, colorectal cancers and finally stomach and miscellaneous gastrointestinal tumors.
Tumor lysis syndrome in patients with hepatic malignancies
We identified 15 published reports on the association between liver cancers and TLS.69-84 Two reports described two patients,70,77 making the total number of reported patients 17. There were two pediatric cases of hepatoblastoma (female and male children aged 7 months) associated with the development of TLS.69,78 One patient with hepatoblastoma had baseline elevation of uric acid.69 TLS developed during surgery in one patient, despite their being on allopurinol,69 and the second patient with hepatoblastoma developed TLS 4 days after the initiation of chemotherapy.78
Fifteen other cases consisted of hepatocellular carcinoma.70-77,79-83 Most of the patients were males; there were two females.77,80 The age ranged from 33 to 79 years, with the mean age being 59.66 years. Baseline elevation of LDH,73,74,79 potassium,74 uric acid,75 and creatinine,73,80 was noted. Other pertinent laboratory values were either normal or not reported.
Five cases of TLS occurred after transarterial chemoembolization of hepatocellular carcinoma.74,75,77,81 TLS developed within 48 hours in these patients. One case of TLS occurred within 48 hours after radiofrequency ablation.72 One case of TLS occurred after chemotherapy and coil embolization, another occurred after only coil embolization,70 and one occurred after chemoem-bolization.81 Chemotherapy-related cases of TLS comprised seven patients.73,76,78-80,82,83 TLS occurrence ranged from 4 days 78 after chemotherapy to 30 days after sorafenib initiation.76 Spontaneous TLS occurred in one patient with hepatocellular cancer.71 A summary of reported cases of TLS in patients with hepatic cancers is presented in Supplementary Table 6.
Tumor lysis syndrome in patients with colorectal cancers
We identified seven published cases of TLS among patients with colorectal malignancies using the search algorithm described above.72,85-90 Five cases consisted of metastatic colon adenocarcinoma,85,88-90 one case of metastatic rectal adenocarcinoma and one case of metastatic small cell cancer of the colon.86,87 Four patients were females.72,85-87 The age ranged from 38 to 82 years with the mean age being 57.57 years.
Some patients had baseline elevation of LDH,85,87-89 potassium,85 phosphorus,85 uric acid,85,89 and creatinine.89 It is important to note that these baseline laboratory abnormalities were not high enough to meet the criteria for laboratory TLS (Table 1). It is also important to note that some reports did not provide any baseline laboratory values and some provided only a few. There was one case of spontaneous TLS.72 All other cases were clearly associated with chemotherapy. However, one patient underwent pelvic radiation and then chemotherapy.85 TLS occurred from as early as 18 hours after therapy to up to 8 days after initiation of chemotherapy. A summary of reported cases of TLS in patients with colorectal cancers is presented in Supplementary Table 7.
Tumor lysis syndrome in patients with gastric malignancies, gastrointestinal stromal tumors and other gastrointestinal cancers
We identified seven published cases of TLS among patients with gastric, gastrointestinal and miscellaneous gastrointestinal malignancies using the search algorithm described above.91-96
Four cases among those were metastatic gastric adenocarcinomas.91,94-96 All of these patients were males. The age ranged from 36 to 69 years with the mean age being 50 years. It is important to mention that some patients had elevated baseline levels of LDH,94-96 and uric acid.94 There was one case of spontaneous TLS,91 and three cases of chemotherapy associated TLS.94-96 TLS presentation ranged from 3 to 7 days after the initiation of chemotherapy.
Two cases of metastatic gastrointestinal stromal tumors complicated by TLS were reported.92,93 One patient was 56 year old male,92 and second was 81 year old male.93 One patient had mild baseline elevation of creatinine and potassium.93 TLS occurrence ranged from 2 days to 1 week of after the initiation of chemotherapy.
One case of metastatic pancreatic cancer complicated by TLS was reported in a 40 year old male.97 This patient had baseline elevation of potassium, phosphorus and LDH, however, the elevation was not high enough to meet the criteria for laboratory TLS. The patient developed TLS 2 days after the initiation of chemotherapy.
A summary of reported cases of TLS in patients with gastric malignancies, gastrointestinal stromal tumors and miscellaneous gastrointestinal tumors is presented in Supplementary Table 8.
Tumor lysis syndrome in patients with neurological malignancies
We identified four published case reports of TLS among patients with neurological malignancies using the search algorithm described above.98-101 One case reported four patients,99 making the total number of patients seven.
There were two cases of metastatic medulloblastoma associated with the development of TLS.98,101 One patient was a 34 year old female,98 and the second was a 23 year old male.101 One patient had baseline elevation of LDH,98 and borderline creatinine, whereas the second had elevated LDH alone.101 One patient was treated with radiation, dexamethasone and vincristine.98 TLS developed 4 days after receiving radiation. The second patient was treated with cisplatin and etoposide.101 TLS developed 2 days after initiation of chemotherapy.
Five other cases of TLS were reported in patients with metastatic neuroblastoma.99,100 Four of the patients were females and one male. The age ranged from 2 day old to 22 months. One patient was noted to have elevated LDH at baseline.100 There were two cases of chemotherapy and radiation induced TLS, two cases of chemotherapy induced TLS,99,100 and one case of radiation induced TLS.99 However, one patient had evidence of laboratory TLS prior to initiation of radiation and chemotherapy according to authors (no actual laboratory values were provided).99 The timing of the development of TLS ranged from during the therapy to 1 week after the initiation of chemotherapy.
A summary of reported cases of TLS in patients with neurological malignancies is presented in Supplementary Table 9.
Tumor lysis syndrome in patients with skin cancers
We identified nine published cases of TLS among patients with skin malignancies using the search algorithm described above.102-110 Two cases consisted of metastatic Merkel cell carcinoma,102,110 while others were metastatic melanoma. One patient with Merkel cell carcinoma was a 65 year old female with baseline elevation of LDH, mild elevation of creatinine (there was evidence of hydronephrosis) and uric acid. The patient was hydrated in conjunction with chemotherapy; nevertheless, they developed TLS on the third day after initiation of chemotherapy.102 The second patient with Merkel cell carcinoma was an 86 year old female who had baseline elevation of LDH and developed TLS on the third day after initiation of chemotherapy.110
The age of the patients with melanoma ranged from 35 years to 76 years with the mean age being 52.7 years. Five patients were males.103-105,108,109 Some patients had baseline elevation of LDH,103,104,106,108,109 uric acid and creatinine.106 All cases of TLS were associated with some form of cancer targeted pharmacological treatment. TLS occurrence ranged from several hours to 4 days after initiation of treatment.
A summary of reported cases of TLS in patients with dermatological malignancies is presented in Supplementary Table 10.
Tumor lysis syndrome in sarcomas
We identified four published reports of TLS among patients with sarcomas using the search algorithm described above.111-114 One report actually described two patients,114 thus, making the total number of reported patients of five. Also, it is important to note that we included one case of uterine leiomyosarcoma into the section of gynecological cancers,54 and gastrointestinal stromal tumors into the section of gastrointestinal cancers.92,93
Age of the patients spanned from 9 years old to 66 years old with a mean age being 20.7 years old. There were three males and two females. All of the patients had evidence of advanced and metastatic disease. One patient was reported to have normal baseline laboratory values,111 one had elevated baseline values of creatinine and phosphorus,112 and another one had baseline values for elevated levels LDH.113 Three cases were associated with preceding chemotherapy.111-113 TLS occurred from 16 hours to 4 days after the initiation of chemotherapy. Two cases were claimed to represent spontaneous TLS.114 Indeed, both patients had baseline elevation of uric acid and phosphorus. However, the phosphorus levels were not high enough to meet the criteria for pediatric TLS.
A summary of reported cases of TLS in patients with sarcomas is presented in Supplementary Table 11.
Tumor lysis syndrome in miscellaneous malignancies
We identified eight published reports of TLS among patients with miscellaneous tumors using the search algorithm described above.71,114-121 Thymoma comprised three of the cases.115,116,120 One patient had metastatic thymoma,116 whereas the other two had advanced non-metastatic thymoma.115,120 Two of the patients were males, aged 16 years,116 and 33 years,115 and one was a 40-year-old female.120 One patient was reported to have baseline elevation of uric acid.116 One case of TLS occurred on the next day after thoracotomy with multiple biopsies.116 Two other cases of TLS were associated with prior chemotherapy and steroids,115,120 and both occurred 2 days after the imitation of therapy.
Two cases of TLS occurred in patients with poorly differentiated metastatic adenocarcinoma of unknown primary.114,118 One patient was a 50-year-old male,114 and the second was a 59-year-old female.118 It is important to note that one of these cases was actually the first reported case of TLS occurring in a patient with solid malignancy.114 Both of these patients developed spontaneous TLS.
Pheochromocytoma with spontaneous TLS in an 80-year-old male,71 a 53-year-old male with metastatic maxillary sinus squamous cell carcinoma with TLS precipitated by surgery and chemoradiation,117 and a 33-year-old female with infantile hemangioma with TLS precipitated by propranolol therapy comprised one case each.119
A summary of reported cases of TLS in patients with miscellaneous tumors is presented in Supplementary Table 12.
Conclusions
TLS is a relatively rare event in patients with solid cancers. Nevertheless, clinicians should keep in mind that patients with solid tumors may develop this potentially deadly syndrome. Based on the current literature review it seems that patients with advanced and metastatic tumors may be at risk for TLS. Other potential risk factors might be the presence of elevated baseline creatinine and decreased renal function, elevated LDH, elevated phosphorus, elevated potassium and elevated uric acid. It is unclear whether liver metastasis represents an individual risk factor for the development of TLS or is a simply marker of advanced disease. Most of the times, TLS occurred in patients with solid cancers as a result of some form of cancer targeted therapy (radiation, chemotherapy or hormonal therapy) and even after biopsies. It is also essential to keep in mind that TLS can occur spontaneously in solid cancers. We propose an approach to the risk stratification for TLS prevention among patients with solid cancers, which is presented in Figure 1. According to this algorithm it is reasonable to consider TLS prophylaxis in particular patient groups as presented in Figure 1. We suggest that clinicians discuss the pros and cons of such prophylaxis with every patient.
Figure 1.
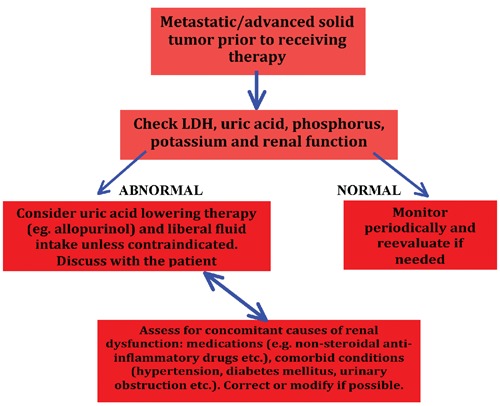
Proposed evaluation for the risk of tumor lysis syndrome and its prevention among patients with solid tumors.
References
- 1.Centers for Disease Control and Prevention. Deaths: preliminary data for 2011. Available from:http://www.cdc.gov/nchs/data/ nvsr/nvsr61/nvsr61_06.pdf
- 2.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11 [DOI] [PubMed] [Google Scholar]
- 3.Wilson FP, Berns JS. Onco-nephrology: tumor lysis syndrome. Clin J Am Soc Nephrol 20127:1730–9 [DOI] [PubMed] [Google Scholar]
- 4.Will A, Tholouli E. The clinical management of tumour lysis syndrome in haematological malignancies. Br J Haematol. 2011;154:3–13 [DOI] [PubMed] [Google Scholar]
- 5.Mika D, Ahmad S, Guruvayoorappan C. Tumour lysis syndrome: implications for cancer therapy. Asian Pac J Cancer Prev. 2012;13:3555–60 [DOI] [PubMed] [Google Scholar]
- 6.McBride A, Westervelt P. Recognizing and managing the expanded risk of tumor lysis syndrome in hematologic and solid malignancies. J Hematol Oncol. 2012;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409–17 [DOI] [PubMed] [Google Scholar]
- 8.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29 [DOI] [PubMed] [Google Scholar]
- 9.Rezaei MK, Nolan NJ, Schwartz AM. Surgical pathology of lung cancer. Semin Respir Crit Care Med. 2013;34:770–86 [DOI] [PubMed] [Google Scholar]
- 10.Persons DA, Garst J, Vollmer R, Crawford J. Tumor lysis syndrome and acute renal failure after treatment of non-small-cell lung carcinoma with combination irinotecan and cisplatin. Am J Clin Oncol. 1998;21:426–9 [DOI] [PubMed] [Google Scholar]
- 11.Feld J, Mehta H, Burkes RL. Acute spontaneous tumor lysis syndrome in adenocarcinoma of the lung: a case report. Am J Clin Oncol. 2000;23:491–3 [DOI] [PubMed] [Google Scholar]
- 12.Sewani HH, Rabatin JT. Acute tumor lysis syndrome in a patient with mixed small cell and non-small cell tumor. Mayo Clin Proc. 2002;77:722–8 [DOI] [PubMed] [Google Scholar]
- 13.Kurt M, Onal IK, Elkiran T, et al. Acute tumor lysis syndrome triggered by zoledronic Acid in a patient with metastatic lung adenocarcinoma. Med Oncol. 2005;22:203–6 [DOI] [PubMed] [Google Scholar]
- 14.Ajzensztejn D, Hegde VS, Lee SM. Tumor lysis syndrome after treatment with docetaxel for non-small-cell lung cancer. J Clin Oncol. 2006;24:2389–91 [DOI] [PubMed] [Google Scholar]
- 15.Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005–6 [DOI] [PubMed] [Google Scholar]
- 16.Shenoy C. Acute spontaneous tumor lysis syndrome in a patient with squamous cell carcinoma of the lung. QJM. 2009;102:71–3 [DOI] [PubMed] [Google Scholar]
- 17.Honda K, Saraya T, Tamura M, et al. Tumor lysis syndrome and acquired ichthyosis occurring after chemotherapy for lung adenocarcinoma. J Clin Oncol. 2011;29:859–60 [DOI] [PubMed] [Google Scholar]
- 18.Vogelzang NJ, Nelimark RA, Nath KA. Tumor lysis syndrome after induction chemotherapy of small-cell bronchogenic carcinoma. JAMA. 1983;249:513–4 [PubMed] [Google Scholar]
- 19.Baumann MA, Frick JC, Holoye PY. The tumor lysis syndrome. JAMA. 1983;250:615. [PubMed] [Google Scholar]
- 20.Hussein AM, Feun LG. Tumor lysis syndrome after induction chemotherapy in small-cell lung carcinoma. Am J Clin Oncol. 1990;13:10–3 [DOI] [PubMed] [Google Scholar]
- 21.Ohnishi T, Mori K, Ohta S, et al. Tumor lysis syndrome in widely metastatic small-cell lung cancer. Int J Clin Oncol. 1997;2:235–7 [Google Scholar]
- 22.Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363–7 [DOI] [PubMed] [Google Scholar]
- 23.Heching N, Bonomi P. Tumor lysis syndrome in metastatic small cell cancer. Proc Am Assoc Cancer Res. 1988;29:179 [Google Scholar]
- 24.Marinella MA. Fatal tumor lysis syndrome and gastric hemorrhage associated with metastatic small-cell lung carcinoma. Med Pediatr Oncol. 1999;32:464–5 [DOI] [PubMed] [Google Scholar]
- 25.Kallab AM, Jillella AP. Tumor lysis syndrome in small cell lung cancer. Med Oncol. 2001;18:149–51 [DOI] [PubMed] [Google Scholar]
- 26.Beriwal S, Singh S, Garcia-Young JA. Tumor lysis syndrome in extensive-stage small-cell lung cancer. Am J Clin Oncol. 2002;25:474–5 [DOI] [PubMed] [Google Scholar]
- 27.Mott FE, Esana A, Chakmakjian C, Herrington JD. Tumor lysis syndrome in solid tumors. Support Cancer Ther. 2005;2:188–91 [DOI] [PubMed] [Google Scholar]
- 28.Jallad B, Hamdi T, Latta S, et al. Tumor lysis syndrome in small cell lung cancer: a case report and review of the literature. Onkologie. 2011;34:129–31 [DOI] [PubMed] [Google Scholar]
- 29.Padhi P, Singh S. Spontaneous tumor lysis syndrome in a patient with metastatic small cell carcinoma of the lung. J Cancer Sci Ther. 2012;4:164–6 [Google Scholar]
- 30.Boikos SA, Forde PM, Chatterjee S, Hann CL. Tumor lysis syndrome in limited-stage small-cell lung cancer. J Thorac Oncol. 2013;8:e61–2 [DOI] [PubMed] [Google Scholar]
- 31.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruddy KJ, Winer EP. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol. 2013;24:1434–43 [DOI] [PubMed] [Google Scholar]
- 33.Cech P, Block JB, Cone LA, Stone R. Tumor lysis syndrome after tamoxifen flare. N Engl J Med. 1986;315:263–4 [DOI] [PubMed] [Google Scholar]
- 34.Stark ME, Dyer MC, Coonley CJ. Fatal acute tumor lysis syndrome with metastatic breast carcinoma. Cancer. 1987;60:762–4 [DOI] [PubMed] [Google Scholar]
- 35.Barton JC. Tumor lysis syndrome in non-hematopoietic neoplasms. Cancer. 1989;64:738–40 [DOI] [PubMed] [Google Scholar]
- 36.Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502–5 [DOI] [PubMed] [Google Scholar]
- 37.Sklarin NT, Markham M. Spontaneous recurrent tumor lysis syndrome in breast cancer. Am J Clin Oncol. 1995;18:71–3 [DOI] [PubMed] [Google Scholar]
- 38.Ustündağ Y, Boyacioğlu S, Haznedaroğlu IC, Baltali E. Acute tumor lysis syndrome associated with paclitaxel. Ann Pharmacother. 1997;31:1548–9 [DOI] [PubMed] [Google Scholar]
- 39.Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol 2000;111349–51 [DOI] [PubMed] [Google Scholar]
- 40.Zigrossi P, Brustia M, Bobbio F, Campanini M. Flare and tumor lysis syndrome with atypical features after letrozole therapy in advanced breast cancer. A case report. Ann Ital Med Int. 2001;16:112–7 [PubMed] [Google Scholar]
- 41.Kurt M, Eren OO, Engin H, Güler N. Tumor lysis syndrome following a single dose of capecitabine. Ann Pharmacother. 2004;38:902. [DOI] [PubMed] [Google Scholar]
- 42.Kawaguchi Ushio A, Hattori M, Kohno N, et al. [Gemcitabine-induced tumor lysis syndrome caused by recurrent breast cancer in a patient without hemodialysis]. Gan To Kagaku Ryoho. 2013;40:1529–32 [Article in Japanese] [PubMed] [Google Scholar]
- 43.Taira F, Horimoto Y, Saito M. Tumor lysis syndrome following trastuzumab for breast cancer: a case report and review of the literature. Breast Cancer 2013 Feb 19. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.DeVita VT, JR, Lawrence TS, Rosenberg SA, DeVita, Hellman, Rosenberg’scancer: principles and practice of oncology. 9th ed.Philadelphia, PA: Lippincott Williams & Wilkins; Ninth, North American Edition edition; 2011 [Google Scholar]
- 45.Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521–4 [DOI] [PubMed] [Google Scholar]
- 46.Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794–5 [DOI] [PubMed] [Google Scholar]
- 47.Yahata T, Nishikawa N, Aoki Y, Tanaka K. Tumor lysis syndrome associated with weekly paclitaxel treatment in a case with ovarian cancer. Gynecol Oncol. 2006;103:752–4 [DOI] [PubMed] [Google Scholar]
- 48.Doi M, Okamoto Y, Yamauchi M, et al. Bleomycin-induced pulmonary fibrosis after tumor lysis syndrome in a case of advanced yolk sac tumor treated with bleomycin, etoposide and cisplatin (BEP) chemotherapy. Int J Clin Oncol. 2012;17:528–31 [DOI] [PubMed] [Google Scholar]
- 49.Cui T, Wang C, He H, et al. A rare case of ovarian Burkitt lymphoma associated tumor lysis syndrome. Eur J Gynaecol Oncol. 2010;31:209–10 [PubMed] [Google Scholar]
- 50.Camarata M, Davies R, Copley S, Blagden S. Tumour lysis syndrome in a patient with intravascular spread from a recurrent epithelial ovarian cancer. BMJ Case Rep 2013;2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol. 1993;51:258–60 [DOI] [PubMed] [Google Scholar]
- 52.Khalil A, Chammas M, Shamseddine A, Seoud M. Fatal acute tumor lysis syndrome following treatment of vulvar carcinoma: case report. Eur J Gynaecol Oncol. 1998;19:415–6 [PubMed] [Google Scholar]
- 53.Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiraizumi Y, Kamoi S, Inde Y, et al. A case of tumor lysis syndrome following chemotherapy for a uterine epithelioid leiomyosarcoma with focal rhabdomyosarcomatous differentiation. J Obstet Gynaecol Res. 2011;37:947–52 [DOI] [PubMed] [Google Scholar]
- 55.Schuman S, Pearson JM, Lucci JA, 3rd, Twiggs LB. Metastatic gestational trophoblastic neoplasia complicated by tumor lysis syndrome, heart failure, and thyrotoxicosis: a case report. J Reprod Med. 2010;55:441–4 [PubMed] [Google Scholar]
- 56.Tanvetyanon T, Choudhury AM. Fatal acute tumor lysis syndrome, hepatic encephalopathy and flare phenomenon following combined androgen blockade. J Urol. 2004;171:1627. [DOI] [PubMed] [Google Scholar]
- 57.Sorscher SM. Tumor lysis syndrome following docetaxel therapy for extensive metastatic prostate cancer. Cancer Chemother Pharmacol. 2004;54:191–2 [DOI] [PubMed] [Google Scholar]
- 58.Wright JL, Lin DW, Dewan P, Montgomery RB. Tumor lysis syndrome in a patient with metastatic, androgen independent prostate cancer. Int J Urol. 2005;12:1012–3 [DOI] [PubMed] [Google Scholar]
- 59.Lin CJ, Hsieh RK, Lim KH, et al. Fatal spontaneous tumor lysis syndrome in a patient with metastatic, androgen-independent prostate cancer. South Med J. 2007;100:916–7 [DOI] [PubMed] [Google Scholar]
- 60.Kaplan MA, Kucukoner M, Alpagat G, Isikdogan A. Tumor lysis syndrome during radiotherapy for prostate cancer with bone and bone marrow metastases without visceral metastasis. Ann Saudi Med. 2012;32:306–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanke CD, Hemmer MP, Witte RS. Acute tumor lysis syndrome with choriocarcinoma. South Med J. 2000;93:916–9 [PubMed] [Google Scholar]
- 62.Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Supp Care Cancer. 2001;9:554–7 [DOI] [PubMed] [Google Scholar]
- 63.Feres GA, Salluh JI, Ferreira CG, Soares M. Severe acute tumor lysis syndrome in patients with germ-cell tumors. Indian J Urol. 2008;24:555–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Alessandro V, Greco A, Clemente C, et al. Severe spontaneous acute tumor lysis syndrome and hypoglycemia in patient with germ cell tumor. Tumori. 2010;96:1040–3 [PubMed] [Google Scholar]
- 65.Kattan J, Culine S, Tavakoli-Razavi T, et al. Acute tumor lysis syndrome in poor-risk germ cell tumors: does it exist? Supp Care Cancer. 1994;2:128–31 [DOI] [PubMed] [Google Scholar]
- 66.Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455–7 [DOI] [PubMed] [Google Scholar]
- 67.Nicholaou T, Wong R, Davis ID. Tumour lysis syndrome in a patient with renal-cell carcinoma treated with sunitinib malate. Lancet. 2007;369:1923–4 [DOI] [PubMed] [Google Scholar]
- 68.Michels J, Lassau N, Gross-Goupil M, et al. Sunitinib inducing tumor lysis syndrome in a patient treated for renal carcinoma. Invest New Drugs. 2010;28:690–3 [DOI] [PubMed] [Google Scholar]
- 69.Rodríguez-Reimúndes E, Perazzo F, Vilches AR. [Tumor lysis syndrome in a patient with a renal carcinoma treated with sunitinib]. Medicina (B Aires). 2011;71:158–60 [Article in Spanish] [PubMed] [Google Scholar]
- 70.Lobe TE, Karkera MS, Custer MD, et al. Fatal refractory hyperkalemia due to tumor lysis during primary resection for hepatoblastoma. J Pediatr Surg. 1990;25:249–50 [DOI] [PubMed] [Google Scholar]
- 71.Burney IA. Acute tumor lysis syndrome after transcatheter chemoembolization of hepatocellular carcinoma. South Med J. 1998;91:467–70 [DOI] [PubMed] [Google Scholar]
- 72.Vaisban E, Braester A, Mosenzon O, et al. Spontaneous tumor lysis syndrome in solid tumors: really a rare condition? Am J Med Sci. 2003;325:38–40 [DOI] [PubMed] [Google Scholar]
- 73.Lehner SG, Gould JE, Saad WE, Brown DB. Tumor lysis syndrome after radiofrequency ablation of hepatocellular carcinoma. AJR Am J Roentgenol. 2005;185:1307–9 [DOI] [PubMed] [Google Scholar]
- 74.Lee CC, Wu YH, Chung SH, Chen WJ. Acute tumor lysis syndrome after thalidomide therapy in advanced hepatocellular carcinoma. Oncologist. 2006;1:87–9 [DOI] [PubMed] [Google Scholar]
- 75.Sakamoto N, Monzawa S, Nagano H, et al. Acute tumor lysis syndrome caused by transcatheter oily chemoembolization in a patient with a large hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2007;30:508–11 [DOI] [PubMed] [Google Scholar]
- 76.Shiba H, Ishida Y, Wakiyama S, et al. Acute tumor lysis syndrome after transarterial chemoembolization for hepatocellular carcinoma. Cancer Sci. 2008;99:2104–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang WS, Yang CH. Sorafenib induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsieh PM, Hung KC, Chen YS. Tumor lysis syndrome after transarterial chemoembolization of hepatocellular carcinoma: case reports and literature review. World J Gastroenterol. 2009;15:4726–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bercovitz RS, Greffe BS, Hunger SP. Acute tumor lysis syndrome in a 7-month-old with hepatoblastoma. Curr Opin Pediatr. 2010;22:113–6 [DOI] [PubMed] [Google Scholar]
- 80.Joshita S, Yoshizawa K, Sano K, et al. A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991–4 [DOI] [PubMed] [Google Scholar]
- 81.Shiozawa K, Watanabe M, Takenaka H, et al. Tumor lysis syndrome after sorafenib for hepatocellular carcinoma: a case report. Hepatogastroenterology. 2010;57:688–90 [PubMed] [Google Scholar]
- 82.Chao CT, Chiang CK. Rasburicase for huge hepatocellular carcinoma with tumor lysis syndrome: case report. Med Princ Pract. 2012;21:498–500 [DOI] [PubMed] [Google Scholar]
- 83.Nishida Y, Fujii H, Hagihara A, et al. [Tumor lysis syndrome after transarterial embolization for hepatocellular carcinoma]. Nihon Shokakibyo Gakkai Zasshi. 2013;110:441–8 [Article in Japanese] [PubMed] [Google Scholar]
- 84.Habib G, Nashashibi M. Fatal tumor lysis syndrome following sorafenib treatment. J Med Cases. 2013;4:269–70 [Google Scholar]
- 85.Boisseau M, Bugat R, Mahjoubi M. Rapid tumour lysis syndrome in a metastatic colorectal cancer increased by treatment (CPT-11). Eur J Cancer 1996;32A:737–8 [DOI] [PubMed] [Google Scholar]
- 86.Nikolic-Tomasevic Z, Jelic S, Popov I, Radosavljevic D. Colorectal cancer: dilemmas regarding patient selection and toxicity prediction. J Chemother. 2000;2:244–51 [DOI] [PubMed] [Google Scholar]
- 87.El Mesbahi O, Beaudoin A, Louvet C, De Gramont A. Tumor lysis syndrome after chemotherapy for colon small cell carcinoma. Rev Med Interne. 2004;25:768–9 [DOI] [PubMed] [Google Scholar]
- 88.Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514–6 [DOI] [PubMed] [Google Scholar]
- 89.Hentrich M, Schiel X, Scheidt B, et al. Fatal tumor lysis syndrome after irinotecan/5-FU/folinic acid/bevacizumab-containing therapy in a patient heavily pretreated for metastatic colon cancer. Acta Oncol. 2008;47:155–6 [DOI] [PubMed] [Google Scholar]
- 90.Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406–8 [DOI] [PubMed] [Google Scholar]
- 91.Woo IS, Kim JS, Park MJ, et al. Spontaneous acute tumor lysis syndrome with advanced gastric cancer. J Korean Med Sci. 2001;16:115–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saylor PJ, Reid TR. Tumor lysis syndrome after treatment of a gastrointestinal stromal tumor with the oral tyrosine kinase inhibitor sunitinib. J Clin Oncol. 2007;25:3544–6 [DOI] [PubMed] [Google Scholar]
- 93.Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with glivec (imatinib mesylate, gleevec, STI571). Sarcoma. 2007;2007:82012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han HS, Park SR, Kim SY, et al. Tumor lysis syndrome after capecitabine plus cisplatin treatment in advanced gastric cancer. J Clin Oncol. 2008;26:1006–8 [DOI] [PubMed] [Google Scholar]
- 95.Kobayashi T, Kuwai T, Yamamoto S, et al. [Acute tumor lysis syndrome in the setting of advanced gastric cancer]. Nihon Shokakibyo Gakkai Zasshi. 2012;109:1372–8 [Article in Japanese] [PubMed] [Google Scholar]
- 96.Vodopivec DM, Rubio JE, Fornoni A, Lenz O. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med. 2012;2012:468452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ling W, Sachdeva P, Wong AS, et al. Unprecedented case of tumor lysis syndrome in a patient with metastatic pancreatic adenocarcinoma. Pancreas. 2012;41:659–61 [DOI] [PubMed] [Google Scholar]
- 98.Tomlinson GC, Solberg LA., Jr.Acute tumor lysis syndrome with metastatic medulloblastoma. A case report. Cancer. 1984;53:1783–5 [DOI] [PubMed] [Google Scholar]
- 99.Hain RD, Rayner L, Weitzman S, Lorenzana A. Acute tumour lysis syndrome complicating treatment of stage IVS neuroblastoma in infants under six months old. Med Pediatr Oncol. 1994;23:136–9 [DOI] [PubMed] [Google Scholar]
- 100.Kushner BH, LaQuaglia MP, Modak S, Cheung NK. Tumor lysis syndrome, neuroblastoma, and correlation between serum lactate dehydrogenase levels and MYCN-amplification. Med Pediatr Oncol. 2003;41:80–2 [DOI] [PubMed] [Google Scholar]
- 101.Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors--a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187–92 [DOI] [PubMed] [Google Scholar]
- 102.Dirix LY, Prove A, Becquart D, et al. Tumor lysis syndrome in a patient with metastatic Merkel cell carcinoma. Cancer. 1991;67:2207–10 [DOI] [PubMed] [Google Scholar]
- 103.Minasian LM, Szatrowski TP, Rosenblum M, et al. Hemorrhagic tumor necrosis during a pilot trial of tumor necrosis factor-alpha and anti-GD3 ganglioside monoclonal antibody in patients with metastatic melanoma. Blood. 1994;83:56–64 [PubMed] [Google Scholar]
- 104.Castro MP, VanAuken J, Spencer-Cisek P, et al. Acute tumor lysis syndrome associated with concurrent biochemotherapy of metastatic melanoma: a case report and review of the literature. Cancer. 1999;85:1055–9 [PubMed] [Google Scholar]
- 105.Stoves J, Richardson D, Patel H. Tumour lysis syndrome in a patient with metastatic melanoma treated with biochemotherapy. Nephrol Dial Transplant. 2001;16:188–9 [DOI] [PubMed] [Google Scholar]
- 106.Habib GS, Saliba WR. Tumor lysis syndrome after hydrocortisone treatment in metastatic melanoma: a case report and review of the literature. Am J Med Sci. 2002;323:155–7 [DOI] [PubMed] [Google Scholar]
- 107.Busam KJ, Wolchok J, Jungbluth AA, Chapman P. Diffuse melanosis after chemotherapy-induced tumor lysis syndrome in a patient with metastatic melanoma. J Cutan Pathol. 2004;31:274–80 [DOI] [PubMed] [Google Scholar]
- 108.Nakamura Y, Nakamura Y, Hori E, et al. Tumor lysis syndrome after transcatheter arterial infusion of cisplatin and embolization therapy for liver metastases of melanoma. Int J Dermatol. 2009;48:763–7 [DOI] [PubMed] [Google Scholar]
- 109.Borne E, Serafi R, Piette F, Mortier L. Tumour lysis syndrome induced by corticosteroid in metastatic melanoma presenting with initial hyperkalemia. J Eur Acad Dermatol Venereol. 2009;23:855–6 [DOI] [PubMed] [Google Scholar]
- 110.Grenader T, Shavit L. Tumor lysis syndrome in a patient with merkel cell carcinoma and provoked pathologic sequence of acute kidney injury, reduced clearance of carboplatin and fatal pancytopenia. Onkologie. 2011;34:626–9 [DOI] [PubMed] [Google Scholar]
- 111.Khan J, Broadbent VA. Tumor lysis syndrome complicating treatment of widespread metastatic abdominal rhabdomyosarcoma. Pediatr Hematol Oncol. 1993;10:151–5 [DOI] [PubMed] [Google Scholar]
- 112.Gold JE, Malamud SC, LaRosa F, Osband ME. Adoptive chemoimmunotherapy using ex vivo activated memory T-cells and cyclophosphamide: tumor lysis syndrome of a metastatic soft tissue sarcoma. Am J Hematol. 1993;44:42–7 [DOI] [PubMed] [Google Scholar]
- 113.Qian KQ, Ye H, Xiao YW, et al. Tumor lysis syndrome associated with chemotherapy in primary retroperitoneal soft tissue sarcoma by ex vivo ATP-based tumor chemo-sensitivity assay (ATP-TCA). Int J Gen Med. 2009;2:1–4 [PMC free article] [PubMed] [Google Scholar]
- 114.Bien E, Maciejka-Kapuscinska L, Niedzwiecki M, et al. Childhood rhabdomyosarcoma metastatic to bone marrow presenting with disseminated intravascular coagulation and acute tumour lysis syndrome: review of the literature apropos of two cases. Clin Exp Metastasis. 2010;27:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crittenden DR, Ackerman GL. Hyperuricemic acute renal failure in disseminated carcinoma. Arch Intern Med. 1977;137:97–9 [PubMed] [Google Scholar]
- 116.Yokoi K, Miyazawa N, Kano Y, et al. Tumor lysis syndrome in invasive thymoma with peripheral blood T-cell lymphocytosis. Am J Clin Oncol. 1997;20:86–9 [DOI] [PubMed] [Google Scholar]
- 117.Trobaugh-Lotrario AD, Liang X, Janik JS, et al. Difficult diagnostic and therapeutic cases: CASE 2. thymoma and tumor lysis syndrome in an adolescent. J Clin Oncol. 2004;22:955–7 [DOI] [PubMed] [Google Scholar]
- 118.Abboud M, Shamseddine A. Maxillary sinus squamous cell carcinoma presenting with fatal tumor lysis syndrome: a case report and review of the literature. Case Rep Oncol. 2009;2:229–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saini N, Pyo Lee K, Jha S, et al. Hyperuricemic renal failure in nonhematologic solid tumors: a case report and review of the literature. Case Rep Med. 2012;2012:314056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cavalli R, Buffon RB, de Souza M, et al. Tumor lysis syndrome after propranolol therapy in ulcerative infantile hemangioma: rare complication or incidental finding? Dermatology. 2012;224:106–9 [DOI] [PubMed] [Google Scholar]
- 121.Lee JY, Lim SH, Lee JY, et al. Tumor lysis syndrome in a solid tumor: a case report of a patient with invasive thymoma. Cancer Res Treat. 2013;45:343–8 [DOI] [PMC free article] [PubMed] [Google Scholar]


