Abstract
Background
In a community sample of low-income African American adolescents, we tested the interactive effects of variation in the mu 1 opioid receptor (OPRM1) gene and the occurrence of stressful life events on symptoms of depression.
Method
Interactive effects of 24 OPRM1 simple nucleotide polymorphisms (SNP) and adolescent report of stressful life events on depression were tested using multilevel regressions. SNPs were dummy coded to test both additive and dominate forms of coding.
Results
Five OPRM1 SNPs showed significant evidence of interaction with stressful life events to alter depression risk (or symptoms) after adjusting for multiple testing and the correlated nature of the SNPs. Follow-up analyses showed significant differences based on OPRM1 genotype at both lower and higher frequencies of stressful life events, suggesting that participants with a copy of the minor allele on OPRM1 SNPs rs524731, rs9478503, rs3778157, rs10485057, and rs511420 have fewer symptoms in low stress conditions but more symptoms in high stress conditions compared to major allele homozygotes.
Limitations
The genetic variants associated with depression in African American adolescents may not translate to other ethnic groups. This study is also limited in that only one gene that functions within a complex biological system is addressed.
Conclusions
This current study is the first to find an interaction between OPRM1 and life stress that is associated with depression. It also addressed an understudied population within the behavioral genetics literature. Further research should test additional genes involved in the opioid system and expand the current findings to more diverse samples.
Keywords: Molecular genetics, Adolescence, Stress, Psychopathology, Depression, Gene x environment interactions
Genetic factors play a significant role in the etiology and temporal stability of depression across childhood and adolescence (Franic, Middeldorp, Dolan, Ligthart, & Boomsma, 2010). Both availability of mu-opioid receptors (Kennedy, Koeppe, Young, & Zubieta, 2006), which activate in response to stressful stimuli (Ide et al., 2010; Wang, Charboneau, Barke, Loh, & Roy, 2002), and variation in the mu-opioid receptor (OPRM1) gene (Kertes et al., 2011) have been linked to depression. The accumulation of life stressors is a known risk factor previously shown to interact with genetic susceptibilities in the prediction of depression (El Hage, Powell, & Surguladze, 2009). The current study examines whether variation in OPRM1 interacts with stressful life events to predict depression.
The adolescent period can be a significant time of change when it comes to depressive symptoms (Conley & Rudolph, 2009; Cyranowski, Frank, Young, & Shear, 2000; Ge, Lorenz, Conger, Elder, & Simons, 1994). Depressive symptoms increase during this period, especially for girls. Increases in stressful life events have also been found to predict increases in depressive symptoms throughout this period (Charbonneau, Mezulis, & Hyde, 2009; Ge, Conger, & Elder, 2001; Johnson, Whisman, Corley, Hewitt, & Rhee, 2012). In order to fully understand the association between stress and depression, it is important to consider the potential genetic and biological mechanisms involved in that association.
Stress on the Genetic and Biological System
Twin research on depression and stressful life events has shown higher heritability estimates for depression among adolescents and preadolescents experiencing more stressful life occurrences (Boardman, Alexander, & Stallings, 2011; Silberg et al., 1999), and provide additional evidence of shared genetic liability among stressful life events and depression, suggesting gene-environment correlation. Within these studies researchers limited their definition of stressful life events to those that the child could be partially responsible for (e.g., “losing a friend through arguments”) in order to focus on life stress that could be most affected by genotype (as opposed to stressful events out of one’s control such as the death of a loved one). The findings from this study support a biological, specifically genetic, role in the occurrence of stressful life events.
Stressful life experiences can also impact our biology. Two biological systems that have been linked to depression and that are influenced by the OPRM1 gene are the hypothalamic-pituitary-adrenal (HPA) axis and the opioid system (Pariante & Lightman, 2008; Tremblay et al., 2005). One of the primary hormones released by the HPA axis and used to measure physiological reactions to stress is cortisol. A history of adverse life events has been found to be associated with a blunted cortisol reaction to a stressor in adults (Elzinga et al., 2008). Cortisol, in turn, is also a predictor of future depression in adolescents (Adam et al., 2010). The opioid system plays an important role in blunting both physical and emotional pain (Drolet et al., 2001). The latter effect is especially crucial to a person’s capacity to cope with stressful events and explains why a gene involved in the opioid system, like OPRM1, might be important for understanding why stress leads to depression in some and not others.
OPRM1, Stress, and Depression
Variability in OPRM1 polymorphisms have been linked to greater activity in the mesocorticolimbic areas of the brain (Filbey et al., 2008). Researchers studied the A118G SNP on OPRM1 and found that having one or more copies of the G allele predicted increased blood flow to the mesocorticolimbic areas. Mesocorticolimbic pathways are an integral part of the brain’s reward system, transporting dopamine to the limbic system and frontal cortex where it regulates motivation and feelings of pleasure. In addition to impacting brain activity, differences in OPRM1 polymorphisms are associated with changes in the HPA axis in both humans and rhesus monkeys (Chong et al., 2006; Pratt & Davidson, 2009; Schwandt et al., 2011; Wand et al., 2002). For both humans and monkeys, high levels of stress and OPRM1 risk variants interact to predict a blunted response from the stress-sensitive hormone cortisol. Variation in OPRM1, specifically within the A118G SNP, is related to expression differences up to 1.5-fold in OPRM1 mRNA and 10-fold in OPRM1 protein for the A118 allele compared to the G118 (Zhang, Wang, Johnson, Papp, & Sadee, 2005). The biological impact of differences in OPRM1 polymorphisms, from the molecular to the brain and HPA axis, presents pathways through which variation on OPRM1 SNPs may influence clinical outcomes, such as depression.
The association between OPRM1 variants and stress is not well documented. As alluded to earlier, OPRM1 genotype predicts a blunted cortisol response to stressful events such as the Trier Social Stress Test, a laboratory based stressor in which participants give a speech in front of confederate judges (Chong et al., 2006). The A118G SNP has also been associated with decreased symptoms of post-traumatic stress disorder in people living with HIV (Nugent, Lally, Brown, Knopik, & McGeary, 2012). Beyond those two studies, questions still exist about how OPRM1 variants alter the impact of stress, especially broader measures of stress, and their association with psychopathology.
Research has found connections between OPRM1 and depression that hold promise for further exploration. In a study of over 100 candidate genes in a sample of adults with alcohol disorders, four SNPs from the OPRM1 gene were associated with symptoms of major depressive disorder (MDD) (Kertes et al., 2011). After adjusting for multiple testing, the rs650245 SNP remained significant. Participants with more copies of the minor allele had more symptoms of depression. The mu-opioid receptor, for which OPRM1 encodes the protein, has also been associated with depression (Kennedy et al., 2006). In a group of 28 women, half diagnosed with MDD, the women with MDD had fewer available mu-opioid receptors compared to the control women.
Current Study
The novel aim of the current study is to test associations of life stressors and variation in OPRM1 with depression. We expect that OPRM1 genotype and frequency of stressful life events will separately predict symptoms of depression and together interact to predict symptoms of depression. Specifically, we predict variation in OPRM1 SNPs will interact with lower frequency stressful life events to predict fewer depressive symptoms and will interact with higher frequency of stressful life events to predict increased depressive symptoms. The current study is the first to explore the effects of both life stress and variation in OPRM1 on depression. Furthermore, we are testing this association with low-income African American adolescents, a population that are understudied in gene-by-environment psychiatric genetic research (Murphy, Wickramaratne, & Weissman, 2009).
METHODS
Data are from the Gene, Environment, Neighborhood Initiative (GENI), a sample of 592 African American adolescents [98.8% AA; 51.2% female; age M = 15.93 (SD = 1.43)] recruited from Mobile, Alabama. Some participants were recruited along with other adolescent family members (M=1.29 children per family). Two hour interviews were conducted between March 2009 and October 2011 with both adolescents and their primary caregiver. Caregivers and adolescent participants gave written consent and assent, respectively, and were compensated for their time. Procedures for this study were approved by the Institutional Review Boards at Northwestern University, Virginia Commonwealth University, University of Illinois at Chicago and the University of Alabama.
Measures
Genotyping
In the GENI sample, a total of 24 SNPs were genotyped across OPRM1. Genotyping was conducted at the Virginia Institute for Psychiatric and Behavioral Genetics (VIPBG) at Virginia Commonwealth University, using TaqMan chemistry and Assays-on-Demand (Applied Biosystems International, Carlsbad CA). Reactions were assembled using an epMotion 5075 liquid handling robot (Eppendorf, Westbury NY), fluorescence signals were read in an LJL Analyst AD plate reader (LJL, Sunnyvale, CA) and genotypes were called using an automated allele scoring platform (van den Oord, Jiang, Riley, Kendler, & Chen, 2003). Primer sequences for VNTRs (available upon request) were designed using PRIMER3 (http://primer3.sourceforge.net/). For SNPs that were previously typed by our group in another sample, these were force-included in the tagging set for the purpose of future comparison. If no TaqMan assay was available for a previously typed SNP, a proxy SNP in complete LD (R2=1.0) with the previously typed SNP was selected and used. A supplementary set of SNPs was then selected (based on HapMap data from the Nigerian Yoruba population) to complete LD tagging within the subset of African American participants in order to capture the additional genetic variability in individuals of African descent.
The genotyping success rate for this gene within this sample was 98.9%. Duplicate genotyping produced concordance rates of 100%. As a measure of quality control, all genetic data were then subjected to a sequence of inclusionary thresholds. First, individual DNA samples yielding a gene-wise genotyping success rate of less than 80% were deemed unreliable, and removed from consideration for inclusion in the analytic dataset. Second, for all remaining data, any individual SNP with a sample-wise genotyping success rate of less than 80% was then excluded from the analytic dataset. Of the 579 participants from whom DNA data were collected, only 4 failed to meet the first threshold; therefore, our final sample size for this analysis was 575. Of the 24 OPRM1 markers genotyped in the GENI sample, none were excluded on the basis of the second criteria. None of the OPRM1 SNPs significantly deviated from within ethnic-group calculations for Hardy-Weinberg equilibrium (p ≤ .001). All SNP chromosomal positions and allele identities are shown with respect to the genomic (+) strand.
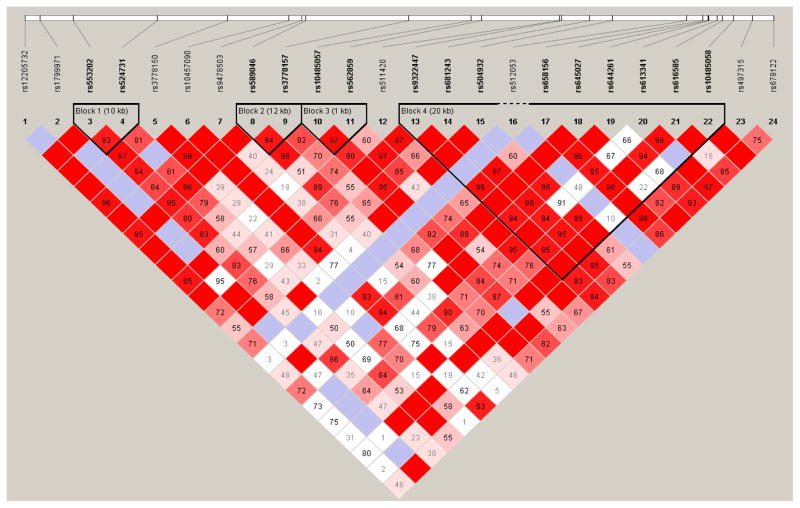
Given the different ancestral histories of populations, allele frequencies and LD differ substantially across populations. Because of this, we limited all analyses and data reported in this paper to the African Americans in our sample to guard against the introduction of bias via population stratification (two participants identifying as “white” and five participants identifying as “mixed” were not included). Haploview (Barrett, Fry, Maller, & Daly, 2005) was used to estimate LD across the full set of genotyped SNPs. Pairwise associations between markers in OPRM1 yielded R2 values ranging from .00 to .81. Likewise, D′ (an alternative estimate of association that is less sensitive to variation in allele frequencies; (Hedrick & Kumar, 2001)) values ranged from .01 to .97. The extent to which inter-SNP correlation exists reflects the degree to which analyses with individual markers represent non-independent tests of association. A multiple testing correction across the SNPs was performed using the web-based software SNPSpD (Nyholt, 2004), which takes into account the number of SNPs genotyped and the LD structure between them (see Figure 1 for LD plot). Based on this test, the effective number of independent marker loci for our analyses was 15.04, resulting in an adjusted significance level of .003.
Figure 1.
OPRM1 LD plot from Haploview
Stressful Life Events
Adolescents completed the Stress Index, a 16-item questionnaire measuring frequency of life transitions, circumscribed events (e. g., property damage from a disaster or a family members illness or death), and violence exposure in the previous year (Attar, Guerra, & Tolan, 1994; Gorman Smith & Tolan, 1998). The Index first asks whether or not an event has occurred in the last year (e. g., “Did your family move to a new home or apartment in the LAST 12 MONTHS?”) and then asks how many times that event occurred (e. g., “How many times did your family move to a new home or apartment in the LAST 12 MONTHS?”) with response options “once”, “twice”, or “three or more times”. Sum scores were calculated based on the total frequency of stressful life events participants experienced.
Diagnostic Interview Schedule for Children (C-DISC-4)
The C-DISC is a widely used assessment of psychiatric diagnoses among adolescents and is administered as a computerized, structured interview by trained lay interviewers. Interviewers were educators, social workers, counselors with experience working with inner city youth, and college interns working on the MYS study. All interviewers went through extensive training according to accepted procedures (Shaffer, Fisher, & Lucas, 2004) and received ongoing supervision by licensed Clinical Psychologists trained in C-DISC administration and in psychological assessment of urban, minority youth. We administered the modules for most major psychiatric disorders, including major depressive disorder (MDD). Number of MDD symptoms (range: 0–20) was taken for the previous 12 months. The acceptable reliability and validity of the computerized DISC 4.0 and earlier versions has been well-described (Shaffer et al., 2004; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000).
Statistical Analyses
Multilevel regression in SPSS (version 21.0) was used to separately test main effects between each SNP and frequency of exposure to stressors on number of MDD symptoms while accounting for potential similarities among adolescent family members. Interactive effects of SNPs and stress exposure on MDD symptoms were then tested. Sex was initially tested as an additional moderator in genotype-by-stressful life event models but was subsequently dropped after being found to be nonsignificant (results not shown). Age and sex were used as covariates in every model (results not shown). SNPs were dummy-coded in order to simultaneously test for both additive and dominant genetic effects within the same model. The advantage of using dummy coding for SNPs is that it allows you to incorporate two interaction terms into your model. If both interaction terms are significant, you have evidence of an additive genetic effect. Alternatively, if only one interaction term is significant, results support a dominant or recessive genetic effect. For testing for additive effects in particular, using dummy codes protects against the false outcomes inherent to using a single cross-product term in genetic interaction analyses (Aliev, Latendresse, Bacanu, Neale, & Dick, 2014). The reason for this inherent problem is that when SNPs are coded additively as 0, 1, and 2, as has been most common in previous GxE designs, this coding creates more unknowns than can be accurately captured by traditional linear regression with a cross-product term. Specifically, additive coding in this way creates two problems: 1) slope difference between genotype 0 and 1 is forced to be the same as the slop difference between genotypes 1 and 2; and 2) all three regression lines are forced to cross at the same point. The use of dummy coding increases the number of parameters and allows us to accurately measure data with three genotype levels.
In order to determine if genetic differences are significant in conditions of low and/or high frequency of stressors, we calculated regions of significance (RoS) (Johnson & Neyman, 1936). Regions of significance identify the levels of life stress at which genotypic differences reflect significant mean differences in MDD symptoms (i.e., the points at the lower and upper end of the graph where the differences between genotypes are significant). We also calculated the proportion of interaction (PoI) (Roisman et al., 2012). The PoI is a measure of differential susceptibility defined within a range of ± 2 SDs on the X variable that is unaffected by sample size. It provides an index of whether or not a crossover interaction is best defined in terms of differential susceptibility, the theory that some individuals do the worst in high stress but the best in low stress, or diathesis stress, the theory that individuals that do poorly in high stress show no difference with other individuals in low stress (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007). Values of PoI closer to .50 are more suggestive of differential susceptibility and values closer to .00 suggest diathesis stress (Roisman et al., 2012). Finally we calculated the proportion affected (PA). The PA estimates the percentage of participants that fall above the crossover point (the value on X at which regression lines for the genotypes cross). If a large percentage of participants do not experience the crossover effect (i.e., there are few participants after the crossover) then the interaction effect is not well explained by the differential susceptibility framework.
Results
Participants had a mean of 5.77 (SD=4.66, range = 0–20) MDD symptoms and a mean frequency of 5.75 (SD=5.23, range = 0–25) stressful life events in the previous year. There was a moderately significant correlation between MDD symptoms and frequency of stressful life events (r = .32, p < .01). Girls had a higher number of MDD symptoms than boys (M = 6.31 vs M = 5.22, p < .01, respectively). There was no difference on frequency of life stressors by gender. There were also no differences by age on either MDD symptoms or frequency of stressful life events. Frequencies for OPRM1 SNPs are presented in Table 1. The main effect of OPRM1 SNPs on frequency of stressful life events was also tested and no significant associations were found (results not shown).
Table 1.
OPRM1 SNP Frequencies
| SNP | Chromosomal position | Alleles | Genotype frequency (%)
|
||
|---|---|---|---|---|---|
| Homozygous for major allele | Heterozygous | Homozygous for minor allele | |||
| rs12205732 | 154400626 | G:A | 80.2 | 19.3 | 0.6 |
| rs1799971 | 154402490 | A:G | 96 | 4 | 0 |
| rs553202 | 154406510 | C:T | 30.3 | 51.2 | 18.5 |
| rs524731 | 154416785 | C:A | 78.6 | 19.5 | 2 |
| rs3778150 | 154425351 | T:C | 66.4 | 29.7 | 3.9 |
| rs10457090 | 154432766 | A:G | 86.3 | 13.7 | 0 |
| rs9478503 | 154434368 | T:C | 62.7 | 32.2 | 5.1 |
| rs589046 | 154434831 | T:C | 29.2 | 53 | 17.7 |
| rs3778157 | 154447394 | T:C | 66 | 30.2 | 3.7 |
| rs10485057 | 154454948 | A:G | 67.4 | 29.7 | 2.9 |
| rs562859 | 154456266 | T:C | 33.8 | 52.4 | 13.8 |
| rs511420 | 154465725 | T:C | 61.9 | 34.9 | 3.2 |
| rs9322447 | 154466013 | A:G | 40.8 | 48.8 | 10.3 |
| rs681243 | 154469433 | C:T | 26.7 | 51.5 | 21.8 |
| rs504932 | 154472161 | A:G | 67 | 29.4 | 3.6 |
| rs512053 | 154481209 | G:T | 97.5 | 2.5 | 0 |
| rs658156 | 154483218 | G:A | 32.6 | 51.1 | 16.4 |
| rs645027 | 154483822 | A:G | 83.2 | 16.7 | 0.2 |
| rs644261 | 154483943 | G:C | 73.9 | 24.3 | 1.8 |
| rs613341 | 154484971 | C:T | 82.8 | 15.9 | 1.3 |
| rs616585 | 154485574 | G:A | 52.1 | 41.8 | 6.1 |
| rs10485058 | 154486907 | A:G | 87.8 | 11.4 | 0.7 |
| rs497315 | 154489237 | A:G | 56.2 | 39.5 | 4.3 |
| rs678122 | 154491795 | T:A | 32.5 | 51 | 16.5 |
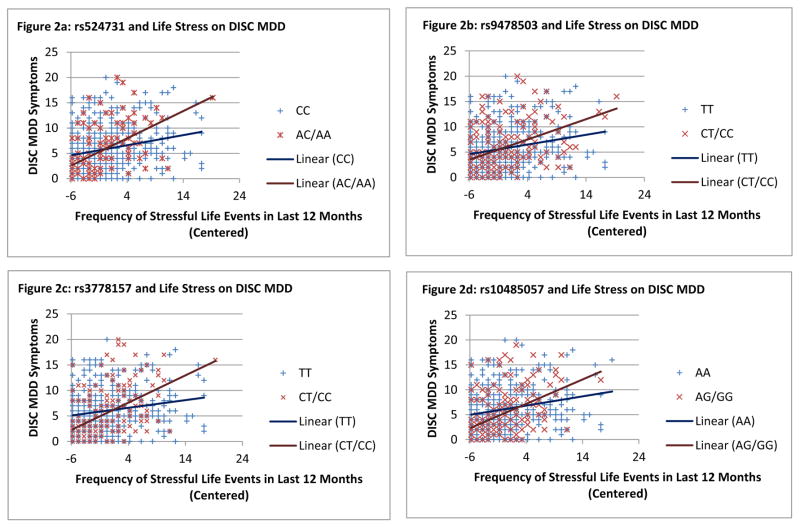
Results for the interactive effects of OPRM1 SNPs and frequency of life stressors are presented in Table 2. There were significant main effects of life stress on MDD symptoms in all models. There were no significant main effects of differences in OPRM1 genotype on MDD symptoms. In terms of interactions, there were no differences found when OPRM1 SNPs were treated as additive (i.e., interaction terms for both dummy codes were not simultaneously significant for any one SNP) but when heterozygous and minor-allele homozygous groups were combined, exposure to stressors significantly interacted with eight SNPs to predict MDD. Significance thresholds were then adjusted to account for multiple testing and linkage disequilibrium among SNPs. This adjustment was made using Li and Ji’s (2005) recommended method of using correlation matrix eigenvalues to adjust for multiple testing in multilocus analyses. Based on that adjustment, the threshold for significance was set at p < .003. Interactions with stress exposure and five SNPs remained significant: rs524731, rs3778157, rs511420, rs10485057, and rs9478503.
Table 2.
Interaction Effects of OPRM1 SNPs and Frequency of Life Stressors on DISC MDD Symptoms
| SNP | Main effect of Frequency of Stressful Life Events | Main effect of OPRM1 (major allele carriers combined)1 | Main effect of OPRM1 (minor allele carriers combined)2 | OPRM1 x Frequency of Stressful Life Events (major allele carriers combined)1 | OPRM1 x Frequency of Stressful Life Events (minor allele carriers combined)2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| β | ρ | β | ρ | β | ρ | β | ρ | β | ρ | |
| rs12205732 | 0.28 | .000 | 0.43 | .948 | 0.90 | .063 | −0.87 | .659 | −0.09 | .341 |
| rs1799971 | 0.26 | .000 | - | - | −0.38 | .691 | - | - | 0.12 | .536 |
| rs553202 | 0.15 | .024 | 0.01 | .978 | −0.39 | .368 | 0.00 | .976 | 0.15 | .063 |
| rs524731 | 0.19 | .000 | 0.17 | .901 | 0.02 | .966 | 0.25 | .383 | 0.33 | .000* |
| rs3778150 | 0.19 | .000 | −1.75 | .075 | 0.56 | .173 | 0.18 | .190 | 0.22 | .007 |
| rs10457090 | 0.27 | .000 | - | - | 1.02 | .063 | - | - | −0.10 | .375 |
| rs9478503 | 0.16 | .000 | −0.46 | .609 | 0.30 | .464 | 0.05 | .778 | 0.24 | .002* |
| rs589046 | 0.31 | .000 | 0.03 | .952 | −0.24 | .572 | −0.13 | .168 | −0.03 | .761 |
| rs3778157 | 0.14 | .001 | 0.23 | .814 | −0.46 | .248 | 0.14 | .458 | 0.36 | .000* |
| rs10485057 | 0.18 | .000 | 0.22 | .843 | −0.79 | .055 | −0.01 | .976 | 0.30 | .000* |
| rs562859 | 0.24 | .000 | −1.10 | .052 | −0.74 | .073 | 0.02 | .891 | 0.01 | .864 |
| rs511420 | 0.15 | .000 | 1.39 | .185 | −0.82 | .037 | −0.13 | .554 | 0.33 | .000* |
| rs9322447 | 0.32 | .000 | −0.57 | .368 | 0.66 | .094 | 0.13 | .226 | −0.12 | .136 |
| rs681243 | 0.23 | .001 | −1.29 | .007 | 0.04 | .919 | 0.04 | .690 | 0.03 | .736 |
| rs504932 | 0.30 | .000 | 0.13 | .900 | −0.38 | .362 | −0.16 | .423 | −0.09 | .257 |
| rs512053 | 0.26 | .000 | - | - | 0.92 | .441 | - | - | 0.32 | .167 |
| rs658156 | 0.21 | .001 | −0.70 | .181 | −0.55 | .183 | 0.21 | .048 | 0.04 | .644 |
| rs645027 | 0.27 | .000 | 6.43 | .143 | 0.82 | .104 | - | - | −0.03 | .770 |
| rs644261 | 0.29 | .000 | 1.50 | .333 | −0.66 | .132 | −0.19 | .492 | 0.08 | .325 |
| rs613341 | 0.21 | .000 | 2.02 | .228 | −0.89 | .086 | 0.00 | .990 | 0.30 | .006 |
| rs616585 | 0.17 | .000 | −0.17 | .825 | −0.70 | .068 | −0.02 | .931 | 0.19 | .009 |
| rs10485058 | 0.28 | .000 | 0.99 | .657 | 1.20 | .043 | 0.60 | .415 | −0.17 | .193 |
| rs497315 | 0.32 | .000 | 0.44 | .666 | −0.69 | .075 | 0.29 | .121 | −0.14 | .063 |
| rs678122 | 0.22 | .001 | −0.64 | .229 | −0.78 | .058 | 0.07 | .538 | 0.06 | .428 |
Notes.
P<.003, significance threshold after adjusting for multiple testing in multilocus analyses.
Results based on first set of dummy codes where genotype was coded as 0, 0, 1.
Results based on second set of dummy codes where genotype was coded as 0, 1, 1.
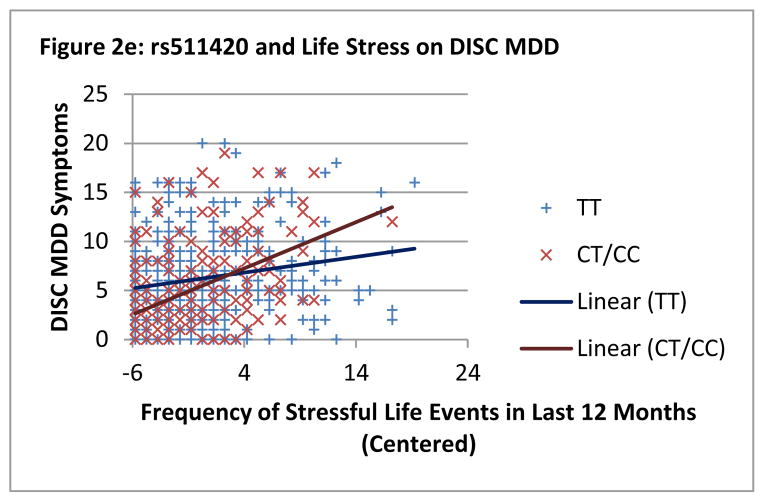
Significant interactions were graphed in order to understand the nature of the interactions (see Figures 2a–2e). For the interactions with all five SNPs, there appeared to be a crossover effect with minor allele carriers who experienced fewer stressful life events demonstrating the fewest MDD symptoms and minor allele carriers who experienced more stressful life events demonstrating the most MDD symptoms. Major allele homozygotes had flatter slopes by comparison. For SNP rs524731, regions of significance indicated that significant genetic differences were found at ≤.59 SDs below the mean and ≥.58 SDs above the mean on frequency of life stressors. PoI was .50, supporting the graphical evidence that minor allele carriers do better in low stress and worse in high stress on MDD symptoms compared to major allele homozygotes. Similar findings for SNPs rs9478503 and rs3778157 were also found (see Table 2). The findings for SNPs rs10485057 and rs511420 are not as strong in terms of support of a differential susceptibility framework as the other three significant SNPs, but results are still in the same direction and support similar conclusions.
Figure 2.
DISCUSSION
Results indicated that for adolescents exposed to fewer stressful life events, having one or two copies of the minor allele on one of the five OPRM1 SNPs is associated with fewer depression symptoms. Conversely, for adolescents exposed to more stressful life events, possessing one or more copies of the minor allele was associated with having more depression symptoms. The results also supported a dominant coding scheme over an additive one for the OPRM1 gene. However, for SNPs with significant interaction effects, the percentage of minor allele homozygotes was five percent of the sample or less, so the lack of support for additive effects may be a function of lack of power to detect them. These findings support a possible framework for the etiology of depression by which changes in life stress interact with pre-existing genetic susceptibilities. Stress can have a powerful effect on the human body with both short and long term biological consequences (Bremner & Vermetten, 2001). Differences in the encoding of mu-opioid receptors may further exacerbate these consequences and result in increases in depressive symptomology.
Whereas previous research has found a main effect of OPRM1 on depression (Kertes et al., 2011), the current findings only supports an effect of OPRM1 on depression dependent on levels of stress. They contribute uniquely to the literature by being the first to support an interaction between OPRM1 and life stress in explaining symptoms of depression. This association holds true across five separate SNPs in the OPRM1 gene. The results underscore the importance of the opioid system in understanding the biological underpinnings of depression. Past research has found a complex etiology for depression that encompasses multiple neurotransmitter systems, including opioids, and structures of the brain (Manji, Drevets, & Charney, 2001). Due to the complexity of the biological systems that have been linked with depression, it is not yet clear exactly how all of those systems function together to result in MDD or if there is a threshold regarding the number of dysregulated systems that cascade into MDD. We also know that depression has a strong genetic component (Boardman et al., 2011; Silberg et al., 1999). The current study has identified a specific gene that may represent a genetic factor involved in the opioid and larger dopaminergic system that may be a risk or protective factor, depending on the environmental stimuli.
Previous research has found that the interaction of life stress with genetic susceptibilities is related to depression (El Hage et al., 2009); results from the current study support this finding. The present findings have two major implications for prevention and intervention work. Allelic differences on OPRM1 that potentially result in changes to opioid levels have the potential to be addressed pharmacologically (Trescot, Datta, Lee, & Hansen, 2008). These findings also illustrate the importance of prevention/intervention work that either takes steps to reduce occurrences of life stress or provides the skills necessary for coping with them. Family interventions for prevention of drug use have been affective for adolescents with risk genotypes (Brody, Beach, Philibert, Chen, & Murry, 2009; Brody, Chen, Beach, Philibert, & Kogan, 2009). Perhaps depression prevention programs that target stress coping skills in adolescents with particular OPRM1 genotypes may have enhanced effectiveness.
The present study is limited in part by the nature of its sample. The genetic variants associated with depression in African American adolescents may not translate to other ethnic groups. At the same time, African Americans have been extremely underrepresented in psychiatric genetic research and their inclusion is critical to assure that they reap the benefits of any resulting health interventions. Research in this area would also be strengthened by a longitudinal perspective on stress. While a retrospective approach to measuring stress is typical in the existing literature, future research should expand the current findings by looking at changes in life stress across multiple time points as well as the effects of more immediate life stressors. This study also has the same limitations that are inherent to candidate gene research (Duncan & Keller, 2011). Primarily, only one gene that functions within a complex biological system is addressed. It is necessary to further study the biological context surrounding the stress response to fully understand how allelic differences on SNPs within the OPRM1 gene alter that biological context. The next steps in addressing this issue would be studying other genes that have a role in the opioid system, as well as genes that potentially impact other neurotransmitters, along with OPRM1. Further research should also address the biological products of the OPRM1 gene, mu-opioid receptors. To fully understand the role of the interaction between OPRM1 and stress on depression, exploring associations between OPRM1 and mu-opioid receptor density may be necessary.
Table 3.
Probing Interaction Effects of OPRM1 SNPs and Frequency of Life Stressors on MDD Symptoms
| SNP | RoS (Frequency of life Stressors) | PoI | PA | Crossover (SD from Mean) | |
|---|---|---|---|---|---|
| Lower Bound (SD from Mean) | Upper Bound (SD from Mean) | ||||
| rs524731 | −.59 | .58 | .50 | .41 | −.01 |
| rs9478503 | −1.08 | .50 | .59 | .49 | −.18 |
| rs3778157 | −.17 | .68 | .39 | .35 | .22 |
| rs10485057 | −.02 | 1.40 | .27 | .24 | .49 |
| rs511420 | −.02 | 1.17 | .29 | .24 | .44 |
Note. RoS, Regions of Significance; PoI, proportion of interaction; PA, proportion affected; Crossover, point on Frequency of Stressful Life Events that regression lines cross.
Acknowledgments
Role of the Funding Source: This project was supported by a grant from the National Institute of Drug Abuse (RO1DA025039). Research supported by NIDA is subject to the National Institute of Health’s (NIH) policy requirement that “authors submit to PubMed Central (PMC), or have submitted on their behalf, their peer-reviewed author manuscripts, to appear on PMC no later than 12 months after final publication”.
Footnotes
Contributors: Greg Swann is responsible for the central hypotheses in this manuscript, all of the statistical analyses, and the majority of writing. Gayle Byck was involved in the conception of the manuscript and consulted on every aspect of the manuscript from initial analyses through to the final stages of writing, as well as cleaning of phenotypic data. Danielle Dick oversaw all aspects of the genotyping as the PI of the genotyping site, from reviewing selection of genes, SNPs, lab progress, and data cleaning of genotypic data. Fazil Aliev and Shawn Latendresse were involved in cleaning the genotypic data and producing the genotypic files for analysis, and associated write-ups of the methods section. Fazil Aliev also consulted on the analytic approach taken. Brien Riley is the director of the molecular lab and was responsible for establishing the protocols and overseeing all aspects of DNA collection, extraction and genotyping. Cuie Sun was involved in DNA receipt, extraction, and genotyping of all samples. Darlene Kertes was involved in the selection of the OPRM1 gene for genotyping, including the selection of all markers for genotyping. Jessica Salvatore provided review and feedback on the organization of the manuscript and analyses. John Bolland is the PI of the Mobile Youth Survey, the parent study of the Gene Environment Neighborhood Initiative (GENI), and contributed significantly to study design and protocol. Brian Mustanski is the PI of GENI and, in addition to being responsible for a great amount of the design and protocol for GENI, also contributed to the current manuscript at every stage of development, including consulting on the hypotheses, analytic approach, and writing. All authors read over the manuscript and helped contribute to its content.
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev F, Latendresse SJ, Bacanu SA, Neale MC, Dick DM. Testing for measured gene-environment interaction: Problems with the use of cross-product terms and a regression model reparameterization solution. Behavior Genetics. 2014 doi: 10.1007/s10519-014-9642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attar BK, Guerra NG, Tolan PH. Neighborhood disadvantage, stressful life events, and adjustments in urban elementary-school children. Journal of Clinical Child Psychology. 1994;23:391–400. [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics (Oxford, England) 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For Better and For Worse: Differential Susceptibility to Environmental Influences. Current Directions in Psychological Science. 2007;16(6):300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Boardman JD, Alexander KB, Stallings MC. Stressful life events and depression among adolescent twin pairs. Biodemography Soc Biol. 2011;57(1):53–66. doi: 10.1080/19485565.2011.574565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: Behavioral and biological consequences. Development and Psychopathology. 2001;13(03):473–489. doi: 10.1017/s0954579401003042. null. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SR, Philibert RA, Chen YF, Murry VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: gene x environment hypotheses tested via a randomized prevention design. Child Development. 2009;80(3):645–661. doi: 10.1111/j.1467-8624.2009.01288.x. CDEV1288 [pii] [DOI] [PubMed] [Google Scholar]
- Brody GH, Chen YF, Beach SR, Philibert RA, Kogan SM. Participation in a family-centered prevention program decreases genetic risk for adolescents’ risky behaviors. Pediatrics. 2009;124(3):911–917. doi: 10.1542/peds.2008-3464. peds.2008-3464 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau AM, Mezulis AH, Hyde JS. Stress and emotional reactivity as explanations for gender differences in adolescents’ depressive symptoms. J Youth Adolesc. 2009;38(8):1050–1058. doi: 10.1007/s10964-009-9398-8. [DOI] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31(1):204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: interacting contributions of puberty and peer stress. Dev Psychopathol. 2009;21(2):593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57(1):21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(4):729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage W, Powell JF, Surguladze SA. Vulnerability to depression: what is the role of stress genes in gene x environment interaction? Psychol Med. 2009;39(9):1407–1411. doi: 10.1017/S0033291709005236. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33(2):227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res. 2008;32(7):1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franic S, Middeldorp CM, Dolan CV, Ligthart L, Boomsma DI. Childhood and Adolescent Anxiety and Depression: Beyond Heritability. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(8):820–829. doi: 10.1016/j.jaac.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology. 1994;30:467–483. [Google Scholar]
- Gorman Smith D, Tolan P. The role of exposure to community violence and developmental problems among inner-city youth. Development and Psychopathology. 1998;10(1):101–116. doi: 10.1017/s0954579498001539. [DOI] [PubMed] [Google Scholar]
- Hedrick P, Kumar S. Mutation and linkage disequilibrium in human mtDNA. Eur J Hum Genet. 2001;9(12):969–972. doi: 10.1038/sj.ejhg.5200735. [DOI] [PubMed] [Google Scholar]
- Ide S, Sora I, Ikeda K, Minami M, Uhl GR, Ishihara K. Reduced emotional and corticosterone responses to stress in mu-opioid receptor knockout mice. Neuropharmacology. 2010;58(1):241–247. doi: 10.1016/j.neuropharm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson &, Neyman Tests of certain linear hypotheses and their application to some educational problems. Statistical Research Memoirs. 1936;1:57–93. [Google Scholar]
- Johnson, Whisman MA, Corley RP, Hewitt JK, Rhee SH. Association between depressive symptoms and negative dependent life events from late childhood to adolescence. J Abnorm Child Psychol. 2012;40(8):1385–1400. doi: 10.1007/s10802-012-9642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63(11):1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Kalsi G, Prescott CA, Kuo PH, Patterson DG, Walsh D, Riley BP. Neurotransmitter and neuromodulator genes associated with a history of depressive symptoms in individuals with alcohol dependence. Alcohol Clin Exp Res. 2011;35(3):496–505. doi: 10.1111/j.1530-0277.2010.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 2005;95(3):221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Wickramaratne P, Weissman MM. Racial and ethnic differences in willingness to participate in psychiatric genetic research. Psychiatr Genet. 2009;19(4):186–194. doi: 10.1097/YPG.0b013e32832cec89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent NR, Lally MA, Brown L, Knopik VS, McGeary JE. OPRM1 and diagnosis-related posttraumatic stress disorder in binge-drinking patients living with HIV. AIDS Behav. 2012;16(8):2171–2180. doi: 10.1007/s10461-011-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74(4):765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pratt WM, Davidson D. Role of the HPA axis and the A118G polymorphism of the mu-opioid receptor in stress-induced drinking behavior. Alcohol Alcohol. 2009;44(4):358–365. doi: 10.1093/alcalc/agp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis-stress: recommendations for evaluating interaction effects. Dev Psychopathol. 2012;24(2):389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Higley JD, Suomi SJ, Heilig M, Barr CS. OPRM1 gene variation influences hypothalamic-pituitary-adrenal axis function in response to a variety of stressors in rhesus macaques. Psychoneuroendocrinology. 2011;36(9):1303–1311. doi: 10.1016/j.psyneuen.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher PW, Lucas CP. The Diagnostic Interview Schedule for Children (DISC) In: Henson M, editor. Comprehensive Handbook of Psychological Assessment. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- Shaffer D, Fisher PW, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Arch Gen Psychiatry. 1999;56(3):225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62(11):1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11(2 Suppl):S133–153. [PubMed] [Google Scholar]
- van den Oord EJ, Jiang Y, Riley BP, Kendler KS, Chen X. FP-TDI SNP scoring by manual and statistical procedures: a study of error rates and types. Biotechniques. 2003;34(3):610–616. 618–620, 622. doi: 10.2144/03343dd04. passim. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26(1):106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Barke RA, Loh HH, Roy S. Mu-opioid receptor mediates chronic restraint stress-induced lymphocyte apoptosis. J Immunol. 2002;169(7):3630–3636. doi: 10.4049/jimmunol.169.7.3630. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280(38):32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]