Abstract
Flavonoid phytochemicals act as both agonists and antagonists of the human estrogen receptors (ERs). While a number of these compounds act by directly binding to the ER, certain phytochemicals, such as the flavonoid compounds chalcone and flavone, elicit antagonistic effects on estrogen signaling independent of direct receptor binding. Here we demonstrate both chalcone and flavone function as cell type-specific selective ER modulators. In MCF-7 breast carcinoma cells chalcone and flavone suppress ERα activity through stimulation of the stress-activated members of the mitogen-activated protein kinase (MAPK) family: c-Jun N-terminal kinase (JNK)1 and JNK2. The use of dominant-negative mutants of JNK1 or JNK2 in stable transfected cells established that the antiestrogenic effects of chalcone and flavone required intact JNK signaling. We further show that constitutive activation of the JNK pathway partially suppresses estrogen (E2)-mediated gene expression in breast, but not endometrial carcinoma cells. Our results demonstrate a role for stress-activated MAPKs in the cell type-specific regulation of ERα function.
Keywords: flavonoids, phytoestrogens, estrogen receptor, mitogen-activated protein kinase, antiestrogens, c-Jun N-terminal kinase (JNK)
Introduction
Classic steroid hormone agonists and antagonists function through direct interaction with their cognate receptor, functioning as a ligand-inducible transcription factor, whereby they activate or inhibit transcriptional activity (1–2). Antiestrogens, such as tamoxifen, antagonize estrogen action by competing with estrogen for binding of the receptor. However, the complexity of tamoxifen’s activity is revealed through studies describing both agonistic and antagonistic effects of this compound in a tissue specific manner (3–4). This mixed action is partially determined by the ability of the ER to activate transcription via its activation domains, AF-1 and AF-2, and to activate tissue specific factors such as cofactors and cellular signaling cascades. In ER-positive breast cancer cell systems, the generation of tamoxifen-refractory cell types can be established while ER status and binding remain unchanged (5–7). These studies suggest that the antiestrogen activity demonstrated by tamoxifen may also be determined by the alternate activation of non-steroid hormone receptor signaling pathways that converge upon the ER. For example, the peptide hormone epidermal growth factor (EGF) and insulin-like growth factor-I (IGF-I) enhance ER function in both ligand-dependent and -independent manners. This activity is regulated by the ability of these growth factors to activate intracellular signaling events such as the Ras-Raf-MEK- signaling pathway (8–10). EGF- and IGF- mediated enhancement of ER function depends in part on activation, which results in subsequent ER phosphorylation (11–12). Additionally, recent reports have demonstrated that other members of the MAPK family including JNK and p38 also can regulate ER function (13–15). Therefore, complexity of the activity of a steroid hormone mimetic is defined by both tissue-and-cell type specific factors as well as alternate signaling events. Added to this complexity is evidence that activation of members of the MAPK cascade occurs not only in response to peptide hormones, but also in response to estrogens and antiestrogens (16–21). We and others have also demonstrated that endocrine-active environmental agents such as organochlorine pesticides, PCBs and flavonoid phytochemicals can affect other cellular signaling cascades (such as MAPK cascades) (22–25).
Given the ability of peptide hormone signaling to enhance steroid hormone function, the possibility exists that certain endocrine disrupting chemicals may also act to enhance or suppress ER function by receptor-binding independent mechanisms. We have previously examined the ability of certain flavonoid phytochemicals to activate or inhibit ER function in both yeast and mammalian cells (26–33). Our studies, and other reports, have identified certain phytochemicals that function as either agonists or antagonists through direct binding of the ER. In contrast, phytochemicals such as kaempferide, flavone, and apigenin can act as ER antagonists independent of direct ERα or ERβ binding (28, 34–37). This suggests that certain phytochemicals, as well as other environmental estrogens, might regulate ER function through mechanisms independent of direct receptor binding. Consistent with this evidence, certain flavonoids can stimulate MAPK-responsive transcription factors in hormone-independent ERnegative cell systems (34–38). These flavinoids, specifically flavone and chalcone, demonstrate anti-viability properties in breast and endometrial cells (XXX). Given the crosstalk between the MAPK cascade and the ER, we proposed that receptor-binding independent antiestrogenic flavonoids might function to regulate members of the MAPK cascade, specifically c-Jun N-terminal kinase (JNK), as a mechanism for their ER antagonistic activity. Here we describe the ability of certain flavonoid phytochemicals to activate the JNK signaling cascade and suppress ER transactivation in a JNK-dependent and cell type specific manner.
MATERIALS & METHODS
Materials
Flavone-(2-phenyl-4H-1-benzopyran-4-one) and chalcone-(1,3--diphenyl-2-propen-1-one) were purchased from INDOFINE Chemical Company Inc. (Somerville, NJ). 4-hydroxy tamoxifen and 17β-estradiol were purchased from Sigma Chemical Co. (St. Louis, MO) and ICI 182,780 was purchased from Tocris (Ellisville, MO). All chemicals were prepared in dimethylsulfoxide (DMSO) and added to the media so the final concentration of solvent did not exceed 0.1%. TNF-α was purchased from R&D Systems (Minneapolis, MN). Mammalian expression constructs containing dominant-negative JNK1 and JNK2 were generously provided by Dr. Roger Davis (University of Massachusetts).
Cell Culture
The ERα-positive MCF-7 breast carcinoma cells (N variant) (39), ERα-positive Ishikawa endometrial carcinoma cells (Ishikawa(+))(38), ERα-negative Human Embryonic Kidney 293 (HEK293) and ERα-negative HepG2 cells were cultured in 150 cm2 culture flasks in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL, Gaithersburg, MD), as well as BME and MEM amino acids, L-glutamine, sodium pyruvate, and penicillin-streptomycin (diluted in the medium to a 1× concentration from either 100× or 50× stocks for a final concentration of 1%), and porcine insulin (10−8 M) (Sigma Chemical Co., St. Louis, MO). The culture flasks were maintained in a cell incubator at a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Cells were placed in phenol red-free Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 5% dextran-coated charcoal-treated FBS for 48 hours prior to plating (5%CS-DMEM) (40–41).
Generation of stably transfected cell lines stable population expressing Vector, DN-JNK1 or DN-JNK2. MCF-7 cells were transfected with 3µg of vector or the dominant-negative JNK1 or JNK2 plasmid using 9µl of lipofectamine. After 5 hours, the transfection media was removed and replaced with 10% FCS-DMEM for 48 hours. Cells were split into T-75 flasks in 10% FCS-DMEM and allowed to attach overnight. The next day the media was removed and replaced with 10% FCS-DMEM containing 750µg/ml of G418. Every three days the media was changed to fresh 10% FCS-DMEM +750µg/ml G418. Following three weeks of selection the MCF-7 cells were examined for expression.
In Vitro JNK Kinase Assay
Cells were harvested in lysis buffer (20mM HEPES, 50mM β–glycerolphosphate, 2mM EGTA, 1mM DTT, 10mM NaF, 1mM Na2VO4, 1% Triton X-100, 10% glycerol, 1mM PMSF, .01mg/ml aprotinin, and .01mg/ml leupeptin) on ice and 300 µg of cell extract was immunoprecipitated for 60 min with antiJNK antibody (Santa Cruz) followed by a 60 min incubation with a 50% protein A Sepharose 4B slurry. The JNK bound beads were washed with a wash buffer (500mM LiCl, 100mM Tris-HCl pH 7.6, 0.1% Triton X-100, and 1mM DTT) and resuspended in 50µl of assay dilution buffer (20mM MOPS, pH 7.2, 25mM β-glycerolphosphate, 5mM EGTA, 1mM sodium orthovanadate, 1mM dithiothreitol). 1µg of c-Jun-GST protein (Upstate Biotechnology, Lake Placid, NY) and 10µl of (γ-32P)ATP (100µCi/10µl) diluted into 90µl of Magnesium/ATP cocktail (500µM ATP and 75mM magnesium chloride in assay dilution buffer) were added to each experimental sample. The reaction was allowed to proceed at 30°C in a shaking incubator for 30 min and terminated with EDTA. 40µl of reaction mixture was placed on PC(P81) paper in a millipore apparatus and washed 5× with 180 mM phosphoric acid. Filters were allowed to air dry and then placed in scintillation fluid and counted using a Beckman LS6500 multi-purpose scintillation counter.
Western Blot Analysis
Stable expression of DN-JNK1 and DN-JNK2 were examined by western blot analysis as described previously (42–43). Briefly, cells were harvested in sonicating buffer (62.5 mM Tris-HCl, pH 6.8, 4% (w/v) SDS, 10% glycerol. 1 mM phenylmethylsulfonyl fluoride (PMSF), 25 mg/ml leupeptin, 25 mg/ml aprotinin) and sonicated for 30 seconds. Following centrifugation at 1,000 × g for 20 minutes, 50 µg of protein was resuspended in sample loading buffer (62.5 M Tris-HCl, pH 6.8, 2% (w/v) SDS, 10% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue), boiled for 3 minutes and electrophoresed on a 10% polyacrylamide gel. The proteins were transferred electrophoretically to a nitrocellulose membrane. The membrane was blocked with PBS-Tween (0.05%) - 5% lowfat dry milk solution at 4°C overnight. The membrane was subsequently incubated with mouse anti-human JNK1,2 (Pharmingen, San Diego, CA) specific monoclonal antibody 1.5µg/µl and incubated for 2 hours at room temperature. Blots were washed in PBS-Tween solution and incubated with goat anti-mouse secondary antibodies conjugated to horseradish peroxidase (1:5000 dilution Oxford, Oxford, MI) for 30 minutes at room temperature. Following four washes with PBS-Tween solution, immunoreactive proteins were detected using the ECL chemiluminescence system (Amersham, Arlington Heights, IL) and recorded by fluorography on Hyperfilm, according to the manufacturer’s instructions. Fluorograms were quantitated by image densitometry using the Molecular Analyst program for data acquisition and analysis (BioRad).
Luciferase Assays
Transient transfections: The cells were plated in 6-well plates at 1 × 106 cells/well in 5%CS-DMEM media and allowed to attach overnight. The next day the cells were transfected for 5 hours in serum/supplement-free DMEM with 2 µg of ERE2-luciferase plasmid which contains two copies of the vitellogenin ERE linked to the luciferase gene. For ERE-luciferase experiments in ERα-negative HEK293 or HepG2 cells, co-transfection with pcDNA3- ERα (500 ng) was used (26, 44–45). For experiments in stable Vec, DN-JNK1 or DN-JNK2 expressing MCF-7 cells were transfected with either pERE2-luciferase using Lipofectamine as above or with an AP-1(7×)-luciferase plasmid (Stratagene, La Jolla, CA) using FuGENE6 (Roche, Indianapolis, IN) according to manufacturer’s instructions. Similarly experiments using stable cells transfected with Gal4-luciferase in combination with Gal4-cJun or Gal4-Elk were performed with FuGENE6 as described previously (46). After 6 hours, the transfection media was removed and replaced with 5%CS-DMEM containing vehicle, 17β-estradiol (1 nM), flavone (25 uM), chalcone (10 uM), or 17β-estradiol plus flavone or chalcone and incubated for 18 hours at 37 °C. After the 18 hours the treatment containing media was removed and 250 µl of 1× lysis buffer (Analytical Luminescence Laboratory, Ann Arbor, MI) was added per well and incubated for 15 min at RT. The cell debris was then pelleted by centrifugation at 15000g for 5 min. The cell extracts were normalized for protein concentration using the Bio-Rad Reagent following the supplied protocol (Bio-Rad Laboratories, Hercules, CA). Luciferase activity for the cell extracts were determined using Luciferase Substrate (Promega, Madison, WI) in a Monolight 2010 luminometer (Analytical Luminescence Laboratory, Ann Arbor, MI).
Statistical Analysis
Data was analyzed using Student’s unpaired t-test with p<0.05 as the limit of statistical Significance (47–49). Experiments comparing multiple concentrations to the control were tested with one way ANOVA with Bonferroni post-test to compare individual concentrations. All statistical analyses were done using GraphPad Prism 5.0 (GraphPad Software).
RESULTS
Cell specific antiestrogenic activity of chalcone and flavone
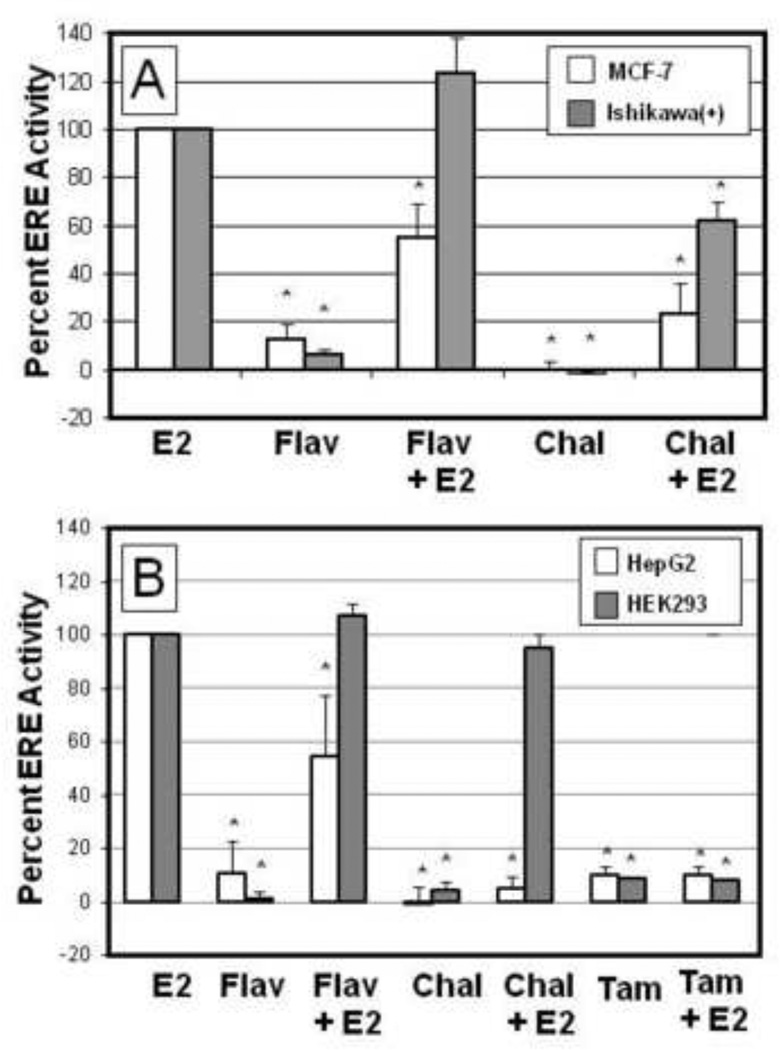
We sought to determine the cell-type specific estrogenic and antiestrogenic activity of the flavonoid phytochemicals flavone and chalcone (Fig 1). Using MCF-7 breast and Ishikawa(+) endometrial carcinoma cell lines which endogenously express ERα as the predominant estrogen receptor subtype (38) we examined the effects of flavone and chalcone on estrogen-stimulated ERE-luciferase activity (Fig 2A). 17β-estradiol (1nM) stimulated a 38.74±19.37 and 12.63±4.27 fold induction of ERE transcriptional activity in MCF-7 and Ishikwa cells, respectively, over control (data not shown). Flavone displayed an estrogenic trend, though not statistically significant, in MCF-7 (19.2±3.4%) and Ishikawa(+) (14.1±3.2%) cells as compared to the reference activity of the major endogenous estrogen, 17b-estradiol, alone (100%) (Fig 2A). In combination with 17β-estradiol, chalcone exhibited antiestrogenic activity in both cell types reducing ERE activity to 28.3± 11.2% in MCF-7 and 65.4±7.1% in Ishikawa(+) cells as compared to estradiol alone (100%). Interestingly flavone acted as an antiestrogen only in the MCF-7 cell line, suppressing ERE-activity to 51.3±13.8% as compared to E2 alone while actually increasing ERE-activity in conjunction with E2 in the Ishikawa(+) cells (120.9±13.6%). To further explore the cell type-specific estrogenic/antiestrogenic activities of chalcone and flavone we used ERα-negative HepG2 and HEK293 cell lines co-transfected with ERE-luciferase and an ERα expression construct (Fig 2B). We demonstrated that again flavone, but not chalcone, exerted a weak agonistic activity in the HepG2 line (20.2±8.6%), an effect that was not observed in the HEK293 cells. Both chemicals exerted antiestrogenic effects in the HepG2 cell line with flavone and chalcone reducing ERE-activity to (59.2±20.9%) and (16.5±7.4%) respectively as compared to E2 alone (100%). Interestingly, the HEK293 cells were refractory to the antiestrogenic activity of both flavone and chalcone. The antiestrogenic effects of these compounds did not correlate with direct competition for receptor binding, suggesting a binding independent mechanism for regulation of ER activity (28).
Figure 1.
Chemical structures of the receptor-binding independent antiestrogenic flavonoids chalcone and flavone.
Figure 2.
Cell specific antiestrogen activity of chalcone and flavone. (A), MCF-7 or Ishikawa(+) cells transfected with ERE-luciferase for 6 hours were treated with vehicle (DMSO), flavones (25 µM), or chalcone (25 µM) alone or in combination with 17β-estradiol for 24 hours and harvested for luciferase assay. (B), ER-α-negative HEK293 or HepG2 cells were transfected with both ERE-luciferase and ER-α followed by treatment with with vehicle (DMSO), Tamoxifen (Tam, 100 nM), flavone (25 µM), or chalcone (25 µM), alone or in combination with 17β-estradiol for 24 hours and harvested for luciferase assay. Data are represented as percent activation as compared to 1nM 17β-estradiol (100%) ± SEM from four independent experiments each in duplicate (*, p< 0.05).
Flavonoid-induced JNK-activation
The ability of estrogens and antiestrogens to activate MAPKs suggests a mechanism by which endocrine-active agents such as flavonoids may exert biological effects. Additionally, environmental agents such as flavonoids and organochlorines also regulate gene expression in part through the activation of specific members of the MAPK signaling cascade. Given that the basal or stimulated activation of members of the MAPK cascade regulate ER-function in both a ligand-dependent and -independent manner, we initially examined the activation of JNK signaling in response to flavone and chalcone.
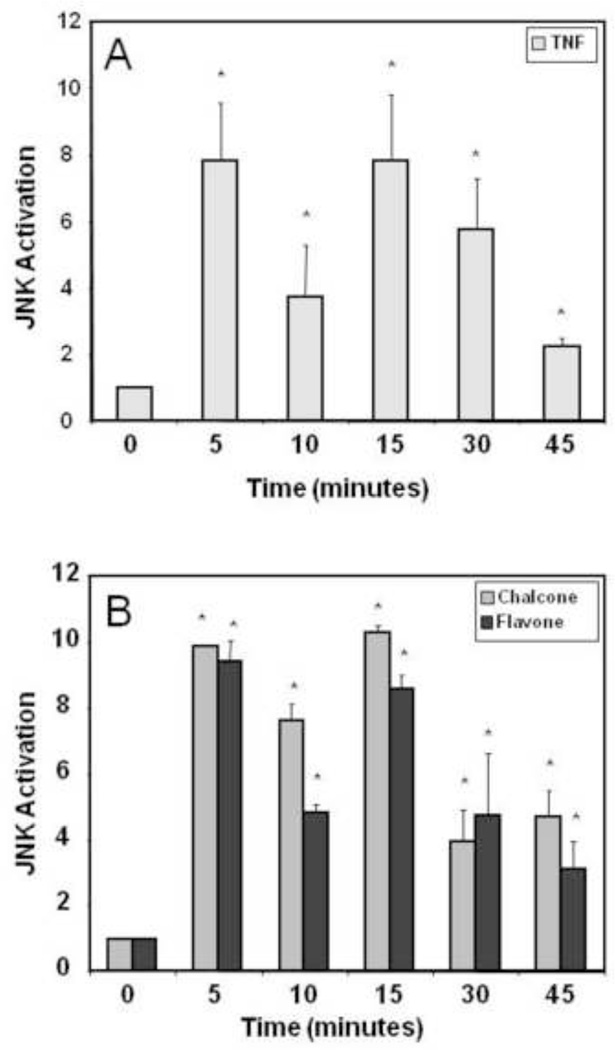
TNF-α rapidly and potently activates JNK in MCF-7 cells (50) and represses ER function in certain systems (51–53). Here, we demonstrate that TNF-α (10 ng/ml) activates JNK in MCF-7 cells in a time dependent biphasic manner with a maximal fold activation of 7.8±1.78 and 7.8±2.03 occurring at 5 and 15 minutes respectively with activity returning to near control levels (2.25±0.25 fold) at 45 minutes (Fig 3A). Flavone and chalcone (Fig 3B) displayed a time dependent activation of JNK similar to TNF-α. At 5 min, chalcone and flavone activated JNK 9.86±0.06 fold and 9.41±0.64 fold, with subsequent increases in JNK activity up to 45min compared to control. These results demonstrate the ability of both chalcone and flavone to potently stimulate JNK activity with a similar duration and magnitude as TNF.
Figure 3.
Activation of JNK by TNF-α and the flavonoid phytochemicals chalcone and flavone. MCF-7 cells were treated with either (A) TNF-α (10 ng/ml), (B) chalcone (25 µM), or flavone (25 µM), for times indicated from 0 to 45 minutes. Cells were harvested and assayed for JNK activity as described in Material & Methods using an in vitro kinase assay. Data are represented as fold activation from control (0 minutes) ± SEM from three independent experiments each in duplicate.
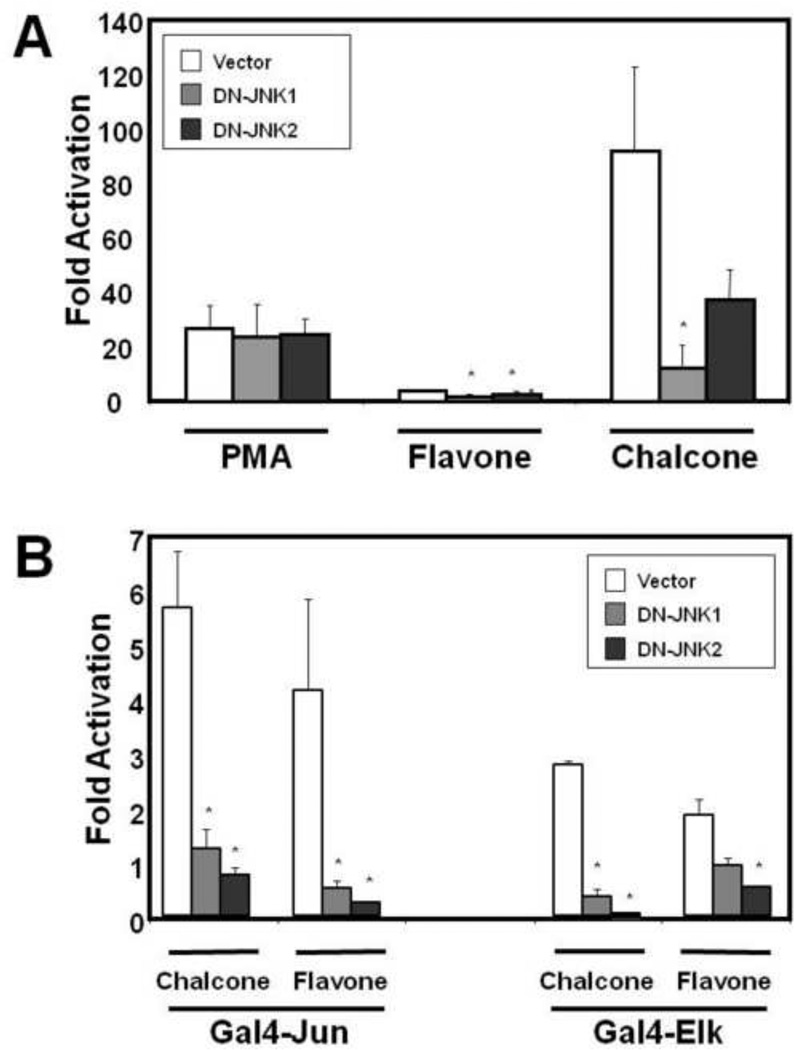
Stable inhibition of JNK-1 and JNK-2 in MCF-7 cells blocks flavonoid-induced activation of AP-1, Elk, and c-Jun
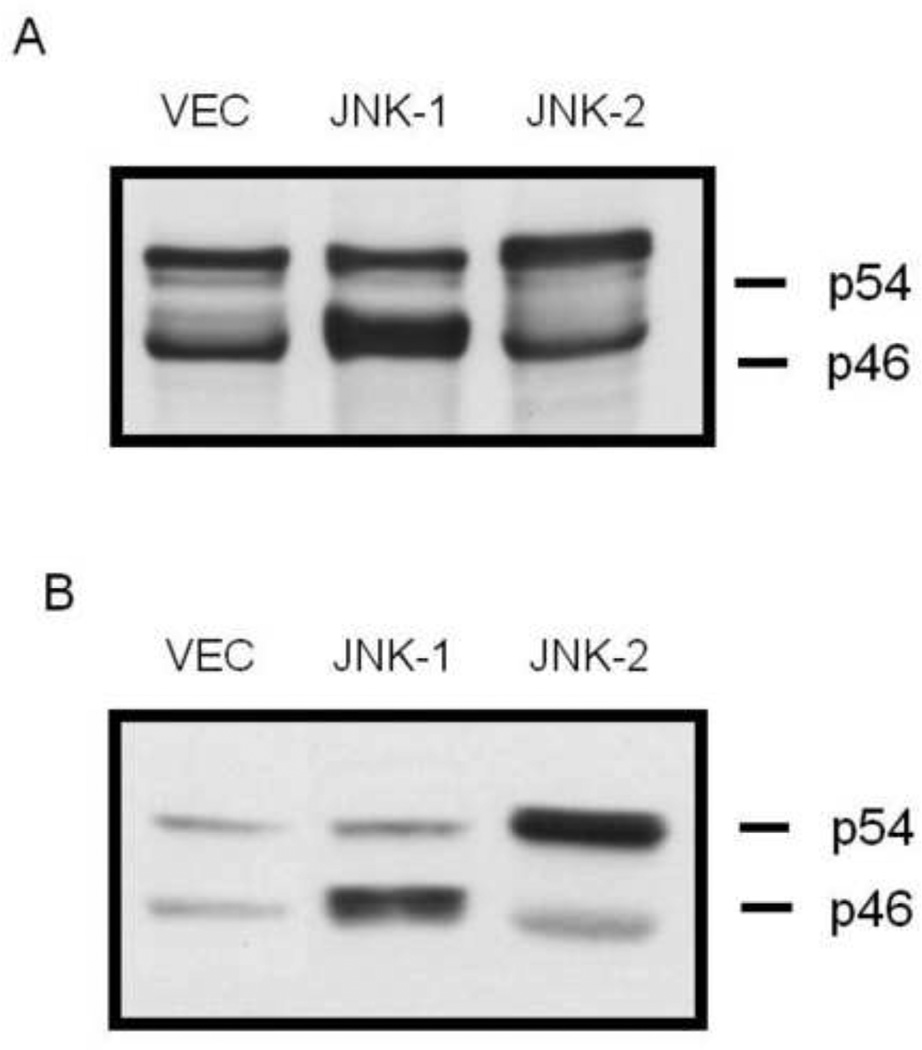
To determine if JNK activation by phytochemicals mediated antagonistic effects of the receptor-binding independent antiestrogenic flavonoids, MCF-7 cells were transfected with either empty vector (Vec) or mammalian expression vector containing either a dominant-negative JNK-1 (DN-JNK1) or a dominant-negative JNK-2 (DN-JNK2) gene and pooled, stable-expressing cell lines were selected. Western blot analysis confirmed stable expression of either DN-JNK1 or DN-JNK-2 (Fig 4A). For comparison to the stable cell lines, MCF-7 cells were transiently-transfected with Vec, DN-JNK1, or DN-JNK2 and expression was confirmed (Fig 4B). Exclusive overexpression of individual isoforms of JNK1 (p46) or JNK2 (p54) can be seen in both stable pools and transiently-transfected cells.
Figure 4.
Stable expression of dominant-negative JNK-1 or JNK-2 isoforms. (A) MCF-7 cells stably (A) or transiently (B) expressing either empty vector (VEC), dominant-negative JNK-1 (DN-JNK-1), or dominant-negative JNK-2 (DN-JNK-2) were generated as described in the Materials & Methods section. Pooled populations were harvested and immunoblot analysis was used to determine expression of either JNK-1 or JNK-1 expression. Molecular weight markers indicate migration of JNK-1 (p46) or JNK-2 (p55) bands.
To confirm that stable expression of either DN-JNK1 or DN-JNK2 suppressed signaling through the JNK cascade, reporter-gene assays responsive to MAPK stimulation were utilized. Stable MCF-7/Vec, MCF-7/DN-JNK1, or MCF-7/DN-JNK2 cells were transfected with an AP-1 responsive luciferase construct and treated with a known activator of the MAPKs, 12-phorbol-myristate-13-acetate (PMA), or with selected JNK-activating flavonoids (Fig 5A). PMA stimulated AP-1-reporter activation in MCF-7/Vec (26.8±8.5 fold), MCF-7/DN-JNK1 (23.7±11.9 fold) or MCF-7/DN-JNK2 (24.3±5.8 fold) cells to a similar extent. The inability of either DN-JNK1 or DN-JNK2 to suppress AP-1 stimulation by PMA is consistent with PMA’s predominant activation of Erk as compared to JNK in these cells (data not shown). In MCF-7/Vec cells, AP-1 stimulation by both flavone (4.0±0.3 fold) and chalcone (92±31.3 fold) was reduced in MCF-7/DN-JNK1 (1.9±0.5 and 12.2±8.2 fold) or MCF-7/DN-JNK2 (2.8±0.8 and 37.5±11.1 fold) cells.
Figure 5.
Regulation of flavonoid-stimulated AP-1, c-Jun, and Elk transcriptional activation by DN-JNK isoform expression. (A), Stable expressing MCF-7/Vec, MCF-7/DN-JNK1, and MCF-7/DN-JNK2 cells were transfected with an AP-1-luciferase reporter construct 6 hours followed by treatment with either vehicle (DMSO), PMA (20 ng/ml), flavone (25 µM) or chalcone (25 µM). (B), Stable expressing MCF-7/Vec, MCF-7/DN-JNK1, and MCF-7/DN-JNK2 cells were transfected with a GAL4-UAS-Luciferase construct in conjunction with either a GAL4-DBD-Jun or GAL4-DBD-Elk plasmid followed by treatment with either vehicle (DMSO), chalcone (25 µM) or flavones (25 µM). Cells were harvested the following day and analyzed for luciferase activity as indicated in the Materials and Methods. Data are represented as fold activation from vehicle control samples for each cell line ±SEM of four experiments in duplicate (AP-1) or as a representative experiment in duplicate (c-Jun, Elk) (*, p< 0.05).
Increased AP-1 activity is induced by MAPK phosphorylation of the transcription factor Elk, resulting in increased expression of c-fos as well as by direct phosphorylation of c-Jun by JNK. Using Gal4 fusions of either Elk or c-Jun, flavone stimulated activity 4.3 and 1.9 fold respectively; while chalcone stimulated 5.8 and 2.9 fold respectively in MCF-7/Vec cells above baseline (Fig 5B). Activation of Gal4-c-Jun by flavone and chalcone was reduced in MCF-7/DN-JNK1 (0.53 and 1.3 fold) and MCF-7/DN-JNK2 (0.8 and 0.3 fold) cells. Similarly, activation of Gal4-Elk by flavone and chalcone was reduced in both MCF-7/DN-JNK1 (1.0 and 0.4 fold) and MCF-7/DN-JNK2 (0.5 and 0.1 fold) cells. The ability of both chalcone and flavone to stimulate AP-1 transcription through c-Jun or Elk activation is correlated with increased JNK activation, and dependent upon an intact JNK 1/2 cascade.
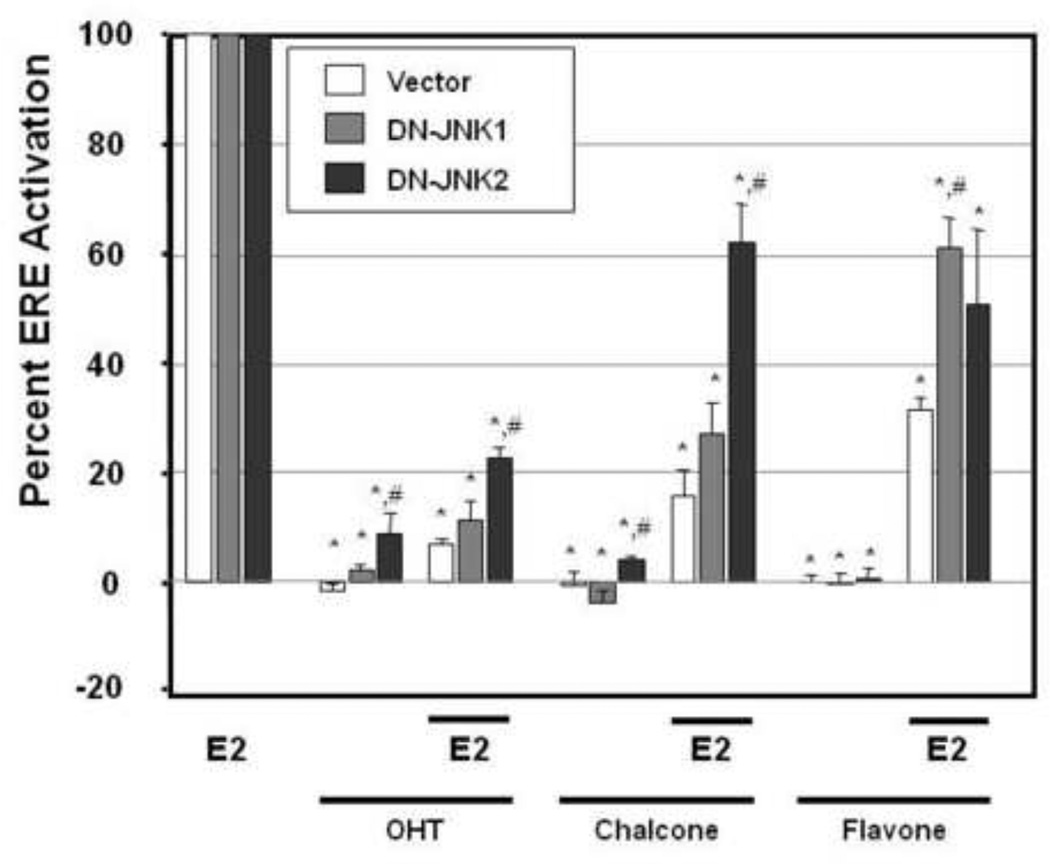
JNK-mediated antiestrogenic effects of flavonoid phytochemicals
The effects of stable expression of Vec, DN-JNK1, or DN-JNK2 on ERE-activity were found to be similar with 17β-estradiol (1nM) stimulating a 14.4±1.6, 14.9±2.1 and 14.3±1.7 fold induction respectively over control (data not shown). In the MCF-7/Vec cells, 4-OH tamoxifen, chalcone, and flavone reduced ER-mediated gene transcription by 93±1.1%, 84±4.7%, and 68.4±2.5% respectively (Figure 6). Stable inhibition of the JNK1 signaling pathway in the MCF-7/DN-JNK1 cells did not significantly (P>0.05) affect chalcone’s inhibition of E2-stimulated transcription which decreased from an 84±4.7% reduction in MCF-7/VEC cells to 72.8±5.6% in the MCF-7/DN-JNK1 cells (Figure 6). In the case of flavone, a significant role (p<0.05) for this signaling pathway is demonstrated with a reduction in its antagonistic activity from a 68.4±2.3% reduction in MCF-7/VEC cells to a 38.7±5.5% reduction in the stable MCF-7/DN-JNK1 cells, suggesting a role for the signaling pathway in the mediation of ER-mediated gene transcription. Similar results were observed in the stable DN-JNK2 expressing MCF-7 cells with chalcone’s inhibition of ER-mediated gene transcription significantly (p< 0.05) decreased from a 84±4.7% reduction in the MCF-7/VEC cells to a 37.9±7.1% reduction in the MCF-7/DN-JNK2 cells. Likewise, a potential role for the JNK2 signaling pathway for flavone is suggested by the statistically significant reduction in flavone’s inhibition of ER-mediated transcription from a 68±2.3% reduction in MCF-7/VEC to a 49.2±13.8% reduction in the MCF-7/DN-JNK2 cells. Inhibition of the JNK1 signaling pathway did not significantly affect the ability of 4-OH tamoxifen to function as an antiestrogen which still inhibited E2-stimulated ERE-activity by 88.3±3.3% in the MCF-7/DN-JNK1 cells. In the MCF-7/DN-JNK2 cells, 4-OH tamoxifen’s antagonism of E2-mediated gene expression was significantly abrogated from a 93±1.1% reduction to a 77.1±1.8% reduction (p<0.05). Interestingly however, stable expression of DN-JNK2 enhanced the agonistic activity with 4-OH tamoxifen alone yielding a statistically significant 9.0±3.7% activation of ERE in MCF-7/DN-JNK2 cells as compared to MCF-7/VEC cells.
Figure 6.
Antiestrogenic effects of chalcone and flavone require intact JNK signaling. Stable expressing MCF-7/Vec, MCF-7/DN-JNK1, and MCF-7/DN-JNK2 cells were transfected with an ERE-luciferase reporter construct using Lipofectamine for 6 hours followed by treatment with either vehicle (DMSO), 4-OH-tamoxifen (100 nM), chalcone (25 µM), or flavones (25 µM) alone or in combination with 17β-estradiol for 24 hours and harvested for luciferase assay. Data are represented as normalized percent activation as compared to 1 nM 17β-estradiol (100%) ± SEM from four independent experiments each in duplicate (*, p< 0.05 compared to E2 control, #, p < 0.05 compared to vector control).
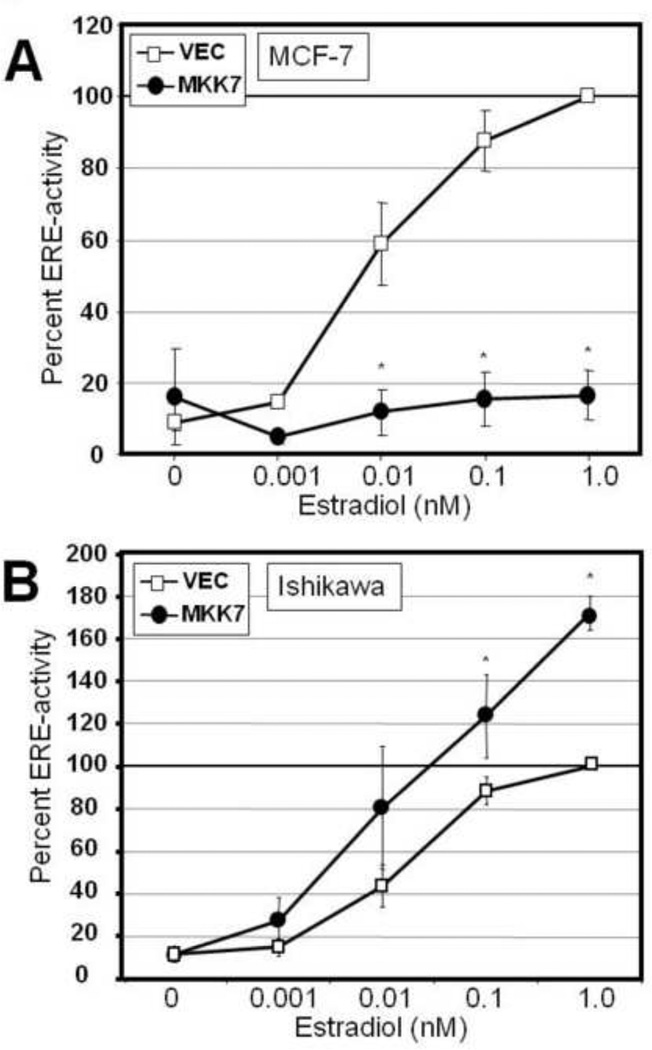
Constitutive activation of MKK7-JNK signaling suppresses E2-mediated gene expression in a cell-type specific manner
To confirm the role of JNK activation in suppression of ER activation, a constitutively-active mitogen-activated protein kinase kinase 7 expression construct (MKK7-CA) was used in MCF-7 (Fig 7A) and Ishikawa(+) cells (Fig 7B). In MCF-7 cells, MKK7-CA predominantly activates the downstream JNK1,2 isoforms, and when transfected along with ERE-luc suppressed a dose dependent E2-stimulated transcriptional activation of ERE-Luc as compared to vector transfected cells (Fig 7A). Interestingly, in Ishikawa(+) cells expression of MKK7-CA potentiated estrogen activation of ERE-luciferase. Thus activation of the MKK7-JNK pathway leads to decreased ER activation in the MCF-7 cells, confirming a role for JNK in suppression of ER function in this system while enhancing ER-activity in the Ishikawa(+) cells. These results demonstrate that similar to the JNK dependent cell type specific effects of flavone, direct activation of MKK7-JNK functions to differentially regulate ER activity in a cell type specific manner.
Figure 7.
Effects of constitutive active MKK7 on estrogen receptor transcriptional activation. MCF-7 cells (A) or Ishikawa(+) cells (B) were transfected with ERE-Luc along with either empty vector (VEC) or constitutive active MKK7 (CA-MKK7) for 6 hours and treated with DMSO or increasing concentration of estradiol (10−12 to 10−9 M) and harvested 18 hours later for luciferase assay. Data are represented as percent ERE-activation normalized to vector transfected and 1 nM E2 treated (100%) ± SEM from four independent experiments each in duplicate (*, p< 0.05).
DISCUSSION
We have previously demonstrated that certain flavonoid phytochemicals including flavone, chalcone, apigenin and kaempferide can negatively regulate ER function independent of direct binding to the ER (28) and are thus classified as binding-independent antiestrogens. Here we demonstrate that flavone and chalcone negatively regulate ER function in ER-α-positive MCF-7 breast and ERα transfected HepG2 liver cancer cells. In contrast, ERα-positive Ishikawa(+) endometrial carcinoma and ERα transfected HEK 293 cells are refractory to the antiestrogenic effects of flavone. Therefore, these cell type specific effects of flavone and chalcone demonstrate the ability of naturally occurring flavonoid phytochemicals to function as selective estrogen receptor modulators or phyto-SERMs. The ability of flavone and chalcone to function as phyto-SERMs through an ER-binding independent manner suggests they regulate cell signaling pathways that converge upon ER function. Recent reports have implicated specific phytochemicals, such as the soy isoflavone genistein and flavonoid apigenin, in the selective activation of MAPK pathways (54–59). In this report we demonstrate that the flavonoid phytochemicals chalcone and flavone are also capable of activation of JNK signaling cascades as measured by both JNK in vitro kinase assay and targeted activation of a Gal4-cJun mediated transcription.
The JNK and p38 signaling pathways have been described as stress response pathways in many cell types and are activated by peptide hormones (TNF-α receptor, Fas ligand receptor, IL-1 receptor, and EGFR) or non-receptor mediated stresses (ex. heat shock, oxidative stress, ionizing UV radiation, tumor promoters, protein synthesis inhibitors, and chemotherapeutic drugs) (60– 64). These pathways have been shown to exert both positive and negative regulatory effects on gene transcription. Our results here suggest that stress-activated MAP-kinases, JNK1 and 2, are critical in regulating the antiestrogenic effects of certain phytochemicals. The suppression of JNK signaling by expression of dominant-negative mutants of JNK-1 or JNK-2 results in an inhibition of the antiestrogenic effects of selected phytochemicals on ERE transcription. Chalcone and flavone both demonstrate the ability to initiate a JNK signaling pathway although with different magnitudes and time courses. Flavone and chalcone require JNK-signaling for their antiestrogenic effects in MCF-7 cells.
Our results demonstrate as a whole, that stress-activated MAP-kinases, JNK1 and 2, are important in regulating the estrogenic and antiestrogenic responses of the ER. We demonstrate the direct involvement of the JNK pathway in the negative regulation of estrogen receptor-mediated gene transactivation. While JNK-activation by Src and MEKK1 has been shown to positively regulate ER function in Hela and Ishikawa(+) cells (15, 65)], other studies have demonstrated negative regulation of ER by members of the Rho GTPase family and p21-activated kinase family which are known activators of JNK signaling (66–67)]. In particular the reported differences in the ability of RAC1, upstream of JNK activation, to either stimulate or repress ER function emphasizes the potential methodologic or cell-type specific factors that influence ER-signaling (15, 66)]. Additionally, work by Bodine et. al. further hints at a potential cell type specific regulation of ER by stress-mediated signaling in which TNFα, a known activator of JNK, suppresses ER activity in MCF-7 cells but not in ERα overexpressing CHO cells even though both cell lines responded to the cytokine with regards to NF-κB activation (52)].
Our results investigating JNK, along with the published reports demonstrating differential effects of p38 on ER function, highlight the complicated task of understanding the MAPK-ER cross-talk and the importance of cellular context. Our data here, using two endogenous ERα-expressing (MCF-7, Ishi(+)) and two artificial ERα expressing (HepG2, HEK293) cell lines, helps better elucidate the cell-type specific nature of estrogen receptor regulation by both specific ligands and signaling pathways. MCF-7 and HepG2 cells respond to flavone and chalcone as ER antagonists while HEK293 and Ishikawa(+) cells are refractory to the effects of flavone and chalcone. The ability of a constitutively active MKK7 to alternately activate or inhibit ER function in Ishikawa(+) and MCF-7 cells respectively further emphasizes the cell-specific nature of the MAPKs in ER regulation. Both Lee et al. and Feng et al. demonstrated an enhancement of ER-activity in a JNK dependent manner which is consistent with our direct MKK7-JNK activation of ERE in Ishikawa(+) cells (15, 65)]. In contrast, Su et al. obtained results like ours, demonstrating a negative regulation of ER by known JNK–activating pathways in MCF-7 cells (66)]. This suggests that JNK or some other signaling pathway(s) are intact and functionally repress ER-activity in the MCF-7 and HepG2 systems but not in HEK293 or Ishikawa(+) cells. Mechanistically the question remains as to how individual MAPKs, and in particular JNK, regulate ER in a cell type specific manner.
Overall, our results here implicate JNK as a component of the antiestrogenic action of the receptor binding-independent phytochemicals flavone and chalcone in a cell specific manner. JNK stress-signaling pathway functions as a cell-type specific regulatory signaling cascade of ER mediated transcription. These results therefore broaden the spectrum of selective estrogen receptor modulators (SERMs) to include both cell-signaling selective ER modulation and phytoestrogen selective ER modulation.
Highlights.
-
▪
Chalcone and flavone have cell-type specific antiestrogenic activity
-
▪
Chalcone and flavone suppress ERα activity through stimulation of MAPKs
-
▪
Constitutive activation of the JNK pathway suppresses ER signaling in breast cancer
-
▪
Constitutive activation of the JNK pathway does not suppresse ER signaling in endometrial cancer
ACKNOWLEDGEMENTS
We wish to thank Dr. Rodger Davis for the generous provision of the DN-JNK1 and DN-JNK2 expression constructs and Dr. Jiahuai Han for the provision of the MKK-7-CA construct. We wish to thank Katinka Vigh, Bruce Hurley, Jeffrey Dyer-Smith and Dr. Daniel Pace for their assistance with this work.
This work was supported by a cooperative agreement with the U.S. Department of Agriculture grant 58-6435-7-019 (J.A.M., M.E.B.); the U.S. Department of Defense Breast Cancer Research Program DAMD17-01-1-0655(M.E.B.), National Institutes of Health Grant DK 059389 (M.E.B.), and The Center for Bioenvironmental Research at Tulane and Xavier Universities (J.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weatherman RV, Fletterick RJ, Scanlan TS. Nuclear-receptor ligands and ligand-binding domains. Annu Rev Biochem. 1999;68:559–581. doi: 10.1146/annurev.biochem.68.1.559. [DOI] [PubMed] [Google Scholar]
- 2.Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun. 2000;270:1–10. doi: 10.1006/bbrc.2000.2214. [DOI] [PubMed] [Google Scholar]
- 3.Jordan VC, Morrow M. Tamoxifen, raloxifene, and the prevention of breast cancer. Endocr Rev. 1999;20:253–278. doi: 10.1210/edrv.20.3.0368. [DOI] [PubMed] [Google Scholar]
- 4.McDonnell DP. The Molecular Pharmacology of SERMs. Trends Endocrinol Metab. 1999;10:301–311. doi: 10.1016/s1043-2760(99)00177-0. [DOI] [PubMed] [Google Scholar]
- 5.Miller MA, Lippman ME, Katzenellenbogen BS. Antiestrogen binding in antiestrogen growth-resistant estrogen- responsive clonal variants of MCF-7 human breast cancer cells. Cancer Res. 1984;44:5038–5045. [PubMed] [Google Scholar]
- 6.Herman ME, Katzenellenbogen BS. Response-specific antiestrogen resistance in a newly characterized MCF- 7 human breast cancer cell line resulting from long-term exposure to trans-hydroxytamoxifen. J Steroid Biochem Mol Biol. 1996;59:121–134. doi: 10.1016/s0960-0760(96)00114-8. [DOI] [PubMed] [Google Scholar]
- 7.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49:4090–4093. [PubMed] [Google Scholar]
- 8.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen Action Via the G Protein-Coupled Receptor, GPR30: Stimulation of Adenylyl Cyclase and cAMP-Mediated Attenuation of the Epidermal Growth Factor Receptor-to-MAPK Signaling Axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 9.Ignar-Trowbridge DM, Pimentel M, Teng CT, Korach KS, McLachlan JA. Cross talk between peptide growth factor and estrogen receptor signaling systems. Environ Health Perspect. 1995;103(Suppl 7):35–38. doi: 10.1289/ehp.95103s735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignar-Trowbridge DM, Pimentel M, Parker MG, McLachlan JA, Korach KS. Peptide growth factor cross-talk with the estrogen receptor requires the A/B domain and occurs independently of protein kinase C or estradiol. Endocrinology. 1996;137:1735–1744. doi: 10.1210/endo.137.5.8612509. [DOI] [PubMed] [Google Scholar]
- 11.Watters JJ, Chun TY, Kim YN, Bertics PJ, Gorski J. Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol Endocrinol. 2000;14:1872–1881. doi: 10.1210/mend.14.11.0551. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Activation of the estrogen receptor through phosphorylation by mitogen- activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 13.Driggers PH, Segars JH, Rubino DM. The proto-oncoprotein Brx activates estrogen receptor beta by a p38 mitogen-activated protein kinase pathway. J Biol Chem. 2001;276:46792–46797. doi: 10.1074/jbc.M106927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol Cell Biol. 2002;22:5835–5845. doi: 10.1128/MCB.22.16.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H, Jiang F, Wang Q, Nicosia SV, Yang J, Su B, Bai W. MEKK1 activation of human estrogen receptor alpha and stimulation of the agonistic activity of 4-hydroxytamoxifen in endometrial and ovarian cancer cells. Mol Endocrinol. 2000;14:1882–1896. doi: 10.1210/mend.14.11.0554. [DOI] [PubMed] [Google Scholar]
- 16.Leung LK, Wang TT. Bcl-2 is not reduced in the death of MCF-7 cells at low genistein concentration. J Nutr. 2000;130:2922–2926. doi: 10.1093/jn/130.12.2922. [DOI] [PubMed] [Google Scholar]
- 17.Prifti S, Mall P, Strowitzki T, Rabe T. Synthetic estrogens-mediated activation of JNK intracellular signaling molecule. Gynecol Endocrinol. 2001;15:135–141. [PubMed] [Google Scholar]
- 18.McClelland RA, Barrow D, Madden TA, Dutkowski CM, Pamment J, Knowlden JM, Gee JM, Nicholson RI. Enhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex) Endocrinology. 2001;142:2776–2788. doi: 10.1210/endo.142.7.8259. [DOI] [PubMed] [Google Scholar]
- 19.Mandlekar S, Kong AN. Mechanisms of tamoxifen-induced apoptosis. Apoptosis. 2001;6:469–477. doi: 10.1023/a:1012437607881. [DOI] [PubMed] [Google Scholar]
- 20.Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, Mandlekar S. Induction of xenobiotic enzymes by the map kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab Rev. 2001;33:255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- 21.Chuang LF, Chuang RY. Heptachlor and the mitogen-activated protein kinase module in human lymphocytes. Toxicology. 1998;128:17–23. doi: 10.1016/s0300-483x(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto J, Hori M, Ichigo S, Morishita S, Tamaya T. Estrogen induces expression of c-fos and c-jun via activation of protein kinase C in an endometrial cancer cell line and fibroblasts derived from human uterine endometrium. Gynecol Endocrinol. 1996;10:109–118. doi: 10.3109/09513599609097900. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama M, Matsumura F. Correlation between the activation of Neu tyrosine kinase and promotion of foci formation induced by selected organochlorine compounds in the MCF-7 model system. J Biochem Mol Toxicol. 1999;13:296–302. doi: 10.1002/(sici)1099-0461(1999)13:6<296::aid-jbt2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Shen K, Novak RF. Differential effects of aroclors and DDT on growth factor gene expression and receptor tyrosine kinase activity in human breast epithelial cells. Adv Exp Med Biol. 1997;407:295–302. doi: 10.1007/978-1-4899-1813-0_44. [DOI] [PubMed] [Google Scholar]
- 25.Shen K, Novak RF. DDT stimulates c-erbB2, c-met, and STATS tyrosine phosphorylation, Grb2- Sos association, MAPK phosphorylation, and proliferation of human breast epithelial cells. Biochem Biophys Res Commun. 1997;231:17–21. doi: 10.1006/bbrc.1996.6039. [DOI] [PubMed] [Google Scholar]
- 26.Burow ME, Boue SM, Collins-Burow BM, Melnik LI, Duong BN, Carter-Wientjes CH, Li S, Wiese TE, Cleveland TE, McLachlan JA. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J Clin Endocrinol Metab. 2001;86:1750–1758. doi: 10.1210/jcem.86.4.7430. [DOI] [PubMed] [Google Scholar]
- 27.Collins BM, McLachlan JA, Arnold SF. The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. Steroids. 1997;62:365–372. doi: 10.1016/s0039-128x(96)00246-2. [DOI] [PubMed] [Google Scholar]
- 28.Collins-Burow BM, Burow ME, Duong BN, McLachlan JA. Estrogenic and antiestrogenic activities of flavonoid phytochemicals through estrogen receptor binding-dependent and -independent mechanisms. Nutr Cancer. 2000;38:229–244. doi: 10.1207/S15327914NC382_13. [DOI] [PubMed] [Google Scholar]
- 29.Khupse RS, Sarver JG, Trendel JA, Bearss NR, Reese MD, Wiese TE, Boue SM, Burow ME, Cleveland TE, Bhatnagar D, Erhardt PW. Biomimetic syntheses and antiproliferative activities of racemic, natural (−), and unnnatural (+) glyceollin I. Journal of medicinal chemistry. 2011;54:3506–3523. doi: 10.1021/jm101619e. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann MC, Tilghman SL, Boue SM, Salvo VA, Elliott S, Williams KY, Skripnikova EV, Ashe H, Payton-Stewart F, Vanhoy-Rhodes L, Fonseca JP, Corbitt C, Collins-Burow BM, Howell MH, Lacey M, Shih BY, Carter-Wientjes C, Cleveland TE, McLachlan JA, Wiese TE, Beckman BS, Burow ME. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. The Journal of pharmacology and experimental therapeutics. 2010;332:35–45. doi: 10.1124/jpet.109.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payton-Stewart F, Khupse RS, Boue SM, Elliott S, Zimmermann MC, Skripnikova EV, Ashe H, Tilghman SL, Beckman BS, Cleveland TE, McLachlan JA, Bhatnagar D, Wiese TE, Erhardt P, Burow ME. Glyceollin I enantiomers distinctly regulate ER-mediated gene expression. Steroids. 2010;75:870–878. doi: 10.1016/j.steroids.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Payton-Stewart F, Schoene NW, Kim YS, Burow ME, Cleveland TE, Boue SM, Wang TT. Molecular effects of soy phytoalexin glyceollins in human prostate cancer cells LNCaP. Molecular carcinogenesis. 2009;48:862–871. doi: 10.1002/mc.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boue SM, Tilghman SL, Elliott S, Zimmerman MC, Williams KY, Payton-Stewart F, Miraflor AP, Howell MH, Shih BY, Carter-Wientjes CH, Segar C, Beckman BS, Wiese TE, Cleveland TE, McLachlan JA, Burow ME. Identification of the potent phytoestrogen glycinol in elicited soybean (Glycine max) Endocrinology. 2009;150:2446–2453. doi: 10.1210/en.2008-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagar S, Islam MA, Das S, Mukherjee A, Saha A. Pharmacophore mapping of flavone derivatives for aromatase inhibition. Molecular diversity. 2008;12:65–76. doi: 10.1007/s11030-008-9077-9. [DOI] [PubMed] [Google Scholar]
- 35.Saxena HO, Faridi U, Kumar JK, Luqman S, Darokar MP, Shanker K, Chanotiya CS, Gupta MM, Negi AS. Synthesis of chalcone derivatives on steroidal framework and their anticancer activities. Steroids. 2007;72:892–900. doi: 10.1016/j.steroids.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed N, Dubuc C, Rousseau J, Benard F, van Lier JE. Synthesis, characterization, and estrogen receptor binding affinity of flavone-, indole-, and furanestradiol conjugates. Bioorganic & medicinal chemistry letters. 2007;17:3212–3216. doi: 10.1016/j.bmcl.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Modzelewska A, Pettit C, Achanta G, Davidson NE, Huang P, Khan SR. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorganic & medicinal chemistry. 2006;14:3491–3495. doi: 10.1016/j.bmc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Frigo DE, Burow ME, Mitchell KA, Chiang TC, McLachlan JA. DDT and Its Metabolites Alter Gene Expression in Human Uterine Cell Lines through Estrogen Receptor-Independent Mechanisms. Environ Health Perspect. 2002;110:1239–1245. doi: 10.1289/ehp.021101239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burow ME, Weldon CB, Chiang TC, Tang Y, Collins-Burow BM, Rolfe K, Li S, McLachlan JA, Beckman BS. Differences in protein kinase C and estrogen receptor alpha, beta expression and signaling correlate with apoptotic sensitivity of MCF-7 breast cancer cell variants. Int J Oncol. 2000;16:1179–1187. doi: 10.3892/ijo.16.6.1179. [DOI] [PubMed] [Google Scholar]
- 40.Antoon JW, Liu J, Ponnapakkam AP, Gestaut MM, Foroozesh M, Beckman BS. Novel D:-erythro N-octanoyl sphingosine analogs as chemo- and endocrine-resistant breast cancer therapeutics. Cancer Chemother Pharmacol. 2010;65:1191–1195. doi: 10.1007/s00280-009-1233-0. [DOI] [PubMed] [Google Scholar]
- 41.Antoon JW, Liu J, Gestaut MM, Burow ME, Beckman BS, Foroozesh M. Design, synthesis, and biological activity of a family of novel ceramide analogues in chemoresistant breast cancer cells. J Med Chem. 2009;52:5748–5752. doi: 10.1021/jm9009668. [DOI] [PubMed] [Google Scholar]
- 42.Burow ME, Weldon CB, Tang Y, McLachlan JA, Beckman BS. Oestrogen-mediated suppression of tumour necrosis factor alpha-induced apoptosis in MCF-7 cells: subversion of Bcl-2 by anti-oestrogens. J Steroid Biochem Mol Biol. 2001;78:409–418. doi: 10.1016/s0960-0760(01)00117-0. [DOI] [PubMed] [Google Scholar]
- 43.Antoon JW, White MD, Slaughter EM, Driver JL, Khalili HS, Elliott S, Smith CD, Burow ME, Beckman BS. Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol Ther. 2011;11:678–689. doi: 10.4161/cbt.11.7.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antoon JW, Meacham WD, Bratton MR, Slaughter EM, Rhodes LV, Ashe HB, Wiese TE, Burow ME, Beckman BS. Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signaling in human breast cancer. J Mol Endocrinol. 2011;46:205–216. doi: 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, Rhodes LV, Ashe HB, Wiese TE, Smith CD, Burow ME, Beckman BS. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151:5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frigo DE, Duong BN, Melnik LI, Schief LS, Collins-Burow BM, Pace DK, McLachlan JA, Burow ME. Flavonoid Phytochemicals Regulate Activator Protein-1 Signal Transduction Pathways in Endometrial and Kidney Stable Cell Lines. J Nutr. 2002;132:1848–1853. doi: 10.1093/jn/132.7.1848. [DOI] [PubMed] [Google Scholar]
- 47.Bratton MR, Antoon JW, Dong BN, Frigo DE, Tilghman S, Collins-Burow B, Elliott S, Tang Y, Melnik LI, Lai L, Beckman BS, Alam J, Hill SM, Rowan B, McLachlan JA, Burow ME. GalphaO Potentiates Estrogen Receptor-alpha Activity Via the Erk Signaling Pathway. J Endocrinol. 2012 doi: 10.1530/JOE-12-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antoon JW, White MD, Burow ME, Beckman BS. Dual inhibition of sphingosine kinase isoforms ablates TNF-induced drug resistance. Oncol Rep. 2012;27:1779–1786. doi: 10.3892/or.2012.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antoon JW, Beckman BS. Anti-proliferative effects of the novel ceramide analog (S)-2-(benzylideneamino)-3-hydroxy-N-tetrade-cylpropanamide in chemoresistant cancer. Bioorganic & medicinal chemistry letters. 2012;22:2624–2628. doi: 10.1016/j.bmcl.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 50.Doman RK, Perez M, Donato NJ. JNK and p53 stress signaling cascades are altered in MCF-7 cells resistant to tumor necrosis factor-mediated apoptosis. J Interferon Cytokine Res. 1999;19:261–269. doi: 10.1089/107999099314199. [DOI] [PubMed] [Google Scholar]
- 51.Danforth DN, Jr, Sgagias MK. Tumour necrosis factor-alpha modulates oestradiol responsiveness of MCF- 7 breast cancer cells in vitro. J Endocrinol. 1993;138:517–528. doi: 10.1677/joe.0.1380517. [DOI] [PubMed] [Google Scholar]
- 52.Bodine PV, Harris HA, Komm BS. Suppression of ligand-dependent estrogen receptor activity by bone- resorbing cytokines in human osteoblasts. Endocrinology. 1999;140:2439–2451. doi: 10.1210/endo.140.6.6612. [DOI] [PubMed] [Google Scholar]
- 53.Speir E, Yu ZX, Takeda K, Ferrans VJ, Cannon RO., 3rd Competition for p300 regulates transcription by estrogen receptors and nuclear factor-kappaB in human coronary smooth muscle cells. Circ Res. 2000;87:1006–1011. doi: 10.1161/01.res.87.11.1006. [DOI] [PubMed] [Google Scholar]
- 54.Croisy-Delcey M, Croisy A, Mousset S, Letourneur M, Bisagni E, Jacquemin-Sablon A, Pierre J. Genistein analogues: effects on epidermal growth factor receptor tyrosine kinase and on stress-activated pathways. Biomed Pharmacother. 1997;51:286–294. doi: 10.1016/S0753-3322(97)83545-7. [DOI] [PubMed] [Google Scholar]
- 55.Chen YR, Wang W, Kong AN, Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J Biol Chem. 1998;273:1769–1775. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- 56.Liu Q, Chen X, Yang G, Min X, Deng M. Apigenin inhibits cell migration through MAPK pathways in human bladder smooth muscle cells. Biocell : official journal of the Sociedades Latinoamericanas de Microscopia Electronica et al. 2011;35:71–79. [PubMed] [Google Scholar]
- 57.Kim SC, Kang SH, Jeong SJ, Kim SH, Ko HS, Kim SH. Inhibition of c-Jun N-terminal kinase and nuclear factor kappa B pathways mediates fisetin-exerted anti-inflammatory activity in lipopolysccharide-treated RAW264.7 cells. Immunopharmacology and immunotoxicology. 2012 doi: 10.3109/08923973.2011.648270. [DOI] [PubMed] [Google Scholar]
- 58.Xie C, Kang J, Li Z, Schauss AG, Badger TM, Nagarajan S, Wu T, Wu X. The acai flavonoid velutin is a potent anti-inflammatory agent: blockade of LPS-mediated TNF-alpha and IL-6 production through inhibiting NF-kappaB activation and MAPK pathway. The Journal of nutritional biochemistry. 2011 doi: 10.1016/j.jnutbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Kim SW, Kim CE, Kim MH. Flavonoids inhibit high glucose-induced upregulation of ICAM-1 via the p38 MAPK pathway in human vein endothelial cells. Biochem Biophys Res Commun. 2011;415:602–607. doi: 10.1016/j.bbrc.2011.10.115. [DOI] [PubMed] [Google Scholar]
- 60.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 61.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 62.Rincon M, Flavell RA, Davis RA. The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic Biol Med. 2000;28:1328–1337. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- 63.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 65.Feng W, Webb P, Nguyen P, Liu X, Li J, Karin M, Kushner PJ. Potentiation of estrogen receptor activation function 1 (AF-1) by Src/JNK through a serine 118- independent pathway. Mol Endocrinol. 2001;15:32–45. doi: 10.1210/mend.15.1.0590. [DOI] [PubMed] [Google Scholar]
- 66.Su LF, Knoblauch R, Garabedian MJ. Rho GTPases as modulators of the estrogen receptor transcriptional response. J Biol Chem. 2001;276:3231–3237. doi: 10.1074/jbc.M005547200. [DOI] [PubMed] [Google Scholar]
- 67.Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP. AR and ER interaction with a p21-activated kinase (PAK6) Mol Endocrinol. 2002;16:85–99. doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]