SUMMARY
Wnt signaling regulates synaptic plasticity and neurogenesis in the adult nervous system, suggesting a potential role in behavioral processes. Here, we probed the requirement for Wnt signaling during olfactory memory formation in Drosophila using an inducible RNA interference approach. Interfering with β-catenin expression in the adult mushroom body neurons specifically impaired long-term memory without altering short-term memory. The impairment was reversible, rescued with expression of a wild-type β-catenin transgene, and correlated with a disruption of a cellular long-term memory trace. Inhibition of wingless, a Wnt ligand, and arrow, a Wnt co-receptor, also impaired long-term memory. Wingless expression in wild type flies was transiently elevated in the brain after long-term memory conditioning. Thus, inhibiting three key components of the Wnt signaling pathway in the adult mushroom bodies impairs long-term memory, collectively indicating that this pathway mechanistically underlies this specific form of memory.
INTRODUCTION
Wnts are a family of secreted, lipid-modified proteins that act as short-range ligands to activate receptor-mediated signaling cascades. Wnt signaling is crucial for many aspects of embryonic development in metazoan organisms including cell proliferation, establishment of tissue polarity, cell movement, cell fate decisions, and stem cell maintenance (Logan and Nusse, 2004; Moon et al., 2004). Wnt-receptor interactions can elicit several intracellular signaling responses. The β-catenin protein plays a pivotal role in the best characterized of these pathways, the canonical pathway. In the absence of Wnt, β-catenin is phosphorylated by glycogen synthase kinase-3β (Gsk-3β) and rapidly degraded by the proteasome pathway. Upon activation of Wnt signaling, β-catenin is stabilized through the inhibition of Gsk-3β and translocates to the nucleus, where it binds the Tcf/Lef family of transcription factors to regulate the expression of Wnt target genes (Figure 1). In addition to this role in transcriptional regulation, β-catenin is also a component of the cadherin complex and plays an important role in regulating cell-cell adhesion (Nelson and Nusse, 2004). Alternatively, Wnt signaling can be executed through non-canonical pathways that result in c-Jun N-terminal Kinase (Jnk) activation (Wnt/PCP pathway) or protein kinase C (Pkc) and calcium/calmodulin-dependent protein kinase II (CaMKII) activation through increased intracellular Ca2+ concentration (Wnt/Ca2+ pathway), respectively (Ciani and Salinas, 2005; Inestrosa and Arenas, 2010).
Figure 1. Schematic of the canonical Wnt signaling pathway.
(A) In the absence of Wnt protein, newly synthesized β-catenin (Armadillo in Drosophila) is sequestered by a “destruction complex” which comprises Adenomatosis polyposis coli (Apc), Axin, glycogen synthase kinase 3β (Gsk3β), and Casein Kinase I (CkI). The phosphorylation of β–catenin protein by Gsk3β and CkI targets it for degradation by the proteasome. This keeps β–catenin at a low level in the cytoplasm. The transcription of Wnt target genes is inhibited through the action of T-cell factor/lymphoid enhancer factor (Tcf/Lef) complex. (B) In the presence of Wnt (Wingless is the homologue for Wnt-1 in Drosophila) protein, Wnt binds to the seven-transmembrane-domain protein Frizzled and its co-receptor, low-density lipoprotein receptor-related protein (Lrp) (Arrow in Drosophila). This triggers the activation of Dishevelled (Dvl) and phosphorylation of the cytoplasmic tail of Lrp. The interaction between activated Dvl, the destruction complex, and the docking of Axin to phosphorylated Lrp promotes the disassembly of the destruction complex, allowing β-catenin to accumulate in the cytoplasm. β-catenin then translocates into the nucleus where it activates the transcription of Wnt target genes by binding to the Tcf/Lef complex. β-catenin also binds to the cytoplamic domain of N-cadherin to regulate the cadherin complex, controlling cell-cell adhesion.
Many components of the Wnt signaling pathway are expressed in adult brain (Shimogori et al., 2004; Tissir and Goffinet, 2006; Cerpa et al., 2008; Maguschak and Ressler, 2008) and participate in synaptic function. The Wnts are involved in activity-dependent dendritic growth and arborization through a transcription independent, adhesion-mediated branch of the canonical pathway (Yu and Malenka, 2003), and through activation of Rac and Jnk of non-canonical pathway (Rosso et al., 2005) in cultured hippocampal neurons. In Drosophila, Wnt signaling mediates activity-dependent structural remodeling of the neuromuscular junction (Ataman et al., 2008). Wingless (Wg), a Wnt1 homologue in flies, is released from synaptic boutons in an activity-dependent manner to regulate plasticity bidirectionally, stimulating a divergent canonical pathway in presynaptic processes leading to cytoskeletal reorganization, and the Frizzled (Fz) nuclear import pathway in postsynaptic processes for assembly of the postsynaptic apparatus (Mathew et al., 2005; Ataman et al., 2008). In cultured hippocampal neurons and slices, the expression and secretion of Wnt is induced by neuronal activity through the activation of CaM-Kinase I and enhanced Creb-dependent transcription (Wayman et al., 2006). Neuronal activity also promotes the mobilization of Wnt receptor, Fz5, to the cell surface and its recruitment to synapses during synaptogenesis in the mouse hippocampus (Sahores et al., 2010). In addition, activity-dependent release of Wnt from synapses has been shown to induce long-term potentiation (LTP) in adult mouse hippocampal slices, whereas inhibition of Wnt signaling impairs LTP (Chen et al., 2006). Moreover, Wnt signaling mediates the global regulation of synapse number in response to experience and age in the adult hippocampus (Stranahan et al., 2008; Gogolla et al., 2009). These studies illustrate the important role of Wnt signaling in structural and functional plasticity of synapses.
The persistent expression of Wnts in the adult brain along with their roles in synaptic function suggests that Wnt signaling may be essential for normal learning and memory. We report here our results of probing the role of Wnt signaling components on olfactory memory formation in Drosophila. We employed a conditional RNA interference approach to inactivate the expression of Wnt signaling components specifically in the mushroom bodies (MB) of adult flies and found that long-term memory (LTM) of odors along with a cellular LTM trace, but not short-term memory (STM), was disrupted in flies with reduced expression of β-catenin (armadillo in Drosophila); arrow (arr), a Wnt co-receptor; and Wingless (Wg), a Wnt ligand.
RESULTS
Armadillo (Arm) is required for LTM
We first examined the expression pattern of Arm in fly brain through whole-mount immunohistochemistry and reporter gene expression under the control of arm-GAL4. We found that that arm is broadly expressed in the adult brain, including in the antennal lobes, MB, and central complex (Figure S1), brain areas known to be important for olfactory memory formation (Davis, 2004; Davis, 2011).
We next tested whether arm is required for olfactory LTM. We took advantage of a spatially restricted and temporally inducible Gene-Switch transgene to specifically silence arm expression with RNAi in the adult MBs. This strategy allows for normal arm expression during development and in adult tissues other than the MBs. The uas-RNAi transgene of the Gene-Switch system is expressed only after feeding flies RU486, and the spatial restriction accomplished by driving the inducible gal4 using a MB enhancer (Mao et al., 2004). We also included uas-dicer2 (dcr-2) in the experimental genotype to enhance the efficiency of the RNAi component (Dietzl et al., 2007). There were no adverse effects of expressing dcr-2 alone in adult MBs on memory formation (Figure S2). When fed on RU486-containing food for five days, the uas-armRNAi/+; P{MB-GeneSwitch}12-1, uas-dcr2/+ flies exhibited impaired LTM tested at 24 hr after 5X spaced training, whereas the 24 hr memory after 5X massed training or 3 min memory after 1X training was not significantly different from the unfed flies of the same genotype (Figure 2A). The LTM deficits cannot be attributed to defects in sensorimotor processes given the normal performance after 5X massed and 1X training (Figure 2A) and normal performance in tests of shock or odor avoidance (Figure S3). Since 5X spaced training produces protein-synthesis dependent LTM, which is both mechanistically and temporally distinct from the memory forms instilled by 5X massed and 1X conditioning (Tully et al., 1994), we conclude that silencing arm expression in the adult MBs produces a specific deficit in protein synthesis-dependent LTM.
Figure 2. Impaired olfactory LTM with arm knockdown in the adult MBs.
(A) Performance of flies expressing armRNAi in the adult MBs. Flies carrying one copy each of P{MB-GeneSwitch}12-1, uas-dcr2, and uas-armRNAi were fed with RU486 to silence arm expression in the adult MBs. These flies exhibited impairment in 24 hr memory after 5X spaced forward conditioning compared to the No-RU fed control (n=12, * P < 0.05). Performance at 24 hr after 5X massed conditioning or at 3 min after 1X conditioning was indistinguishable between RU-fed and No-RU flies (n=6 for each group). N. S., no significant difference. (B) Performance of uas-armRNAi/+; P{MB-GeneSwitch}12-1, uas-dcr2/+ flies kept on regular food for 9 days (No-RU group), or on the RU486-containing food for 5 days and then on regular food for 4 days (RU to No-RU group), or on the RU486-containing food for 9 days (RU group). The RU to No-RU group performed significantly better than the RU group while there was no significant difference between the RU to No-RU and No-RU groups (n=8 for all groups, * P < 0.05). (C) Performance of flies expressing armRNAi in the adult MBs compared to those co-expressing armRNAi with a uas-arm transgene. These flies also carried one copy of P{MB-GeneSwitch}12-1 and one copy of uas-dcr2. Flies expressing only armRNAi exhibited a significant decrement in performance compared to flies of the same genotype that remained uninduced (n=12 for each group, * P < 0.05). In addition, flies expressing both armRNAi and uas-arm in the adult MBs exhibited performance levels that were indistinguishable from flies in which transgene expression remained uninduced. (D) Effects of over-expression of uas-arm on LTM. Performance of flies with induced expression of uas-arm in the adult MBs. The P{MB-GeneSwitch}12-1/uas-arm flies were fed with RU486 for five days to induce the expression of arm in the adult MBs. The 24 hr performance index after 5X spaced conditioning of the RU-fed flies was indistinguishable from that of the No-RU control (n=6 for all groups).
The specific effect of this disruption on LTM but not STM suggests that Wnt signaling participates in an active way for LTM formation rather than having some non-specific effect on behavior. To further address this issue, we tested whether the LTM impairment was reversible. A non-specific effect of arm knockdown in the adult MBs might be expected to produce non-reversible changes in the MB neurons due to cell-death or the loss or rearrangement of fibers or synapses. A specific mechanistic role for arm in LTM in which the pathway is normally activated or modulated by conditioning would likely produce reversible effects. The uas-armRNAi/+; P{MB-GeneSwitch}12-1, uas-dcr2/+ flies were first fed on RU486-containing food for 5 days to induce transgene expression, and then transferred to regular food without RU486 for another 4 days to restore arm expression in the MBs. Flies withdrawn from the RU-containing food regained 24 hr LTM performance that was indistinguishable from the No-RU group (Figure 2B). These data indicate that the behavioral impairment produced by arm silencing was reversible and lead to the conclusion that Arm participates in the ongoing physiology of MB neurons for normal olfactory LTM.
To further confirm that the observed LTM impairment observed in uas-armRNAi/+; P{MB-GeneSwitch}12-1, uas-dcr2/+ flies fed with RU486 was due to the specific reduction in arm expression, we tested whether co-expression of a uas-arm transgene could offset the effect of uas-armRNAi. Indeed, the co-expression of uas-arm with uas-armRNAi rescued the LTM score to normal levels (Figure 2C). The expression of uas-arm in adult MBs alone had no effect on LTM (Figure 2D). Thus, the LTM defects in uas-armRNAi/+; P{MB-GeneSwitch}12-1, uas-dcr2/+ flies after induction were caused by the silencing of arm expression. These data (Figure 2D) further indicate that the normal abundance of Arm in the MBs is not rate limiting for LTM expression.
We assayed the silencing efficiency of armRNAi by quantitative RT-PCR using head total RNA from flies expressing the transgene under the control of c155-gal4, a pan-neuronal driver. The relative level of arm mRNA was reduced to ~30–40% of that found in either of the parental control lines (Figure S4A). This is likely an underestimate of the potency of armRNAi since the transgene was expressed only in brain neurons and the head is a complex tissue containing muscle, fat body, and other types of cells.
Arm is required for the formation of a LTM trace in the MBs
Our prior studies have identified a LTM trace that forms in the α branch of the α/β MB neurons after spaced forward conditioning (Yu et al., 2006). This branch-specific memory trace is detectable between 9 and 24 hr after conditioning, is registered as an increased calcium influx in response to the presentation of the conditioned odor, and is dependent on normal protein synthesis and the function of amnesiac, Creb, and 26 other genes involved in LTM (Yu et al., 2006; Akalal et al., 2011). The perfect correspondence between the conditions required for the formation of this memory trace and long-term behavioral memory indicate that the memory trace is intimately tied to LTM and may help guide behavior after spaced conditioning. Given the role of Arm in LTM, we asked whether it is also required for the expression of the LTM trace.
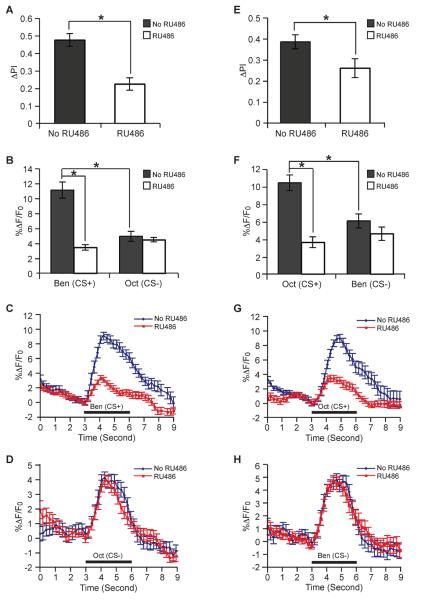
We first constructed MB-GCaMP, a transgene that expresses the GCaMP1.6 calcium reporter from a minimal heat shock promoter under the control of the 247 bp MB enhancer from the Dmef2 gene (Mao et al., 2004). This allows for the expression of GCaMP in the MBs independent of the gal4:uas system. We then co-expressed the MB-GCaMP transgene with uas-armRNAi and uas-dcr2/+, the latter transgenes being driven by P{MB-GeneSwitch}12-1. This “within genotype” experimental design allows neural activity in the MBs to be monitored with G-CaMP fluorescence in a single genotype under two conditions: flies fed RU486 or those that remain unfed. Flies with induced expression of armRNAi in the adult MBs exhibited a significant decrement in 24 hour LTM after 5X spaced training using benzaldehyde as the CS+ compared to flies of the same genotype that were uninduced (Figure 3A). A small fraction of the behaviorally trained flies were removed prior to testing and utilized for functional imaging of the LTM trace in the α branch of the MB. Consistent with the pronounced deficit in behavioral LTM, there was pronounced deficit in CS+-evoked calcium response in the α branch of the MB neurons of these flies as well (Figure 3B–C). In contrast, the calcium response to octanol (CS−) in the induced flies was indistinguishable from that of uninduced control (Figure 3B and 3D). Similar results were obtained using octanol as CS+ (Figure 3E–H). We conclude that the expression of arm in the adult MBs is required for the formation of the LTM trace as well as for long-term behavioral memory. Thus, Wnt signaling underlies long-term behavioral memory as well as the cellular memory trace that is critical for behavioral memory.
Figure 3. Expression of armRNAi blocks the formation of a LTM trace.
The flies used to obtain data for this figure were uas-armRNAi/+; P{MB-GeneSwitch}12-1,uas-dcr2/MB-GCamP and were trained using 5X spaced forward conditioning. (A) Performance of flies with and without RU486 feeding at 24 hr after conditioning using benzaldehyde (Ben) as the CS+ and 3-octanol (Oct) as the CS−. The RU-fed group showed a significantly reduced performance index compared to the unfed group of the same genotype (n=8, * P < 0.05). A small fraction of the behaviorally trained flies were removed prior to testing and used for functional imaging (panels B–D). (B) Average peak calcium responses in the α branch of the α/β MB neurons to the CS+ (Ben) and CS− (Oct) at 24 hr after conditioning with benzaldehyde as the CS+. There was a significant difference in response to the CS+ between the RU-fed and unfed groups (* P < 0.05). There was no significant difference in response to the CS− between the RU-fed and unfed groups (n=9 and 10 for no RU and RU groups respectively). (C) Group time course for calcium responses in the α branch of the α/β MB neurons during the presentation of the CS+ (Ben) at 24 hr after conditioning with benzaldehyde as the CS+ (n=9 and 10 for no RU and RU groups respectively). The graph was made using the data from the same flies used for the bar graph in panel (B). (D) Group time course for calcium responses in the α branch of the α/β MB neurons across time during the presentation of the CS− (Oct) at 24 hr after conditioning with benzaldehyde as the CS+ (n=9 and 10 for no RU and RU groups respectively). The graph was made using the data from the same flies used for the bar graph in panel (B). (E) Performance of flies with and without RU486 feeding at 24 hr after conditioning using 3-octanol (Oct) as the CS+ and benzaldehyde (Ben) as the CS−. The RU-fed group showed a significantly reduced performance index compared to the unfed group of the same genotype (n=8, * P < 0.05). A small fraction of the behaviorally trained flies were removed prior to testing and used for functional imaging (panels F–H). (F) Average peak calcium responses in the α branch of the α/β MB neurons to the CS+ (Oct) and CS− (Ben) at 24 hr after conditioning with octanol as the CS+. There was a significant difference in response to the CS+ between the RU-fed and unfed groups (* P < 0.05). There was no significant difference in response to the CS− between the RU-fed and unfed groups (n=9 and 8 for no RU and RU groups respectively). (G) Group time course for calcium responses in the α branch of the α/β MB neurons across time during the presentation of the CS+ (Oct) at 24 hr after conditioning with octanol as the CS+ (n=9 and 8 for no RU and RU groups respectively). The graph was made using the data from the same flies used for the bar graph in panel (F). (H) Group time course for calcium responses in the α branch of the α/β MB neurons across time during the presentation of the CS− (Ben) at 24 hr after conditioning with octanol as the CS+ (n=9 and 8 for no RU and RU groups respectively). The graph was made using the data from the same flies used for the bar graph in panel (F).
Other key components of Wnt signaling are also required for LTM
The discovery that Arm is required for LTM expression prompted the question of whether other components of the Wnt signaling pathway function in LTM expression. In essence, does the complete Wnt signaling pathway participate in LTM formation, or does Arm function through a mechanism that is independent of Wnt signaling? The first step in the Wnt signaling pathway is the interaction between Wnt proteins and the receptor protein Fz and the co-receptor, low-density lipoprotein receptor-related protein 5/6 (Lrp5/6) (Figure 1). There are four Fz homologues in flies, which may have redundant functions in Wnt pathway activation (Bhat, 1998; Bhanot et al., 1999; Chen and Struhl, 1999), so we focused on testing a role for arr, the only known Drosophila gene encoding a homologue of Wnt co-receptor, Lrp5/6.
We utilized the same “within genotype” experimental strategy for conditional RNAi knockdown specifically to the MBs of adult flies. Induced expression of arrRNAi6707 in the adult MBs impaired 24 hour LTM produced by spaced conditioning, whereas 24 hour memory after 5X massed training or 3 min memory after 1X training was unaffected (Figure 4A). Similar LTM defects were observed with arrRNAi6708, a second independent transgenic line that was available carrying the same arr RNAi construct (Figure 4B). There was no effect of expression of these RNAi's on the flies' performance in odor and shock avoidance tests (Figure S3B, C). The knock down efficiency on arr mRNA was equivalent for the two arr RNAi lines as tested by quantitative RT-PCR (Figure S4B). These results force the conclusion that reducing expression of the Wnt co-receptor Arr, specifically in the adult MB, impairs LTM expression without altering other temporally- or mechanistically-distinct forms of olfactory memory.
Figure 4. Impaired olfactory LTM with arrow (arr) or wingless (wg) knockdown in the adult MBs.

(A) Performance of flies expressing arrRNAi6707 in the adult MBs. The P{MB-GeneSwitch}12-1, uas-dcr2/uas-arrRNAi6707 flies were fed on either RU486-containing or no-RU food. The RU-fed group exhibited a significant lower performance score at 24 hr after 5X spaced conditioning (n=12, * P < 0.05). There was no significant difference (N. S.) in performance scores at 24 hr after 5X massed conditioning or at 3 min after 1X conditioning between RU-fed and No-RU groups (n=6 for each group). (B) Performance of flies expressing arrRNAi6708 in the adult MBs. The RU-fed group exhibited a significant lower performance score at 24 hr after 5X spaced conditioning (n=12, * P < 0.05). There was no significant difference in performance scores at 24 hr after 5X massed conditioning or at 3 min after 1X conditioning between RU-fed or No-RU groups (n=6). (C) Performance of flies expressing wgRNAi in the adult MBs. The RU-fed P{MB-GeneSwitch}12-1, uas-dcr2/uas-wgRNAi flies showed a significantly lower performance score at 24 hr after 5X spaced conditioning compared to the No-RU control (n=12, * P < 0.05). There was no significant difference (N. S.) in performance scores at 24 hr after 5X massed conditioning or at 3 min after 1X conditioning between RU-fed and No-RU groups (n=6).
We next asked whether Wnt pathway ligands are involved in LTM. Since Wingless (Wg), a Wnt1 homologue, has been shown to play an important role in regulating neuronal plasticity at the neuromuscular junction in flies (Mathew et al., 2005), we decided to investigate the effect of wingless inactivation on LTM. Inducible RNAi-mediated knock-down of wg in the adult MBs led to impaired 24 hr LTM after spaced conditioning, but had no effect on 24 hr memory after massed training or 3 min memory after 1X conditioning (Figure 4C). Shock and odor avoidance tests of the induced flies were indistinguishable from the uninduced control (Figure S3D). The efficiency of the wgRNAi transgene in silencing wg expression was assayed by quantitative RT-PCR and found to be effective (Figure S4C). We conclude that the conditional knockdown of the Wnt pathway ligand, wg, in the adult MBs produces a specific deficit in protein synthesis-dependent LTM.
The expression of wg is transiently increased after spaced conditioning
Since LTM formation requires de novo protein synthesis that depends partly on transcriptional regulation, we asked whether wg mRNA expression might be regulated after spaced conditioning in wild type flies. We trained w(CS10) flies with a 5X spaced conditioning protocol, isolated total RNA from whole heads at various time points after training, and measured the levels of wg mRNA in these samples by quantitative RT-PCR. The wg mRNA was significantly increased by about 20% at 30 and 45 min after training compared to the untrained control (Figure 5A). Although this increase in expression seems small, it is probably larger in a subset of tissues that mediate olfactory learning. Olfactory learning is mediated by the olfactory nervous system and this system comprises a small fraction of the tissue comprising the whole head (Davis, 2004; Davis, 2011). We replicated this increase at 30 min after conditioning in a separate experiment and followed its subsequent return to baseline by 60 min using extended time points in the assay (Figure 5B). No increase of wg expression was observed in fly heads after 1X forward, 5X massed forward, or 5X spaced backward training (Figure 5C). Given that spaced forward conditioning alone induces protein synthesis-dependent LTM, we conclude that the transient increase in wg expression is likely to be specifically related to and important for this form of memory.
Figure 5. Increased expression of wg mRNA after spaced forward conditioning.

The w(CS10) flies were trained using 5X spaced forward, 5X spaced backward, 5X massed forward, and 1X forward conditioning protocols. Total RNA samples were isolated from the heads of these flies at various time points after conditioning and assayed by quantitative RT-PCR relative to an untrained control group. (A) Relative wg mRNA level in the heads of trained flies at 15, 30, and 45 min after 5X spaced forward conditioning. The expression of wg mRNA was significant higher in heads at both 30 and 45 min compared to the untrained control (* P < 0.05). There was no significant difference in wg mRNA expression between the control and trained flies collected at 15 min after conditioning. (n=5 for all groups). (B) In a separate experiment, the relative wg mRNA level in the heads of trained flies at 30, 60, and 90 min after 5X spaced forward conditioning. There was a significant increase in wg expression at 30 min after 5X spaced conditioning compared to the untrained control group (* P < 0.05), replicating the results from panel A. There were no significant differences in wg expression between the control and trained flies collected at 60 or 90 min after conditioning. (n=5 for all groups). (C) Relative wg mRNA level in the heads of trained flies at 45 min after 5X spaced backward, 5X massed forward, and 1X forward conditioning. No significant differences in wg mRNA level were observed in the heads of trained flies at 45 min after conditioning compared to the untrained control group (n=5, N. S., no significant difference).
DISCUSSION
We provide multiple lines of evidence that the Wnt signaling pathway is functionally involved and required for the expression of LTM. Conditional RNAi strategies that offered both time and space control for reducing the mRNA levels of three different molecules involved in Wnt signaling, β-catenin, Wingless, and Arrow, showed that each of these molecules participates specifically in the processes underlying LTM in the adult MB neurons. We have also utilized similar strategies to knockdown N-cadherin, a cell adhesion molecule regulated by β-catenin, and found the same LTM deficits as with other molecules involved in Wnt signaling (data not shown). Our behavioral data align nicely with fear conditioning results of Maguschak and Ressler (2008) who showed that an amygdala-specific deletion of β-catenin in adult mice prevented consolidation. In addition, we demonstrated that a LTM trace that forms in the MBs only after spaced conditioning is eliminated when β-catenin levels are reduced, consistent with a role for Wnt signaling in long-term behavioral memory and the physiological processes underlying long-term memory trace formation. Furthermore, the expression level of wg mRNA is transiently elevated after spaced conditioning. These molecular results are consistent with those of Chen et al., 2006, Wayman et al., 2006, and Tabatadze et al., 2012, who showed that the expression of the ligands Wnt-3a, Wnt-2, Wnt-7, and Wnt-5a are elevated with increased neuronal activity or spatial memory.
This study was prompted by our previous discovery that a casein kinase Iγ homologue (CkIγ), gilgamesh (gish), is required for short-term memory (STM) in Drosophila (Tan et al., 2010). We followed the lead that CkIγ-mediated phosphorylation of the cytoplasmic tail of Lrp5/6 (Arrow) is crucial for Wnt/β-catenin signaling (Davidson et al., 2005) and predicted that disruption of the Wnt signaling pathway would perturb STM. However, we surprisingly found that knockdown of the four Wnt signaling components leaves STM intact. The likely explanation to reconcile this is that Gish has other roles important for STM formation besides a role in LTM through the phosphorylation of the Arrow receptor.
How does Wnt signaling in the MB neurons mediate the formation of LTM? Since the normal expression of β-catenin, Wg, and Arr is required in the set of MB neurons defined by P{MB-GeneSwitch}12-1 and Wg is a short-range ligand, we favor a model in which the Wnt ligand, Wg, participates in an autocrine fashion in the MB neurons. Spaced conditioning, which produces long-term behavioral memory, but not massed nor single cycle conditioning, leads to the transient increase in wg expression in the MB neurons, perhaps as a step downstream of Creb. The subsequent secretion of Wg by the MB neurons activates the Frizzled/Arrow receptor (Figure 1B) leading to the accumulation of β-catenin in the MB neurons. β-catenin, in turn, orchestrates transcriptional changes in the MB neurons required for LTM as well as the breaking and re-making of cell contacts through N-cadherin function necessary for the reorganization of synapses for LTM storage. Recently, ribonucleoprotein particles containing synaptic protein transcripts have been shown to exit the nucleus through a nuclear envelope budding process in response to Wnt signaling at the Drosophila neuromuscular junction (Speese et al., 2012). Wnt dependent nuclear budding could provide the initial step for transporting RNAs to synapses for local protein synthesis and long-term memory formation.
EXPERIMENTAL PROCEDURES
Animal Husbandry
Fly stocks were obtained from Drosophila stock centers and out-crossed to w(CS10) for six generations to normalize the genetic background (Supplemental Experimental Procedures). Flies were cultured on standard medium at 25°C, 60% relative humidity, and a 12 hr light/dark cycle. RU486 was administered at a final concentration of 200 μM.
Quantitative RT-PCR
Total RNA was isolated from fly heads using the TRIZOL reagent (Invitrogen) and reverse transcribed into cDNA using SuperScript III first-strand synthesis system (Invitrogen). For each cDNA sample, quantitative PCR was performed in triplicate using the TaqMan gene expression master mix (Applied Biosystems). The relative level of wg in each sample was normalized to the level of a eukaryotic 18S rRNA control developed by Applied Biosystems.
Behavior
Drosophila olfactory classical conditioning and testing was performed under dim red light at 25°C and 60% relative humidity as previously described (Akalal et al., 2011; Tan et al., 2010). Flies were trained to a CS+ and CS− odor with the CS+ paired with electric shock pulses (Supplemental Experimental Procedures). A 1X conditioning protocol was used to generate STM and a 5X conditioning protocol used to generate protein synthesis-dependent LTM (spaced) or protein synthesis-independent LTM (massed) (Tully et al., 1994).
Functional Imaging
Functional imaging experiments were performed as described previously (Yu et al., 2006). At selected times after conditioning, one or two flies were aspirated from the vial and mounted in a pipette tip. A small area of dorsal head cuticle was removed and the opening covered with a small piece of plastic wrap. The flies were then mounted beneath a 20X objective lens of a confocal microscope and imaged using a 488 nm excitation laser line. Odorants were diluted in mineral oil and delivered from a micropipette in an air stream at a rate of 100 ml/min. The calcium response to the CS+ odor was assayed first by imaging at 5 frames per sec with a 3 sec odor exposure. After a 5 min interval, the calcium response to the CS− odor was assayed in an identical way. Quantification of the response was made from the pixels representing the dorsal tip of the α lobe in each image. The Fo value was calculated for each pixel within the region of interest as the fluorescence prior to odor application averaged over five successive frames. The ΔF was calculated for each pixel as the difference between the highest average intensity during the 3 sec odor application across five successive frames and Fo.
Statistics
Data were analyzed with XLSTAT. All data presented represent the mean ± the standard error of the mean. One-way ANOVA was followed by Bonferroni-Dunn to test statistical significance.
Supplementary Material
HIGHLIGHTS
Wnt signaling in adult mushroom bodies is required for long-term memory
Wnt signaling is required for formation of a long-term cellular memory trace
Wingless expression becomes transiently elevated after LTM conditioning
ACKNOWLEDGEMENTS
This work was supported by NIH grant NS19904 to R.L.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akalal DB, Yu D, Davis RL. The long-term memory trace formed in the Drosophila α/β mushroom body neurons is abolished in long-term memory mutants. J. Neurosci. 2011;31:5643–5647. doi: 10.1523/JNEUROSCI.3190-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development. 1999;126:4175–4186. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- Bhat KM. frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell. 1998;95:1027–1036. doi: 10.1016/s0092-8674(00)81726-2. [DOI] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J. Biol. Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- Chen CM, Struhl G. Wingless tranduction by the Frizzled and Frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long tem potentiation. J. Biol. Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. Wnts in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. β-catenin is required for memory consolidation. Nat. Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Swtich. Proc. Natl. Acad. Sci. USA. 2004;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. Wnt and beta-catenin signaling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogeneis. Development. 2010;137:2215–2225. doi: 10.1242/dev.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J. Comp. Neurol. 2004;473:496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, Moore MJ, Budnik V. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol. Aging. 2008;31:1937–1949. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Tomas C, McGonigal R, Lin B, Schook A, Routtenberg A. Wnt transmembrane signaling and long-term spatial memory. Hippocampus. 2012;22:1228–1241. doi: 10.1002/hipo.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Yu D, Pletting J, Davis RL. Gilgamesh is required for rutabaga-independent olfactory learning in Drosophila. Neuron. 2010;67:810–820. doi: 10.1016/j.neuron.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Expression of planar cell polarity genes during development of the mouse CNS. Eur. J. Neurosci. 2006;23:597–607. doi: 10.1111/j.1460-9568.2006.04596.x. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal DB, Davis RL. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.