Abstract
Background
This study aimed to evaluate the association of recurrent molecular alterations in prostate cancer, such as ERG rearrangements and phosphatase and tensin homolog gene (PTEN) deletions, with oncologic outcomes in patients with prostate cancer treated with brachytherapy.
Methods
Ninety-two men underwent I-125 brachytherapy with a 145 Gy delivered dose between 2000 and 2008. Pretreatment prostate biopsies were analyzed by immunohistochemistry (IHC) and FISH for ERG rearrangement and overexpression, PTEN deletion, and expression loss. Univariable and multivariable Cox-regression analyses evaluated association of ERG and PTEN status with biochemical recurrence (BCR).
Results
Within a median follow-up of 73 months, 11% of patients experienced BCR. Of 80 samples with both IHC and FISH performed for ERG, 46 (57.8%) demonstrated rearrangement by FISH and 45 (56.3%) by IHC. Of 77 samples with both IHC and FISH for PTEN, 14 (18.2%) had PTEN deletion by FISH and 22 (28.6%) by IHC. No significant associations were found between ERG, PTEN status, and clinicopathologic features. Patients with concurrent ERG rearrangement and PTEN deletion demonstrated significantly worse relapse-free survival rates compared with those with ERG or PTEN wild type (P < 0.01). In multivariable Cox regression analysis adjusted for the effects of standard clinicopathologic features, combined ERG rearranged and PTEN deletion was independently associated with BCR (HR = 2.6; P = 0.02).
Conclusions
Concurrent ERG rearrangement and PTEN loss was independently associated with time to BCR in patients undergoing brachytherapy. Future studies are needed to validate prostate cancer molecular subtyping for risk stratification.
Impact
Identifying patients in the ERG-rearranged/PTEN-deleted molecular subclass may improve treatment personalization.
Introduction
Prostate cancer is a clinically heterogeneous disease; in Europe, 92,000 men were estimated to have died of advanced prostate cancer in 2012 (1), whereas a significant proportion of men had indolent disease that would not have affected their lifespan.
Brachytherapy can provide local radiation delivery in or near tumors while potentially minimizing the adverse effects and toxicities (2–4). The response to brachytherapy is quite variable, with 5-year biochemical recurrence-free survival rates ranging from 71% to 96% (3, 4). Preoperative nomograms from large studies (5–8) have helped improve risk stratification significantly. Nevertheless, 20% to 40% of patients with intermediate risk prostate cancer will fail primary treatment (9). Recent studies suggest that the variability in clinical outcomes may reflect molecular and genetic heterogeneity, which has led to the search for prognosis-specific genetic alterations (10).
Furthermore, the discovery of different molecular subclasses of prostate cancer (11–14) may help transition to more precise treatment regimens and modalities as demonstrated in other cancers. In breast cancer, the identification of clinically relevant molecular subtypes has led to the development of targeted management strategies, such as trastuzumab (15) for those expressing human epidermal growth factor receptor 2, and the use of PARP inhibitors for the treatment of triple-negative breast cancer who demonstrate BRCA1 mutations (16). Recent studies have also identified differential responses to radiation therapy according to molecular subtypes in breast cancer (17, 18).
A major advance has been the discovery of recurrent fusions between androgen-regulated genes and ETS family transcription factors in a majority of prostate cancers, most commonly as a fusion of TMPRSS2 gene and transcription factor ERG (19, 20). The TMPRSS2:ERG fusion has been associated with deletions in several tumor suppressor genes including the phosphatase and tensin homolog gene (PTEN; refs. 21 and 22), which normally acts to deactivate phosphoinositide 3-kinase–dependent signaling.
ETS gene rearrangements and PTEN deletions have now been found to be common molecular events and may be important in prostate carcinogenesis. PTEN deletions are found in approximately 40% of prostate cancer specimens, and have been associated with advanced disease and poorer prognosis (21, 22–26). The relationship between ETS rearrangements and clinical outcomes has been inconsistent. In general, population-based studies of watchful-waiting cohorts have found ETS rearrangements to be associated with poorer prognosis (27), whereas retrospective radical prostatectomy cohorts have found conflicting associations (10, 28–30). In a recent study of a watchful-waiting cohort, PTEN loss and ETS gene rearrangements were found to be associated with poorer cancer-specific survival (31). Several studies indicate that PTEN status may influence response to radiation therapy (32, 33), whereas ERG status may not be associated. Although suggested to provide a response advantage (34), the association between PTEN loss and ETS gene rearrangements has not been formally studied in patients undergoing radiation therapy previously.
The major objective of this study was to characterize the association of PTEN deletions and ERG fusions with oncologic outcomes in patients with prostate cancer treated with brachytherapy.
Materials and Methods
Patient population and specimen collection
This institutional review board–approved study included 92 men with a positive biopsy for prostate cancer treated with interstitial brachytherapy (I-125 permanent implant with a delivered dose of 145 Gy) between 2000 and 2008 from Santa Chiara Hospital in Trento, Italy, Santa Maria del Carmine Hospital in Rovereto, Italy, and Bolzano Hospital in Bolzano, Italy. One third of the patients received short-term neoadjuvant androgen deprivation therapy (ADT), either bicalutamide or flutamide, for 4 to 6 months pre-brachytherapy. Patients were assigned to risk groups (low, intermediate, and high) based upon clinical stage, initial biopsy Gleason Score, and prostate-specific antigen (PSA) levels as per the National Comprehensive Cancer Network (35). Biochemical relapse (BCR) was defined according to the Phoenix criteria (PSA nadir + 2 ng/mL; refs. 36). International Prostate Symptom Scores (IPSS) were collected before initiating brachytherapy (Table 1).
Table 1.
Study cohort demographics
| Number of patients | 92 | |
|---|---|---|
| Age (y) (mean ± SD) | 65.8 ± 5 | |
| cT, N (%) | cT1 | 50 (54) |
| cT2/3 | 42 (46) | |
| PSAi (ng/mL), N (%) | <4 | 7 (8) |
| 4 ≤ x< 10 | 59 (64) | |
| >10 | 26 (28) | |
| Risk group, N (%) | L | 55 (60) |
| I | 32 (35) | |
| H | 5 (5) | |
| Gleason score, N (%) | 6 | 68 (74) |
| 7 | 16 (17) | |
| 8 | 8 (9) | |
| International prostate symptom score, N (%) |
<8 | 80 (87) |
| ≥ 8 | 12 (13) | |
| Volume transrectal ultrasound (cc) (mean ± SD) |
34 ± 9 | |
| BCR, N (%) | No event | 82 (89) |
| Event | 10 (11) | |
| RFS (mo) (mean ± SD) | No event | 72 ± 28 |
| Event | 50 ± 33 | |
| OS (mo) (mean ± SD) (median) | No event | 72 ± 28 (70) |
| Event | 90 ± 30 (96) | |
| Hormonal therapy pre-implant | No | 58 (63) |
| Yes | 34 (37) |
For this study, all hematoxylin and eosin (H&E)–stained sections (12 for each patient) from formalinfixed paraffin-embedded pretreatment biopsies were centrally reviewed by 2 study pathologists (P. Dalla Palma and M. Barbareschi) who were blinded to clinicopathologic parameters and patient outcomes. For each patient, a paraffin block, which was representative of the highest Gleason score, was selected for IHC and FISH evaluations.
Immunohistochemistry analysis
Two 4 μm sections were prepared from each block for immunostaining for ERG and PTEN. Rabbit monoclonal antibodies were utilized (ERG: EPR3864, Ventana, at 1:100 dilution; PTEN: 138G6, Cell Signaling Technology, at 1:25) with an automatic immunostainer (Leica Bond MAX, Leica Biosystem), with antigen retrieval (Bond Polymer Refine Detection, Leica Biosystem). Two pathologists performed a semiquantitative evaluation of nuclear ERG expression using a Fourtier grading system: negative (0), weakly (1+), moderately (2+), and strongly (3+) positive. Any positive staining with >5% of total tumor cells was considered positive for ERG expression (ERG+). ERG expression of endothelial cells was utilized as the positive internal control of the immunohistochemical reaction (37). Cytoplasmic and nuclear PTEN expression was scored with the same grading system as ERG. Each tumor focus was scored as negative or positive for PTEN protein by comparing staining in malignant glands and adjacent benign glands and/or stroma, which provided an internal positive control. Cases lacking PTEN expression in all or some tumor cells in presence of positive internal controls in the surrounding benign glands and/or stroma were classified as PTENdel. Tissue quality was adequate for ERG and for PTEN immunohistochemistry (IHC) assessments for 86 patients (93.5%). IHC scoring was blinded with respect to FISH results.
FISH analysis
Two 4-μm-thick tissue sections from each block were cut for FISH analysis. ERG rearrangement status was determined by 2 observers using a dual-color break-apart interphase FISH assay as previously described (19, 38). Briefly, ERG rearrangement status was determined using differentially labeled probes spanning the centromeric (BAC clone RP11-24A11, labeled red) and telomeric (BAC clone RP11-372O17, labeled green) regions of ERG. PTEN deletion was detected using a gene-specific probe (BAC clone CTD-2047N14) and a reference probe, located at 10q25.2 (RP11-431P18). Deletion was defined as fewer than 2 copies of the gene specific probe per nucleus in the presence of 2 reference signals. All clones were tested on metaphase spreads. At least 100 nuclei were evaluated per tissue biopsy using a fluorescence microscope (Olympus BX51; Olympus Optical). Tissue quality was adequate for ERG rearrangement and PTEN loss status evaluation in 82 cases (89.1%).
Statistical analysis
TMPRSS2:ERG gene rearrangement leading to the overexpression of ERG protein expression as determined by IHC or FISH will be referred to as ERG+, PTEN deletions will be referred to as PTENdel, and the respective molecular subclasses are referred to as ERG+/PTENdel, ERGwt/PTENdel, ERG+/PTENwt, and ERGwt/PTENwt. Differences in variables with a continuous distribution across categories were assessed using the Mann–Whitney U test (2 categories). The Fisher exact test and the χ2 test were used to evaluate the association between categorical variables. Univariable recurrence-free and cancer-specific survival probabilities were estimated using the Kaplan–Meier method. Differences were assessed using the log-rank test. Uni- and multivariable Cox regression analyses addressed factors associated with disease recurrence, cancer-specific, and all-cause mortality. Multivariable analysis was done using forward step-wise logistic regression. Multivariable analyses were performed using ERG and PTEN status by IHC, as status by FISH was not significantly associated with time to relapse-free survival on univariable analysis (P = 0.09). All tests were 2-sided, with a P value of <0.05 considered = to be statistically significant. All analyses were performed with SPSS 20 (SPSS Inc., IBM Corp.).
Results
Clinical characteristics
The patient characteristics are listed in Table 1. Of the 92 men, 5% (5/92) and 35% (32/92) had high-risk and intermediate-risk disease. Overall, 11% (10/92) of the patients developed BCR with a median overall follow-up of 73 months (range 1–138 months). In total, 37% (34/92) underwent neoadjuvant ADT.
Comparison of IHC and FISH for PTEN and ERG
Of the 92 patients, 80 men (87%) had both IHC and FISH performed for ERG rearrangement and 77 (83.7%) for PTEN deletion status. ERG+ frequency was 57.8% (46/80) when evaluated by FISH and 56.3% (45/80) by IHC, with a concordance of 97.8% (P < 0.01). ERG IHC staining was generally either diffusely positive (2+ or 3+ intensity) or completely negative. For the 77 men that had IHC and FISH for PTEN, 22 (28.6%) had PTENdel by IHC and an additional 14 (18.2%) had hemizygous loss of PTEN by FISH, with 4 exhibiting PTENdel by FISH and IHC (P = 1.0).
In comparing the 86 men that had IHC performed for both ERG and PTEN, 18 (20.9%) were ERG+/PTENdel, 5 (5.8%) were ERGwt/PTENdel, and 30 (34.9%) were ERG+/PTENwt (P = 0.01). A representative ERG+/PTENdel prostate biopsy is shown in Fig. 1. For the 82 men that had FISH performed for PTEN and ERG, 7 (8.5%) were ERG+/PTENdel, 7 (8.5%) were ERGwt/PTENdel, and 39 (47.6%) were ERG+/PTENwt.
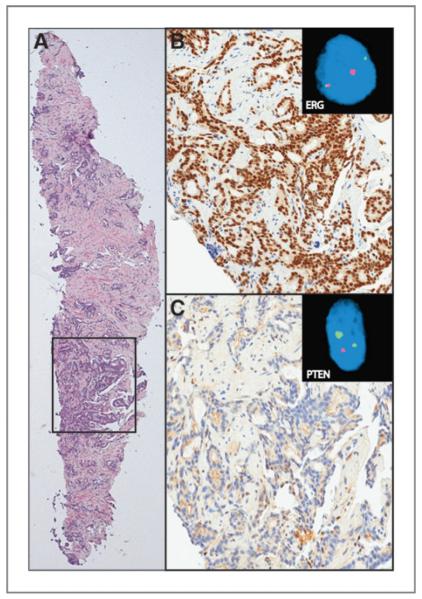
Figure 1.
Detection of ERG and PTEN status by IHC and FISH. An ERG+/PTENdel biopsy is represented. A, H&E of needle biopsy showing prostatic adenocarcinoma, Gleason score 7. B, strong, diffuse nuclear ERG IHC staining of tumor glands. ERG break-apart FISH assay showing ERG translocation (inset). C, absence of PTEN IHC staining in tumor glands. FISH assay showing hemizygous PTEN loss (inset).
Association of PTEN and ERG with clinicopathologic features
Rearrangement of ERG by FISH or expression of ERG protein by IHC did not differ according to patient age, PSA, clinical stage, risk-factor grouping, use of neoadjuvant ADT, biopsy Gleason score, or pre-brachytherapy IPSS scores (all P > 0.05). The deletion of PTEN by FISH or IHC was also not associated with any of the previously mentioned clinicopathologic features (all P > 0.05; Table 2).
Table 2.
Association of ERG and PTEN IHC status with clinicopathologic features
| ERG IHC |
PTEN IHC |
ERG/PTEN IHC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neg | Pos | P-value | Neg | Pos | P-value | Neg/no loss | Pos/no loss | Neg/loss | Pos/loss | P-value | ||
| Age | ≤ Median | 23 | 26 | 0.58 | 35 | 13 | 0.94 | 20 | 15 | 3 | 10 | 0.86 |
| > Median | 16 | 23 | 28 | 10 | 13 | 15 | 2 | 8 | ||||
| PSAi (ng/mL) | < 4 | 3 | 4 | 0.97 | 4 | 2 | 0.93 | 1 | 3 | 1 | 1 | 0.46 |
| 4 ≤ x < 10 | 24 | 31 | 40 | 14 | 20 | 20 | 4 | 10 | ||||
| > 10 | 12 | 14 | 19 | 7 | 12 | 7 | 0 | 7 | ||||
| cT | 1 | 24 | 22 | 0.21 | 35 | 10 | 0.32 | 19 | 16 | 4 | 6 | 0.21 |
| 2/3 | 15 | 27 | 28 | 13 | 14 | 14 | 1 | 12 | ||||
| Risk group | H | 3 | 2 | 0.62 | 2 | 3 | 0.18 | 2 | 0 | 1 | 2 | 0.44 |
| I | 12 | 19 | 22 | 9 | 11 | 11 | 1 | 8 | ||||
| L | 24 | 28 | 39 | 11 | 20 | 19 | 3 | 8 | ||||
| Gleason score |
6 | 29 | 36 | 0.68 | 49 | 14 | 0.13 | 26 | 23 | 2 | 12 | 0.18 |
| 7 | 6 | 10 | 11 | 5 | 5 | 6 | 1 | 4 | ||||
| 8 | 4 | 3 | 3 | 4 | 2 | 1 | 2 | 2 | ||||
Association of PTEN and ERG with oncologic outcomes
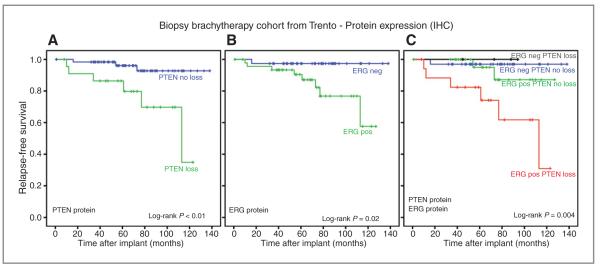
The median follow-up time was 73 months. Within the follow-up, 10 (11%) developed BCR, and 2 (2.2%) died of disease. From Kaplan–Meier analysis, the actuarial recurrence-free survival was significantly lower for those with moderate and high-risk disease compared with low-risk (log rank P-value < 0.01) diseases. Those who were PTENdel by IHC displayed significantly shorter times to recurrence (P < 0.01; Fig. 2A), as did ERG+ patients by FISH and IHC (P = 0.02; Fig. 2B). Estimated times to recurrence-free survival were not significantly associated with PTENdel by FISH.
Figure 2.
Prostate cancer relapse-free survival according to PTEN and ERG IHC status. Kaplan–Meier curves are reported with respect to recurrence-free survival for PTEN loss identified by IHC (A), for ERG+ prostate cancer identified by IHC (B), and for their combination (C).
We hypothesized that ERG and PTEN status could be used as classifiers to define molecular subgroups. ERG+/PTENdel patients identified by IHC had significantly lower rates of recurrence-free survival than ERGwt/PTENwt patients (log-rank P-value <0.01; Fig. 2C), whereas ERG+/PTENdel by FISH was not significantly associated with time to relapse-free survival (P = 0.09). Use of ADT was not associated with BCR or survival in this cohort. In a subset analysis of only those with Gleason 6 on prostate biopsy, ERG+/PTENdel patients exhibited significantly shorter times to BCR than ERGwt/PTENdel or ERGwt/PTENwt patients (P = 0.03). The 2 patients who died of disease were ERG+/PTENdel.
In multivariable Cox regression analysis, adjusting for age and biopsy Gleason score, PTENdel by IHC remained independently associated with BCR [HR = 1.80; P-value = 0.01; 95% confidence interval (CI), 1.51–24.2]. The ERG+/PTENdel subtype was also independently associated with BCR (HR = 2.60, P-value = 0.02; 95% CI, 1.62–111.9).
Discussion
Recent discoveries in the genomic landscape and molecular pathways of prostate cancer (10, 12–14) have helped spur the search for molecularly distinct subclasses of prostate cancer that may have differential responses to various therapies. This represents the first known study to investigate the association between the ERG+/PTENdel subtype and biochemical recurrence in patients with prostate cancer treated with brachytherapy. ERG+/PTENdel patients exhibited shorter times to BCR compared with ERGwt/PTENdel or ERG+/PTENwt. After adjusting for disease characteristics, ERG+/PTENdel subtype was independently associated with BCR in patients that underwent brachytherapy.
Prior studies have reported that the lack of ETS gene fusions and lack of PTEN loss (ERGwt/PTENwt) were associated with good prognosis in patients undergoing radical prostatectomy or in a conservatively treated watchful waiting cohort (39–41). However those who were ERG+/PTENdel had faster BCR rates in the prostatectomy cohort (41), whereas the ERGwt/PTENdel patients had significantly lower survival rates than ERG+/PTENdel patients in the watchful waiting cohort (31). This discrepancy may reflect differences in the outcomes measured or study sampling methods, but may also reflect true differences in the response to different treatment modalities among distinct molecular subtypes. Larger sample sizes across different treatment modalities will help to further characterize the importance of molecular subtypes in prostate cancer.
To our knowledge, few studies have interrogated the influence of molecular subclasses of prostate cancer on brachytherapy treatment response, specifically. In a recent publication, Dal Pra and colleagues (42) looked at ERG status alone in pretreatment biopsies in patients with prostate cancer treated by image-guided radiotherapy (IGRT), and identified no association between ERG status and biochemical-free relapse rate. Another study reported that tumors with c-MYC amplification alone, or combined with PTEN loss, were prognostic for BCR after IGRT (32).
ETS gene fusions and PTEN deletions do not exist in isolation but have been found to have complex interactions altering androgen receptor signaling. Chen and colleagues (22) reported that ETS positive cancers that lose PTEN exhibit partial restoration of androgen receptor transcription resulting in early-onset invasive prostate cancer, in contrast to the suppression of androgen receptor when there is loss of PTEN in ETS negative samples. Several other studies have demonstrated the subclonal loss of PTEN in prostate cancers (13, 40, 43), whereas ETS rearrangements tend to occur homogenously in both metastatic and primary prostate cancer samples, indicating that often PTEN deletion occurs as a relatively late event compared with ETS fusions in prostate carcinogenesis. These data indicate that patients with ETS gene rearrangements that develop loss of PTEN exhibit a distinct molecular environment, with potentially differing responses to treatments (44). In support of this observation, a recent study found that PARP inhibition using rucaparib was able to sensitize cells that exhibited PTEN loss and ETS rearrangements to low-dose radiation (34).
Several groups have explored the biologic mechanisms by which ERG rearrangement and PTEN deletion may confer radiation resistance. A recent study showed that ERG confers radioresistance through increased DNA damage response efficiency, by interacting with PARP1 and increasing its activity (45). Similarly, it has been suggested that loss of PTEN function delays the repair of radiation-induced double-stranded breaks (46).
In addition, in our study, we confirm that there is a high concordance between IHC and FISH for the detection of ERG rearrangements, as previously reported (37, 47). However, PTEN assessment is less concordant between PTEN protein loss by IHC and PTEN genomic loss by FISH. This is because of the fact that loss of PTEN protein expression may be caused by variable genomic and epigenomic mechanisms, such as inversions and mutations of PTEN, recently described rearrangements disrupting PTEN-interacting proteins such as MAGI2 (14) or post-translational inactivation, all of which were not detectable by FISH (48, 49).
There are several limitations to consider in our study. The study was retrospective in design with the inherent biases and confounders of all retrospective studies. Inherent in prostate cancer studies is inter- and intratumoral heterogeneity, which can confound the association of outcomes with molecular subclasses. This study has a relatively small sample size, and the current findings should be substantiated in independent studies on larger cohorts. In addition, there is significant heterogeneity in management strategies with neoadjuvant ADT, and may influence the times to BCR.
Conclusions
Concurrent ERG rearrangement and loss of PTEN, which seems to represent a biologically relevant molecular subclass, was independently associated with time to BCR and worse prognosis in patients undergoing brachytherapy. Identifying patients in this subclass may predict failure to radiotherapy and may therefore improve treatment personalization by suggesting alternative management strategies. Larger prospective studies are needed to validate the molecular subtyping of prostate cancer for risk stratification.
Acknowledgments
The authors thank T.Y. MacDonald for her assistance in performing FISH assays.
Grant Support This study was supported by the Fondazione Trentina per la Ricerca sui Tumori (F. Demichelis and C. Cantaloni), by the Associazione Italiana per la Ricerca sul Cancro (AIRC, IG 13562), and by the Early Detection Research Network (5U01 CA11275-07 for M.A. Rubin, J. Fontugne, and J.M. Mosquera).
Footnotes
Disclosure of Potential Conflicts of Interest J.M. Mosquera has a commercial research grant from Ventana Medical Systems, Inc. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions Conception and design: O. Caffo, M.A. Rubin, G. Fellin, J.M. Mosquera, F. Demichelis
Development of methodology: P. Dalla Palma, F. Demichelis
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J. Fontugne, C. Cantaloni, O. Caffo, E. Hanspeter, G. Mazzoleni, G. Fellin, J.M. Mosquera, M. Barbareschi, F. Demichelis
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J. Fontugne, D. Lee, C.E. Barbieri, P. Dalla Palma, J.M. Mosquera, M. Barbareschi, F. Demichelis
Writing, review, and/or revision of the manuscript: J. Fontugne, D. Lee, C.E. Barbieri, O. Caffo, M.A. Rubin, G. Fellin, J.M. Mosquera, M. Barbareschi, F. Demichelis
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): D. Lee, E. Hanspeter, G. Mazzoleni, F. Demichelis
Study supervision: P. Dalla Palma, J.M. Mosquera, M. Barbareschi, F. Demichelis
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Ferrer M, Guedea F, Suarez JF, De Paula B, Macias V, Marino A, et al. Quality of life impact of treatments for localized prostate cancer: Cohort study with a 5-year follow-up. Radiother Oncol. 2013;108:306–13. doi: 10.1016/j.radonc.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Peinemann F, Grouven U, Bartel C, Sauerland S, Borchers H, Pinkawa M, et al. Permanent interstitial low-dose-rate brachytherapy for patients with localised prostate cancer: a systematic review of randomised and nonrandomised controlled clinical trials. Eur Urol. 2011;60:881–93. doi: 10.1016/j.eururo.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Peinemann F, Grouven U, Hemkens LG, Bartel C, Borchers H, Pinkawa M, et al. Low-dose rate brachytherapy for men with localized prostate cancer. Cochrane Database Syst Rev. 2011:CD008871. doi: 10.1002/14651858.CD008871.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715–7. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ, Jr, Yossepowitch O, Vickers AJ, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–5. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briganti A, Joniau S, Gontero P, Abdollah F, Passoni NM, Tombal B, et al. Identifying the best candidate for radical prostatectomy among patients with high-risk prostate cancer. Eur Urol. 2012;61:584–92. doi: 10.1016/j.eururo.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg MS, Karnes RJ, Kaushik D, Rangel L, Bergstralh EJ, Boorjian SA. Risk stratification of patients with extraprostatic extension and negative lymph nodes at radical prostatectomy: identifying optimal candidates for adjuvant therapy. J Urol. 2013;190:1735–41. doi: 10.1016/j.juro.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 9.Nichol AM, Warde P, Bristow RG. Optimal treatment of intermediate-risk prostate carcinoma with radiotherapy: clinical and translational issues. Cancer. 2005;104:891–905. doi: 10.1002/cncr.21257. [DOI] [PubMed] [Google Scholar]
- 10.Barbieri CE, Bangma CH, Bjartell A, Catto JW, Culig Z, Gronberg H, et al. The mutational landscape of prostate cancer. Eur Urol. 2013;64:567–76. doi: 10.1016/j.eururo.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–9. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–77. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Siva-chenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 16.O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–8. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 18.Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Over-gaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–26. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 19.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 20.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–9. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 21.Carver BS, Tran J, Gopalan A, Chen Z, Shaikh S, Carracedo A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41:619–24. doi: 10.1038/ng.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Chi P, Rockowitz S, Laquinta PJ, Shamu T, Shukla S, et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat Med. 2013;19:1023–9. doi: 10.1038/nm.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, White TA, MacKenzie AP, Clegg N, Lee C, Dumpit RF, et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci U S A. 2011;108:17087–92. doi: 10.1073/pnas.1108745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weischenfeldt J, Simon R, Feuerbach L, Schlangen K, Weichenhan D, Minner S, et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23:159–70. doi: 10.1016/j.ccr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Krohn A, Diedler T, Burkhardt L, Mayer PS, De Silva C, Meyer-Korn-blum M, et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am J Pathol. 2012;181:401–12. doi: 10.1016/j.ajpath.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Yoshimoto M, Trpkov K, Duan Q, Firszt M, Corcos J, et al. Detection of ERG gene rearrangements and PTEN deletions in unsuspected prostate cancer of the transition zone. Cancer Biol Ther. 2011;11:562–6. doi: 10.4161/cbt.11.6.14376. [DOI] [PubMed] [Google Scholar]
- 27.Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–9. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 28.Darnel AD, Lafargue CJ, Vollmer RT, Corcos J, Bismar TA. TMPRSS2-ERG fusion is frequently observed in Gleason pattern 3 prostate cancer in a Canadian cohort. Cancer Biol Ther. 2009;8:125–30. doi: 10.4161/cbt.8.2.7134. [DOI] [PubMed] [Google Scholar]
- 29.Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Sun C, Chen Y, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene. 2005;24:3847–52. doi: 10.1038/sj.onc.1208518. [DOI] [PubMed] [Google Scholar]
- 30.Saramaki OR, Harjula AE, Martikainen PM, Vessella RL, Tammela TL, Visakorpi T. TMPRSS2:ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res. 2008;14:3395–400. doi: 10.1158/1078-0432.CCR-07-2051. [DOI] [PubMed] [Google Scholar]
- 31.Reid AH, Attard G, Ambroisine L, Fisher G, Kovacs G, Brewer D, et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br J Cancer. 2010;102:678–84. doi: 10.1038/sj.bjc.6605554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zafarana G, Ishkanian AS, Malloff CA, Locke JA, Sykes J, Thoms J, et al. Copy number alterations of c-MYC and PTEN are prognostic factors for relapse after prostate cancer radiotherapy. Cancer. 2012;118:4053–62. doi: 10.1002/cncr.26729. [DOI] [PubMed] [Google Scholar]
- 33.Rosser CJ, Tanaka M, Pisters LL, Tanaka N, Levy LB, Hoover DC, et al. Adenoviral-mediated PTEN transgene expression sensitizes Bcl-2-expressing prostate cancer cells to radiation. Cancer Gene Ther. 2004;11:273–9. doi: 10.1038/sj.cgt.7700673. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee P, Choudhary GS, Sharma A, Singh K, Heston WD, Cieski J, et al. PARP inhibition sensitizes to low dose-rate radiation TMPRSS2-ERG fusion gene-expressing and PTEN-deficient prostate cancer cells. PLoS ONE. 2013;8:e60408. doi: 10.1371/journal.pone.0060408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 36.Roach M, 3rd, Hanks G, Thames H, Jr., Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 37.Park K, Tomlins SA, Mudaliar KM, Chiu YL, Esgueva R, Mehra R, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–8. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–41. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 39.Nagle RB, Algotar AM, Cortez CC, Smith K, Jones C, Sathyanarayana UG, et al. ERG overexpression and PTEN status predict capsular penetration in prostate carcinoma. Prostate. 2013;73:1233–40. doi: 10.1002/pros.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimoto M, Ding K, Sweet JM, Ludkovski O, Trottier G, Song KS, et al. PTEN losses exhibit heterogeneity in multifocal prostatic adenocarcinoma and are associated with higher Gleason grade. Mod Pathol. 2013;26:435–47. doi: 10.1038/modpathol.2012.162. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimoto M, Joshua AM, Cunha IW, Coundry RA, Fonseca FP, Ludkovski O, et al. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol. 2008;21:1451–60. doi: 10.1038/modpathol.2008.96. [DOI] [PubMed] [Google Scholar]
- 42.Dal Pra A, Lalonde E, Sykes J, Warde F, Ishkanian A, Meng A, et al. TMPRSS2-ERG status is not prognostic following prostate cancer radiotherapy: implications for fusion status and DSB repair. Clin Cancer Res. 2013;19:5202–9. doi: 10.1158/1078-0432.CCR-13-1049. [DOI] [PubMed] [Google Scholar]
- 43.Gumuskaya B, Gurel B, Fedor H, Tan HL, Weier CA, Hicks JL, et al. Assessing the order of critical alterations in prostate cancer development and progression by IHC: further evidence that PTEN loss occurs subsequent to ERG gene fusion. Prostate Cancer Prostatic Dis. 2013;16:209–15. doi: 10.1038/pcan.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demichelis F, Attard G. A step toward functionally characterized prostate cancer molecular subtypes. Nat Med. 2013;19:966–7. doi: 10.1038/nm.3285. [DOI] [PubMed] [Google Scholar]
- 45.Hans S, Brenner JC, Salboch A, Jackson W, Speers C, Wilder-Romans K, et al. Targeted radiosensitization of ETS fusion-positive prostate cancer through PARP1 inhibition. Neoplasia. 2013;15:1207–17. doi: 10.1593/neo.131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pappas G, Zumstein LA, Munshi A, Hobbs M, Meyn RE. Adenoviral-mediated PTEN expression radiosensitizes non-small cell lung cancer cells by suppressing DNA repair capacity. Cancer Gene Ther. 2007;14:543–9. doi: 10.1038/sj.cgt.7701050. [DOI] [PubMed] [Google Scholar]
- 47.Chaux A, Albadine R, Toubaji A, Hicks J, Meeker A, Platz EA, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol. 2011;35:1014–20. doi: 10.1097/PAS.0b013e31821e8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhalla R, Kunju LP, Tomlins SA, Christopherson K, Cortez C, Carskadon S, et al. Novel dual-color immunohistochemical methods for detecting ERG-PTEN and ERG-SPINK1 status in prostate carcinoma. Mod Pathol. 2013;26:835–48. doi: 10.1038/modpathol.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lotan TL, Gurel B, Sutcliffe S, Esopi D, Liu W, Xu J, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011;17:6563–73. doi: 10.1158/1078-0432.CCR-11-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]