Abstract
OBJECTIVES
This study sought to evaluate the relationship between microalbuminuria (MA) and the development and progression of atherosclerosis, as assessed by incident and progression of coronary artery calcification (CAC).
BACKGROUND
MA is associated with an increased risk of cardiovascular disease, but the mechanism by which MA imparts this increased risk is not known.
METHODS
The MESA (Multi-Ethnic Study of Atherosclerosis) study is a prospective cohort study of 6,814 self-identified White, African-American, Hispanic, or Chinese participants free of clinical cardiovascular disease at entry. Of the 6,775 individuals with available urine albumin data, we excluded 97 subjects with macroalbuminuria and 1,023 with missing follow-up CAC data. The final study population consists of 5,666 subjects.
RESULTS
At baseline, individuals with MA were more likely to have CAC >0 compared with those without MA (62% vs. 48%, p < 0.0001). During a mean follow-up of 2.4 ± 0.8 years, those with MA and no CAC at baseline were more likely to develop CAC (relative risk [RR]: 2.05, 95% confidence interval [CI]: 1.41 to 3.02, p < 0.0001) as compared with those without MA in demographic-adjusted analyses. After multivariant adjustment, the relationship was attenuated but remained statistically significant (RR: 1.76, 95% CI: 1.19 to 2.61, p = 0.005). Among those with CAC at baseline, those with versus those without MA had a 15 (95% CI: 8 to 22, p < 0.0001) volume units higher median increase in CAC in demographic-adjusted analyses. After multivariant adjustment, MA remained associated with incident CAC (RR: 1.76, 95% CI: 1.19 to 2.61, p = 0.005) and with progression of CAC (median increase in CAC volume score of 9 [95% CI: 2 to 16, p = 0.009]), relative to those without MA.
CONCLUSIONS
This large multiethnic, population-based study of asymptomatic individuals demonstrates an increased risk of incident CAC as well as greater CAC progression among those with MA. Further study is needed to determine the degree to which MA precedes and predicts progression of atherosclerosis and how this information can be used to reduce cardiovascular events.
Keywords: coronary artery calcium, microalbuminuria, risk prediction, coronary heart disease, Multi-Ethnic Study of Atherosclerosis
Analysis of cohort studies including more than 15,000 participants have demonstrated a progressive increase in cardiovascular events with increasing urine albumin excretion in diabetic, nondiabetic, symptomatic, and asymptomatic individuals (1–3). Additionally, a reduction in urine albumin excretion at 1 year was associated with a reduction in the risk of the composite end point of cardiovascular death, myocardial infarction, or stroke in 8,206 subjects treated with antihypertensive medications (4).
The mechanism by which increased urine albumin excretion contributes to cardiovascular risk is not entirely clear. Studies have demonstrated that various measures of endothelial function correlate with microalbuminuria (MA) (defined as excretion of 30 to 300 mg albumin/day) in older adults and diabetic patients (5–7). Recently, Kramer et al. have demonstrated that the presence of MA is associated with a higher burden of coronary artery calcification (CAC) in the MESA (Multi-Ethnic Study of Atherosclerosis) study (8). However, data on whether MA is associated with progression of atherosclerosis are sparse.
CAC is a measure of atherosclerosis that has been shown to predict future cardiovascular events in multiple asymptomatic populations (9–18), and limited data suggest that progression of CAC may predict future cardiovascular disease risk with greater accuracy than a single measure of CAC (19,20).
The MESA study provides a unique opportunity to study the association of increasing degree of urine albumin excretion with progression of CAC in a large, gender-balanced asymptomatic cohort (21). We hypothesized that asymptomatic individuals with MA would have higher degrees of CAC progression and that this relationship will be independent of other coronary heart disease (CHD) risk factors. Establishing a relationship between MA and CAC progression would add to our understanding of how MA contributes to cardiovascular risk. It would support the hypothesis that MA is a global measure of vascular health (22)—that is, a summation measure of the impact that known and/or unknown vascular insults (hypertension, dyslipidemia, smoking, etc.) have on an individual’s vasculature.
METHODS
MESA is a prospective epidemiologic study of the prevalence, risk factors, and progression of subclinical cardiovascular disease in a multiethnic cohort. The study design and methods has been previously published (21). Briefly, 6,814 participants aged 45 to 84 years who identified themselves as White, African American, Hispanic, or Chinese were recruited from 6 U.S. communities (Forsyth County, North Carolina; Northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles County, California) in 2000 to 2002. These participants were free of clinical cardiovascular disease (myocardial infarction, angina, stroke, transient ischemic attack, heart failure, atrial fibrillation, revascularization, valve replacement, pacemaker or defibrillator implantation, or taking nitroglycerin).
An approximately equal number of men and women were recruited according to pre-specified age and race/ethnicity strata. All participants gave informed consent, and the study protocol was approved by the institutional review board at each site.
Medical history, anthropometric measurements, and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000 to August 2002) as previously described (21). Urine albumin and creatinine were measured at the Clinical Chemistry Laboratory at Fletcher Allen Health Care (Burlington, Vermont). Urine albumin and creatinine were measured by nephelometry and the rate Jaffe reaction, respectively. Spot urine albumin (µg/ml)-to-creatinine (mg/ml) ratios (UACRs) were calculated for all participants except those with missing urine data (n = 39). Participants were classified as those with MA (30 to 300 mg albumin/g creatinine) and those with normal UACR (<30 mg/g). This study does not evaluate the relationship between chronic kidney disease and CAC, and therefore, those with macroalbuminuria, UACR >300 mg/g, were excluded from the study.
Measurement of CAC
CAC was measured using either electron-beam tomography (EBT) (3 sites) or multidetector computed tomography (MDCT) (3 sites). Participants were scanned twice consecutively, and each scan was read by a single trained physician-reader independently at a centralized reading center (Harbor-UCLA Medical Center/ Los Angeles Biomedical Research Institute, Torrance, California). The methodology for acquisition and interpretation of the scans, as well as reproducibility of the readings, has been reported previously (23). The results from the 2 scans were averaged to provide a more accurate point estimate of the amount of calcium present. Calcium scores were adjusted using a standard calcium phantom that was scanned along with the participant. The phantom contained 4 bars of known calcium density, and was used to calibrate the X-ray attenuation level between measurements conducted on different machines (24). Any detectable calcium was defined as an Agatston CAC score greater than 0 (25); a minimum focus of calcification was based on at least 4 contiguous voxels, which resulted in identification of calcium of 1.15 mm3 for the MDCT scanners and 1.38 mm3 for the EBT scanners. The nominal section thickness was 3.0 mm for EBT scanners and 2.5 mm for MDCT.
To quantify CAC progression, a second CAC measurement was performed on one-half of the cohort (randomly selected) at a second exam (September 2002 to January 2004) and on the other half of the cohort at a third exam (March 2004 to July 2005), an average of 1.6 and 3.2 years after the first scan, respectively (overall average time between scans of 2.4 years). The distribution of CAC in MESA at baseline by age, gender, and race has been published previously (26).
Statistical analysis
All participants with both a baseline and a follow-up CAC measurement were included in the analysis. The presence of CAC was defined as an Agatston score >0. Progression of CAC was defined in 2 ways as previously described by Kronmal et al. (27); incident CAC defined as detectable CAC at the follow-up examination (either examination 2 or 3) in a participant free of detectable CAC at examination 1; and change in CAC volume score in participants who had detectable CAC at examination 1.
The association of incident CAC and median increase in CAC volume with presence of MA as well as increasing UACR as a continuous variable (natural logarithmic transformation) was assessed. Relative risk regression was used to model the probability of incident-detectable CAC among those free of CAC at examination 1. The probability of incident CAC was modeled as a function of covariates using a generalized linear model with log link and binomial error distribution. Relative risk regression was used rather than logistic regression because the incidence of new calcification was >10%, so the odds ratio is an overestimate of the relative risk. To estimate the progression of CAC among those with detectable CAC at baseline, we used median regression analyses. These 2 end points were modeled separately and stratified on race/ethnicity. Risk factors considered include age, gender, MESA site, follow-up duration, body mass index (height/meter2), hypertension, diabetes mellitus, cigarette smoking, family history of CHD, cholesterol levels, and use of cholesterol-lowering medications.
Two sets of multivariable models were examined in a hierarchical fashion: 1) adjusted for age, gender, race, MESA site, and duration of follow-up; and 2) adjusted for age, gender, race, MESA site, follow-up duration, body mass index, hypertension, diabetes mellitus, cigarette smoking, family history of CHD, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, and cholesterol-lowering medications. Baseline CAC was not included in our multivariant analysis because inclusion would lead to overcorrection/ overmodeling because the factors that produce baseline CAC are certainly contributory to CAC progression. Effect modification by scan type, systolic blood pressure as a continuous variable, creatinine level, and estimated glomerular filtration rate (Cockroft and Gault equation) was evaluated. Race was not adjusted for race-specific analyses. An interaction term with MA and race/ethnicity was created to assess for interaction.
We used chi-square tests for categorical variables and t test for continuous variables to test for differences between participants with and without MA to assess for baseline differences in demographics and cardiovascular risk factors.
All statistical analyses were completed using STATA software version 8 (College Station, Texas).
RESULTS
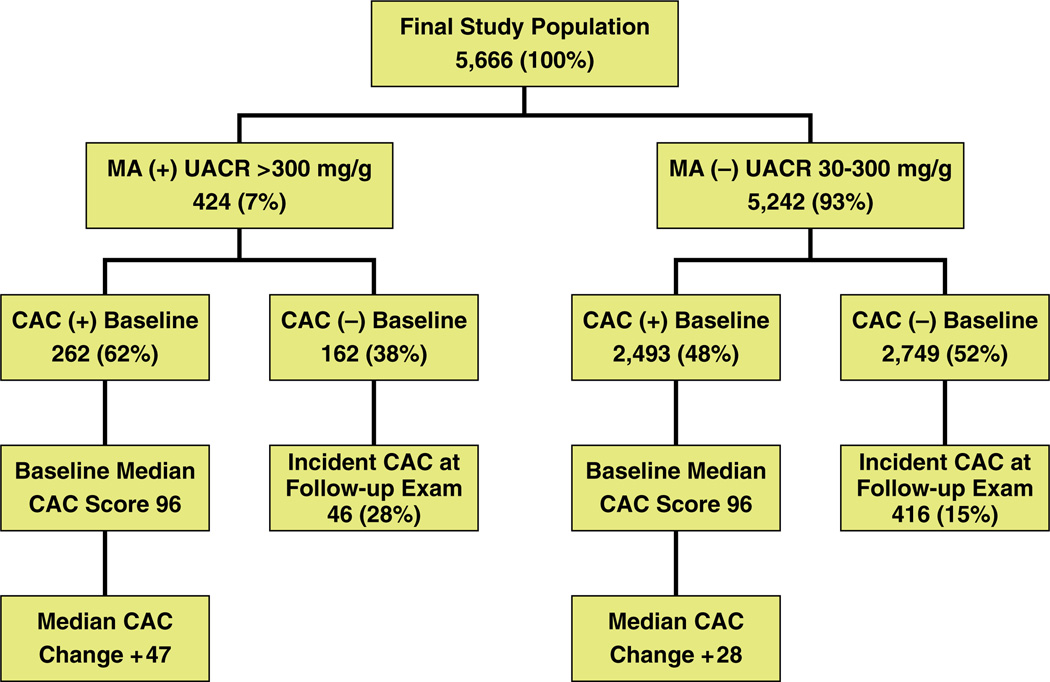
The current study included the 6,775 individuals that had data available on UACR. We excluded 97 subjects with macroalbuminuria; in addition, 1,023 with missing follow-up computed tomography were not considered in the final study analysis. The final study population consists of 5,666 MESA participants (mean age 62 ± 10 years; 48% males). Overall, 424 (7%) individuals had UACR in the range of 30 to 300 mg/g (with MA) (Fig. 1).
Figure 1. Study Population Subgroups According to Baseline MA and CAC as Well as Follow-Up CAC Progression.
This figure illustrates the breakdown of those with versus those without microalbuminuria (MA) at baseline, whether coronary artery calcium (CAC) was identified in these subjects, the percentage of subjects without CAC at baseline who developed CAC on follow-up, and the median increase in CAC among those with CAC at baseline. At baseline, a greater percentage of CAC was identified among those with MA (62%) versus those without MA (48%). A greater likelihood of developing incident CAC at follow-up among those with (28%) versus those without (15%) MA, and a greater median progression of CAC among those with CAC at baseline and MA (+47) versus those without MA (+28) were identified.
Table 1 describes the baseline characteristics between those with and without MA (UACR <30 mg/g). Whites were less likely to have MA than Chinese, African-American, and Hispanic study participants. Those with MA were older and more likely to have hypertension, diabetes mellitus, higher triglycerides, lower HDL, higher body mass index, more likely to be on cholesterol-lowering medications, have a higher serum creatinine and lower estimate glomerular filtration rate but less likely to have a family history of heart attack than those without MA (Table 1).
Table 1.
Baseline Characteristics of Study Population
| UACR <30 mg/g (n = 5,242) |
Microalbuminuria (n = 424) |
p Value | |
|---|---|---|---|
| Age (years) | 62 ± 10 | 67 ± 10 | <0.0001 |
| Gender (male) | 47% | 51% | 0.12 |
| Race | |||
| Whites | 94% | 6% | |
| Chinese | 90% | 10% | <0.0001 |
| African Americans | 90% | 10% | |
| Hispanics | 90% | 10% | |
| Current smoker | 13% | 13% | 0.825 |
| Hypertension | 42% | 74% | <0.0001 |
| Systolic blood pressure (mm Hg) | 125 ± 20 | 140 ± 23 | <0.0001 |
| Diabetes mellitus | 11% | 37% | <0.0001 |
| Family history of heart attack | 43% | 38% | 0.017 |
| LDL-C (mg/dl) | 117 ± 31 | 115 ± 34 | 0.07 |
| Triglycerides (mg/dl)* | 128 ± 77 | 158 ± 154 | <0.0001 |
| HDL-C (mg/dl) | 51 ± 15 | 48 ± 13 | <0.0001 |
| BMI (kg/m2) | 28 ±5 | 30 ±6 | <0.0001 |
| Lipid-lowering meds | 16% | 20% | <0.0001 |
| Creatinine | 0.94 ± 0.20 | 1.00 ± 0.32 | <0.0001 |
| Estimated GFR (Cockroft and Gault) | 87 ± 27 | 83 ± 34 | 0.02 |
| CAC >0 | 48% | 62% | <0.0001 |
| Baseline volume score (among those with CAC >0)* | 266 ± 488 | 384 ± 674 | <0.0001 |
| Baseline Agatston score (among those with CAC >0)* | 234 ± 407 | 339 ± 562 | <0.0001 |
Tests for association done on the mean of log-transformed data.
BMI = body mass index; CAC = coronary artery calcification; GFR = glomerular filtration rate; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol.
At baseline, individuals with MA were more likely to have CAC >0 compared with those with normal UACR (62% vs. 48%, p < 0.0001) (Fig. 1). In addition, among those with CAC, both volume score and Agatston score were higher in those with MA (p < 0.01). Among the 2,911 participants (51%) without detectable CAC at baseline, 462 (16%) went on to develop incident CAC over an average of 2.4 ± 0.8 years of follow-up. The incidence of CAC (per 100 person-years) was significantly higher among those with MA; this feature was observed in all racial/ethnic groups (Fig. 1 and Fig. 2).
Figure 2. Incident CAC per 100 Person-Years According to MA and Stratified by Race/Ethnicity.
Overall, those with baseline microalbuminuria (MA) (solid bars) had greater incident coronary artery calcium (CAC) as compared with those without MA (open bars). In this univariant analysis, stratified by self-identified race, the trend of greater incident CAC among those with versus those without MA persisted.
The relative risks for incident CAC (among those without CAC at baseline) was elevated for those with MA and those with increasing levels of log-transformed UACR (Tables 2 and 3). In our study, compared with participants with UACR <30 mg/g, those with MA had a higher risk of incident CAC (relative risk [RR]: 2.05, 95% confidence interval [CI]: 1.41 to 3.02, p < 0.0001) in the analysis adjusted for demographic factors and site (model 1). After further adjustment for CHD risk factors, the relationship was somewhat attenuated but remained statistically significant (RR: 1.76, 95% CI: 1.19 to 2.61, p = 0.005). As shown in Table 3, continuous UACR (log-transformed) was also significantly associated with incident CAC, with a relative risk ratio of 1.15 (95% CI: 1.03 to 1.30). In analyses stratified by ethnicity, the relative risk of incident CAC was not consistently greater by MA and increasing levels of UACR (logtransformed) among any of the ethnic groups studied (Tables 2 and 3), and no interaction between race and MA for incident CAC was detected (data not shown). However, a significant interaction was present for an increased risk of incident CAC with increasing levels of UACR (log-transformed) among Chinese as compared with Whites (p = 0.02). Adjustment for type of scanner used to access CAC, systolic blood pressure as a continuous variable, creatinine, or estimate glomerular filtration rate had no significant impact on the results.
Table 2.
RRR (95% CI) of Incident CAC With Presence of MA
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| MA (Baseline) | Incident CAC (FU) | RRR (95% CI) | p Value | RRR (95% CI) | p Value | |
| Overall (n = 2,911) | 162 (6%) | 462 (16%) | 2.05 (1.41–3.02) | <0.0001 | 1.76 (1.19–2.61) | 0.005 |
| Whites (n = 1,001) | 23 (2%) | 176 (18%) | 1.36 (0.50–3.67) | 0.55 | 1.09 (0.37–3.19) | 0.87 |
| Chinese Americans (n = 348) | 24 (7%) | 39 (11%) | 4.08 (1.61–10.37) | <0.0001 | 2.77 (0.96–7.9) | 0.059 |
| African Americans (n = 899) | 68 (7%) | 142 (16%) | 2.43 (1.37–4.36) | 0.003 | 2.22 (1.22–4.03) | 0.009 |
| Hispanics (n = 663) | 47 (7%) | 105 (16%) | 1.48 (0.70–3.14) | 0.31 | 1.16 (0.51–2.61) | 0.72 |
Model adjusted for age, gender, race, *MESA site, and follow-up duration. Model 2 adjusted for age, gender, race, *MESA site, follow-up duration, body mass index, hypertension, diabetes mellitus, cigarette smoking, family history of CHD, LDL, HDL, triglycerides, and cholesterol-lowering medications. Adjustment for type of scanner used to access CAC, systolic blood pressure as a continuous variable, creatinine, or estimated glomerular filtration rate had no significant impact on the results. *Race was not adjusted for race-specific analyses.
CHD = coronary heart disease; CI = confidence interval; MA = microalbuminuria; MESA = Multi-Ethnic Study of Atherosclerosis; RRR = relative risk ratio; other abbreviations as in Table 1.
Table 3.
RRR (95% CI) of Incident CAC With Increasing Levels of UACR (Log-Transformed)
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| MA (Baseline) | Incident CAC (FU) | RRR (95% CI) | p Value | RRR (95% CI) | p Value | |
| Overall (n = 2,911) | 162 (6%) | 462 (16%) | 1.24 (1.11–1.38) | <0.0001 | 1.15 (1.03–1.30) | 0.016 |
| Whites (n = 1,001) | 23 (2%) | 176 (18%) | 1.09 (0.87–1.37) | 0.43 | 1.00 (0.80–1.26) | 0.97 |
| Chinese Americans (n = 348) | 24 (7%) | 39 (11%) | 1.62 (1.19–2.21) | 0.002 | 1.45 (1.02–2.05) | 0.038 |
| African Americans (n = 899) | 68 (7%) | 142 (16%) | 1.24 (1.04–1.47) | 0.019 | 1.16 (0.96–1.41) | 0.13 |
| Hispanics (n = 663) | 47 (7%) | 105 (16%) | 1.25 (1.01–1.56) | 0.039 | 1.17 (0.91–1.51) | 0.23 |
Model 1 adjusted forage, gender, race, *MESA site, and follow-up duration. Model 2 adjusted forage, gender, race, *MESA site, follow-up duration, body mass index, hypertension, diabetes mellitus, cigarette smoking, family history of CHD, LDL, HDL, triglycerides, and cholesterol-lowering medications. Adjustment for type of scanner used to access CAC, systolic blood pressure as a continuous variable, creatinine, or estimated glomerular filtration rate had no significant impact on the results. *Race was not adjusted for race-specific analyses.
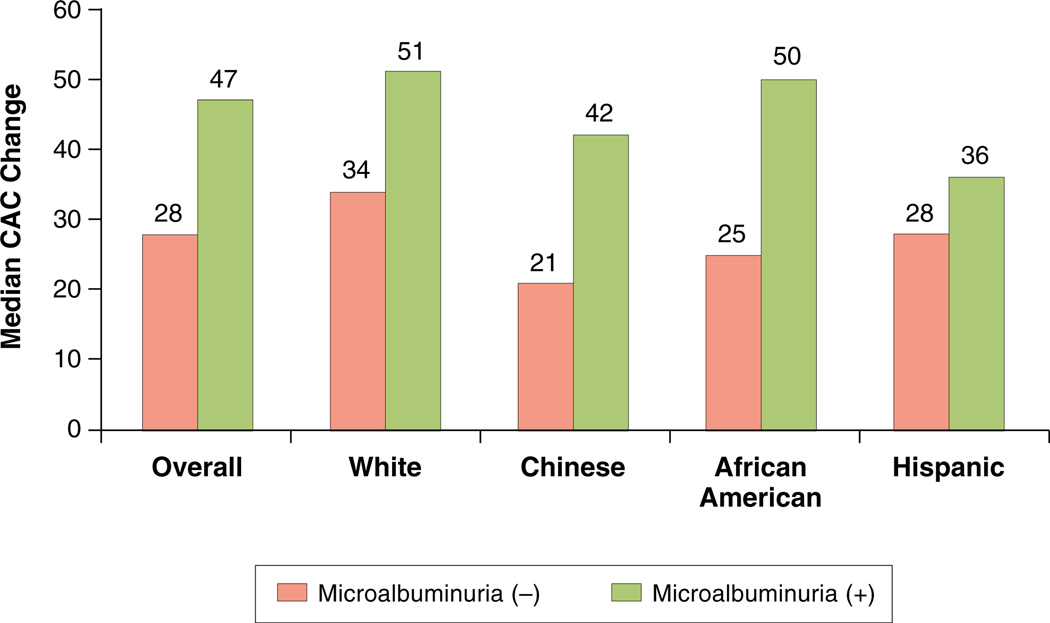
Among those with CAC at baseline, the median absolute progression of CAC was significantly higher by presence of MA (Fig. 1 and Fig. 3). The absolute median CAC increase in those with and without MA was 47 and 28, respectively (p < 0.0001). From median regression analyses (Table 4), median progression among those with MA was 15.0 (95% CI: 8.1 to 21.9, p < 0.0001) units higher (volume) than those without MA in demographicadjusted analyses; the respective change was reduced to 9.4 units (95% CI: 2.3 to 16.5, p = 0.009) in the fully adjusted analyses (model 2). Although the association between median increase in coronary calcium volume and MA/log-transformed UACR remained statistically significant only in African-American participants in the fully adjusted model (Tables 4 and 5). However, no interaction between race and MA or UACR (log-transformed) for progression of CAC was detected (data not shown). Adjustment for type of scanner used to access CAC, systolic blood pressure as a continuous variable, creatinine, or estimate glomerular filtration rate had no significant impact on the results.
Figure 3. Median CAC Change According to MA and Stratified by Race/Ethnicity Among Those With CAC >0 at Baseline.
Overall, those with baseline coronary artery calcium (CAC) and with baseline microalbuminuria (MA) (solid bars) had greater progression of CAC as compared with those without MA (open bars). In this univariant analysis, stratified by self-identified race, the trend of greater CAC progression among those with versus those without MA persisted.
Table 4.
Multivariable Analysis of Absolute Progression of Coronary Calcium With Presence of MA
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| β* (95% CI) Volume CAC Scores |
p Value | β* (95% CI) Volume CAC Scores |
p Value | |
| Overall (n = 2,755) | 15.01 (8.13–21.90) | <0.0001 | 9.39 (2.30–16.48) | 0.009 |
| Whites (n = 1,256) | 18.54 (5.84–31.23) | 0.004 | 3.27 (—12.19–18.74) | 0.68 |
| Chinese Americans (n = 328) | 16.46 (0.60–32.32) | 0.042 | 13.86 (—3.92–31.56) | 0.13 |
| African Americans (n = 627) | 17.75 (3.42–32.08) | 0.015 | 8.43 (—6.31–23.17) | 0.27 |
| Hispanics (n = 544) | 9.45 (—5.31–24.20) | 0.21 | 7.12 (—5.33–19.57) | 0.26 |
Regression coefficients are derived from median regression analysis. Model 1 adjusted for age, gender, race, MESA site, and follow-up duration. Model 2 adjusted for age, gender, race, MESA site, follow-up duration, body mass index, hypertension, diabetes mellitus, cigarette smoking, family history of CHD, LDL, HDL, triglycerides, and cholesterol-lowering medications. Race was not adjusted for race-specific analyses. Adjustment for type of scanner used to access CAC, systolic blood pressure as a continuous variable, creatinine, or estimated glomerular filtration rate had no significant impact on the results.
Table 5.
Multivariable Analysis of Absolute Progression of Coronary Calcium With Increasing Levels of UACR (Log-Transformed)
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| β* (95% CI) Volume CAC Scores |
p Value | β* (95% CI) Volume CAC Scores |
p Value | |
| Overall (n = 2,755) | 4.90 (2.92–6.89) | <0.0001 | 3.06 (0.77–5.35) | 0.009 |
| Whites (n = 1,256) | 4.49 (1.38–7.60) | 0.005 | 1.19 (—3.75–6.13) | 0.64 |
| Chinese Americans (n = 328) | 7.16 (1.66–12.65) | 0.011 | 4.09 (—2.98–11.15) | 0.26 |
| African Americans (n = 627) | 8.24 (3.68–12.80) | 0.019 | 4.70 (0.46–8.94) | 0.03 |
| Hispanics (n = 544) | 3.49 (—1.22–8.20) | 0.15 | 1.68 (—2.44–5.80) | 0.42 |
Regression coefficients are derived from median regression analysis. Model 1 adjusted for age, gender, race, MESA site, and follow-up duration. Model 2 adjusted for age, gender, race, MESA site, follow-up duration, body mass index, hypertension, diabetes mellitus, cigarette smoking, family history of CHD, LDL, HDL, triglycerides, and cholesterol-lowering medications. Race was not adjusted for race-specific analyses. Adjustment for type of scanner used to access CAC, systolic blood pressure as a continuous variable, creatinine, or estimated glomerular filtration rate had no significant impact on the results.
DISCUSSION
The present study examined the association between MA and development of CAC as well as progression of CAC in a large, community-based sample of asymptomatic individuals. MA is associated with cardiovascular events and appears to be a measure of vascular health (22). Our finding extends these observations by demonstrating that in individuals free of CHD, MA is associated with a greater incidence and degree of progression of CAC compared with those without MA, independent of traditional CHD risk factors, creatinine, or estimate glomerular filtration rate.
MA has been independently associated with multiple markers of subclinical atherosclerosis, including left ventricular hypertrophy, carotid artery wall intima–media thickness, pulse wave velocity, carotid plaque number, and carotid and coronary artery calcium (28–30). Additionally, we have previously demonstrated an association between MA and CAC in the MESA population (8). However, only scant data exist regarding the influence of MA in the progression of subclinical atherosclerosis. The Tromoso study, a prospective study of 4,037 subjects without diabetes who were followed for an average of 7 years, demonstrated a positive relationship between baseline MA and the incidence and progression of plaque (size and number) in the carotid arteries using ultrasonography (31). After controlling for traditional cardiovascular disease risk factors, MA was also associated with both the number of new plaques and the total plaque area at follow-up among 2,203 subjects without any identifiable carotid plaque at baseline. The PREDICT (The Progress of Coronary Heart Disease in Type 2 Diabetes as Measured by Coronary Calcium Score From Electron Beam Computed Tomography) study (32) evaluated 202 type 2 diabetic patients without known clinical cardiovascular disease with sequential CAC scans. After a mean follow-up of 4 years, UACR was positively associated with CAC progression after controlling for baseline CAC score (32). Costacou et al. (33) demonstrated a positive association between MA and CAC progression in a study of 222 type 1 diabetics with CAC scans at baseline and 4 years later. This relationship remained significant after controlling for baseline CAC, duration of diabetes diagnosis, body mass index, and non-HDL cholesterol. Insulin dose, hypertension, systolic blood pressure, white blood cell count, and angiotensin-converting enzyme inhibitor use did not impact model fit, and results were unchanged when restricted to the 183 patients free of clinically recognized cardiovascular disease.
Our study results are consistent with the findings from the studies highlighted in the previous text and add to current literature by demonstrating that MA is associated with incident CAC and CAC progression among low-risk individuals with and without detectable CAC at baseline. Among those with CAC = 0 at baseline, we observed nearly a 2-fold increased incidence of CAC among those with MA. These associations were attenuated but remain significant even after adjustment for CHD risk factors, and creatinine or estimated glomerular filtration rate had no significant impact on the results. Among those with detectable coronary atherosclerosis at baseline, by coronary calcium measures, the presence and degree of MA was associated with the degree of coronary atherosclerosis progression (coronary calcium) after taking into account traditional CHD risk factors. Creatinine or estimated glomerular filtration rate had no significant impact on this relationship. No significant interaction between MA, as it relates to incident CAC and CAC progression, and ethnic groups was found. Only 1 race/MA interaction was detected for an increased risk of incident CAC with log UACR among Chinese as compared with Whites. This may be due to smaller sample size within the subethnic groups. Further study will be needed to determine whether MA is a more sensitive and/or specific marker of the progression of atherosclerosis among any specific ethnic/racial group.
The mechanisms for the increased incidence and accelerated progression of coronary atherosclerosis in the presence of MA are unclear. Several studies, including data from our study, have established MA as an independent risk factor for cardiovascular disease in prospective analyses (1–3,34). This independent relationship remains true with respect to renal insufficiency, which in itself is a risk factor for cardiovascular disease. This was most clearly demonstrated in an analysis of over 14,000 individuals with over 13 years of follow-up in the NHANES III (National Health and Nutrition Examination Survey III) study, which established estimated glomerular filtration rate and MA to be independent risk factors for cardiovascular disease events (34). MA may be the result of vascular damage to the glomerulus. Although this damage to the kidney is reflected as albumin in the urine, the process of vascular damage resulting in atherosclerosis is likely ongoing in all vascular beds, including the heart. Thus, MA may be a cumulative measure of the impact of multiple factors on vasculature health. MA has been shown to correlate with depressed vascular function, including the coronary artery vasculature as measured by vasodilatory response to various stimuli (5,6).
In our study, MA preceded the detection of atherosclerosis by CAC in the majority of participants. A limitation of our study is that although UACR is a dynamic measure impacted by multiple factors, including antihypertensive medications, only a single UACR was used. It is not known whether repetitive UACR measures would have lead to an even larger proportion of MA preceding CAC detection. Speculation as to why MA precedes CAC include the glomerulus may be more sensitive to vascular damage than the coronary arteries, or MA may reflect the lag time between the development of atherosclerosis and calcification of arthrosclerotic plaque. It is also possible that MA is a more sensitive measure of atherosclerotic damage in the glomerulus than detection of calcification by computerized tomography in the coronary arteries. Regardless, these findings support the notion that MA is an early measure of systemic vascular health/damage. It is important to note that we have used CAC progression as a measure of atherosclerosis progression in our study. Although extensive and robust data exist on the prognostic value of CAC severity in predicting adverse cardiovascular outcome, less data exist as to whether CAC progression may provide additional information regarding the heightened risk of future events. A study of 495 asymptomatic patients with baseline CAC examined the impact of CAC progression at 2 years on incident myocardial infarctions (19). Progression of coronary artery calcium on the follow-up scan was associated with a 17-fold increased relative risk (p < 0.0001) of a myocardial infarction as compared with those without progression of coronary calcium. Further studies are needed to more clearly delineate the role of CAC progression in risk prediction and evaluation of treatment efficacy. In addition, it is also yet to be seen among participants with MA whether a differential outcome will exist according to the severity of CAC progression.
Our study findings raise 3 important questions. First, should we assess the presence of MA in all asymptomatic patients? Given that the association between MA and increased cardiovascular (CV) risk is well established, and the reduction in urine albumin excretion at 1 year was associated with a reduction in CV risk in 1 study, screening for MA to help identify patients that are at increased risk for CV disease is reasonable if the results will lead to a therapeutic change (prescribing aspirin, statin, angiotensin-converting enzyme inhibitor). It should be noted that currently, no professional society guidelines exist for MA screening to identify individuals at increased CV risk. Second, can we use MA to select which patients should undergo CAC testing? Currently, an American Heart Association scientific statement gives a tepid endorsement (class IIb, Level of Evidence: B) for CAC testing in intermediate-risk patients, to refine clinical risk prediction, and to select patients for more aggressive target values for lipid-lowering therapies (35). The presence or absence of MA can potentially aid in the decision to proceed or forgo CAC testing based on the threshold for treatment in an individual patient. The third question our study finding poses is can we use MA to determine which patients on whom we should perform follow-up CAC testing in the future? Although in our large, population-based study we demonstrate an association between MA and CAC progression, we believe additional data confirming these findings and more thoroughly defining the impact of CAC progression of clinical outcomes are needed before clinical utility recommendations can be made about MA testing and sequential CAC testing.
In summary, this large, population-based study demonstrates an increased risk of incident CAC as well as greater CAC progression among those with MA. Further study is needed to determine the degree to which MA precedes and predicts progression of atherosclerosis and how this information can be used to reduce cardiovascular events.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by r01 hl071739 and contracts n01-hc-95159 through n01-hc-95165 and n01-hc-95169 from the National Heart, Lung, and Blood Institute. Dr. DeFilippis is supported by a National Research Service Award (nrsa) Training Grant (t32-hl-07227). Dr. Budoff is on the speakers bureau for General Electric (<$10,000/year).
ABBREVIATIONS AND ACRONYMS
- CAC
coronary artery calcification
- CHD
coronary heart disease
- EBT
electron-beam tomography
- HDL
high-density lipoprotein
- MA
microalbuminuria
- UACR
urine albumin-to-creatinine ratio
REFERENCES
- 1.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman S, Wikstrand J, Hartford M, Berglund G. Urinary albumin excretion—a predictor of risk of cardiovascular disease. A prospective 10-year follow-up of middle-aged nondiabetic normal and hypertensive men. Am J Hypertens. 1996;9:770–778. doi: 10.1016/0895-7061(96)00102-1. [DOI] [PubMed] [Google Scholar]
- 3.Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 5.Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103:1869–1874. doi: 10.1161/01.cir.103.14.1869. [DOI] [PubMed] [Google Scholar]
- 6.Cosson E, Pham I, Valensi P, Paries J, Attali JR, Nitenberg A. Impaired coronary endothelium-dependent vasodilation is associated with microalbuminuria in patients with type 2 diabetes and angiographically normal coronary arteries. Diabetes Care. 2006;29:107–112. doi: 10.2337/diacare.29.1.107. [DOI] [PubMed] [Google Scholar]
- 7.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340:319–323. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- 8.Kramer H, Jacobs DR, Jr, Bild D, et al. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 9.Arad Y, Goodman KJ, Roth M, New-stein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 10.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 11.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 12.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 13.LaMonte MJ, FitzGerald SJ, Church TS, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421–429. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 14.O’Malley PG, Taylor AJ, Jackson JL, Doherty TM, Detrano RC. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am J Cardiol. 2000;85:945–948. doi: 10.1016/s0002-9149(99)00906-6. [DOI] [PubMed] [Google Scholar]
- 15.Raggi P, Cooil B, Callister TQ. Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J. 2001;141:375–382. doi: 10.1067/mhj.2001.113220. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 18.Wong ND, Hsu JC, Detrano RC, Diamond G, Eisenberg H, Gardin JM. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495–498. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 19.Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24:1272–1277. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 20.Shemesh J, Apter S, Stolero D, Itzchak Y, Motro M. Annual progression of coronary artery calcium by spiral computed tomography in hypertensive patients without myocardial ischemia but with prominent atherosclerotic risk factors, in patients with previous angina pectoris or healed acute myocardial infarction, and in patients with coronary events during follow-up. Am J Cardiol. 2001;87:1395–1397. doi: 10.1016/s0002-9149(01)01561-2. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Bogojevic Z, Bakris GL. Albuminuria and cardiovascular risk. Heart Fail Clin. 2006;2:53–59. doi: 10.1016/j.hfc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JC, Kronmal RA, Carr JJ, et al. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005;235:403–414. doi: 10.1148/radiol.2352040515. [DOI] [PubMed] [Google Scholar]
- 25.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 26.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 27.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 28.Freedman BI, Langefeld CD, Lohman KK, et al. Relationship between albuminuria and cardiovascular disease in Type 2 diabetes. J Am Soc Nephrol. 2005;16:2156–2561. doi: 10.1681/ASN.2004100884. [DOI] [PubMed] [Google Scholar]
- 29.Tsioufis C, Dimitriadis K, Antoniadis D, Stefanadis C, Kallikazaros I. Interrelationships of microalbuminuria with the other surrogates of the atherosclerotic cardiovascular disease in hypertensive subjects. Am J Hypertens. 2004;17:470–476. doi: 10.1016/j.amjhyper.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama H, Aoki T, Imahori M, Kuramitsu M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int. 2004;66:448–454. doi: 10.1111/j.1523-1755.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen L, Jenssen T, Johnsen SH, et al. Albuminuria as risk factor for initiation and progression of carotid atherosclerosis in non-diabetic persons: the Tromso Study. Eur Heart J. 2007;28:363–369. doi: 10.1093/eurheartj/ehl394. [DOI] [PubMed] [Google Scholar]
- 32.Elkeles RS, Godsland IF, Rubens MB, Feher MD, Nugara F, Flather MD. The progress of coronary heart disease in Type 2 diabetes as measured by coronary calcium score from electron beam computed tomography (EBCT): the PREDICT study. Atherosclerosis. 2008;197:777–783. doi: 10.1016/j.atherosclerosis.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Costacou T, Edmundowicz D, Prince C, Conway B, Orchard TJ. Progression of coronary artery calcium in type 1 diabetes mellitus. Am J Cardiol. 2007;100:1543–1547. doi: 10.1016/j.amjcard.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astor BC, Hallan SI, Miller ER3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 35.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]