Abstract
Objectives
To assess barriers to colorectal cancer screening among urban publicly insured women and to evaluate how barriers among underscreened urban women have changed between 2001 and 2007-2008.
Methods
Eligible women were selected using Medicaid Managed Care Organization (MMCO) administrative data. MMCO outreach staff interviewed women by phone between October 2007 and February 2008, and assessed their barriers to colorectal cancer screening. We compared the results of these interviews with interviews conducted in 2001 with women in community health center waiting rooms.
Results
Thirty percent of overdue women had never heard of either colonoscopy or sigmoidoscopy, and 55% had never heard of home fecal occult blood testing (FOBT). Among overdue women who had heard of colonoscopy or sigmoidoscopy, 33% reported misconceptions and 28% reported worry as a barrier. No clinician recommendation was the most commonly reported barrier to home FOBT (44%) and was also reported as a barrier to endoscopy by 22% of women. Between 2001 and 2007-2008, the proportion of women reporting that they had not received a clinician's recommendation for endoscopy or home FOBT increased significantly.
Conclusions
A lack of information, no clinician recommendation, misconceptions, and worry persist as barriers to colon cancer screening among this underscreened urban population. An increased focus on clinician recommendation and patient education about stool-based as well as endoscopic screening methods could lead to greater screening compliance.
Keywords: colorectal cancer, screening, barriers, women's health, urban population
INTRODUCTION
In early 2010, members of the National Institutes of Health (NIH) State-of-the-Science Conference on Colorectal Cancer Screening recognized that while slow but steady progress has been made in increasing colorectal cancer screening rates, disparities persist among minorities, those born outside the United States, and those with limited English proficiency.1-5
We assessed barriers to colon cancer screening among women in New York City in the spring and summer of 2001 and again in 2007-2008, as part of successive projects to develop and disseminate a successful patient-directed cancer screening intervention known as prevention care management (PCM)6 (NIH grants R01-CA87776 and R01-CA119014). Just prior to our 2001 interviews, national colon cancer screening practices began to change, with a strong shift toward use of screening colonoscopy. In 2000, the American College of Gastroenterology recommended colonoscopy as its preferred screening strategy7 and, in 2001, screening colonoscopy was added as a covered benefit for average-risk Medicare patients. In 2003, between our 2 sets of interviews, the New York City Department of Health and Mental Hygiene launched a public health initiative known as Take Care New York, which included a highly successful component focusing on increasing colon cancer screening rates, primarily through colonoscopy.8,9 While flexible sigmoidoscopy and the home fecal occult blood test (FOBT) were commonly used for screening through the late 1990s, the use of home FOBT, and particularly sigmoidoscopy, has declined3,4,10,11 since 2001, and colonoscopy has become the most frequently recommended and utilized colon cancer screening test.1,3-4,12
A comparison of our interview data from 2007-2008 with that from 2001 provides the opportunity to explore whether and how barriers to colorectal cancer screening reported by urban women from underscreened groups changed during this time, potentially influenced by national screening trends, as well as a successful local public health campaign. We report the results of our 2007-2008 interviews here and compare them with our 2001 findings.
METHODS
Problem Conceptualization
In 2001, we interviewed women recruited from community health center waiting rooms in New York City about barriers to home FOBT and sigmoidoscopy, and used the results to inform the development of the PCM telephone support intervention.13 After this intervention was found to be successful at increasing cancer screening rates,6 we moved on to test whether prevention care management could be effectively delivered through Medicaid managed-care organizations (MMCOs) to their eligible female members. Using MMCO enrollment lists allowed us to expand the sample to include women who never or rarely receive health care, who would have been missed by the waiting room recruitment strategy of our previous study. In 2007-2008, before launching prevention care management among MMCO-enrolled women, we explored barriers to home FOBT, sigmoidoscopy, and colonoscopy among this somewhat different population. The results of these interviews were used to inform the prevention care management/MMCO intervention, to be delivered by MMCO staff to eligible female enrollees.
Setting
In 2006, 17 health plans provided Medicaid Managed Care and Family Health Plus (New York State's public health insurance program for low-income adults who do not qualify for Medicaid) to residents of New York City. We recruited 3 MMCOs to participate in this study, each of which provided claims and administrative data used to select eligible members and staff to conduct the interviews.
Participants and Research Design
Female Medicaid and Family Health Plus members who were 50 to 64 years old, had been continuously enrolled with a participating MMCO for at least 12 months, received primary care from 1 of 20 participating practices, and were overdue for colon cancer screening as per MMCO claims data were eligible for this study. We used codes from baseline claims data to exclude women with a history of cancer (breast, cervical, colorectal, or lung) or who had been under active cancer treatment within the past 6 months.
To select the final sample for the interview, we stratified eligible women into 4 lists, by preferred language (English or Spanish) as reflected in MMCO administrative data, and by whether or not their claims data included a recent ambulatory care visit. This ensured representative samples of both English and Spanish speakers, and of women who had recently received health care as well as those who had not done so. Each list was randomized using a random number generator. Working from the top of these lists, MMCO clinical out-reach staff made 3 attempts to reach each woman by phone, then continued down each list until the desired sample size had been reached. We aimed to complete 50 interviews at the 2 larger MMCOs and 25 at the third, for a total sample of 125 completed interviews.
The study was approved by the Committee for the Protection of Human Subjects at Dartmouth College, Clinical Directors Network's institutional review board and all relevant MMCO and practice institutional review boards. Women who were reached by phone were informed that they could decline to participate in the interview without compromising their health care or insurance coverage. Those who completed the interview were provided with a $20 gift card as compensation for their time and effort.
Data Collection
The Clinical Directors Network's project director trained MMCO outreach staff to conduct and record the interviews, using a structured script and protocol based on our prior work.13 In 2001, we asked women about barriers to home FOBT and sigmoidoscopy. By 2007-2008, colonoscopy had become more common among this population, so in our second set of interviews we asked about barriers to home FOBT and to both colonoscopy and sigmoidoscopy. We pilot tested the revised interview among Clinical Directors Network and MMCO staff, and with 7 MMCO patients who met all eligibility criteria except that they did not receive care at a participating practice. The interview was translated into Spanish, back translated into English by a bilingual member of Clinical Directors Network's staff, and reviewed by bilingual staff at Clinical Directors Network, Dartmouth Medical School, and participating MMCOs. Outreach staff conducted telephone interviews between October 2007 and February 2008, in either English or Spanish, based on interviewee preference. Interview responses were recorded on paper forms, then double-entered into an Access database (Microsoft Corp, Redmond, Washington).
Analysis
Analyses were conducted using STATA version 11. Proportions were calculated for each response, and comparisons between colonoscopy/sigmoidoscopy combined and home FOBT were made using continuity corrected χ2 tests.
When comparing interview results from 2001 with those from 2007-2008, we compare barriers to sigmoidoscopy reported in 2001 with barriers to sigmoidoscopy and/or colonoscopy reported in 2007-2008.
Measures
Cancer screening status was derived from claims data and patient self-report, based on US Preventive Services Task Force guidelines14 and Healthcare Effectiveness Data and Information Set (HEDIS) 2008 technical specifications.15 Women were considered upto-date for colon cancer screening if they had received a home FOBT within the prior 12 months, a sigmoidos-copy or double-contrast barium enema within 5 years, or a colonoscopy within 10 years.
RESULTS
Interviews of Women Enrolled in Medicaid Managed-Care Organizations, 2007-2008
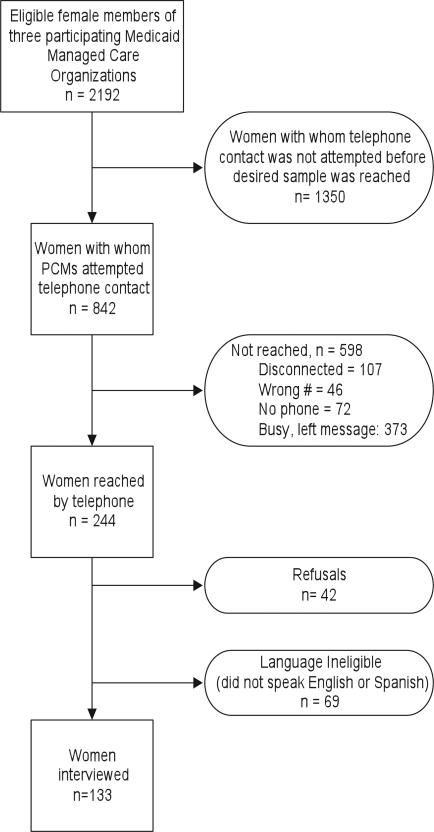
Interviewers made a total of 1240 telephone calls to 842 women (Figure), in order to complete 133 interviews (16% completion rate). This exceeded our planned sample of 125 because interviews were completed with a small number of women who responded to outreach staff messages after the sample had been reached. Of the completed interviews, 41% were in Spanish and 59% were in English (Table 1). A majority of women (56%) were born outside of the United States, 29% self-identified as African American, and half self-identified as Hispanic. About 37% of women had less than a high school education, and 6% had a college degree or higher. Three-quarters of the women had a usual provider; 54% described their health as good, very good, or excellent; and 85% had received a routine medical checkup within the last 2 years. While all women were overdue for colon cancer screening according to their claims data, 27% of those interviewed reported that they were up-to-date, most by colonoscopy (18%) or home FOBT (9%). This discrepancy between claims data and self-report may reflect recent tests for which a claim had not yet been processed, inaccuracies in patient recall and reporting,2,16 tests received while a patient was uninsured or insured by another health plan, or tests received outside of the country or from a nonbilling provider such as a free mobile mammography van.
Figure.
Eligible Female Medicaid Managed-Care Members Reached, Not Reached, and Interviewed, 2007-2008
Table 1.
Characteristics of Medicaid-Insured Women Interviewed in 2007-2008
| Demographics (n=133) | ||
|---|---|---|
| Mean age, y (SD) | 56.4 (4.1) | |
| No. | % | |
| Language | ||
| Interview in Spanish | 54 | 41 |
| Race | ||
| Black/African American | 38 | 29 |
| White | 36 | 27 |
| Mixed | 24 | 18 |
| Unknown/missing/refused | 35 | 26 |
| Ethnicity | ||
| Hispanic | 66 | 50 |
| Birthplace | ||
| United States (including Puerto Rico) | 58 | 44 |
| Education level achieved | ||
| Some high school or less | 49 | 37 |
| High school graduate | 53 | 40 |
| Some college | 21 | 16 |
| College grad or grad school | 8 | 6 |
| Missing/refused | 2 | 2 |
| Health Status | ||
| Self-assessment of health | ||
| Very good or excellent | 33 | 25 |
| Good | 39 | 29 |
| Fair or poor | 60 | 45 |
| Don't know/missing | 1 | 1 |
| Up to date for screening by self-report | ||
| Colorectal any testq | 35 | 27 |
| Colonoscopy within 10 y | 24 | 18 |
| Sigmoidoscopy within 5 y | 1 | 1 |
| Unspecified endoscopy within 5 y | 2 | 2 |
| Home FOBT within 12 mo | 12 | 9 |
| Body mass index | ||
| Underweight/normal (BMI <25) | 31 | 23 |
| Overweight (BMI 25-30) | 47 | 35 |
| Obese (BMI ≥30) | 38 | 29 |
| Missing height and/or weight | 17 | 13 |
| Health Care Utilization | ||
| Has a usual provider | 100 | 75 |
| Last routine checkup, y | ||
| 0-2 | 113 | 85 |
| 2-5 | 15 | 11 |
| >5 | 3 | 2 |
| Don't know | 2 | 2 |
a n = 132 after excluding 1 woman, who reported a history of colorectal cancer.
We use Woolf's framework to report specific barriers (Table 2),17 breaking them down into knowledge, acceptance, ability, and reinforcement barriers. Barriers to colonoscopy/sigmoidoscopy are reported only for women who had no history of endoscopy and were currently overdue for colon cancer screening. Barriers to home FOBT are reported for women who may have had home FOBT previously but who were currently overdue for colon cancer screening. Before asking a woman about barriers to a colon cancer screening test, the interviewer asked if she had heard of the test. Additional barriers are only reported for women who had heard of each test (n = 64 for endoscopy, n = 41 for home FOBT).
Table 2.
Barriers to Endoscopy and Home Fecal Occult Blood Testing Reported by Overdue Women, 2007-2008a
| Endoscopyb | Home FOBTc | ||||
|---|---|---|---|---|---|
| No. | % | No. | % | p Value | |
| Have you ever heard of this test? | |||||
| Yes | 64 | 70.3 | 41 | 44.6 | .001 |
| No | 27 | 29.7 | 51 | 55.4 | |
| What are the reasons why you have not had 1 of these tests?d | |||||
| Knowledge barriers | |||||
| Misconceptions | 21 | 32.8 | 4 | 9.8 | .007 |
| No knowledge of test | 3 | 4.7 | 2 | 4.9 | .964 |
| Acceptance barriers | |||||
| Don't want test | 8 | 12.5 | 4 | 9.8 | .666 |
| Fatalism | 2 | 3.1 | 0 | 0.0 | .253 |
| Individual attitudes and beliefs | 2 | 3.1 | 4 | 9.8 | .153 |
| Worry (general or about pain) | 18 | 28.1 | 4 | 9.8 | .024 |
| Ability barriers | |||||
| Access barriers | 3 | 4.7 | 2 | 4.9 | .964 |
| Competing priorities, including chronic conditions | 7 | 10.9 | 7 | 17.1 | .367 |
| Other: insurance issues, forgot something | 1 | 1.6 | 1 | 2.4 | .749 |
| Reinforcement barriers | |||||
| No clinician's recommendation | 14 | 21.9 | 18 | 43.9 | .017 |
Abbreviation: FOBT, fecal occult blood test.
Screening status was assessed using claims and self-reported data.
Endoscopy barriers were assessed among overdue women who had never had either colonoscopy or sigmoidoscopy (n = 91).
Home FOBT barriers were assessed among women who may or may not have had prior home FOBT but who were currently overdue (n = 92).
Barriers are reported only for women who answered yes when asked if they had heard of the test.
Knowledge barriers were high among this sample, particularly for home FOBT. About 30% of women had never heard of either colonoscopy or sigmoidoscopy, while significantly more (55%) had never heard of home FOBT. Misconceptions about endoscopy—most commonly that the test is not needed unless there are symptoms (12 women, 18.8%) or that these screening tests are simply not medically necessary (9 women, 14.1%)— were also reported by 33% of women, while only 10% reported such misconceptions for home FOBT (p < .01).
Worry was the most commonly reported acceptance barrier. More than a quarter of women (28%) were worried about endoscopy, with 11 women (17.2%) specifically worried about pain. Significantly fewer women were worried about home FOBT (10%, p < .05). A small number of women just did not want to have the tests (13% for endoscopy and 10% for home FOBT), and 10% reported individual attitudes and beliefs as a barrier to home FOBT.
Competing priorities, including chronic health conditions, were the most frequently reported ability barrier (11% for endoscopy, 17% for home FOBT). Access and other ability barriers among this insured population were low.
Finally, significantly more women reported that their clinician had not recommended the home FOBT (44%) than reported the same for endoscopy (22%, p < .05).
Comparison of 2001 and 2007-208 Interviews
Our interview sample from 2001 was similar to our 2007-2008 sample in terms of primary language (41% of interviews were conducted in Spanish) and ethnicity (51% identified as Hispanic), but included a higher percentage of African Americans13 (44%); more women who had not graduated from high school (61%); and fewer women who described their health as good, very good, or excellent (41%) (Christina Robinson, unpublished data). In contrast to our 2007-2008 sample, women interviewed in 2001 were recruited from community health center waiting rooms rather than from MMCO enrollee lists, and thus varied by health insurance status; most were publicly insured (72% Medicaid, 28% Medicare), 5% were insured through an employer, and 11% had no insurance (Christina Robinson, unpublished data).
In 2001, we did not exclude women based on up-to-date status; 14% of interviewed women reported being up-to-date by sigmoidoscopy and twice as many (28%) reported being up-to-date by home FOBT. In contrast, by 2007-2008, twice as many women reported being upto-date by colonoscopy or sigmoidoscopy (18%) as by home FOBT (9%).
More women had heard of colonoscopy or sigmoidoscopy in 2007-2008 (70.3%) than had heard of sigmoidoscopy in 2001(59.8%), and a similar trend was seen for home FOBT, with 44.6% reporting that they had heard of the test in 2007-2008, up from 38.5% of women13 in 2001 (Table 3). Misconceptions around endoscopic tests were reported by about a third of women in both sets of interviews, while misconceptions around home FOBT were significantly higher in 2001 (36.7%) than in 2007-2008 (9.8%, p < .005).13
Table 3.
Comparison of Barriers to Colorectal Cancer Screening Tests Reported by Overdue Women in 2001 and in 2007-2008
| Endoscopya | Home FOBT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2001 | 2007-2008 | 2001 | 2007-2008 | |||||||
| No. | % | No. | % | p Value | No. | % | No. | % | p Value | |
| Have you ever heard of this test?b | 52 | 59.8 | 64 | 70.3 | .187 | 20 | 38.5 | 41 | 44.6 | .592 |
| Yes | ||||||||||
| No | 35 | 40.2 | 27 | 29.7 | 32 | 61.5 | 51 | 55.4 | ||
| Barriers to screeningc | ||||||||||
| Knowledge barriers | ||||||||||
| Misconceptions about test | 26 | 29.5 | 21 | 32.8 | .667 | 22 | 36.7 | 4 | 9.8 | .002 |
| Acceptance barriers | ||||||||||
| Worry/fear | 13 | 14.8 | 18 | 28.1 | .044 | 1 | 1.7 | 4 | 9.8 | .066 |
| Don't like idea/don't want | 3 | 3.4 | 8 | 12.5 | .033 | 3 | 5.0 | 4 | 9.8 | .355 |
| Reinforcement barriers | ||||||||||
| No clinician recommendation | 9 | 10.2 | 14 | 21.9 | .048 | 8 | 13.3 | 18 | 43.9 | .001 |
Women were asked about barriers to sigmoidoscopy in 2001, and to sigmoidoscopy and colonoscopy in 2007-2008.
In 2001, data on whether women had heard of the test was missing for 1 woman for endoscopy and for 8 women for home FOBT.
Barriers from 2001 are reported for unscreened women whether they had heard of the test or not (n = 88 for endoscopy, n= 60 for home FOBT). Barriers from 2007-2008 are reported only for overdue women who had heard of the test.
More women reported acceptance barriers in our more recent interviews, with significantly more women reporting worry as a barrier for endoscopy13 in 2007-2008 (28.1%) than in 2001 (14.8%, p ≤ .05). Ability barriers (not shown) were very low in 2001, with only 2 women (4%) reporting access barriers, and no women reporting competing priorities for either test.13
For both endoscopy and home FOBT, significantly more women reported the absence of a clinician recommendation in 2007-2008 than in 2001. While the percent of women reporting no clinician recommendation for endoscopy doubled (10.2% in 2001, 21.9% in 2007-2008, p < .05), it more than tripled for home FOBT13(13.3% in 2001, 43.9% in 2007-2008, p ≤ .001).
DISCUSSION
While colonoscopy screening rates nationally1 and in New York City18 have increased in recent years, boosted by local public health initiatives such as Take Care New York, limited patient knowledge about both endoscopic and stool-based colorectal cancer screening tests and the absence of clinician recommendations for these tests remain commonly reported barriers to screening.
Fewer women reported that they had never heard of endoscopic tests in 2007-2008 than in 2001, but misconceptions and worry about endoscopy remain common, highlighting the need for additional patient education. While the percent of women reporting that they'd never heard of home FOBT also decreased from 61.5% in 2001 to 55% in 2007-2008, among this population of unscreened women more than half remain unaware of home stool testing as an alternative. Data suggest that some underscreened populations prefer the home FOBT to colonoscopy19,20 and that providing patients with information on home FOBT as well as colonoscopy could improve screening rates, particularly among minorities, less-acculturated individuals, and those with lower incomes.19-25 During the last 2 years, there has been renewed interest in home stool testing, especially with studies showing the effectiveness of the fecal immuno-chemical test,12,26-28 and the development of fecal DNA tests.29 The American College of Gastroenterology's 2008 recommendation is one example of the growing movement toward recommending alternative colorectal screening tests.26 As Woolf has suggested, “redefining the best test as the one the patient wants may save the most lives.”30
All women in our 2007-2008 sample had health insurance coverage through Medicaid or Family Health Plus, and three-quarters of them reported having a usual care provider, removing these 2 potential barriers to cancer screening. Nonetheless, the percentage of women reporting no clinician recommendation was significantly higher in 2007-2008 than in 2001 for both endoscopy (from 10.2% to 21.9%, p < .05) and home FOBT (from 13.3% to 43.9%, p ≤ .001). Numerous studies have documented the crucial role that a clinician's recommendation plays in colorectal cancer screening, particularly among minority patients.21,31-39 If a public service announcement prompts a woman to improve her health by changing her diet or quitting smoking, she can take action on her own without a clinician's recommendation. In contrast, if the same announcement recommends that she use a home stool kit or get a colonoscopy, it is much more difficult for her to take action on her own in the absence of a clinician's recommendation or referral.40
The most effective strategies for increasing colon cancer screening rates among underserved populations will likely be multidimensional, combining provider-, patient-, and systems-level interventions that recommend and encourage patients to review all valid screening tests.11 In the face of limited clinician time,41 there is also strong evidence that patient navigators or lay health workers can successfully provide these recommendations as well as additional education and follow-up support.2,6,35,42-47
Limitations of both sets of interviews include our reliance on self-reported data and small sample sizes. Interviews in 2007-2008 were conducted only among women with a working phone who spoke either English or Spanish. Because many women in 2007-2008 had not heard of the colon cancer screening tests, the number of women reporting specific barriers to screening was low, particularly for home FOBT. Self-reported data, although considered to be quite accurate for colon cancer screening,48 can contain inaccuracies or errors.2,16,37
Limitations of our comparison between 2001 and 2007-2008 interview data include differences in the populations sampled and in the specific questions included in each interview. Some differences in reported barriers between 2001 and 2007-2008 may reflect differences between the 2 samples: women who rarely or never receive health care are represented in the 2007-2008 sample, but not in the earlier study, which recruited women from community health center waiting rooms. Women interviewed in 2007-2008 who rarely, if ever, went to the doctor would have had fewer opportunities to receive either patient education or a clinician's recommendation for cancer screening, potentially making these barriers more common. However, 85% of our 2007-2008 sample did report having a routine checkup within 2 years, during which they could have received both education and screening recommendations. Because we asked only about sigmoidoscopy in 2001 and about both sigmoidoscopy and colonoscopy in 2007-2008, differences between the 2 sets of interviews could reflect barriers to colonoscopy alone that would not have been reported in the 2001 interviews.
In conclusion, our data suggest that the Medicaid population may benefit from a renewed emphasis on clinician recommendations for colon cancer screening, including information on stool-based tests as well as colonoscopy. Encouraging clinicians to take a more active role in recommending colon cancer screening to their patients, presenting all recommended screening methods as acceptable approaches, and providing patients with follow-up support to these recommendations could help further increase colon cancer screening rates among underscreened urban populations, resulting in higher early detection rates, earlier stage at detection, and a reduction in colon cancer health disparities.
ACKNOWLEDGMENTS
We wish to acknowledge the staff at the participating Medicaid managed-care organizations: Tania Gordon, Miguel Negron, Dianna Coles, Maurice Sahar, and Jahnhoy Smith at Metroplus; Rosanna Quezada, Cristina Sierra, Clyde Jackson, Ana Sintigo, Januaria Urena, Cynthia Pena, Dorine Levin, Korneliya Pluchik, and Eric Wentz at Health Plus; and Kimberly Falkenstein, Marina Azarova, Daisy Huang, Sharonda Thompson, and Michelle Wheatcraft at Americhoice; as well as Tzyy Jye Lin and Xiaoxi Yao at Clinical Directors Network, who both provided invaluable support to the study.
Funding/Support: This work was supported by the National Cancer Institute (RO1CA119014).
Role of the Sponsor: The National Cancer Institute had no role in the design, conduct, or reporting of the study.
Footnotes
Disclaimer: None of the authors have any direct or indirect financial interests in connection with the content of this paper.
REFERENCES
- 1.Steinwachs D, Allen JD, Barlow WE, et al. National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):663–667. doi: 10.7326/0003-4819-152-10-201005180-00237. [DOI] [PubMed] [Google Scholar]
- 2.Holden DJ, Jonas DE, Porterfield DS, et al. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 3.Klabunde CN. Trends in the Use and Quality of Colorectal Cancer Screening in the US NIH State of the Science Conference. Bethesda, MD: 2010. [Google Scholar]
- 4.Shavers VL, Jackson MC, Sheppard VB. Racial/ethnic patterns of uptake of colorectal screening, National Health Interview Survey 2000-2008. J Natl Med Assoc. 2010;102(7):621–635. doi: 10.1016/s0027-9684(15)30640-4. [DOI] [PubMed] [Google Scholar]
- 5.Soneji S, Iyer SS, Armstrong K, et al. Racial disparities in stage-specific colorectal cancer mortality: 1960-2005. Am J Public Health. 2010;100(10):1912–1916. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich AJ, Tobin JN, Cassells A, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006;144(8):563–571. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rex DK, Johnson DA, Lieberman DA, et al. Am J Gastroenterol. 4. Vol. 95. American College of Gastroenterology; 2000. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. pp. 868–877. [DOI] [PubMed] [Google Scholar]
- 8.Stevens LM. A Practical Guide to Increasing Screening Colonoscopy. Cancer Prevention and Control Program, Bureau of Chronic Disease Prevention and Control, New York City Department of Health and Mental Hygiene; New York, NY: 2006. [Google Scholar]
- 9.Neugut AI, Lebwohl B. Screening for colorectal cancer: the glass is half full. Am J Public Health. 2009;99(4):592–594. doi: 10.2105/AJPH.2008.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neugut AI, Lebwohl B. Colonoscopy vs sigmoidoscopy screening: getting it right. JAMA. 2010;304(4):461–462. doi: 10.1001/jama.2010.1001. [DOI] [PubMed] [Google Scholar]
- 11.Doubeni CA, Laiyemo AO, Young AC, et al. Primary care, economic barriers to health care, and use of colorectal cancer screening tests among medicare enrollees over time. Ann Fam Med. 2010;8(4):299–307. doi: 10.1370/afm.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allison JE. FIT: a valuable but underutilized screening test for colorectal cancer-it's time for a change. Am J Gastroenterol. 2010;105(9):2026–2028. doi: 10.1038/ajg.2010.181. [DOI] [PubMed] [Google Scholar]
- 13.Ogedegbe G, Cassells AN, Robinson CM, et al. Perceptions of barriers and facilitators of cancer early detection among low-income minority women in community health centers. J Natl Med Assoc. 2005;97(2):162–170. [PMC free article] [PubMed] [Google Scholar]
- 14.Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 15.HEDIS 2008 Technical Specifications. Vol. 2. National Committee for Quality Assurance; Washington, DC: 2007. [Google Scholar]
- 16.DuBard CA, Schmid D, Yow A, et al. Recommendation for and receipt of cancer screenings among medicaid recipients 50 years and older. Arch Intern Med. 2008;168(18):2014–2021. doi: 10.1001/archinte.168.18.2014. [DOI] [PubMed] [Google Scholar]
- 17.Woolf SH. Overcoming the barriers to change: screening for colorectal cancer. Am Fam Physician. 2000;61(6):1621–1622, 1628. [PubMed] [Google Scholar]
- 18.Cohen L, Desai E, Guerrero Z, et al. Take Care New York: Fourth Year Progress Report. New York City Department of Health and Mental Hygiene; New York, NY: 2008. [Google Scholar]
- 19.DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. J Gen Intern Med. 2008;23(2):169–174. doi: 10.1007/s11606-007-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46(9 suppl 1):S10–S16. doi: 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 21.Woolf SH. National Institutes of Health State-of-the-Science Conference. Bethesda, MD: 2010. Patient and Physician Barriers to Colorectal Cancer Screening. [Google Scholar]
- 22.Ling BS, Moskowitz MA, Wachs D, Pearson B, Schroy PC. Attitudes toward colorectal cancer screening tests. J Gen Intern Med. 2001;16(12):822–830. doi: 10.1111/j.1525-1497.2001.10337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168(12):1317–1324. doi: 10.1001/archinte.168.12.1317. [DOI] [PubMed] [Google Scholar]
- 24.Doubeni CA, Laiyemo AO, Klabunde CN, et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010;38(2):184–191. doi: 10.1016/j.amepre.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxwell AE, Danao LL, Crespi CM, et al. Disparities in the receipt of fecal occult blood test versus endoscopy among Filipino American immigrants. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1963–1967. doi: 10.1158/1055-9965.EPI-07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008 [corrected]. Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 27.van Dam L, Kuipers EJ, van Leerdam ME. Performance improvements of stool-based screening tests. Best Pract Res Clin Gastroenterol. 2010;24(4):479–492. doi: 10.1016/j.bpg.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Park DI, Ryu S, Kim YH, et al. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol. 2010;105(9):2017–2025. doi: 10.1038/ajg.2010.179. [DOI] [PubMed] [Google Scholar]
- 29.Itzkowitz SH. Incremental advances in excremental cancer detection tests. J Natl Cancer Inst. 2009;101(18):1225–1227. doi: 10.1093/jnci/djp273. [DOI] [PubMed] [Google Scholar]
- 30.Woolf SH. The best screening test for colorectal cancer—a personal choice. N Engl J Med. 2000;343(22):1641–1643. doi: 10.1056/NEJM200011303432211. [DOI] [PubMed] [Google Scholar]
- 31.Taylor V, Lessler D, Mertens K, et al. Colorectal cancer screening among African Americans: the importance of physician recommendation. J Natl Med Assoc. 2003;95(9):806–812. [PMC free article] [PubMed] [Google Scholar]
- 32.Paskett ED, Katz ML. Why Disparities Matter in Colorectal Cancer Screening.. National Institutes of Health State-of-the-Science Conference; Bethesda, MD. 2010. [Google Scholar]
- 33.Walsh JM, Kaplan CP, Nguyen B, et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004;19(2):156–166. doi: 10.1111/j.1525-1497.2004.30263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costanza ME, Luckmann R, Stoddard AM, et al. Applying a stage model of behavior change to colon cancer screening. Prev Med. 2005;41(3-4):707–719. doi: 10.1016/j.ypmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 35.O'Malley AS, Beaton E, Yabroff KR, et al. Patient and provider barriers to colorectal cancer screening in the primary care safety-net. Prev Med. 2004;39(1):56–63. doi: 10.1016/j.ypmed.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Klabunde CN, Meissner HI, Wooten KG, et al. Comparing colorectal cancer screening and immunization status in older Americans. Am J Prev Med. 2007;33(1):1–8. doi: 10.1016/j.amepre.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guessous I, Dash C, Lapin P, et al. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010;50(1-2):3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Shokar NK, Carlson CA, Weller SC. Factors associated with racial/ethnic differences in colorectal cancer screening. J Am Board Fam Med. 2008;21(5):414–426. doi: 10.3122/jabfm.2008.05.070266. [DOI] [PubMed] [Google Scholar]
- 39.Borum ML, Shafa S, Hatara MC, et al. The primary care physician may have a more critical role in colorectal cancer screening in African Americans when compared to non-African Americans. J Natl Med Assoc. 2009;101(7):734. doi: 10.1016/s0027-9684(15)30987-1. [DOI] [PubMed] [Google Scholar]
- 40.Brawarsky P, Brooks DR, Mucci LA. Correlates of colorectal cancer testing in Massachusetts men and women. Prev Med. 2003;36(6):659–668. doi: 10.1016/s0091-7435(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 41.Yarnall KS, Pollak KI, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen LA, Santos S, Jandorf L, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6(4):443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Jandorf L, Gutierrez Y, Lopez J, et al. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82(2):216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christie J, Itzkowitz S, Lihau-Nkanza I, et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100(3):278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 45.Green AR, Peters-Lewis A, Percac-Lima S, et al. Barriers to screening colonoscopy for low-income Latino and white patients in an urban community health center. J Gen Intern Med. 2008;23(6):834–840. doi: 10.1007/s11606-008-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazargan M, Ani C, Bazargan-Hejazi S, et al. Colorectal cancer screening among underserved minority population: discrepancy between physicians’ recommended, scheduled, and completed tests. Patient Educ Couns. 2009;76(2):240–247. doi: 10.1016/j.pec.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Nash D, Azeez S, Vlahov D, et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83(2):231–243. doi: 10.1007/s11524-006-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baier M, Calonge N, Cutter G, et al. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev. 2000;9(2):229–232. [PubMed] [Google Scholar]