Abstract
Prostaglandins (PGs) are bioactive lipids that modulate a broad spectrum of biologic processes including reproduction and circulatory homeostasis. Although reproductive functions of mammals are influenced by PGs at numerous levels, including ovulation, fertilization, implantation, and decidualization, it is not clear which PGs are involved and whether a single mechanism affects all reproductive functions. Using mice deficient in 1 of 4 prostaglandin E2 (PGE2) receptors — specifically, the EP2 receptor — we show that Ep2–/– females are infertile secondary to failure of the released ovum to become fertilized in vivo. Ep2–/– ova could be fertilized in vitro, suggesting that in addition to previously defined roles, PGs may contribute to the microenvironment in which fertilization takes place. In addition to its effects on reproduction, PGE2 regulates regional blood flow in various vascular beds. However, its role in systemic blood pressure homeostasis is not clear. Mice deficient in the EP2 PGE2 receptor displayed resting systolic blood pressure that was significantly lower than in wild-type controls. Blood pressure increased in these animals when they were placed on a high-salt diet, suggesting that the EP2 receptor may be involved in sodium handling by the kidney. These studies demonstrate that PGE2, acting through the EP2 receptor, exerts potent regulatory effects on two major physiologic processes: blood pressure homeostasis and in vivo fertilization of the ovum.
Introduction
Prostaglandins (PGs) are well-recognized mediators of many important biologic processes (1). They exert their effects through binding to a family of G protein–coupled receptors (2). Among PGs, prostaglandin E2 (PGE2) is unique in that it acts through 4 different receptors (EP1–EP4), each with distinct but overlapping tissue distributions that activate different intracellular signaling pathways (3). It has been postulated that this complexity underlies the broad spectrum of physiologic responses that can be mediated by PGE2 and that specific receptors will be associated with each of these responses (4).
A role for PGs in reproductive physiology has been recognized for many years. PGs are present throughout the female reproductive tract, and the entire reproductive process is believed to be under the influence of these lipid mediators (5). Early studies showed that several aspects of female reproduction could be affected by inhibitors of PG synthesis (6, 7). Furthermore, genes encoding prostanoid receptors are expressed in both a temporal and cell-specific fashion during key events of early pregnancy (8, 9). More recently, mice deficient in cyclooxygenase-2 (COX-2), the rate-limiting enzyme in synthesis of all prostanoids during pregnancy, including PGE2, have been generated. These mice are infertile, with abnormalities in ovulation, fertilization, implantation, and decidualization (10). However, because COX-2 is critical for the production of PGE2, PGD2, PGF2, PGI2 (prostacyclin), and thromboxane A2, these studies did not identify the particular prostanoid or receptor critical to each of the reproductive functions disrupted in these animals. Some insight into the mechanism by which prostanoids mediate their effects is beginning to emerge from the study of animals deficient in specific receptors. For example, mice deficient in the receptor for PGF (FP receptor) demonstrated failure of parturition but showed no abnormalities in ovulation, fertilization, or implantation (11).
Surprisingly, no decreased fertility has been reported for the EP3-, EP4-, thromboxane (TP)-, or prostacyclin (IP)-receptor–deficient mice, despite the expression of these prostanoid receptors in the reproductive tract (8, 9, 12–16).
PGE2 also has potent effects on the cardiovascular system. A role for PGE2 in blood pressure homeostasis has been recognized for years, but its effects are complex because it acts on multiple tissues important to the maintenance and control of blood pressure (17). Although it is clear that blood pressure homeostasis and many aspects of female reproduction may be regulated by PGs, the precise contribution of the individual receptors that mediate the actions of PGE2 remains to be defined. Utilizing mice deficient in the EP2 receptor, we show that this PG receptor plays a critical role in successful fertilization of the released ovum and the maintenance of circulatory homeostasis.
Methods
Animal welfare.
The use of experimental animals was in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines of the University of North Carolina–Chapel Hill and Duke University.
Generation of Ep2–/– mice.
Genomic clones were isolated from a 129/Sv mouse genomic library with an Ep2 cDNA probe and their identity confirmed by sequence analysis. A targeting vector was constructed in which the DNA encoding amino acids 246–601 was replaced by a neomycin-resistant gene and electroporated into 129/Ola–derived E14TG2a embryonic stem (ES) cells, and neomycin- and ganciclovir-resistant colonies were identified using standard methods (18). DNA isolated from ES cell colonies was digested with the restriction enzyme HindIII and analyzed by Southern blot to identify clones with a targeted Ep2 allele. Chimeras derived from targeted ES cells were mated with 129/SvEv mice, and offspring carrying the targeted allele were identified by Southern blot analysis. These heterozygotes were intercrossed to produce mice homozygous for the Ep2 mutation.
Analysis of Ep2 RNA expression.
Total uterine RNA was isolated from 7-week-old Ep2–/– and Ep2+/+ mice using RNAzol (Tel- Test Inc., Friendswood, Texas, USA) according to the manufacturer’s specifications. Ep2 and actin cDNA were synthesized by reverse transcription, and PCR amplification was carried out using the following oligonucleotide primers: Ep2-1F (5′-GTGGCCCTGGCTCCCGAAAGTC-3′), Ep2-2R (5′-GGCAAGGAGCATATGGCGAAGGTG-3′),actin-1F(5′-TAAGGCCAACCGTGAAAAGATGAC-3′), and actin-2R (5′-ACCGCTCGTTGCCAATAGTGATG-3′). The PCR products were analyzed by gel electrophoresis and staining with ethidium bromide.
Blastocyst transfer.
Ep2–/– and Ep2+/+ females (8–22 weeks old) were mated with wild-type vasectomized males and checked daily for vaginal plugs. Fourteen to 20 C57BL/6 blastocysts were transferred into the uteri of recipient females on day 3.5 of pseudopregnancy, and the foster mothers were checked daily for pregnancy and delivery.
Ovulation and fertilization.
Ep2–/– and Ep2+/+ mice were mated with wild-type males, and inspection for vaginal plugs was performed daily. On the day after finding a vaginal plug, mice were sacrificed and oviducts were flushed with 100–200 μL of CO2-independent media to recover eggs or embryos.
In vitro fertilization.
Female mice were superovulated by injecting 5 IU pregnant mare’s serum intraperitoneally and then, 48 hours later, injecting 5 IU human chorionic gonadotropin (hCG) intraperitoneally. Twelve hours after hCG was injected, mice were sacrificed and the ova were collected and incubated in HTF media for 4–6 hours at 37°C with sperm collected from the epididymides of proven male mice. The ova were then washed and cultured for an additional 24 hours, at which time the number of unfertilized eggs and 2-cell embryos was determined.
Blood pressure measurements.
Resting systolic blood was measured in conscious female mice (3–4 months old) using a computerized noninvasive tail-cuff system (Visitech Systems, Apex, North Carolina, USA). The validity of this system has been demonstrated previously (19). Mice were adapted to the system for 5 days, after which blood pressure was recorded daily for 5 consecutive days after 2 weeks of either a normal (0.4% NaCl) or high-salt diet (6% NaCl; Harlan Teklad Laboratory, Madison, Wisconsin, USA), ad libitum, with free access to water.
Plasma electrolytes.
Blood was collected by cardiac puncture into cold EDTA-coated tubes, and plasma samples were immediately placed at –80°C. Plasma sodium levels were determined by flame photometry.
Twenty-four–hour urine collections for PGE2 determination.
Urine was collected over a 24-hour period using a mouse metabolic cage, and stored at –20°C immediately upon completion of the study period. Urinary PGE2 was determined by enzyme immunoassay according to the manufacturer’s protocol (Cayman Chemicals, Ann Arbor, Michigan, USA).
Plasma renin activity.
Plasma renin concentration was determined by radioimmunoassay according to the manufacturer’s protocol (Du Pont NEN Research Products, Boston, Massachusetts, USA).
Renin mRNA.
Kidneys were harvested from Ep2–/– and Ep2+/+ mice, frozen in liquid nitrogen, and immediately placed at –80°C. Total RNA was extracted by homogenization with RNAzol (Tel-Test Inc.) according to the manufacturer’s specifications. Renin mRNA was measured by RNase protection assay as described previously (20).
Statistical analysis.
Data are presented as mean ± SEM. Statistical significance was assessed by using χ2 analysis and the unpaired Student’s t test.
Results
Generation of Ep2–/– mice.
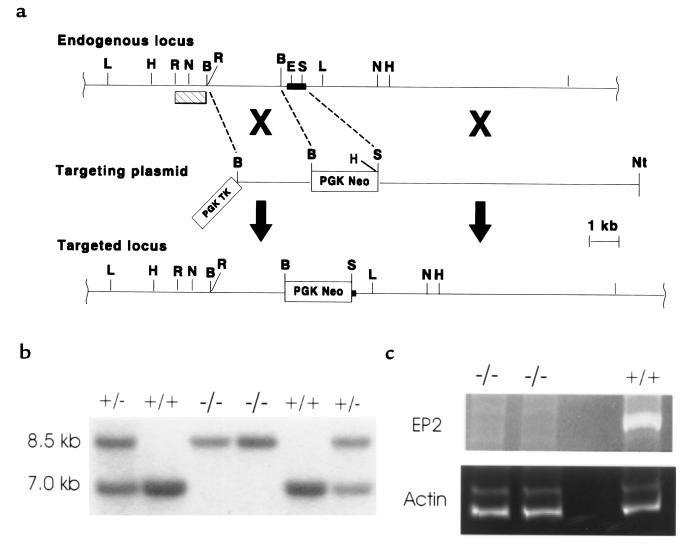
Mouse ES cells in which the Ep2 gene was disrupted by homologous recombination were generated using the scheme shown in Figure 1a. Chimeric animals created with these targeted cells were mated with 129/SvEv mice to generate heterozygotes for the mutant allele on the 129 background. Heterozygous Ep2+/– mice were intercrossed and Ep2–/– offspring were born at expected frequencies — 25.2% (Ep2+/+), 50% (Ep2+/–), and 24.7% (Ep2–/–) — indicating that this receptor does not have a unique role in intrauterine development or perinatal survival (Figure 1b). The appearance, behavior, and longevity of Ep2–/– mice did not differ from that of wild-type littermates, with some mice living longer than 18 months. Gross and histologic appearance of all major organs, including the kidney, uterus, and ovaries, did not differ between Ep2–/– and wild-type animals. Loss of Ep2 expression was verified by RT-PCR analysis of RNA from Ep2–/– animals (Figure 1c).
Figure 1.
Disruption of the gene encoding the EP2 receptor. (a) Restriction maps of the targeting construct, endogenous locus, and targeted locus. The dark filled box represents exon sequences of the Ep2 gene. The open boxes represent phosphoglycerate kinase-thymidine kinase (PGK-TK) and PGK-Neo selection cassettes. Homologous recombination of the targeting vector with the endogenous locus results in deletion of a 355-bp segment of DNA that is predicted to encode 3 transmembrane domains and 2 intracellular loops of mature EP2 protein. The hatch filled box represents the probe used to detect homologous recombination events by Southern blot analysis. Relevant restriction sites are abbreviated by the following: B, BamHI; E, EagI; H, HindIII; L, BglI; N, NsiI; Nt, NotI; R, EcoRI; S, SacII. Note that not all HindIII sites are mapped. (b) Southern blot analysis of DNA obtained from tail biopsies of offspring from Ep2+/– heterozygote breedings. HindIII-digested DNA was analyzed using a 1.1-kb EcoRI probe corresponding to DNA outside the targeted region of the Ep2 gene. This probe detects a 7-kb band derived from the endogenous locus and an 8.5-kb band from the targeted locus. (c) RT-PCR amplification of Ep2 total RNA from uteri of Ep2–/– and Ep2+/+ mice. Ep2-specific cDNA was synthesized by RT-PCR. The left lanes represent cDNA from 2 Ep2–/– animals; the lane on the far right represents cDNA from an Ep2+/+ animal. To ensure that the failure to detect an Ep2-specific DNA fragment in the mRNA obtained from Ep2–/– animals was not due to the absence of RNA, the same samples were subjected to RT-PCR using actin-specific primers.
Fertility of the EP2-deficient animals.
Whereas Ep2–/– males were fertile, fertility was severely impaired in Ep2–/– females. After 12 weeks of mating, only 6 of 34 (18%) Ep2–/– females achieved pregnancy and delivered litters, compared with 39 of 42 (93%) Ep2+/– females (P = 0.001). This reduced fertility of the Ep2–/– females did not reflect a decrease in the frequency of mating, as determined by the presence of vaginal plugs.
Blastocyst transfer.
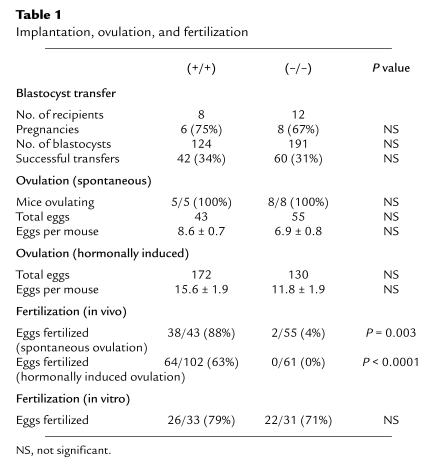
To determine if reproductive failure was due to defective implantation of the embryos, wild-type blastocysts were transferred into the uteri of Ep2–/– and Ep2+/+ animals on day 3.5 of pseudopregnancy. As shown in Table 1, eight successful pregnancies were achieved through blastocyst transfer into Ep2–/– females, with 60 out of 191 blastocysts developing into embryos (31% efficiency). These results are not significantly different from those obtained in Ep2+/+ females (Table 1). Gestational time was similar for both groups of animals (17.2 ± 0.2 vs. 17.8 ± 0.5 days), as was the care of the pups.
Table 1.
Implantation, ovulation, and fertilization
To confirm the previously observed infertility, the same female mice used for blastocyst transfer were then mated with fertile males. Whereas 8 of 8 Ep2+/+ females became pregnant, successful pregnancies developed in only 3 of the 12 Ep2–/– mice (P = 0.004). Furthermore, litter sizes of those Ep2–/– females achieving pregnancy were significantly smaller than litters from Ep2+/+ females (5.2 ± 0.8 vs. 1.3 ± 0.3; P = 0.004). These experiments demonstrate that the effects of the Ep2 mutation on fertility are not due to defective implantation, decidualization, or parturition.
Ovulation and fertilization.
We next examined ovulation and fertilization in Ep2–/– and Ep2+/+ mice. Histologic examination of the ovaries revealed normal numbers of primary and mature follicles in the 2 mouse lines. As shown in Table 1, all Ep2–/– females ovulated, and the number of eggs released per mouse was similar to wild-type controls (6.9 ± 0.8 vs. 8.6 ± 0.7; P = 0.13). Furthermore, administration of gonadotropic hormones stimulated release of eggs to a similar extent in the 2 groups of animals (11.8 ± 1.9 vs. 15.6 ± 1.9; P = 0.165)
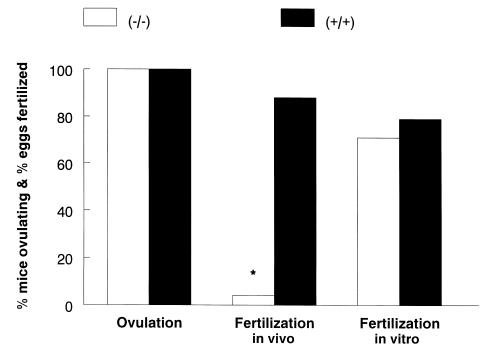
Fertilization, however, was severely impaired in Ep2–/– mice. Only 4% (2/55) of eggs from 8 Ep2–/– mice were fertilized, compared with 88% (38/43) of eggs from 5 Ep2+/+ mice (P < 0.0001) (Figure 2). Morphologically, eggs and their cumulus complexes from Ep2–/– animals were indistinguishable from those of wild-type controls. Motile sperm were present in the oviducts of Ep2–/– females and wild-type females 12 hours postcoitus, suggesting that alterations in sperm transport were not the underlying cause of fertilization failure for the Ep2–/– ovum. To exclude the possibility that fertilization failure was secondary to delayed ovulation, experiments were repeated with mice in which ovulation was hormonally induced. Whereas 64 of 102 eggs released from 6 Ep2+/+ females were fertilized, none of the 61 eggs released from 5 Ep2–/– females were fertilized (P < 0.001).
Figure 2.
Ovulation and fertilization in Ep2–/– and Ep2+/+ mice. Female Ep2–/– (n = 8) and Ep2+/+ (n = 5) mice were mated with wild-type males, and ovulation and in vivo fertilization were determined by counting eggs and embryos 2 days following coitus. For in vitro fertilization, eggs from mice of both genotypes were collected 12 hours after ovulation and cultured with sperm obtained from wild-type males. Fertilization was scored by counting the number of 2-cell embryos 24 hours later. *P < 0.0001.
To determine whether fertilization failure was secondary to abnormalities of the ovum, in vitro fertilization was performed with ova from Ep2–/– and Ep2+/+ mice. Seventy-one percent of eggs obtained from 4 Ep2–/– females were successfully fertilized in vitro, similar to in vitro fertilization rates in control eggs (Figure 2).
Blood pressure regulation.
To define the role of the EP2 receptor in regulating blood pressure, we compared systolic blood pressure in conscious Ep2–/– and Ep2+/+ mice. In animals maintained on a normal diet (0.4% NaCl), systolic blood pressure was reduced significantly in the Ep2–/– mice compared with wild-type controls (109 ±3 vs. 122 ± 5 mmHg; P = 0.04) (Table 2). To determine whether this blood pressure difference could be overcome by providing excess sodium in the diet, we fed Ep2–/– and Ep2+/+ mice a high-salt diet containing 6% NaCl. This alteration of dietary NaCl content had no effect on blood pressure in the wild-type mice. In contrast, blood pressure increased significantly in Ep2–/– mice on a high-salt diet, from 109 ± 3 to 117 ± 3 mmHg (P = 0.01). Plasma sodium levels and urinary PGE2 excretion were no different between Ep2–/– and Ep2+/+ animals (data not shown).
Table 2.
Average resting systolic blood pressure of Ep2–/– and Ep2+/+ mice
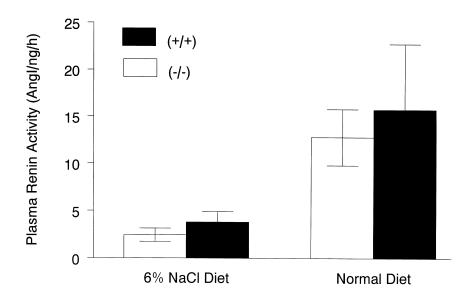
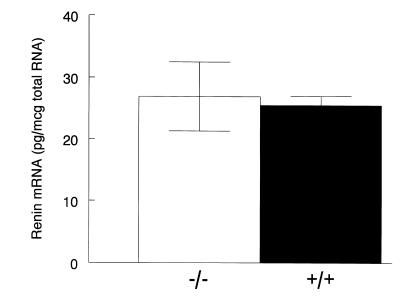
To evaluate the effect of the Ep2 mutation on regulation of the renin-angiotensin system, and its potential contribution to altered blood pressure regulation, we also measured plasma renin activity (PRA) in the 2 groups of mice on the different dietary NaCl regimens. Figure 3 shows the changes in PRA measured in mice on normal and high-salt diets. Despite the differences in blood pressure between Ep2–/– and Ep2+/+ mice on a normal (0.4% NaCl) diet, PRAs were virtually identical (12.4 ± 3.0 [n = 8] vs. 15.8 ± 7.0 [n = 7] Ang I/ng/h; P = 0.63) (Figure 3). Renin mRNA levels were also similar between genotypes (26.9 ± 5.6 vs. 25.5 ± 1.5 pg/μg total RNA) (Figure 4). After 10 days on the high-salt diet, PRA was suppressed appropriately in both Ep2–/– and Ep2+/+ mice (2.4 ± 0.7 [n = 5] vs. 3.8 ± 1.1 [n = 6] Ang I/ng/h; P = 0.34) (Figure 3).
Figure 3.
PRA in Ep2–/– and Ep2+/+ mice on normal and high-salt diets. Plasma was prepared from Ep2–/– (n = 8) and Ep2+/+(n = 7) mice on a normal diet (0.4% NaCl), and PRA was determined by radioimmunoassay. PRA was also measured in Ep2–/– (n = 5) and Ep2+/+(n = 6) mice after 10 days on a high-salt diet (6% NaCl). Data represent mean ± SEM.
Figure 4.
Renin mRNA levels in Ep2–/– and Ep2+/+ mice. RNA was obtained from the kidneys of Ep2–/– (n = 8) and Ep2+/+(n = 7) mice on a normal diet (0.4% NaCl), and renin mRNA levels were determined by RNase protection assay. Data represent mean ± SEM.
To determine if the reproductive failure of Ep2–/– females was related to the reduced blood pressure of these animals, fertilization was examined after 10 days on a high-salt diet (6% NaCl). Only 3% of eggs from Ep2–/– mice were fertilized, compared with 93% of eggs from Ep2+/+ mice (n = 6; P < 0.0001). These results suggest that the impaired fertilization in Ep2–/– females is independent of the hemodynamic changes seen in these animals.
Discussion
We report here on the generation of mice deficient in the expression of the PGE2 EP2 receptor. Although these mice are generally healthy, female Ep2–/– mice have significantly reduced pregnancy rates, and when these animals do become pregnant, they deliver smaller litters than Ep2+/–and wild-type controls. We show that this decrease in fertility is primarily due to a significantly lower fertilization rate in the Ep2–/– females. This reduction in fertilization is not due to an intrinsic defect of the ovum, because the in vitro fertilization rates of the Ep2–/– and wild-type ova are identical. In addition to this decreased fertility, we show that Ep2–/– animals have reduced blood pressure on normal diets and that this hypotension can be partially ameliorated by increased dietary salt.
Although all 4 EP receptors are expressed in the uterus, reduced fertility has not been reported for either the EP3- or EP4-deficient mice (13, 16). Although the fertility of the EP2-deficient mouse is decreased, implantation and decidualization is identical to that seen in wild-type animals. Decreased implantation or decidualization was not reported for the FP-, IP-, or TP-receptor–deficient mice (11, 12, 21). These observations are somewhat surprising given the abnormalities of these reproductive functions in the COX-2–deficient mice. A number of explanations for these findings can be put forth. First, it is possible that PGD2 or PGE2 activation of EP1 is important for implantation. It is also possible that, despite the seemingly unique pattern of expression of the various prostanoid receptors, their functions overlap enough that implantation is not affected by the loss of a single receptor. Alternatively, a yet to be identified COX-2 product might be important for implantation.
Although ovulation is primarily triggered by a surge of luteinizing hormone, a role for PGs in this process has also been suggested. For example, inhibitors of PG synthesis, such as indomethacin, can prevent follicular rupture, and this effect can be overcome by administration of PGE2 (22). Similarly, mice deficient in COX-2 demonstrated impaired ovulation (10). Our results indicate that these effects of PGs on ovulation are not mediated through the EP2 receptor, because both natural and hormonally induced ovulation in Ep2–/– females were no different from wild-type controls.
Although PGs are produced throughout the female reproductive tract and by ovulated cumulus cell-oocyte complexes, their precise role in the fertilization process remains unclear (23). Again, direct evidence for a role of prostanoids in fertilization came from studies of the COX-2–deficient mice. The results presented here are consistent with the hypothesis that at least one function of prostanoids in fertilization is mediated by PGE2 through the EP2 receptor. To define the mechanism by which loss of the EP2 receptor leads to decreased fertilization, a number of experiments were carried out. To determine if decreased fertilization was due to delayed or premature release of the ovum, ovulation was induced by administration of hCG, and the appearance of the eggs in the oviduct, as well as the rate of fertilization, was examined. Although the appearance of the ampulla in the oviduct was similar, the ova released after the animals were treated with hCG still failed to become fertilized. In addition, ova and their cumulus complexes from Ep2–/– animals were morphologically indistinguishable from those from wild-type controls. Therefore, it seems unlikely that the decreased fertilization of eggs from Ep2–/– mice results from an alteration in the timing of ovulation.
The fertilization process requires successful transport of sperm to the oviducts, where they can be sequestered for interaction with the ova. PGs have been shown to facilitate sperm transport through the female reproductive tract, not only by their actions on sperm but also by their effects on female reproductive tissue, including oviductal smooth muscle (24). Numerous sperm were found in the oviducts of Ep2–/– females within 12 hours of coitus, making it unlikely that alteration in sperm transport is the cause of fertilization failure in the EP2-deficient animals.
Together, these findings suggest that the reduced fertilization seen in these animals could be a function of PGs intrinsic to the ovum and cumulus cells, or that EP2-mediated PGE2 functions are essential for establishing an oviductal microenvironment conducive to successful fertilization. To distinguish between these 2 possibilities, we compared in vitro and in vivo fertilization of the Ep2–/– ovum. Successful in vitro fertilization of ova from Ep2–/– animals does not support the former hypothesis. These results are consistent with studies showing that maturation of the ovum is independent of PGs (25, 26). Previous studies have also suggested that ovum viability is unaffected by inhibitors of PG synthesis in vitro (5).
Our studies are consistent with the hypothesis that PGE2 acts through the EP2 receptor to define a microenvironment in the oviduct conducive to fertilization. Although such a function in reproductive physiology has not previously been ascribed to these lipids, it is consistent with the diverse actions of PGs in numerous organ systems. For example, PGs have been shown to modify ion transport in a number of epithelia (27, 28). It is possible that the ionic composition of the oviductal fluid is altered in the Ep2–/– animals and that this, in turn, leads to reduced fertilization.
PGE2 also has potent effects on the cardiovascular system. A role for PGE2 in blood pressure homeostasis has been recognized for years, but these actions are complex, involving regulation of vascular tone and sodium balance (17). To examine the role of the EP2 receptor in blood pressure regulation, we measured systolic blood pressure in conscious Ep2–/– animals. On a normal diet (0.4% NaCl), systolic blood pressure was reduced by 13 mmHg in the EP2-deficient mice compared with controls. To determine whether this difference in blood pressure could be overcome by providing excess sodium in the diet, Ep2–/– and Ep2+/+ mice were fed high-salt diets containing 6% NaCl (wt/wt). This increase in dietary NaCl content had no effect on blood pressures in the wild-type mice. In contrast, on the high-salt diet, blood pressure increased significantly in Ep2–/– mice to levels that were not significantly different than controls. Thus, reduced blood pressure in the Ep2–/– mice is sodium sensitive and can be overcome with dietary sodium loading. This suggests that the absence of EP2 receptors produces an abnormality in sodium handling by the kidney. EP2 receptor expression in the kidney has been demonstrated by both RT-PCR and Northern analysis (29, 30), although its cellular functions in the kidney have not been clearly elucidated.
Our findings of reduced blood pressure in the Ep2–/– animals may seem somewhat paradoxical given the known vasorelaxant actions of PGE2 (31, 32). However, whereas acute infusions of PGE2 cause vasodilation, chronic infusions of PGE2 increase blood pressure through direct stimulation of renin secretion (33). Furthermore, inhibitors of PG synthesis have been shown to lower blood pressure and renin levels in patients with renovascular hypertension (34). It is well established that PGs can stimulate renin release through actions on the juxtaglomerular apparatus; hormones that increase intracellular cAMP levels stimulate renin release by these specialized cells (35). Because 2 EP receptors, EP2 and EP4, are Gs protein–coupled, it has been suggested that one or both of these receptors may mediate this action of PGE2 (28, 36).
To determine whether abnormal regulation of the renin-angiotensin system might contribute to altered blood pressure regulation and salt sensitivity in Ep2–/– mice, we measured PRA in these animals. Despite their lower blood pressures while on a normal diet, neither PRA nor renin mRNA was elevated in the Ep2–/– animals (Figures 3 and 4). This differs from other lines of mice harboring targeted mutations that lower blood pressure, where PRA and renin mRNA are consistently elevated as a compensatory mechanism (20, 37). These findings suggest that renin responses are significantly impaired in EP2-deficient mice. Failure to stimulate PRA may contribute to the reduced blood pressure seen in these animals while they are on a normal diet.
The contribution of PGs to many important biologic processes has been recognized for years. Recently, mice carrying homozygous null mutations for genes encoding enzymes in the PG biosynthetic pathway and in PG receptors themselves have furthered our understanding of the role that these lipid mediators play in various aspects of reproduction (10, 11). The role of PGs in maintaining circulatory homeostasis has been more controversial and less understood. Here we define critical roles for the EP2 PGE2 receptor in blood pressure regulation and female reproduction. Specifically, we present evidence supporting the hypothesis that PGE2, acting though the EP2 receptor, is important for establishment of the microenvironment required for successful fertilization of the released ovum. We also show that the EP2 receptor is essential for normal blood pressure homeostasis. Mice lacking this receptor are relatively hypotensive, and this hypotension can be partially reversed by salt loading. Finally, compensatory increases in renin are not seen in these animals, suggesting that the EP2 receptor may be involved in PG-stimulated renin release. Continued investigations into the specific roles of the receptors that mediate the actions of PGs will further our understanding of these important lipid mediators and potentially may lead to the development of more specific and less toxic therapies for a variety of disease processes.
Note added in proof.
During the review process of this manuscript, an analysis of EP2 receptor–deficient mice was independently reported in the February 1999 issue of Nature Medicine (5:217–220).
Acknowledgments
The authors thank Cun-Yu Wang for his work on the targeting construct; Anne Latour for her work in tissue culture; Betsy Drake and Jane Farley (The Jackson Laboratory, Bar Harbor, Maine, USA)for their advice on in vitro fertilization; and M. Solle, J.E. Fabre, and J. Snouwaert for their helpful comments on the manuscript. This work was supported in part by National Institutes of Health grant HL-58554.
Footnotes
Stephen L. Tilley and Laurent P. Audoly contributed equally to this work.
References
- 1.Oates JA, et al. Clinical implications of prostaglandin and thromboxane A2 formation. N Engl J Med. 1988;319:689–698. doi: 10.1056/NEJM198809153191106. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RA, Smith WL, Narumiya S. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 3.Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta. 1995;1259:109–120. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- 4.Ashby B. Interactions among prostaglandin receptors. Receptor. 1994;4:31–42. [PubMed] [Google Scholar]
- 5.Hayashi S, Noda Y, Mori T. Analysis of the role of prostaglandins in the fertilization process. Eur J Obstet Gynecol Reprod Biol. 1988;29:287–297. doi: 10.1016/0028-2243(88)90069-x. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy TG. Evidence for a role for prostaglandins in the initiation of blastocyst implantation in the rat. Biol Reprod. 1977;16:286–291. doi: 10.1095/biolreprod16.3.286. [DOI] [PubMed] [Google Scholar]
- 7.Tsafriri A, Lindner HR, Zor U, Lamprecht SA. Physiologic role of prostaglandins in the induction of ovulation. Prostaglandins. 1972;2:1–10. doi: 10.1016/0090-6980(72)90024-x. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z-M, et al. Potential sites of prostaglandin actions in the periimplantation mouse uterus: differential expression and regulation of prostaglandin receptor genes. Biol Reprod. 1997;56:368–379. doi: 10.1095/biolreprod56.2.368. [DOI] [PubMed] [Google Scholar]
- 9.Katsuyama M, et al. Distinct cellular localization of the messenger ribonucleic acid for prostaglandin E receptor subtypes in the mouse uterus during pseudopregnancy. Endocrinology. 1997;138:344–350. doi: 10.1210/endo.138.1.4885. [DOI] [PubMed] [Google Scholar]
- 10.Lim H, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto Y, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 12.Murata T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen M, et al. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 14.Hirata M, et al. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 15.Namba T, et al. Mouse thromboxane A2 receptor: cDNA cloning, expression and Northern blot analysis. Biochem Biophys Res Commun. 1992;184:1197–1203. doi: 10.1016/s0006-291x(05)80009-9. [DOI] [PubMed] [Google Scholar]
- 16.Fleming EF, et al. Urinary concentrating function in mice lacking EP3 receptors for prostaglandin E2. Am J Physiol. 1998;275:F955–F961. doi: 10.1152/ajprenal.1998.275.6.F955. [DOI] [PubMed] [Google Scholar]
- 17.Lee JB, Attallah AA. Renal prostaglandins. Nephron. 1975;15:350–368. doi: 10.1159/000180520. [DOI] [PubMed] [Google Scholar]
- 18.Mohn, A., and Koller, B.H. 1995. Genetic manipulation of embryonic stem cells. In DNA cloning 4. D.M. Glover and B.D. Hames, editors. Oxford University Press. New York, NY. 143–184.
- 19.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 20.Oliver MI, Best CFKHS, Arendshorst WJ, Smithies O, Coffman TM. Angiotensin II responses in AT1A receptor-deficient mice: a role for AT1B receptors in blood pressure regulation. Am J Physiol. 1997;272:F515–F520. doi: 10.1152/ajprenal.1997.272.4.F515. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DW, et al. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest. 1998;102:1194–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsafriri, A. 1995. Ovulation as a tissue remodelling process. In Tissue renin-angiotensin systems. A.K. Mukhipadhyay and M.K. Raizada, editors. Plenum Press. New York, NY. 121–140.
- 23.Schuetz AW, Dubin NH. Progesterone and prostaglandin secretion by ovulated rat cumulus cell-oocyte complexes. Endocrinology. 1981;108:457–463. doi: 10.1210/endo-108-2-457. [DOI] [PubMed] [Google Scholar]
- 24.Mandl JP. The effect of prostaglandin E1 on rabbit sperm transport in vivo. J Reprod Fertil. 1972;31:263–269. doi: 10.1530/jrf.0.0310263. [DOI] [PubMed] [Google Scholar]
- 25.Poulos A, Voglmayr JK, White IG. Phospholipid changes in spermatozoa during passage through the genital tract of the bull. Biochim Biophys Acta. 1973;306:194–202. doi: 10.1016/0005-2760(73)90225-7. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi S, Noda Y, Matsumoto H, Mori T. Fertilization of unovulated mature eggs following indomethacin administration in mice. Gamete Res. 1987;18:291. doi: 10.1002/mrd.1120180403. [DOI] [PubMed] [Google Scholar]
- 27.Al-Bazzaz F, Yadava VP, Westenfelder C. Modification of Na and Cl transport in canine tracheal mucosa by prostaglandins. Am J Physiol. 1981;240:F101–F105. doi: 10.1152/ajprenal.1981.240.2.F101. [DOI] [PubMed] [Google Scholar]
- 28.Breyer MD, et al. Regulation of renal function by prostaglandin E receptors. Kidney Int. 1998;54(Suppl. 67):S88–S94. doi: 10.1046/j.1523-1755.1998.06718.x. [DOI] [PubMed] [Google Scholar]
- 29.Nemoto K, Pilbeam CC, Bilak SR, Raisz LG. Molecular cloning and expression of the rat prostaglandin E 2 receptor of the EP2 subtype. Prostaglandins. 1997;54:713–725. doi: 10.1016/s0090-6980(97)00145-7. [DOI] [PubMed] [Google Scholar]
- 30.Katsuyama M, et al. The mouse prostaglandin E receptor EP2 subtype: cloning expression and Northern blot analysis. FEBS Lett. 1995;372:151–156. doi: 10.1016/0014-5793(95)00966-d. [DOI] [PubMed] [Google Scholar]
- 31.Lee JB, Covino BG, Takman BH, Smith ER. Renomedullary vasodepressor substance, medullin: isolation, chemical characterization, and physiological properties. Circ Res. 1965;17:57–77. doi: 10.1161/01.res.17.1.57. [DOI] [PubMed] [Google Scholar]
- 32.Daniels EG, Hinman JW, Leach BE, Muirhead EE. Identification of prostaglandin E2 as the principal vasodepressor lipid of rabbit renal medulla. Nature. 1967;215:1298–1299. doi: 10.1038/2151298a0. [DOI] [PubMed] [Google Scholar]
- 33.Hockel GM, Cowley AW. Role of the renin-angiotensin system in prostaglandin E2-induced hypertension. Hypertension. 1980;2:529–537. doi: 10.1161/01.hyp.2.4.529. [DOI] [PubMed] [Google Scholar]
- 34.Imanishi M, et al. Aspirin lowers blood pressure in patients with renovascular hypertension. Hypertension. 1989;14:461–468. doi: 10.1161/01.hyp.14.5.461. [DOI] [PubMed] [Google Scholar]
- 35.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 36.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol. 1996;271:F659–F669. doi: 10.1152/ajprenal.1996.271.3.F659. [DOI] [PubMed] [Google Scholar]
- 37.Tanimoto K, et al. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. [PubMed] [Google Scholar]