SUMMARY
Distinct subclasses of retinal ganglion cells (RGCs) mediate vision and non-image forming functions such as circadian photoentrainment. This distinction stems from studies that ablated melanopsin-expressing intrinsically photosensitive (ip)RGCs and showed deficits in non-image forming behaviors, but not image vision. However, we show that the ON alpha RGC, a conventional RGC type, is intrinsically photosensitive in mammals. In addition to their classical response to fast changes in contrast through rod/cone signaling, melanopsin expression allows ON alpha RGCs to signal prior light exposure and environmental luminance over long periods of time. Consistent with the high contrast sensitivity of ON alpha RGCs, mice lacking either melanopsin or ON alpha RGCs have behavioral deficits in contrast sensitivity. These findings indicate a surprising role for melanopsin and ipRGCs in vision.
INTRODUCTION
The visual system can be grossly divided into two systems: the image forming visual system, which involves conscious perception of images, and the non-image forming visual system, which includes functions that occur outside of conscious awareness such as circadian photoentrainment and the pupillary light reflex. The image forming visual system relies on rod and cone photoreceptors to detect light. These signals are then processed in the retina and relayed via conventional retinal ganglion cells (RGCs) to visual nuclei such as the dorsal lateral geniculate nucleus (dLGN) for further processing. The non-image forming visual system, however, relies almost exclusively on a distinct subset of RGCs that are intrinsically photosensitive (ip)RGCs and express melanopsin (Berson et al., 2002; Guler et al., 2008; Hattar et al., 2002). These photoreceptive neurons project to brain regions such as the suprachiasmatic nucleus (SCN), which mediates circadian photoentrainment, and the olivary pretectal nucleus (OPN), which drives the pupillary light reflex (Hattar et al., 2006). Because genetic ablation of ipRGCs results in loss of non-image forming behaviors, but retention of image forming vision via conventional RGCs, the logical assumption has been that RGCs driving non-image and image-forming behaviors are distinct (Guler et al., 2008). However, here we show that a well-described RGC type, the ON alpha (A type) cell, characterized by large soma and dendritic field sizes (Boycott and Wassle, 1974), expresses melanopsin, is intrinsically photosensitive, and encodes irradiance over long periods. We also demonstrate that in ground squirrels the ON alpha-like RGCs, which have a transient rod/cone driven response, are also intrinsically photosensitive. Moreover, mice lacking melanopsin (Opn4−/−), or with ON alpha RGCs ablated (Opn4Cre/+; Brn3bzDTA/+; (Chen et al., 2011), are defective in contrast detection, consistent with the demonstrated high contrast sensitivity of alpha RGCs (Enroth-Cugell and Robson, 1966; Shapley and Victor, 1978; Zaghloul et al., 2003). These findings reveal a functional role for melanopsin signaling in contrast detection in the presence of fully functional rod/cone signaling pathways.
RESULTS
ON alpha RGCs are intrinsically photosensitive
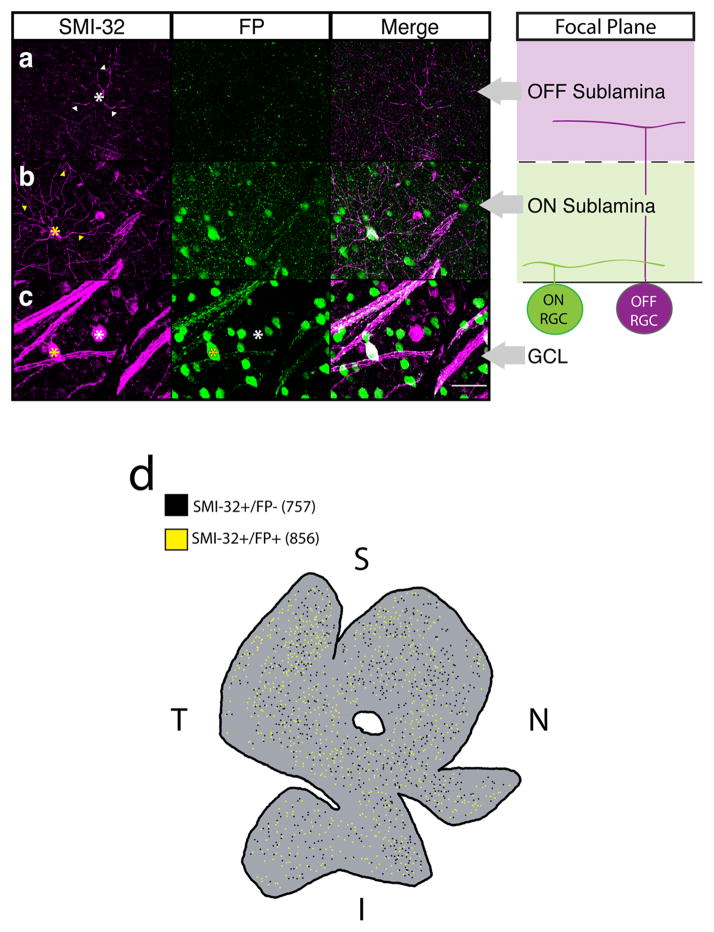
We found that a subset of fluorescently labeled ipRGCs (Opn4Cre/+;Brainbow-1.0 mice; (Ecker et al., 2010; Livet et al., 2007) with large somas (≥ 20 μm) are immunopositive for the non-phosphorylated form of the neurofilament heavy chain protein (SMI-32, Fig. 1c), which strongly labels alpha RGCs, in addition to weakly labeling other conventional RGC subtypes (Coombs et al., 2006; Lin et al., 2004). This is surprising because alpha cells project to image forming brain regions and have high contrast sensitivity, which is essential for image formation. Alpha cells are divided into two populations that arborize in either the ON or OFF sublamina of the inner plexiform layer (IPL). These morphological divisions correlate with physiological responses to light increments (ON-center RGCs) or decrements (OFF-center RGCs) respectively (Famiglietti and Kolb, 1976; Kuffler, 1953). We found that only SMI-32 positive RGCs with dendrites stratifying in the ON sublamina expressed the melanopsin reporter (Fig. 1b,c). Conversely, SMI-32 positive cells that stratified in the OFF sublamina were consistently negative for the fluorescent reporter (Fig. 1a,c). However, we observed no colocalization when we immunostained for melanopsin and SMI-32, even with the most sensitive melanopsin antibody (Ecker et al., 2010; Provencio et al., 2002); Supplemental Fig. 1), confirming previous reports (Coombs et al., 2006; Lin et al., 2004). These surprising results show that ON, but not OFF, alpha cells co-label with a melanopsin reporter.
Fig. 1. A subset of SMI-32 immunopositive RGCs label with a melanopsin reporter.
(a–c) Retinas from adult Opn4Cre/+;Brainbow-1.0 mice in which all ipRGCs are labeled with FPs (Green) were immunostained for the alpha RGC marker SMI-32 (Magenta). Far right panels show the focal plane in which z-stacks in a–c were taken relative to the soma location or dendrite stratification of ON and OFF RGCs within the retina. (a) SMI-32+FP− cells (white *) stratified in the OFF sublamina of the IPL (white arrowheads). (b) SMI-32+FP+ cells (yellow *) stratified in the ON sublamina of the IPL (yellow arrowheads). (c) ipRGCs with the largest somas always colocalized with SMI-32 (yellow *), but not all SMI-32+ cells colocalized with FP (white *). (d) Wholemount Opn4Cre/+;Brainbow-1.0 retina immunostained for SMI-32. Both SMI-32+/FP+ (856, yellow dots) and SMI-32+/FP− (757, black dots) cells are found across all retinal quadrants (Coverage Factors: ON = 5.1 ± 1.3, n = 3 retinas, OFF = 4.9 ± 0.9, n = 3 retinas). Scale bar (a–c) = 50 μm. I = inferior retina, S = superior retina, T = temporal retina, N = nasal retina. GCL: ganglion cell layer, FP: fluorescent protein, RGC: retinal ganglion cell.
Nearly half of SMI-32 labeled cells (n = 1715/3646 cells from N = 3 mice) were positive for the melanopsin fluorescent reporter in whole-mount retinas from Opn4Cre/+;Brainbow-1.0 mice. The fluorescent protein positive and negative populations were distributed evenly throughout the retina (Fig. 1d). Previously, five different subtypes of ipRGCs were identified by labeling with the Opn4Cre reporter system on the basis of their morphological properties (Ecker et al., 2010). One distinctive subtype, M4, has been described as having “alpha-like morphology” and has the largest soma size of all ipRGC subtypes, dendrites that stratify in the ON sublamina, and does not stain with the most sensitive antibody against melanopsin unless amplification techniques are used (Supplemental Fig. 1; Coombs et al., 2006; Ecker et al., 2010; Estevez et al., 2012; Lin et al., 2004). As described above, SMI-32 positive RGCs expressing the melanopsin reporter share all of these properties. This supports the hypothesis that ON alpha RGCs are the same cell population as those previously designated M4 ipRGCs.
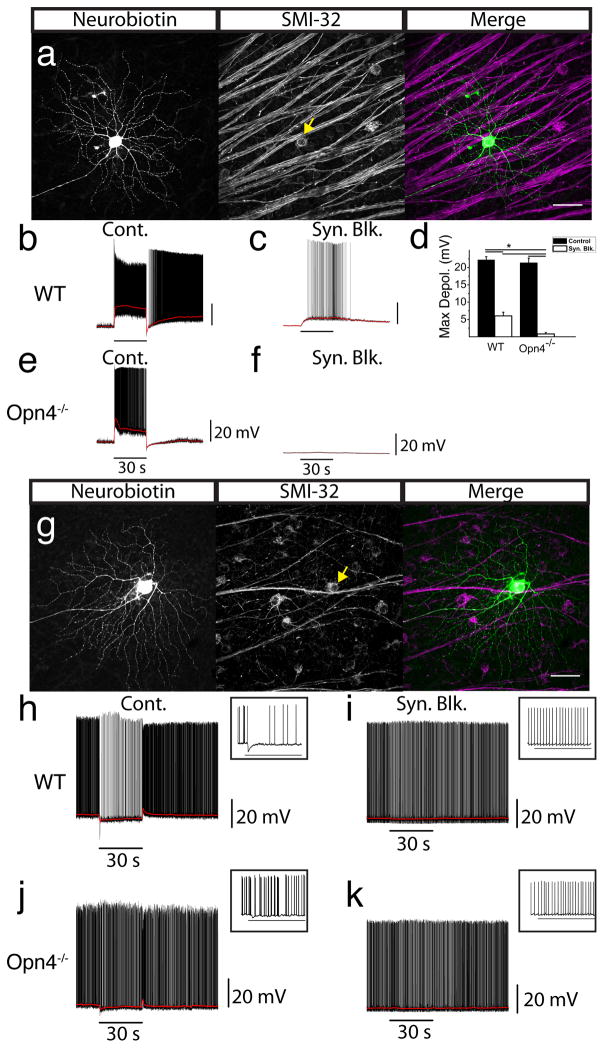
Though it has been shown that M4 ipRGCs are “alpha-like” (Ecker et al., 2010; Estevez et al., 2012), it remains unclear whether all ON alpha RGCs are intrinsically photosensitive. To directly test this, we performed whole cell recordings in wild type (WT) retinas from putative alpha RGCs randomly selected based on soma size (≥20 μm; Pang et al., 2003). We encountered both ON and OFF-center alpha RGCs, which responded with a fast depolarization or hyperpolarization, respectively, to a 30s bright, full-field 480 nm light stimulus (Fig. 2b,h). Following application of a cocktail of pharmacological agents to block synaptic transmission of rod and cone signals, ON (31/31; Fig. 2c), but not OFF (0/8; Fig. 2i) alpha RGCs responded intrinsically to light, showing that ON alpha RGCs are intrinsically photosensitive. We then filled each recorded cell with Neurobiotin and immunostained them for SMI-32. All recorded ON alpha RGCs were intrinsically photosensitive (n =31, Fig. 2a–c), had large dendritic field diameter (397.7 ± 51.77 μm, n = 8), large soma diameter (31.47 ± 3.73 μm, n = 8) and were positive for SMI-32 (Fig. 2a; Coombs et al., 2006; Lin et al., 2004). Importantly, ON RGCs that were not intrinsically photosensitive were SMI-32 negative (3/3) and had morphologies dissimilar to alpha RGCs. OFF alpha RGCs, in contrast, were all SMI-32 positive but none were intrinsically photosensitive (n = 8; Fig. 2g).
Fig. 2. ON, but not OFF, alpha cells are intrinsically photosensitive.
Cells with the largest soma sizes were randomly targeted for whole cell recordings. (a) Cell recorded in panel b filled with Neurobiotin (left panel) and coimmunostained for SMI-32 (middle panel). Merged image (right panel) shows colocalization of Neurobiotin (green) and SMI-32 (magenta). (b) Whole cell current clamp recording of light response from ON alpha RGC in WT retina. (c) Cell from b recorded in the presence of a cocktail of synaptic blockers. (d) Mean ± SEM maximum depolarization in ON alpha (SMI-32+) RGCs from WT (n = 8) and Opn4−/− (n = 5) mice under control conditions (black bars) and then in the presence of synaptic blockers (white bars). (e) Whole cell current clamp recording from ON alpha RGC in Opn4−/− retina. (f) Cell from e recorded in the presence of synaptic blockers. (g) Cell recorded from in panel h filled with Neurobiotin (left panel) and coimmunostained for SMI-32 (middle panel). Merged image (right panel) shows colocalization of Neurobiotin (green) and SMI-32 (magenta). (h) Whole cell current clamp recording from OFF alpha RGC in a WT mouse. (i) Cell from h recorded in the presence of a cocktail of synaptic blockers. (j) Whole cell current clamp recording from OFF alpha RGC in Opn4−/− retina. (k) Cell from j recorded in the presence of synaptic blockers. (a,g) Scale bars 50 μm. (b–e, h–k) All cells were stimulated with 30s, full-field, 480 nm stimulus. Horizontal bars indicate 30 s light stimulus. (h–k) Inset shows first ~3.5s of response following light onset. * indicates p < 0.01. RGC: retinal ganglion cell.
The onset of light responses of ON alpha cells were significantly slower (change in 10-90% RT = 18.9 ± 2.9 s, n = 8, t-test, p < 0.01; Fig. 2c) and smaller in the presence of synaptic blockers (maximum depolarization = 6.0 ± 1.1 mV, n = 8) compared to control conditions (maximum depolarization = 22.2 ± 0.9 mV n = 8, one-way ANOVA, p < 0.01; Fig. 2d). Furthermore, their membrane potential remained elevated throughout the duration of the recording, even minutes after light offset, with only 1/8 cells decaying to 37% of maximum within the recording period. These properties implicate melanopsin-phototransduction in driving these intrinsic responses.
To test whether the intrinsic light responses of ON alpha RGCs is melanopsin-dependent, we recorded from ON alpha RGCs in the retinas of Opn4−/− mice. Under control conditions, ON alpha RGCs in Opn4−/− mice responded to light (maximum depolarization = 21.3 ± 1.4 mV, n = 5) similarly to ON alpha cells in WT mice (maximum depolarization = 22.2 ± 0.9 mV, n = 8, one-way ANOVA, p > 0.05; Fig. 2d,e). However, in contrast to the intrinsic response of WT mice (6.0 ± 1.1 mV, n = 8; Fig. 2c), the light response of ON alpha RGCs was completely abolished in Opn4−/− mice upon application of synaptic blockers, (0.8 ± 0.4 mV, n = 5, one-way ANOVA, p < 0.01; Fig. 2d,f) showing that the intrinsic photosensitivity of ON alpha RGCs is melanopsin-dependent. The synaptic response of OFF alpha RGCs in Opn4−/− mice (n = 3) was similar to WT (Fig. 2h,j), and no intrinsic response was detected these cells (Fig. 2k). Each recorded ON and OFF cell in Opn4−/− mice was SMI-32 positive, indicating that we were sampling the same alpha RGC population in Opn4−/− retinas as in WT.
The ON alpha cells of the mouse show sustained rod/cone driven responses to light, which differ from the transient rod/cone-driven response of the classically identified cat ON alpha RGCs (Cleland and Levick, 1974). Therefore, we examined whether the ON alpha-like RGCs of the diurnal ground squirrel (Ictidomys tridecemlineatus), which has a functional melanopsin gene (Supplemental Fig. 2g; Provencio et al., 2000) are intrinsically photosensitive. We randomly selected RGCs with the largest somas, and confirmed ON alpha-like morphology, positive staining for SMI-32, and stratification in layer S4/S5 of the IPL by dye filling (Supplemental Fig. 2a–c,f; Linberg et al., 1996). ON alpha-like RGCs in squirrel responded to light with a transient inward current at light onset (Cleland et al., 1973; Jakiela and Enroth-Cugell, 1976; Supplemental Fig. 2d). Upon application of synaptic blockers, we found that ON alpha-like RGCs had a sluggish and sustained inward current resembling a melanopsin-mediated light response (Supplemental Fig. 2e; n = 4), which persisted throughout the duration of the 30s light stimulus and recovered slowly following stimulus offset (Supplemental Fig. 2e; n = 4). This indicates that the intrinsic photosensitivity of ON alpha-like RGCs exists in a diurnal mammal with a cone-dominated retina, and may thus be found across a variety of species.
Melanopsin influences ON alpha RGC signaling
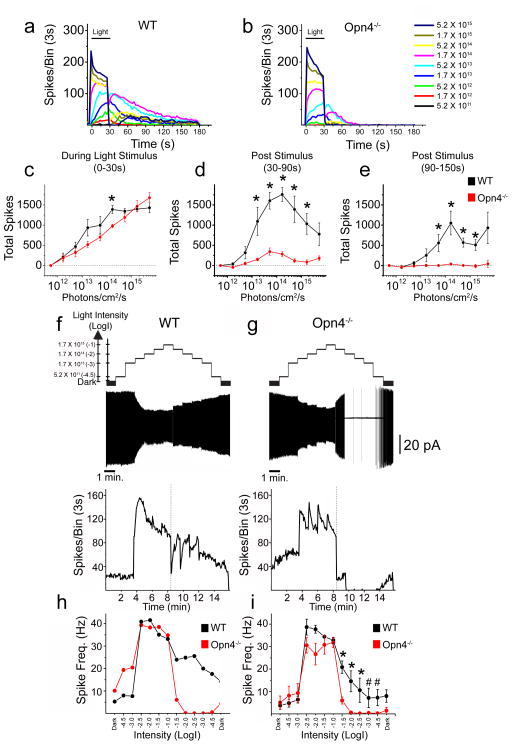
To determine whether the intrinsic, melanopsin-based photoresponse contributes physiologically to ON alpha RGC signaling, we compared responses of ON alpha RGCs of WT and Opn4−/− mice to 30s stimuli of increasing irradiance (Fig. 3a,b; Supplemental Fig. 3). In WT and Opn4−/− mice, ON alpha RGCs (n = 5 each) responded to increasing intensities of light with increasing spike frequencies during the 30s light stimulus, reaching maximum average firing levels of >40 Hz (Fig. 3a,c; Supplemental Fig. 3). However, a major difference between WT and Opn4−/− animals was observed following cessation of the light stimulus. 4/5 ON alpha RGCs in WT mice showed continued elevation of spike frequencies following stimulus offset (Fig. 3a; Supplemental Fig. 3), and were utilized for subsequent analyses. We found that the total number of spikes in the first 60s following stimulus offset in WT ON alpha RGCs was intensity dependent (Fig. 3a,d), reaching maximum spike output at 1.7 × 1014 photons/cm2/s (Fig. 3d). In contrast, cells in Opn4−/− mice showed markedly reduced levels of firing during this same period, though cells still showed slight increases in spike output between 5.2 × 1012 and 5.2 × 1013 photons/cm2/s (Fig. 3b,d). A dramatic difference between ON alpha cell responses in WT and Opn4−/− retinas was evident when we quantified the total number of spikes between 60 and 120 s following stimulus offset. As stimulus intensity increased, ON alpha RGCs continued to show steadily increasing post stimulus firing levels through 1.7 × 1014 photons/cm2/s in WT mice (Fig. 3e), while cells in Opn4−/− mice showed no appreciable increases (Fig. 3e). The fact that ON alpha cells signal irradiance even after the light stimulus has ceased enables ON alpha RGCs to keep a record of lighting history.
Fig. 3. Alpha RGCs signal long-term lighting history and irradiance.
ON alpha RGCs of WT and Opn4−/− mice were recorded extracellularly in loose patch or cell attached configuration. (a,b) Responses (spikes/3s bin) of WT (a) and Opn4−/− (b) ON alpha RGCs to increasing intensities of 480 nm light for 30s (horizontal black bar) at various intensities. (c) Mean ± SEM total spikes recorded during 30s light stimulus for ON alpha RGCs in WT (black, n = 4) and Opn4−/− (red, n = 5) retinas. (d) Mean ± SEM total spikes of ON alpha RGCs recorded 0–60s following light offset in WT (black) and Opn4−/− (red) retinas. (e) Mean ± SEM total spikes of ON alpha RGCs recorded 60–120s following light offset in WT (black) and Opn4−/− (red) retinas. (f,g) ON alpha RGCs were exposed to 1 minute steps of increasing and then decreasing irradiance from 5.2 × 1011 to 1.7 × 1015 photons/cm2/s. Top panels show representative examples of WT (f) and Opn4−/− (g) ON alpha cells exposed to this light stimulus. Bottom panels show spikes/3s bin of same WT (f) or Opn4−/− (g) cells on the same timescale. Dotted line indicates point at which light intensities began to decrease. (h) Spike frequency for single WT (black) and Opn4−/− (red) cells shown in f and g over 1 minute at each light intensity (x axis = Log(intensity)). (i) Mean ± SEM spike frequency (Hz) for WT (black, n = 4) and Opn4−/− (red, n = 4) ON alpha cells over 1 minute at each light intensity (x axis = Log(intensity)). Horizontal scale bar (f,g) represents 1 minute of recording time and vertical scale bar (f,g) represents 20pA. * p < 0.05, # p = 0.057 (Mann-Whitney U test).
As melanopsin is implicated in signaling ambient light intensity, we next recorded spike output from ON alpha RGCs in WT and Opn4−/− retinas as the intensity of a 480 nm background light was steadily increased and then decreased at 1 min intervals with no intervening dark (see supplemental methods, Fig. 3f,g; Barlow and Levick, 1969). We found that ON alpha RGCs in WT (n = 4) and Opn4−/− (n = 4) mice responded to the onset of illumination increases and were able to maintain elevated firing levels throughout the light stimulation (Fig. 3f,g). As the light intensity was stepped down, ON alpha RGCs in WT mice responded to decreases in luminance with a fast initial offset of spiking followed by a robust rebound in spiking that was maintained throughout the remainder of the light pulse (Fig. 3f, h–i). However, cells in Opn4−/− mice demonstrated complete cessation of firing by the second light decrement step (Fig. 3g–i). This repression was maintained throughout steps of decreasing intensity, consistent with the characteristics of an ON center RGC. Thus, while rods and cones mediate the response to sustained increases in illumination, melanopsin-mediated phototransduction enables ON alpha RGCs to signal ambient environmental irradiance regardless of whether the intensity has been increased or decreased.
Melanopsin is required for full contrast sensitivity
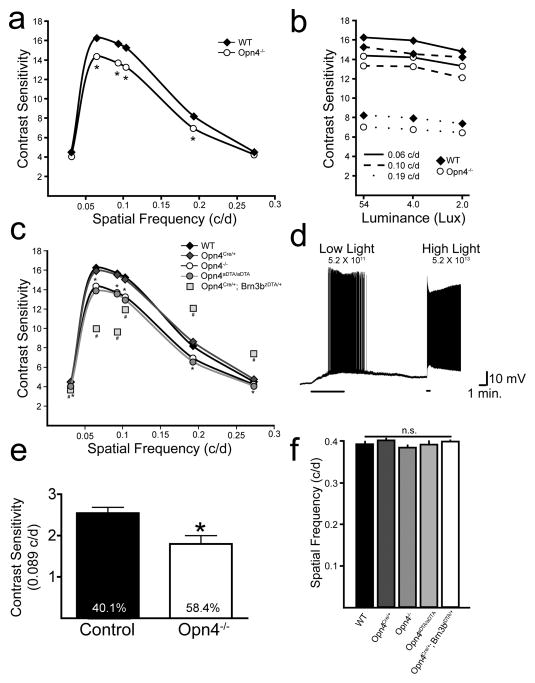
The contribution of melanopsin to the responses of ON alpha RGCs, which have high contrast sensitivity (Enroth-Cugell and Robson, 1966; Shapley and Victor, 1978; Zaghloul et al., 2003), raises the possibility that Opn4−/− animals could have deficits in image-forming functions. We used optokinetic tracking (OKT; Douglas et al., 2005; Prusky et al., 2004) as an assay to measure contrast sensitivity and spatial frequency thresholds in WT (N = 8) and Opn4−/− (N = 8) animals (Fig. 4a–b). Consistent with a role for melanopsin in alpha cell signaling, Opn4−/− mice showed deficits in contrast sensitivity when compared to WT mice (Fig. 4a). These were observed at the peak of the contrast sensitivity curve (0.064 c/d; one-way ANOVA, p < 0.001) as well as spatial frequencies of 0.092 c/d (one-way ANOVA; p < 0.001), 0.103 c/d (one-way ANOVA; p < 0.001) and 0.192 c/d (one-way ANOVA; p < 0.01; Fig. 4a). Reduced contrast sensitivity in Opn4−/− animals was also observed at various spatial frequencies as screen luminance was decreased from 54 Lux to 2 Lux (p < 0.05; Fig. 4b). Consistent with the behavioral contrast sensitivity deficits at low light intensities, we were able to record melanopsin-mediated responses at similar, low light intensities in ON alpha RGCs in the isolated, dark-adapted retina (5.2 × 1011 photons/cm2/s; n = 6/6 cells; Figure 4d). We also measured contrast sensitivity in control (n = 8) and Opn4−/− (n = 5) mice in a visual cortex dependent assay: the visual water task (VWT; Prusky and Douglas, 2004). At 0.089 c/d, Opn4−/− mice showed a significant reduction in contrast sensitivity compared to control mice (Fig 4e; t-test; p < 0.01), consistent with the OKT results. The reduction in visual function in Opn4−/− mice was specific to contrast sensitivity because spatial frequency thresholds in WT and Opn4−/− mice did not differ (one-way ANOVA, p > 0.05; Fig. 4f; (Guler et al., 2008). Importantly, these findings also demonstrate that RGCs (such as ON alpha RGCs or other ipRGCs) that do not project to the accessory optic system, which is necessary for OKT, are nonetheless capable of modulating the optokinetic reflex (Fig. 4a–c).
Fig. 4. Melanopsin phototransduction and non-M1 ipRGCs are involved in contrast detection.
(a) Contrast sensitivity plot of OKT response (each data point is average of both eyes; N = 8 mice) for WT and Opn4−/− animals. (b) Reduced contrast sensitivity in Opn4−/− mice relative to WT was maintained over a 25-fold decrease in luminance. *Opn4−/− mice showed significant deficits (p < 0.05; two-way ANOVA, Bonferroni post-hoc test) in contrast sensitivity relative to WT at all spatial frequencies and luminances tested. (c) Contrast sensitivity plot of OKT response (each data point is average of both eyes; N = 5–8 mice) for WT (replotted from a), Opn4−/− (replotted from a), Opn4Cre/+, Opn4aDTA/aDTA, and Opn4Cre/+; Brn3bzDTA/+ animals. *Opn4aDTA/aDTA mice differ from WT at each spatial frequency but they do not differ from Opn4−/− mice. #Opn4Cre/+; Brn3bzDTA/+ animals differ from Opn4Cre/+ at all spatial frequencies and differ from Opn4−/− mice at all spatial frequencies except the lowest (0.031; two-way ANOVA, Bonferroni post-hoc test, significance concluded when p < 0.05). (d) Intrinsic light response of dark-adapted ON alpha RGC recorded in current clamp mode in a cocktail of synaptic blockers for 5 minutes at dim light intensity comparable to the 2 lux stimulation used in b (5.2 × 1011 photons/cm2/s) and 30s at bright light (5.2 × 1013 photons/cm2/s). 6/6 dark-adapted ON alpha RGCs showed intrinsic light responses at the low light intensities. (e) Contrast sensitivity measured in the VWT from control and Opn4−/− mice at 0.089 c/d. The contrast threshold in %contrast is denoted inside the bar for both genotypes. (f) WT, Opn4−/−, Opn4Cre/+, Opn4aDTA/aDTA, and Opn4Cre/+; Brn3bzDTA/+ animals do not differ in spatial frequency thresholds for OKT response (one-way ANOVA). “Contrast Sensitivity” is defined as 100/[% Contrast at Threshold] (e.g. a threshold of 50% contrast would result in a contrast sensitivity of 2). Data in all graphs are plotted as Mean ± SEM, but SEM is too small to visualize when plotted on this scale in a–c. OKT: optokinetic tracking. VWT: visual water task.
To delineate the role of ipRGC subtypes in contrast sensitivity, we examined whether the ablation of M1 ipRGCs caused further deficits in contrast sensitivity using the Opn4aDTA/aDTA mice, which have normal ON alpha RGC distribution but lack M1 ipRGCs (Supplemental Fig. 4). Opn4aDTA/aDTA mice showed normal spatial frequency thresholds (one-way ANOVA, p > 0.05) and no deficits in contrast sensitivity beyond those observed in Opn4−/− animals (Fig. 4c,f). It is therefore unlikely that the contrast sensitivity deficits observed in Opn4−/− animals are due to changes in retinal dopamine release since M1 ipRGCs are the only subtype of ipRGC that form a plexus with the dopaminergic amacrine cells (Vugler et al., 2007).
ON alpha RGC deletion causes severe deficits in contrast sensitivity
To determine the contribution of the intrinsically photosensitive ON alpha RGCs themselves to contrast sensitivity, we utilized animals in which ON alpha RGCs are ablated (Opn4Cre/+; Brn3bzDTA/+; Chen et al., 2011). We found that SMI-32 positive cells were reduced by approximately 50% in these animals and those remaining were predominantly OFF stratified (t-test, p < 0.05; Supplemental Fig. 4 and data not shown). These animals also lack other non-M1 ipRGC subtypes as well as the M1 ipRGCs that project to the OPN (Chen et al., 2011). Importantly, the Opn4Cre/+; Brn3bzDTA/+ animals, which exhibit normal circadian photoentrainment, contain 200 remaining M1 ipRGCs that arborize with dopaminergic amacrine cells and still contain melanopsin (Chen et al., 2011). Opn4Cre/+; Brn3bzDTA/+ animals showed severe contrast sensitivity deficits beyond those observed in Opn4−/− animals at the contrast sensitivity curve peak (0.064, 0.092, and 0.103 c/d; Fig. 4c), indicating that non-M1 ipRGCs contribute to contrast sensitivity. As published previously, Opn4Cre/+; Brn3bzDTA/+ animals show normal spatial frequency thresholds (one-way ANOVA, p > 0.05; Fig. 4f; Chen et al., 2011), demonstrating the specificity of the non-M1 ipRGC contribution to contrast sensitivity. In addition, the number of choline acetyl transferase positive amacrine cells in both the ganglion cell and inner nuclear layers was similar between Opn4Cre/+; Brn3bzDTA/+ animals and control animals (Supplemental Fig. 4), indicating that these deficits are not due to defects in the direction selective circuitry. Interestingly, at higher spatial frequencies, Opn4Cre/+; Brn3bzDTA/+ animals had enhanced contrast sensitivity (Fig. 4c). The spatial frequencies where contrast sensitivity deficits were observed in Opn4Cre/+; Brn3bzDTA/+ mice match the spatial frequencies over which ON alpha RGCs respond to a sine wave grating stimulus in the isolated retina (Estevez et al., 2012), making it likely that the contrast sensitivity deficits in these animals arises as a result of ablation of the intrinsically photosensitive ON alpha RGCs.
DISCUSSION
ON alpha RGCs are intrinsically photosensitive and melanopsin signaling influences the physiological properties of these conventional RGCs. Opn4−/− mice show defects in contrast sensitivity in both a subcortical optokinetic tracking task and a visual cortex-dependent task, providing a behavioral role for melanopsin in image forming vision, even in the context of functional rods and cones. This decrease in contrast sensitivity in Opn4−/− mice does not occur through M1 ipRGCs because ablation of M1 ipRGCs results in no further contrast sensitivity deficits beyond those seen in melanopsin knockout animals. ON alpha RGCs were demonstrated to have high contrast sensitivity in vitro in other rodent models (Zaghloul et al., 2003), which suggests that melanopsin enhances behavioral contrast sensitivity at least in part through the ON alpha RGCs. Our data indicate that robust melanopsin responses can be driven in ON alpha RGCs at lower light intensities than previously appreciated, consistent with previous reports of melanopsin responses in ipRGCs at dim light (Wong, 2012), and consistent with a behavioral deficit in contrast sensitivity in mice lacking melanopsin at intensities of 2 lux (Fig. 4b).
The severe contrast sensitivity deficits observed in mice where all non-M1 ipRGCs are ablated provide the first behavioral function identified for non-M1 ipRGCs, and implicate this small population of ipRGCs in setting the thresholds for contrast detection. These results are consistent with recently identified non-M1 ipRGC connections to the dLGN in the mouse and primate (Dacey et al., 2005; Ecker et al., 2010; Estevez et al., 2012), as well as widespread melanopsin-derived light responses in 40% of light sensitive neurons in the dLGN of mice (Brown et al., 2010). Collectively, these results show that melanopsin expression in non-M1 cells is not simply an evolutionary remnant, but plays a crucial role in setting the threshold of contrast detection.
Experimental Procedures
Animals
All animals were handled in accordance with guidelines of the Animal Care and Use Committees of Johns Hopkins University, the University of Minnesota, the National Eye Institute or Cornell University. Specific details regarding strains used in each experiment as well as other methods can be found in Supplemental Experimental Procedures.
Electrophysiology
All electrophysiological analyses were performed as described previously (Schmidt and Kofuji, 2011; and see Supplemental Methods). Light stimulation was full field band-pass filtered 480 nm light of 1.66 × 1016 photons/cm2/s for mouse retina recordings and 1.94 × 1016 photons/cm2/s for ground squirrel retina recordings.
Immunohistochemistry
Immunohistochemical studies were performed as described previously (Li and DeVries, 2004; Schmidt and Kofuji, 2009).
Statistical Analyses
Statistical analyses were performed using Origin 7.5 (MicroCal) or GraphPad Prism. Statistical comparison of means was performed using a Student’s t test or one-way ANOVA with Tukey’s post hoc test or a Mann-Whitney U test and significance was concluded when p < 0.05. Data are presented as mean ± SEM.
Behavioral Analyses
Optokinetic tracking experiments were performed as described previously (Prusky et al., 2004; OptoMotry©, CerebralMechanics, Lethbridge, Alberta). The visual water task was performed essentially as described previously (Prusky et al., 2004; Prusky et al., 2000) but with printed instead of electronic grating stimuli.
Supplementary Material
HIGHLIGHTS.
All ON alpha RGCs, a conventional RGC type, are intrinsically photosensitive.
ON alpha RGCs signal irradiance and light history via melanopsin phototransduction.
Melanopsin phototransduction is required for contrast sensitivity.
Non-M1 ipRGCs influence contrast sensitivity necessary for image formation.
Acknowledgments
We would like to thank Drs. Marnie Halpern, Rejji Kuruvilla, Haiqing Zhao, Robert F. Miller, and Stewart Hendry for comments on the manuscript. We would also like to thank Dr. Cara Altimus, Ms. Diane Vig, and Mr. Nathan Schmidt for technical assistance. This work was funded by National Institutes of Health grants GM076430 (S.H.) and EY022543 (T.S.), the NIH Intramural Research Program (W.L.), and the Burke Foundation (G.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barlow HB, Levick WR. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969;202:699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wassle H. The morphological types of ganglion cells of the domestic cat’s retina. J Physiol. 1974;240:397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Levick WR. Brisk and sluggish concentrically organized ganglion cells in the cat’s retina. J Physiol. 1974;240:421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Levick WR, Sanderson KJ. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973;228:649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966;187:517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, Auferkorte ON, Demb JB, Berson DM. Form and function of the m4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J Neurosci. 2012;32:13608–13620. doi: 10.1523/JNEUROSCI.1422-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakiela HG, Enroth-Cugell C. Adaptation and dynamics in X-cells and Y-cells of the cat retina. Exp Brain Res. 1976;24:335–342. doi: 10.1007/BF00235001. [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nat Neurosci. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Linberg KA, Suemune S, Fisher SK. Retinal neurons of the California ground squirrel, Spermophilus beecheyi: a Golgi study. J Comp Neurol. 1996;365:173–216. doi: 10.1002/(SICI)1096-9861(19960205)365:2<173::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Characterization of mouse cortical spatial vision. Vision Res. 2004;44:3411–3418. doi: 10.1016/j.visres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res. 2000;40:2201–2209. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. An isolated retinal preparation to record light response from genetically labeled retinal ganglion cells. J Vis Exp. 2011 doi: 10.3791/2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Victor JD. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugler AA, Redgrave P, Semo M, Lawrence J, Greenwood J, Coffey PJ. Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Exp Neurol. 2007;205:26–35. doi: 10.1016/j.expneurol.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci. 2012;32:11478–11485. doi: 10.1523/JNEUROSCI.1423-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.