Abstract
The role of adrenal hexose-6-phosphate dehydrogenase in providing reducing equivalents to P450 cytochrome steroidogenic enzymes in the endoplasmic reticulum is uncertain. Hexose-6-phosphate dehydrogenase resides in the endoplasmic reticulum lumen and co-localizes with the bidirectional enzyme 11β-hydroxysteroid dehydrogenase 1. Hexose-6-phosphate dehydrogenase likely provides 11β-hydroxysteroid dehydrogenase 1 with NADPH electrons via channeling. Intracellularly, two compartmentalized reactions generate NADPH upon oxidation of glucose-6-phosphate: cytosolic glucose-6-phosphate dehydrogenase and microsomal hexose-6-phosphate dehydrogenase. Because some endoplasmic reticulum enzymes require an electron donor (NADPH), it is conceivable that hexose-6-phosphate dehydrogenase serves in this capacity for these pathways. Besides 11β-hydroxysteroid dehydrogenase 1, we examined whether hexose-6-phosphate dehydrogenase generates reduced pyridine nucleotide for pivotal adrenal microsomal P450 enzymes. 21-hydroxylase activity was increased with glucose-6-phosphate and, also, glucose and glucosamine-6-phosphate. The latter two substrates are only metabolized by hexose-6-phosphate dehydrogenase, indicating that requisite NADPH for 21-hydroxylase activity was not via glucose-6-phosphate dehydrogenase. Moreover, dihydroepiandrostenedione, a non-competitive inhibitor of glucose-6-phosphate dehydrogenase, but not hexose-6-phosphate dehydrogenase, did not curtail activation by glucose-6-phosphate. Finally, the most compelling observation was that the microsomal glucose-6-phosphate transport inhibitor, chlorogenic acid, blunted the activation by glucose-6-phosphate of both 21-hydroxylase and 17-hydroxylase indicating that luminal hexose-6-phosphate dehydrogenase can supply NADPH for these enzymes. Analogous kinetic observations were found with microsomal 17-hydroxylase. These findings indicate that hexose-6-phosphate dehydrogenase can be a source, but not exclusively so, of NADPH for several adrenal P450 enzymes in the steroid pathway. Although the reduced pyridine nucleotides are produced intra-luminally, these compounds may also slowly transverse the endoplasmic reticulum membrane by unknown mechanisms.
Keywords: Adrenal, Microsome, Hexose-6-phosphate dehydrogenase, Steroidogenesis
1. Introduction
There are at least two cellular sub-compartmentalized enzymes which, upon oxidation of glucose-6-phosphate (G6P), provide pyridine reducing equivalents (NADPH and NADH) for other downstream reactions: cytosolic glucose-6-phosphate dehydrogenase (G6PDH) and endoplasmic hexose-6-phosphate dehydrogenase (H6PDH). H6PDH has been localized to the lumen of the endoplasmic reticulum (ER), spatially and perhaps kinetically linked to 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) [1,2]. An elevated NADPH/NADP pyridine nucleotide redox ratio must be maintained for reductase activation of inert 11-oxosteroids. Although other NADPH-producing enzymes have been found within the ER, namely, malic enzyme and isocitrate dehydrogenase, neither appears to kinetically interact with 11β-HSD1 as does H6PDH [3,4].
H6PDH is a bifunctional enzyme kinetically linked with 11β-HSD1 that is also functionally related to the cytosolic G6PDH. However, unlike its cytosolic counterpart, H6DPH will metabolize a variety of other hexose substrates [5]. H6PDH not only metabolizes G6P, but also catalyzes the hydrolysis of 6-phosphogluconolactone, thereby serving as the first two enzymes of the oxidative segment of the pentose pathway [6].
11β-HSD1 is a bidirectional enzyme which has both reductase and dehydrogenase activities and is robustly expressed in liver, adrenal and adipose tissue [2,7]. The directional flux is determined by the mass action ratio of pyridine nucleotides [8]. These compounds exist in concentrations far exceeding the actual steroid reactants and dictate the net-flux interconversion of inactive ketosteroids (i.e., cortisone and dehydrocorticosterone) to their active hydroxy derivatives (cortisol and corticosterone). Nonetheless, other enzymes within the ER require reduced pyridine nucleotides, such as the P450 steroidogenic enzymes 17-hydroxylase (CYP17) and 21-hydroxylase (CYP21), functioning as monooxygenases to reduce molecular oxygen [9]. In the ER the NADPH electrons are transferred by the tandem of P450 oxidoreductase and allosteric cytochrome b5. CYP17 has been found in microsomes obtained from adrenal glands, liver, gonads and placenta [9,10] whereas CYP21 has been localized to microsomes in the adrenal cortex and kidney [11–13].
The nutrient G6P transverses the intact microsomal membrane by a specific G6P transporter [14–16]. G6P is the physiologic substrate for H6PDH as well as G6PDH, but there are several key kinetic distinctions. Only H6PDH, being not as specific as G6PDH, will oxidize other hexose phosphoesters such as glucosamine-6-phosphate (Gln-6-P) and 2-deoxyglucose-6-phosphate [1]. In addition, DHEA is a well recognized non-competitive inhibitor of hepatic G6PDH [17–21], but does not alter H6PDH [22,23]. While nearly all previous studies have explored the role of H6PDH as it pertains to its kinetic interaction with 11β-HSD1 in adipose and liver tissue, our studies extend to the adrenal glucocorticoid synthetic pathway.
Based on the experimental findings herein, H6PDH can provide NADPH for both 21- and 17-hydroxylase, although it remains uncertain to what extent insofar as other enzymatic avenues for NADPH provision co-exist.
2. Materials and methods
2.1. Materials
[3H]-17-hydroxyprogesterone and [3H]-progesterone were purchased from American Radiolabeled Chemicals (St. Louis, MO, USA). Other compounds and general chemicals were from Sigma–Aldrich (St. Louis, MO, USA). The G6PDH antibody (Rabbit anti human G6PDH) was from Santa Cruz Biotechnology (Dallas, TX).
2.2. Preparation of adrenal microsomes
All animal procedures were undertaken with approval and oversight of the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama Birmingham.
Microsomes were isolated from adrenals of Yorkshire pigs (30–40 kg body weight). Care was taken to excise cortex by visible inspection. All tissue samples were homogenized in an ice-bath with 4 vol. of 0.25 M sucrose and 50 mM Tris/HCl, pH 7.3. The homogenate was centrifuged for 10 min at 1000 g. The supernatant portion was removed and centrifuged for 20 min at 10,000 g. Thereafter, the subsequent supernatant was centrifuged for 60 min at 100,000 g. The resulting pellet was washed twice with the same buffer. The microsomes were resuspended at a protein concentration of 15–20 mg/ml (measured using the Bio-Rad protein assay). To shed cytosolic enzymes such as G6PDH or other contaminants which may possibly adhere to microsomal membranes, the microsomes were washed with polyethylene glycol (7% (w/v) final concentration) as previously described [3,24] and resuspended in MOPS–KCl buffer (100 mM KCl, 20 mM NaCl, 3.5 mM MgCl2 and 20 mM MOPS, pH 7.2). Intactness of microsomes was confirmed by using a method with glucose dehydrogenase [25].
2.3. Purification of glucose-6-phosphate dehydrogenase and effect of G6PDH antibody
G6PDH was purified according to the method previously described by Clarke et al. [6]. Porcine adrenal gland cells were solubilized and centrifuged to remove mitochondria. The cytosolic supernatant was then applied to a 2′,5′-ADP-sepharose column. The G6PDH was eluted with a washing buffer that included 50 μM NADP.
In some experiments, G6PDH antibody (Santa Cruz Biotechnology, Dallas, TX) was incubated at a protein concentration of 10 μg/ml for 20 min at 37 °C in MOPS–KCl buffer (pH 7.2) with purified G6PDH or adrenal microsomes before specific enzyme assays.
2.4. Measurement of microsomal NADPH production
A sensitive micro-method was adopted to assay NADPH through the conversion of radio-labeled α-ketoglutarate to glutamate [26]. Stoichiometrically, the oxidative deamination of 1 mol of glutamate generates 1 mol of NADPH (or NADH) — the same stoichiometric relationship holds true for the reverse reaction. Ten microliters of microsomes (1 mg/ml) was pre-incubated for 15 min in MOPS–KCl buffer (100 mM KCl, 20 mM NaCl, 3.5 mM MgCl2 and 20 mM MOPS, pH 7.2). The reaction was initiated with 90 μl of 50 mM HEPES– NaOH buffer (pH 7.4) reaction mixture (containing 50 mM ammonium acetate,1 mM ADP, 0.5 μCi [14C]-α-ketoglutarate, 0.8 μM α-ketoglutarate, 12 Units/ml glutamate dehydrogenase, 12 Units/ml G6PDH). After incubating for 30 min at 37 °C, the reactions were arrested with cold water and 2 M HCl. To separate the unreacted [14C]-α-ketoglutarate, samples were placed on anion exchange Dowex-50 columns. The reaction product was eluted with 3 mL of 2 M NH4OH; the [14C]-glutamate was quantified by a scintillation counter.
2.5. Measurement of G6DPH with various phosphoesters
G6PD activity was measured spectrophotometrically by monitoring G6P dependent NADP reduction at 340 nm. The reaction was initiated by adding 1 mM G6P into an enzyme solution containing 1 mM NADP in Tris–HCl buffer, pH8. The reaction was linear over 10 min at room temperature. The protein concentration in the reaction was 50–60 μg/ml [6]. One international unit of G6PD activity is defined as the reduction of 1 μmol NADP/min.
2.6. Measurement of 21-hydroxylase and 17-hydroxylase activities
The microsomal 21-hydroxylase and 17-hydroxylase activities were assayed by measuring conversion of 17-hydroxyprogesterone (17OH-P) to 11-deoxycortisol and the loss of radiolabeled progesterone to products, respectively. As for microsomal 21-hydroxylase, insofar as the terminal step to cortisol occurs in the mitochondria, nearly all the converted [3H]-17 hydroxyprogesterone appears in the 11-deoxycortisol band. As for 17-hyroxylase, besides promoting 21-carbon cortisol flux, it also generates 19-carbon androgen precursors through its intrinsic lyase activity. Consequently, the absolute loss of radiolabeled progesterone, rather than assaying all the products, best represents 17-hydroxylase activity. Parenthetically, approximately 90–95% of the [3H]-progesterone substrate was converted to the combined radioactivity found in 17OH-P and 11-deoxycortisol. This is consistent with previous experiments using porcine adrenal microsomes wherein CYP17 exhibits proportionally modest lyase activity vs. robust 17-hydroxylation [27].
The reactions were conducted at 37 °C for 15 min in MOPS–KCl buffer (pH 7.2) containing 1 mM NADP+, 5 μM 17-hydroxyprogesterone and 0.2 μCi/ml [3H]-17-hydroxyprogesterone (21-hydroxylase assay), or 5 μM progesterone and 0.2 μCi/ml [3H]-progesterone (17-hydroxylase assay). The reaction was started by the addition of microsomes (0.5 mg protein/ml) and terminated by submerging the tubes in ice and simultaneously adding three volumes of ethyl acetate (21-hydroxylase assay) or nine volumes of dichloromethane (17-hydroxylase assay). Steroids were then extracted and separated on silicon-coated thin layer chromatography plates (Whatman Inc., Piscataway, NJ) using chloroform:ethanol (95:5) as solvent system for 21-hydroxylase measurement or chloroform:ethyl acetate (4:1) for 17-hydroxylase measurement. Results are expressed as pmol/mg protein/min. For the experiments in which DHEA was added, it was dissolved in DMSO 0.1% (v/v).
2.7. Statistical analysis
Data are presented as mean ± SD (n). The significance of differences between groups was determined by Student's t-test and analysis of variance. P < 0.05 was considered significant. All statistical analyses were performed with GraphPad Prism software (San Diego, CA, USA).
3. Results
3.1. Effect of G6P on NADPH production by intact adrenal microsomes
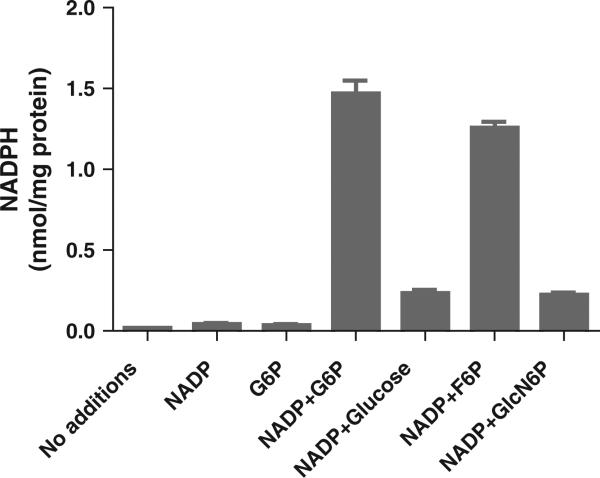
For 15 min, isolated intact adrenal microsomes were incubated without and with G6P and/or NADP; thereafter, NADPH was measured. In the absence of either metabolite (control), NADPH production was negligible. With NADP, a trace amount of NADPH was detected. When 1 mM NADP and 1 mM G6P were combined, NADPH production rose to 1.47 nmol/mg protein (Fig. 1). To a far lesser extent, high (600 mM) glucose concentration likewise increased microsomal NADPH.
Fig. 1.
Effect of G6P and high glucose concentration on NADPH production by intact adrenal microsomes. Isolated intact adrenal microsomes were incubated according to the methods for 15 min as follows: no additions (control), 1 mM NADP, 1 mM G6P, 1 mM NADP + 1 mM G6P, 1 mM NADP + 600 mM glucose, 1 mM NADP + 1 mM F6P, 1 mM NADP + 6.6 mM GlcN6P. Results are given as a mean ± SD, n = 3. Statistical differences were by unpaired t test (NADP versus NADP + G6P or versus NADP + glucose or NADP + F6P or NADP + GlcN6P were all p < .001). There was no statistical difference between the control, NADP or G6P alone groups.
3.2. Purified adrenal glucose-6-phosphate dehydrogenase activity
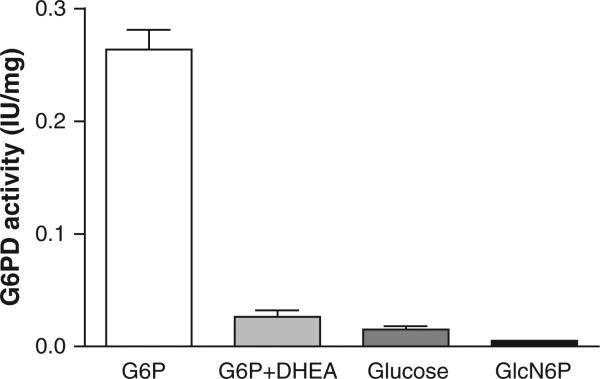
Purified porcine adrenal glucose-6-phosphate dehydrogenase activity was measured with various substrates. Whereas activity was brisk with 1 mM G6P (0.26 IU/mg protein) it was 90% inhibited by DHEA (100 μM). Glucose, on the other hand, only marginally activated G6PDH (6% compared to G6P) while Gln-6-P (6.6 mM) was in essence inert (Fig. 2). These experiments employed 600 mM glucose based on the high Km of H6DPH for glucose and near zero activation was anticipated below 10 mM [1,28].
Fig. 2.
Purified adrenal glucose-6-phosphate dehydrogenase activity: effect of various substrates. Purified porcine adrenal glucose-6-phosphate dehydrogenase activity was measured as per methods using the following substrates/modifiers: G6P (1 mM), G6P (1 mM) + DHEA (100 μM), glucose (600 mM) or glucoseamine-6-phosphate (Gln-6-P) (6.6 mM). All incubations included 1 mM NADP. Results are presented as a mean ± SD, n = 4. All statistical comparisons (by unpaired t test) versus the G6P group were signifi-cant at p < .001.
3.3. Effect of various hexose phosphoester substrates on 21-hydroxylase and 17-hydroxylase activity
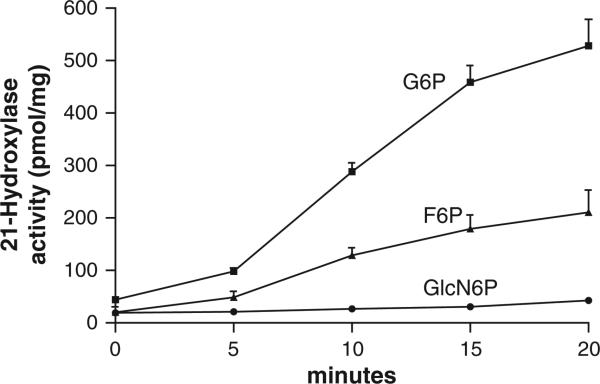
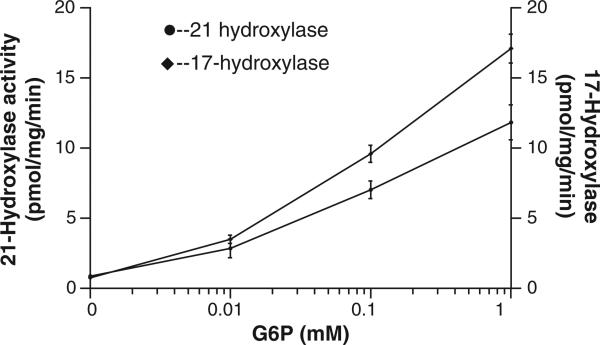
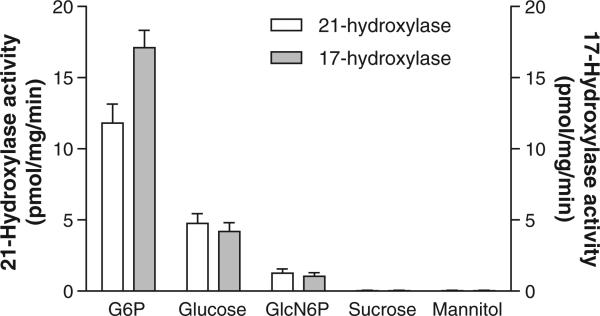
NADPH is required for the conversion of [3H]-17-OH progesterone to [3H]-11-deoxycortisol. Fig. 3 shows that F6P and G6P served as excellent substrates, increasing [3H]-11-deoxycortisol production 10 and 12 fold, respectively. This finding corroborates earlier evidence by our lab [29] and others [30] that both of these hexose-phosphoesters can stimulate 11βHSD1. In the case of F6P, the stimulation is indirect, that is, via conversion to G6P by microsomal isomerase [30]. Gln-6-P (6.6 mM), in contrast, increased production by 2 fold, suggesting that cytosolic or membrane adherent G6PDH is unlikely providing NAPDH in adrenal ER (i.e. the purified enzyme had negligible activity with Gln-6-P as a substrate per Fig. 2). The activities of 21-hydroxylase and 17-hydroxylase were directly related to G6P concentration (Fig. 4). Glucose at 600 mM stimulated both enzymes to a small degree at this high, non-physiologic concentration (Fig. 5). To rule out a non-specific osmotic effect, both sucrose and mannitol were also tested [25]. At 600 mM, both compounds were ineffective in modifying both 21- and 17-hydroxylases activities.
Fig. 3.
Effect of hexose phosphate substrates on 21 hydroxylase activity. Isolated intact adrenal microsomes were incubated with either 1 mM G6P, 1 mM F6P or 6.6 mM glucoseamine-6-phosphate (GlcN6P) and 21-hyroxylase activity was measured over 20 min in the presence of 1 mM NADP. In each experiment, NADP was at 1 mM. Results are expressed as a rate (pmol/mg) mean ± SD, n = 4.
Fig. 4.
21-Hydroxylase and 17-hydroxylase activity in porcine adrenal microsomes. Isolated intact adrenal microsomes were incubated for 15 min with increasing concentrations of G6P and 21-hydroxylase and 17-hydroxylase activities measured as per methods with 1 mM NADP. Results are presented as mean ± SD, n = 4.
Fig. 5.
Effect of various substrates and osmotic agents on 21-hydroxylase and 17-hydroxylase activity in porcine adrenal microsomes. Isolated intact adrenal microsomes were incubated for 15 min with G6P (1 mM), glucose (600 mM), GlucN6P (6.6 mM), sucrose (600 mM) and mannitol (600 mM) and the activities of 21-hydroxylase and 17-hydroxylase measured as per methods with 1 mM NADP. Results are given as mean ± SD, n = 4.
3.4. DHEA inhibition of 21-hyroxylase activity
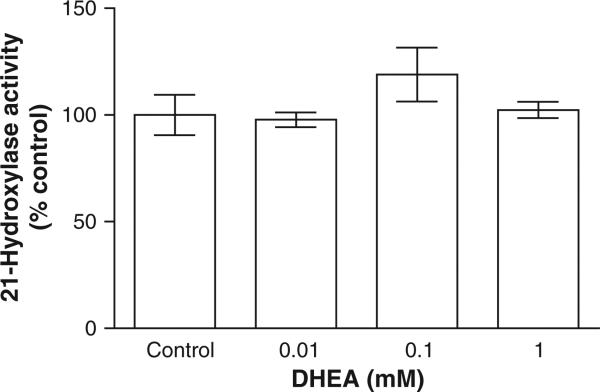
When DHEA – a known non-competitive G6PDH inhibitor – was added at up to 1 mM, the identical concentration as G6P, there was no inhibition detected (Fig. 6). Insofar as DHEA at 0.1 mM profoundly reduced partially purified G6PDH activity (see Fig. 2), the preservation of G6P activation of 21-hydroxylase despite the presence of 1 mM DHEA supports H6PDH as a provider of NADPH, not cytosolic G6PDH. Moreover, polyclonal anti-G6PDH inhibited purified adrenal G6P activity by 70–85%, yet had no effect on the activation of 21-hydroxylase by G6P when added to the intact microsomal experiments (data not shown). If residual cytosolic G6PDH remained, despite washing the microsomes, the addition of the anti-G6PDH should have attenuated, even to a limited degree, the G6P activation of 21-hydroxylase — evidence again that G6PDH provided the obligate NADPH.
Fig. 6.
Effect of dihydroepiandrostenedione (DHEA) on 21-hydroxylase activity in adrenal microsomes. Isolated intact adrenal microsomes were incubated for 15 min with 1 mM G6P, 1 mM NADP and between 0 and 1 mM DHEA (an inhibitor of G6PDH) according to methods. Results are given as a mean ± SD, n = 4. There were no statistically significant differences.
3.5. Effect of inhibition of G6P microsomal transport on the activation of 21- and 17-hyroxylase activities
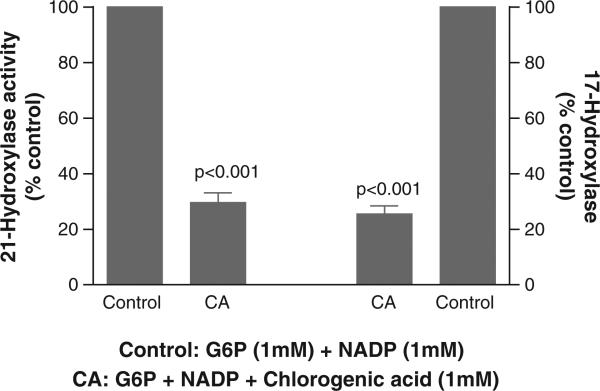
When chlorogenic acid at 1 mM was added to the 15 minute reaction, there was, respectively, a 70% and 75% reduction in the G6P stimulation of 21-hydroxylase and 17- hydroxylase (Fig. 7). Because of the ER luminal orientation of H6PDH, which is fully dependent on the transport of the substrate G6P, this data again reinforces the capability of H6PDH to supply NADPH for these two P450 cytochrome enzymes. Considering the possibility that 1 mM chlorogenic acid could directly inhibit these enzymes, they were assayed as previously described except the nucleotide NADP was replaced by NADPH, thereby eschewing the need for the G6P transporter-H6PDH tandem to generate NADPH and the microsomes were rendered permeable. Under these assay conditions using CHAPSO (a zwitter ionic, nondenaturing detergent) permeabilized microsomes, with NADPH but without G6P, chlorogenic acid (CA) did not alter the activity of either CYP enzymes. Compared to basal rates (=100%, no added CA), the effect of added CA on 21-hydroxylase activity was 96%, 99.9% and 97% of basal with 0.1 mM, 0.5 mM and 1 mM CA, respectively. In sum, CA did not directly alter the activity of either CYP enzyme.
Fig. 7.
Effect of chlorogenic acid (CA), an inhibitor of microsomal G6P transport, on G6P-stimulated 21- and 17-hyroxylase activities. Isolated intact adrenal microsomes were incubated for 15 min. The control group included 1 mM G6P and 1 mM NADP. The CA group contained 1 mM chlorogenic acid (CA) with 1 mM G6P and 1 mM NADP. Both 21-hydroxylase and 17-hydroxylase activities were measured as per methods. Results are expressed relative to the control for each enzyme and as a mean ± SD.
4. Discussion
The role of adrenal hexose 6-phosphate dehydrogenase in providing reducing equivalents to P450 cytochrome steroidogenic enzymes in the endoplasmic reticulum (ER) is uncertain. Insofar as numerous ER enzymes require an electron donor (NADPH), it is conceivable that H6DPH serves in that capacity for several steroid pathways.
H6PDH resides in the ER lumen and co-localizes with the bidirectional enzyme 11β-HSD1. Given this spatial protein-to-protein interaction with 11β-HSD1, it is likely that H6PDH provides 11β-HSD1 with NADPH electrons via substrate channeling. The enzymatic source(s) providing reducing equivalents for microsomal steroid-synthesizing P450 enzymes is ill-defined. Hexose-6-phosphate dehydrogenase has been found throughout adrenal tissue [22]. Previous studies have convincingly demonstrated that H6PDH is likely the major source of reduced pyridine nucleotides for 11β-HSD1 within the ER lumen of liver and fat [4,7,31,32]. However, few studies have explored its role in adrenal tissue, particularly as pertains to disparate P450 steroidogenic enzymes.
In adrenocortical cells, reduced pyridine nucleotides generated from enzymatic conversion of G6P can be provided by either microsomal H6PDH or cytosolic G6PDH. In our enzyme studies, any residual G6PDH not initially eliminated in the preparation of the washed micro-somes, yet possibly adherent to the outer ER membrane, was expunged by polyethylene glycol washes. The activity of adrenal 21-hydroxylase and 17-hydroxylase was assayed using G6P, Gln-6-P, and glucose as substrates. There was significant increase in enzymatic activity with each. H6PDH can metabolize glucose, but only at very high non-physiologic concentrations given its Km [28,33]. Indeed, at 600 mM, glucose stimulated both 21- and 17-hydroxylases. This was not due to a non-specific osmotic effect because neither 600 mM mannitol nor sucrose modified enzyme activity.
In microsomes prepared from H6DPH knockout mice, only 11β-HSD1 dehydrogenase activity – as opposed to reductase – was detected with consequent impaired active steroid recovery [34]. If G6PDH was the primary source of NADPH for reductase activity for 11β-HSD1 in peripheral tissues, or for steroid synthesis in adrenal tissue, there would be no activation of the hypothalamic–pituitary–adrenal (HPA) axis. Yet H6PDH deficient mice have glucocorticoid deficiency with a corresponding increase in the HPA axis, as evidence by increased circulating ACTH [35,36]. H6PDH may serve a pivotal role in not only supplying NADPH for co-localized luminal 11β-HSD1, but also, under certain conditions, for the provision of electrons to other key P450 enzymes within adrenal microsomes.
To further corroborate that the NADPH was generated by H6DPH rather than G6DPH, the specific enzymatic inhibition by DHEA of the latter was explored [22,23]. DHEA is a well recognized inhibitor of G6PDH [18,20,21]; for example, at only 0.05 mM, DHEA inhibits hepatic G6PDH in vitro Vmax activity by nearly 75%[22,23]. Results from our study confirm inhibition of purified porcine adrenal G6PDH with DHEA (an 89% inhibition at 1 mM DHEA). Moreover, using 10 mM G6P as substrate, a previous study showed 95% inhibition of G6PDH by DHEA using adrenal cortex tissue [37]. These inhibition results are in stark contrast to our findings in intact microsomes: no attenuation of the G6P activation of CYP21 and CYP17 by a high concentration of DHEA. Also, using intact microsomes, the addition of anti-G6PDH antibody to the assay did not alter the G6P stimulation of 21-hydroxylase.
Finally, the possible role of H6PDH in the provision of NADPH for two ER enzymes is supported by the effect of G6P transport inhibition. If indeed cytosolic G6PDH is, as previously opined, the sole provider of reduced pyridine nucleotides, then chlorogenic acid should have had a nugatory effect on the activation by G6P of the P450 enzymes — this was certainly not the case. The observation that the G6P transport inhibitor, chlorogenic acid (CA), significantly blunted the activation of both CYP21 and CYP17 by G6P corroborates that H6PDH can be an auxiliary enzyme for NADPH production. And, of note, CA did not directly inhibit either enzyme when NADPH replaced NADP in the absence of G6P in permeabilized microsomes.
As is the case with most cytochrome P450 enzymes, the topo-graphical orientation of 21-hydroxylase has a cytosolic orientation [38,39]. This topology is antithetical to that of 11β-HSD1 wherein its N-terminal transmembrane domain begets a luminal orientation of the catalytic moiety [40,41]. This enzyme depends on H6PDH as its NADPH source to promote reductase over dehydrogenase activity. Therefore, at present, it is hard to reconcile the ER luminal locus of H6PDH, the ensuing luminal production of NADPH following the ingress of G6P, and the eventual activation of a cytosolic oriented cytochrome P450 enzyme. Nevertheless, it is not unprecedented that some ER enzymes may have a dual membrane orientation, as in the case with diacyglycerol acyltransferase I and some oligomeric enzymes [42].
Furthermore, for 21-hydroxylase, based on rotational diffusion studies and other methods, using protease digestion, the anchoring of the enzyme to the ER membrane appears to be unusually deeply embedded [43,44]. Does this atypical membrane attachment dispose or facilitate luminal pyridine nucleotide contact with CYP21? Or, perhaps, do pyridine nucleotides cross the ER membrane, albeit in a limited fashion? Over brief periods (<10–15 min) of incubation, these nucleotides appear to be impermeant to the microsomal membrane, whereas with more prolonged incubations, and with membranes proven to be still intact, there is clearly a slow penetrance of NADPH [45–49]. Many experimental observations from unrelated studies can be adduced wherein pyridine nucleotides unmistakably gain access to the microsomal lumen, even in the absence of detergents or permeabilizing agents. These studies involve primarily microsomes from rat liver, but similar data is found in HEK-293 cell microsomes [32]. Based on measurements of accumulated radioactivity following incubation of liver microsomes and radio labeled G6P, it was concluded that NADPH can slowly penetrate the membranes [45]. In humans with severe G6PDH deficiency, and despite a basal mean adrenal enzyme activity 13% of normal [50], there is no evidence of impaired cortisol synthesis or clearance. Compared to 43 controls, 10 subjects with G6PDH enzyme deficiency had normal basal and ACTH-stimulated serum concentrations. This data suggests, at least in humans, alternative sources of the requisite NADPH for steroid synthesis, either via the oxidative decarboxylation of malate, isocitrate dehydrogenase or endoplasmic H6PDH. Another possibility which has been proposed is that there is an ER membrane electron shuttle, yet to be discovered, much like what occurs in mitochondria. Of comparable weight, a transporter for ATP exists in the ER [51].
5. Conclusions
In conclusion, in intact microsomes, H6DPH can provide the reducing electrons for at least two adrenal P450 enzymes involved in glucocorticoid synthesis. Several findings buttress this premise. Firstly, unique phosphoesters and high concentrations of glucose, which are not (or minimally) utilized by cytosolic G6PDH, increase the enzyme activity of 21-hydroxylase and 17-hydroxylase. Secondly, upon addition of G6P to intact microsomes, no curtailment of G6P-stimulation of CYP21 and CYP17 was noted with a G6PDH inhibitor, DHEA. Thirdly, upon blocking the ingress of G6P in intact microsomes, the stimulation of both CYP21 and CYP17 was substantially curbed. In sum, H6PDH, besides its kinetic interaction with 11β-HSD1, may play a role in electron transfer for multiple P450 steroidogenic enzymes.
Abbreviations
- H6PDH
hexose-6-phosphate dehydrogenase
- G6PDH
glucose-6-phosphate dehydrogenase
- ER
endoplasmic reticulum
- 11β-HSD1
11 beta hydroxysteroid dehydrogenase 1
- CYP21
21-hydroxylase
- CYP17
17-hydroxylase
- G6P
glucose-6-phosphate
- F6P
fructose-6-phosphate
- Gln-6-P
glucosamine-6-phosphate
- DHEA
dihydroepiandrostenedione
References
- 1.Beutler E, Morrison M. J. Biol. Chem. 1967;242:5289–5293. [PubMed] [Google Scholar]
- 2.Senesil S, Csala M, Marcolongo P, Fulceri1 R, Mandl J, Banhegyi1 G, Benedetti1 A. Biol. Chem. 2010;391:1–8. doi: 10.1515/BC.2009.146. [DOI] [PubMed] [Google Scholar]
- 3.Margittai É, Bánhegyi G. Arch. Biochem. Biophys. 2008;471:184–190. doi: 10.1016/j.abb.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Mick GJ, Maser E, McCormick K. Biochem. J. 2011;437:109–115. doi: 10.1042/BJ20102069. [DOI] [PubMed] [Google Scholar]
- 5.Marcolongo P, Senesi S, Guinti R, Csala M, Fulceri R, Bánhegyi G, Benedetti A. J. Steroid Biochem. Mol. Biol. 2011;126:57–64. doi: 10.1016/j.jsbmb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Clarke JL, Mason PJ. Arch. Biochem. Biophys. 2003;415:229–234. doi: 10.1016/s0003-9861(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt KN, Walker EA, Stewart PM. Endocrinology. 2005;146:2539–2543. doi: 10.1210/en.2005-0117. [DOI] [PubMed] [Google Scholar]
- 8.McCormick KL, Wang X, Mick GJ. J. Biol. Chem. 2006;281:341–347. doi: 10.1074/jbc.M506026200. [DOI] [PubMed] [Google Scholar]
- 9.Payne AH, Hales DB. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 10.Yanase T, Kagimoto M, Suzuki S, Hashiba K, Simpson ER, Waterman MR. J. Biol. Chem. 1989;264:18076–18082. [PubMed] [Google Scholar]
- 11.Sasano H, White PC, New MI, Sasano N. Endocrinology. 1988;122:291–295. doi: 10.1210/endo-122-1-291. [DOI] [PubMed] [Google Scholar]
- 12.Oliw EH, Kinn AC, Kvist U. J. Biol. Chem. 1988;263:7222–7227. [PubMed] [Google Scholar]
- 13.Griffing GT, Holbrook M, Melby JC, Alberta J, Orme-Johnson NR. Am. J. Med. Sci. 1989;298:83–88. doi: 10.1097/00000441-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Fulceri R, Bellomo G, Gamberucci A, Scott HM, Burchell A, Benedetti A, Biochem J. 1992;286:813–817. doi: 10.1042/bj2860813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster JD, Nordlie RC. Exp. Biol. Med. (Maywood) 2002;227:601–608. doi: 10.1177/153537020222700807. [DOI] [PubMed] [Google Scholar]
- 16.Marcolongo P, Fulceri R, Giunti R, Margittai E, Banhegyi G, Benedetti A. FEBS Lett. 2012;586:3354–3359. doi: 10.1016/j.febslet.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki Y, Honda A. Curr. Pharm. Des. 2006;12:3411–3421. doi: 10.2174/138161206778194015. [DOI] [PubMed] [Google Scholar]
- 18.Frolova AI, O'Neill K, Moley KH. Mol. Endocrinol. 2011;25:1444–1455. doi: 10.1210/me.2011-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apostolova G, Schweizer RA, Balazs Z, Kostadinova RM, Odermatt A. Am. J. Physiol. Endocrinol. Metab. 2005;288:E957–E964. doi: 10.1152/ajpendo.00442.2004. [DOI] [PubMed] [Google Scholar]
- 20.Gordon G, Mackow MC, Levy HR. Arch. Biochem. Biophys. 1995;318:25–29. doi: 10.1006/abbi.1995.1199. [DOI] [PubMed] [Google Scholar]
- 21.Marks PA, Banks J. Proc. Natl. Acad. Sci. U. S. A. 1960;46:447–452. doi: 10.1073/pnas.46.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandula B, Srivastava SK, Beutler E. Arch. Biochem. Biophys. 1970;141:155–161. doi: 10.1016/0003-9861(70)90118-9. [DOI] [PubMed] [Google Scholar]
- 23.Oka K, Takahashi T, Hori SH. Biochim. Biophys. Acta. 1981;662:318–325. doi: 10.1016/0005-2744(81)90045-0. [DOI] [PubMed] [Google Scholar]
- 24.Yang MX, Cederbaum AI. Arch. Biochem. Biophys. 1994;315:438–444. doi: 10.1006/abbi.1994.1522. [DOI] [PubMed] [Google Scholar]
- 25.Bublitz C, Steavenson S. Biochim. Biophys. Acta. 1988;965:90–92. doi: 10.1016/0304-4165(88)90155-9. [DOI] [PubMed] [Google Scholar]
- 26.Sener A, Malaisse WJ. Anal. Biochem. 1990;186:236–242. doi: 10.1016/0003-2697(90)90073-i. [DOI] [PubMed] [Google Scholar]
- 27.Yanagibashi K, Hall PF. J. Biol. Chem. 1986;261:8429–8433. [PubMed] [Google Scholar]
- 28.Brink NG. Acta Chem. Scand. 1953;7:1081–1089. [Google Scholar]
- 29.McCormick KL, Wang X, Mick GJ, Steroid Biochem J. Mol. Biol. 2008;111:18–23. doi: 10.1016/j.jsbmb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Senesi S, Legeza B, Balazs Z, Csala M, Marcolongo P, Kereszturi E, Szelenyi P, Egger C, Fulceri R, Mandl J, Giunti R, Odermatt A, Banhegyi G, Benedetti A. Endocrinology. 2010;151:4830–4839. doi: 10.1210/en.2010-0614. [DOI] [PubMed] [Google Scholar]
- 31.Czegle I, Piccirella S, Senesi S, Csala M, Mandl J, Banhegyi G, Fulceri R, Benedetti A. Mol. Cell. Endocrinol. 2006;248:24–25. doi: 10.1016/j.mce.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Dzyakanchuk AA, Balazs Z, Nashev LG, Amrein KE, Odermatt A. Mol. Cell. Endocrinol. 2009;301:137–141. doi: 10.1016/j.mce.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Hino Y, Minakami S. J. Biol. Chem. 1982;257:2563–2568. [PubMed] [Google Scholar]
- 34.Zielinska AE, Walker EA, Stewart PM, Lavery GG. Mol. Cell. Endocrinol. 2011;336:213–218. doi: 10.1016/j.mce.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Rogoff D, Ryder JW, Black K, Yan Z, Burgess SC, McMillan DR, White PC. Endocrinology. 2007;148:5072–5080. doi: 10.1210/en.2007-0593. [DOI] [PubMed] [Google Scholar]
- 36.Lavery GG, Walker EA, Draper N, Jeyasuria P, Marcos J, Shackleton CH, Parker KL, White PC, Stewart PM. J. Biol. Chem. 2006;281:6546–6551. doi: 10.1074/jbc.M512635200. [DOI] [PubMed] [Google Scholar]
- 37.Frederiks WM, Kummerlin IP, Bosch KS, Vreeling-Sindelarova H, Jonker A, Van Noorden CJ. J. Histochem. Cytochem. 2007;55:975–980. doi: 10.1369/jhc.7A7222.2007. [DOI] [PubMed] [Google Scholar]
- 38.Black SD. FASEB J. 1992;6:680–685. doi: 10.1096/fasebj.6.2.1537456. [DOI] [PubMed] [Google Scholar]
- 39.Monier S, VanLuc P, Kreibich G, Sabatini DD, Adesnik M, Cell Biol J. 1988;107:457–470. doi: 10.1083/jcb.107.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frick C, Atanasov AG, Arnold P, Ozols J, Odermatt A. J. Biol. Chem. 2004;279:31131–31138. doi: 10.1074/jbc.M313666200. [DOI] [PubMed] [Google Scholar]
- 41.Mziaut H, Korza G, Hand AR, Gerard C, Ozols J. J. Biol. Chem. 1999;274:14122–14129. doi: 10.1074/jbc.274.20.14122. [DOI] [PubMed] [Google Scholar]
- 42.Wurie HR, Buckett L, Zammit VA. J. Biol. Chem. 2011;286:36238–36247. doi: 10.1074/jbc.M111.251900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kominami S, Tagashira H, Ohta Y, Yamada M, Kawato S, Takemori S. Biochemistry. 1993;32:12935–12940. doi: 10.1021/bi00210a048. [DOI] [PubMed] [Google Scholar]
- 44.Ohta Y, Kawato S, Tagashira H, Takemori S, Kominami S. Biochemistry. 1992;31:12680–12687. doi: 10.1021/bi00165a019. [DOI] [PubMed] [Google Scholar]
- 45.Gerin I, Van Schaftingen E. FEBS Lett. 2002;517:257–260. doi: 10.1016/s0014-5793(02)02640-6. [DOI] [PubMed] [Google Scholar]
- 46.Hino Y, Minakami S, Biochem J. 1982;92:547–557. doi: 10.1093/oxfordjournals.jbchem.a133963. [DOI] [PubMed] [Google Scholar]
- 47.Mazur CS, Kenneke JF, Goldsmith MR, Brown C. Drug Metab. Dispos. 2009;37:1801–1805. doi: 10.1124/dmd.109.027615. [DOI] [PubMed] [Google Scholar]
- 48.Romanelli A, St-Denis JF, Vidal H, Tchu S, van de Werve G. Biochem. Biophys. Res. Commun. 1994;200:1491–1497. doi: 10.1006/bbrc.1994.1619. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi T, Hori SH. Biochim. Biophys. Acta. 1978;524:262–276. doi: 10.1016/0005-2744(78)90163-8. [DOI] [PubMed] [Google Scholar]
- 50.So PL, Chan TK, Lam SK, Teng CS, Yeung RT, Todd D. Metabolism. 1973;23:1443–1448. doi: 10.1016/0026-0495(73)90259-x. [DOI] [PubMed] [Google Scholar]
- 51.Berninsone P, Hirschberg CB. Ann. N. Y. Acad. Sci. 1998;842:91–99. doi: 10.1111/j.1749-6632.1998.tb09636.x. [DOI] [PubMed] [Google Scholar]