Abstract
Exotic predators have driven the extinction of many island species. We examined impacts of feral cats on the abundance and anti-predator behaviours of Aegean wall lizards in the Cyclades (Greece), where cats were introduced thousands of years ago. We compared populations with high and low cat density on Naxos Island and populations on surrounding islets with no cats. Cats reduced wall lizard populations by half. Lizards facing greater risk from cats stayed closer to refuges, were more likely to shed their tails in a standardized assay, and fled at greater distances when approached by either a person in the field or a mounted cat decoy in the laboratory. All populations showed phenotypic plasticity in flight initiation distance, suggesting that this feature is ancient and could have helped wall lizards survive the initial introduction of cats to the region. Lizards from islets sought shelter less frequently and often initially approached the cat decoy. These differences reflect changes since islet isolation and could render islet lizards strongly susceptible to cat predation.
Keywords: anti-predator behaviour, flight initiation distance, phenotypic plasticity, feral cat, Podarcis erhardii, island endemics
1. Introduction
Loss of biodiversity is a major global problem, with more than 30% of the world's species being threatened with extinction [1]. Island species appear to be disproportionally affected [2], and the introduction of exotic predators has been the driving factor behind many of these extinctions [3]. Island ecosystems often have few or no predator species [4] and many island vertebrates lack adaptations against predators. Anti-predator defences can cost energy and time that might otherwise be used for growth, foraging, mating and reproduction [5], and evolutionary theory predicts their gradual loss in the absence of substantial predation [6].
Feral cats (Felis silvestris catus) are dietary generalists and efficient predators that have adapted to a wide range of adverse environments [7]. They have been introduced to nearly 179 000 islands worldwide [8] and have been implicated in 8.2% of extinctions and 13.9% of declines for endangered birds, mammals and reptiles on islands globally [7]. Owing to their wide distribution and detrimental effects on local biodiversity, feral cats have been listed as one of the world's 100 worst invasive species [9]. Cats were introduced to eastern Mediterranean islands more than 9500 years ago [10,11] and to Aegean islands (e.g. Thera, Kea) during the Bronze Age [12]. Thus, this region provides the opportunity to evaluate the long-term impacts of feral cats on native wildlife populations.
Many lizard species avoid capture by fleeing and by sometimes shedding their tails. Flight initiation distance (FID), defined as the distance between the prey and a predator at which the animal initiates an escape, increases with predation risk and increasing distance to refuge [13]. Ease of caudal autotomy should also balance benefits and costs [14]. Because intact tails indicate social status and also play important roles in locomotion, courtship, defence and as lipid storage sites [15], the costs of caudal autotomy may outweigh the benefits under conditions of relaxed predation [16]. On small islands with few or no predators, the most expensive behaviours are predicted to be lost first [5].
The Mediterranean Basin is a biodiversity ‘hotspot’ and harbours more than 461 endemic taxa of reptiles and amphibians [2]. In this region, multiple island reptiles are either endangered or already extinct and invasive predators are implicated in several of these extinctions [14,17]. We conducted a combined field and experimental study of the effects of introduced predators on a widespread lizard species. We examined the current effects of cat predation on lizards' defensive behaviours by comparing sites with heavy predation pressure (many cats, close to villages) to sites with relaxed predation (few cats, far away from human settlements) on the same island. To understand potential historical changes, we also compared the reactions of lizards from sites with cats to those from cat-free isolated islets.
2. Material and methods
(a). Study sites and species
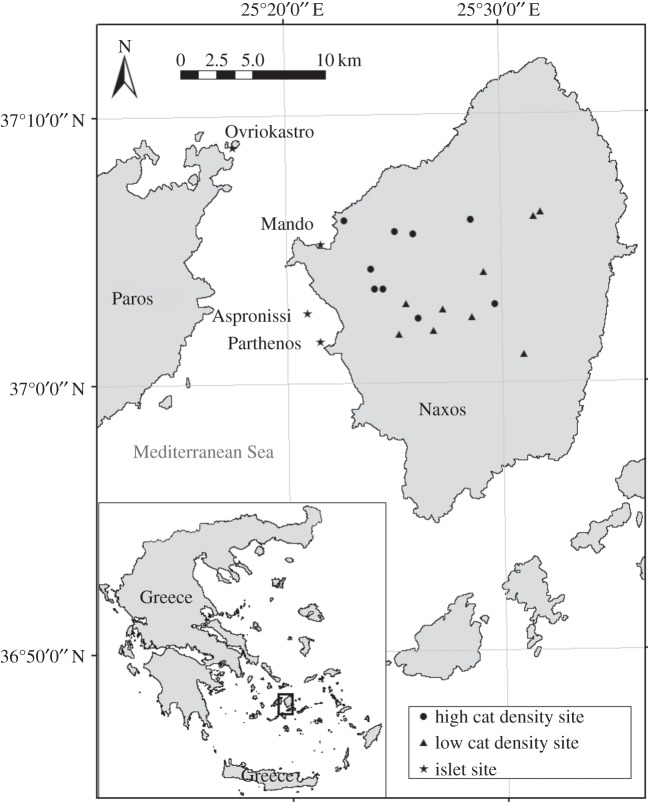
This study was conducted during summer 2011 on 18 sites on the large island of Naxos (Aegean Sea, Greece) and on four satellite islets (Ovriokastro, Aspronissi, Parthenos and Mando; figure 1). Naxos represents the core of an island block that has been separated from the European landmass since the Middle Pleistocene [18]. The first three study islets were connected to Naxos during the last ice age and became separated by rising sea levels 5600–6100 years ago [19]. By contrast, Mando Island was separated from Naxos by a storm in 2006. Of the 18 study sites on Naxos, nine were selected close (less than 300 m) to villages as high cat population density sites, while the other nine sites were ecologically similar more than 500 m away from villages, selected as low cat density sites (figure 1). The study animals were Aegean wall lizards (Podarcis erhardii, Lacertidae), a small, terrestrial and diurnal insectivore species, common throughout the ecosystems of the Aegean. These lizards often inhabit dry stone walls, a ubiquitous anthropogenic landscape feature. Cats often attack lizards in the Aegean islands [4], and it is quite common to see a cat with a dead lizard hanging from its mouth (B Li, A Belasen, J Foufopoulos 2011, personal observation).
Figure 1.
Map of the study area (Naxos and surrounding islets, Cyclades Cluster, Greece). High cat density sites are indicated with dots, whereas low cat density sites are shown with triangles and islets with stars.
(b). Population estimation for cats and lizards
We measured the density of cats along 1 km long road transects by moving at about 4 km h−1 and searching 50 m on both sides using a hand-held spotlight (Cyclops CYC-9WS; Cyclops Solutions, Grand Prairie, TX, USA) [20]. Surveys were conducted at the same time (21.00–00.30) during clear and windless nights. We visited each site three times on non-consecutive nights through the month of June.
For each of the Naxos locations, lizard densities were surveyed for a focal 100 m long wall segment by walking along the wall at 1 m distance and recording the number of lizards seen either on the wall or on the nearby ground. We repeated the survey at the same time on three consecutive days. To evaluate possible effects of habitat on lizard behaviour and ecology, we measured canopy coverage, understory vegetation coverage and understory biomass, arthropod populations, aspect, slope, wall height and wall direction.
(c). Measurements of lizard anti-predator behaviour
We determined FIDs by collecting data on at least 30 focal animals from each site. Measurements were taken during the same time period (09.00–11.00 and 15.00–17.00) for all sites during sunny and windless days (20–25°C) in June 2011. After sighting, animals were approached at a standard speed (45 m min−1). The distance at which the lizard initiated the escape, the distance covered to reach a refuge and the sex of the focal animal were recorded. (Males and females can be distinguished by sight in this species.)
To conduct laboratory autotomy tests and to quantify laboratory escape behaviour measurements, 20 individuals (10 males and 10 females) were captured from each of three high cat density sites, three low cat density sites and the four islets. Because previous autotomy may affect the ease of tail-shedding [21], we limited our sample to individuals with intact tails. Animals were housed in plastic terraria allowing for normal thermoregulation and were allowed to acclimatize to captivity conditions for 2 days.
To quantify ease of tail-shedding, we followed the standardized laboratory procedure of Pérez-Mellado et al. [14]: we grasped the base of the tail 20 mm from the cloaca with a pair of calipers and depressed the tail to half its thickness for 20 s. The autotomy rate for a population was calculated as percentage of individuals that shed their tails.
To quantify responses specifically towards cats under controlled conditions, we approached lizards from different populations using a mounted cat decoy on a low-wheeled platform in a laboratory arena. A concealed observer could use a 3 m long pole to push the decoy towards the lizard (see the electronic supplementary material for the illustration).
The arena was an open-top corridor (35 cm width × 3 m length × 30 cm height) attached to an open-top trapezoid enclosure (50 cm width × 60 cm length × 30 cm height), and separated by a glass pane that prevented escape but enabled the lizard to observe a black cat decoy approaching in the corridor. The floor of the enclosure was covered with sand, which was changed between trials to obscure olfactory cues. Two hollow bricks were placed at the corners of the enclosure and acted as refuges for the lizards. A 100 W incandescent light bulb 8 cm above the centre of the enclosure maintained stable species-appropriate temperatures. In preliminary trials, we determined that a 10 min period was sufficient for lizards to acclimate to the arena. After this acclimation period, the decoy was pushed along the corridor towards the enclosure at a constant speed of 25 cm s−1. We recorded (i) whether the focal animal initially fled, approached or stayed still when it noted the approaching decoy, (ii) whether it escaped into a refuge and (iii) the distance to the decoy at which a lizard initiated its escape. All trials were done during normal lizard activity periods (9.00–16.00), once per day for three consecutive days and were recorded using an overhead digital video camera (Sony HDR-CX550).
(d). Statistical analyses
Proportion data were arcsine transformed for further analysis. Generalized linear models with Poisson error function (Wald tests) were used to analyse cat and lizard densities for different sites. One-way ANOVA was used to test the difference in anti-predator behaviours except when two-way repeated measures ANOVA was used to analyse how FID changed across the three presentations of the cat decoy to lizards from different predation regimes.
3. Results
(a). Comparisons of high and low cat density sites
On average, 16.3 ± 2.5 cats km−2 were seen at high cat density sites, whereas no cats were ever detected during surveys at the low cat density sites (Wald χ2 = 10.493, p = 0.001). High and low cat density sites were very similar in canopy cover, understory coverage, understory biomass, mean arthropod biomass and height of their dry stone walls (see the electronic supplementary material).
Lizard densities were more than twice as high at low as at high cat density sites (10.33 ± 3.88 versus 4.85 ± 3.35 individuals per 100 m of wall; Wald χ2 = 50.951, p < 0.001). Lizard densities were highly repeatable: the results of all surveys were highly correlated with each other (e.g. r = 0.767, p < 0.01, between the first and the second survey). Across all sites, lizard density was negatively correlated with cat density (r = −0.54, p = 0.021, Spearman).
(b). Autotomy rates
Lizards from populations exposed to more cats shed their tails more easily during standardized trials (figure 2; F2,7 = 109.04, p < 0.001).
Figure 2.
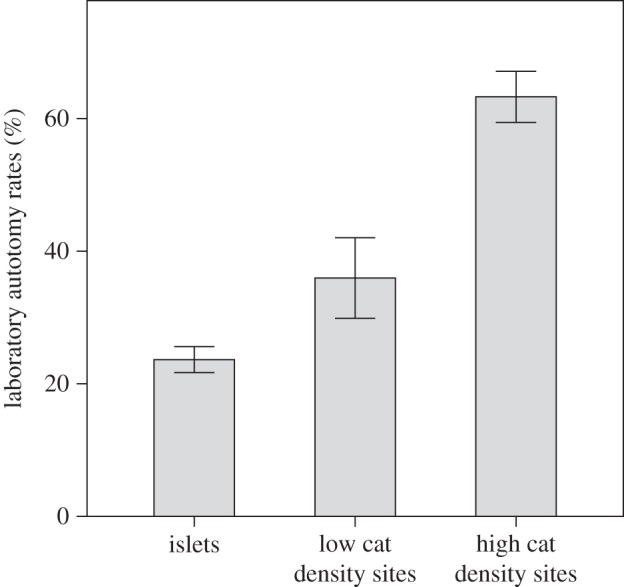
Laboratory autotomy rates, expressed as percentage of animals in a population that shed their tails. Data pooled for all lizard populations living under the same predation regimes (mean ± 2 s.e.).
(c). Field flight initiation distances
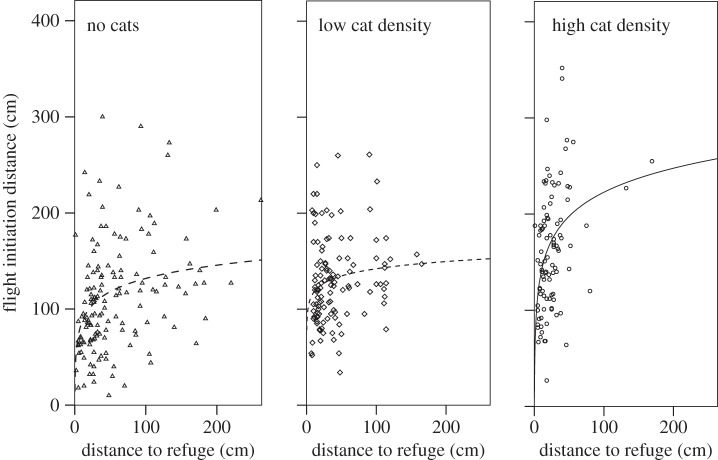
At all sites, lizards that were farther from a refuge fled at greater distances from an approaching threat, though the strength of this relationship varied between predation regimes (no cats: r = 0.311, n = 145, p < 0.001; low cat density: r = 0.192, n = 131, p < 0.028; high cat density: r = 0.402, n = 101, p < 0.001; Pearson; figure 3). Lizards from different predation regimes differed in both distance to refuge (F2,374 = 18.208, p < 0.001) and FID (F2,398 = 31.257, p < 0.001; figure 4). Lizards from high cat density sites generally stayed closer to refugia and had greater FID than lizards from low cat density sites, and especially than lizards from islet populations.
Figure 3.
The pattern between FID and refuge distance in the field for high cat density sites, low cat density sites and islets (no cats). Logarithmic curves fit the data best and are shown in the figures.
Figure 4.
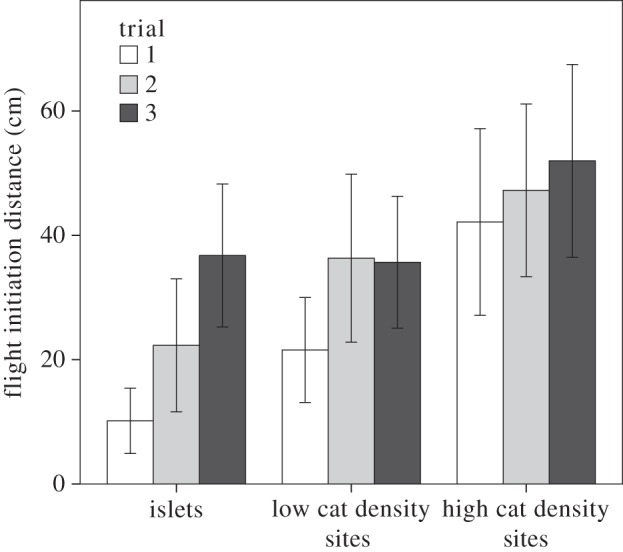
FID for three trials of a cat predation simulation. Figure shows means for each trial ± 2 s.e.
(d). Cat predation experiment
Lizards from sites with more cats fled at greater distances during standardized presentations of a cat decoy (F2,114 = 8.136, p < 0.001). FIDs increased with each successive trials (F2,114 = 7.608, p < 0.001; figure 4). FIDs increased across the three trials in roughly the same way for lizards from all three predation regimes (interaction F4,228 = 1.371, p = 0.245). It is interesting to note that lizards from islets by the third trial had FIDs similar to those from the first trial for lizards from high predation areas.
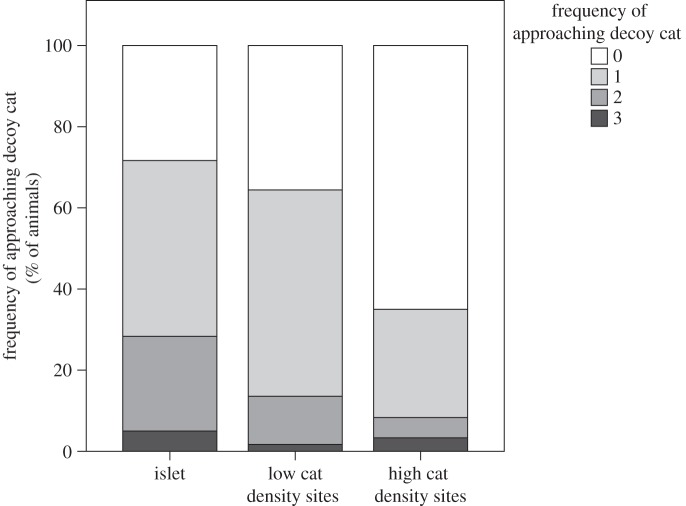
Significantly more lizards from islet populations (60%) did not seek refuge at all relative to lizards from low (20.0%) or high (21.7%) cat density sites (F2,7 = 6.050, p = 0.030). A substantial fraction of lizards initially moved towards the approaching decoy instead of fleeing, which was unexpected. Overall 75.7% of lizards from the islets, 64.4% of lizards from low cat density sites and 35.0% of lizards from high cat density sites approached the mounted decoy at least once. Lizards from lower predation regimes were more likely to approach a cat decoy than were lizards from higher predation regimes (F2,7 = 5.878, p = 0.032; figure 5).
Figure 5.
The percentage of the frequency of approach behaviour over the course of the three predation simulation trials. 0, never approached cat; 1, approached once; 2, approached twice; 3, approached cat decoy on all three trials.
(e). Rapidity of loss of anti-predator behaviour
Lizards from predator-free Mando, which was separated from Naxos only 5 years before this study, offer an opportunity to evaluate the rapidity with which anti-predator behaviours are being lost. FIDs of Mando lizards (145.7 cm) were significantly longer than those for other islets populations (95.0 cm) and similar to Naxos populations (144.6 cm). By contrast, laboratory autotomy rates of Mando lizards (25.0%) were more similar to those of other islets (24.5%) rather than any of the Naxos populations (49.3%).
4. Discussion
On Naxos, wall lizard densities were less than half as great at sites with many cats as sites with few cats while these sites were similar in ecological factors that are thought to be important ecological determinants of lizard density: understory coverage, understory biomass, canopy cover, arthropod biomass and wall height. We believe that the underlying reason is that cats are fed from the villagers and thus avoid wandering far away from the human settlements. Therefore, our results indicate heavy predation by cats and strong current selection by cats on the anti-predator defences of wall lizards.
The effect of cats was localized in our study, because cats were found almost exclusively around human habitations. Although feral cats can have large home ranges (130 ha for females and 497 ha for males) on islands isolated from human settlement [22], home ranges are usually much smaller for cats feeding from human settlements (10 ha for females and 15 ha for males) [23]. Lizards constitute only a small portion of the diet of cats, with cats on Mediterranean islands mostly maintained by food deliberately or accidentally provided by humans [7,24]. Although we never observed cats far away from human habitations, predation pressure no doubt exists on Naxos even away from villages. First, feral cats are known both to be cryptic and to roam widely and have been shown to affect prey populations significantly even at low densities [25]. Second, other native predators, such as stone martens (Martes foina), have the potential to affect lizard populations. By contrast, the islets are too small to sustain populations of mammalian predators.
In addition to altering lizard densities, cats also had strong effects on lizard anti-predator behaviours. Lizards at high cat density sites had longer FIDs and a strongly elevated ease of caudal autotomy. In line with other studies [4,14], we found that all populations from Naxos shed their tails more easily than the islet populations. The reduction in caudal autotomy in relaxed predation environments is best explained by the high costs of this anti-predator defence.
Our results show that some of the defences of wall lizards are plastic. Lizards from all three types of predation regimes (islets without cats, low cat density sites and high cat density sites) increased their FIDs between the first and the third exposure to a cat decoy (figure 4). In the islet group FIDs more than doubled from the first to the third trial. This plasticity in anti-predator behaviour could allow populations that have lived without predators for more than 6000 years to respond to predation threats, especially if given the benefit of multiple encounters.
Despite close proximity and likely gene flow between sites with high and low cat densities, lizards from low cat density sites generally acted less like lizards from high cat density sites and more like lizards from islets in their distance to refuge, FID and tail autotomy. These results suggest that wall lizards retain ancestral plasticity in anti-predator behaviour that allows them to change their behaviour in response to current predation levels. Results from other ancient invasions, such as predation from dingoes on mammalian species such as wallabies [26] in Australia, should be compared to see if these effects are general.
The islet populations differed from lizards from both low and high cat density sites in several interesting ways that may indicate independent evolution since isolation. In the field, islet lizards roamed further away from refuges than Naxos animals. In the laboratory, about 60% of islet lizards did not seek a refuge during at least one of the predation presentations. Furthermore, more than 70% of all islet lizards actually approached the moving cat decoy before fleeing. Similar neophilic behaviour is evident in the field, where islet lizards will quickly investigate new objects, as we observed when we set down items such as buckets, fishing poles or backpacks during fieldwork. On small islets, lizard population densities are very high and neophilic behaviour is likely part of constantly searching for scarce food items [27,28]. Lizards that approach novel objects or remain exposed on approach could be very susceptible to invasive cats.
Whereas the laboratory predation trials reveal the ability of lizards to quickly regain fear behaviours, a comparison of the lizards from Mando also highlights the ease with which such behaviours can be lost. Mando lizards became isolated from larger Naxos (and predators) only about 5 years ago. While FIDs of these animals still resemble those of nearby Naxos, tail autotomy rates already resemble those of the islets. This contrast between different anti-predator responses matches predictions from evolutionary theory that in the face of relaxed predation ‘expensive’ behaviours such as caudal autotomy will be lost much sooner than ‘cheap’ behaviours like longer FIDs [29,30].
Predation regime is known to shape the defensive repertoire of lizards [31,32]. In our work, higher cat densities led to longer FIDs and higher autotomy rates in both the laboratory and the field. Behavioural plasticity allows Aegean wall lizards to adapt to changing predation levels. From a conservation perspective, such plasticity could allow endangered lacertids to be trained to avoid predators. Such programmes have taught animals slated for re-introduction to recognize predators and to respond more strongly in order to bolster their survival in nature [29,33,34]. Studies in more taxa and other systems would shed light in the generality of these findings.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Ana Marcela Chara, Maureen Lynch and Colin Donihue for assistance and advice. We also thank two anonymous reviewers for feedback that greatly improved the presentation of this research.
Data accessibility
Datasets can be accessed through Dryad by doi:10.5061/dryad.fm146.
Funding statement
We acknowledge the University of Michigan School of Natural Resources and Environment and the Rackham Graduate School for funding support.
References
- 1.Workman JG. 2013. 2012 Nature+towards nature-based solutions. IUCN Annual Report 2012. Gland, Switzerland: IUCN; (https://portals.iucn.org/library/efiles/edocs/2013-017.pdf) [Google Scholar]
- 2.Cox N, Chanson J, Stuart SN. 2006. The status and distribution of reptiles and amphibians of the Mediterranean Basin. Gland, Switzerland: IUCN. [Google Scholar]
- 3.Primack RB. 1995. A primer of conservation biology. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 4.Pafilis P, Foufopoulos J, Poulakakis N, Lymberakis P, Valakos ED. 2009. Tail shedding in island lizards [Lacertidae, Reptilia]: decline of antipredator defenses in relaxed predation environments. Evolution 63, 1262–1278. ( 10.1111/j.1558-5646.2009.00635.x) [DOI] [PubMed] [Google Scholar]
- 5.Blumstein DT. 2002. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 29, 685–692. ( 10.1046/j.1365-2699.2002.00717.x) [DOI] [Google Scholar]
- 6.McNab BK. 1994. Energy conservation and the evolution of flightlessness in birds. Am. Nat. 144, 628–642. ( 10.1086/285697) [DOI] [Google Scholar]
- 7.Bonnaud E, et al. 2011. The diet of feral cats on islands: a review and a call for more studies. Biol. Invas. 13, 581–603. ( 10.1007/s10530-010-9851-3) [DOI] [Google Scholar]
- 8.Medina FM, et al. 2011. A global review of the impacts of invasive cats on island endangered vertebrates. Glob. Change Biol. 17, 3503–3510. ( 10.1111/j.1365-2486.2011.02464.x) [DOI] [Google Scholar]
- 9.Lowe S, Browne M, Boudjelas S, De Poorter M. 2000. 100 of the world's worst invasive alien species: a selection from the global invasive species database. Auckland, New Zealand: Invasive Species Specialist Group. [Google Scholar]
- 10.Serpell JA. 2000. Domestication and history of the cat. In The domestic cat: the biology of its behaviour (eds Turner DC, Bateson P.), pp. 179–192. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.Driscoll CA, Clutton-Brock J, Kitchener AC, O'Brien SJ. 2009. The taming of the cat. Sci. Am. 300, 68–75. ( 10.1038/scientificamerican0609-68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels DW. 1999. Classical cats: the rise and fall of the sacred cat. New York, NY: Routledge. [Google Scholar]
- 13.Cooper WE., Jr 2009. Optimal escape theory predicts escape behaviors beyond flight initiation distance: risk assessment and escape by striped plateau lizards Sceloporus virgatus. Curr. Zool. 55, 123–131. [Google Scholar]
- 14.Pérez-Mellado V, Corti C, Lo Cascioa P. 1997. Tail autotomy and extinction in Mediterranean lizards. A preliminary study of continental and insular populations. J. Zool. 243, 533–541. ( 10.1111/j.1469-7998.1997.tb02799.x) [DOI] [Google Scholar]
- 15.Maginnis TL. 2006. The costs of autotomy and regeneration in animals: a review and framework for future research. Behav. Ecol. 17, 857–872. ( 10.1093/beheco/arl010) [DOI] [Google Scholar]
- 16.Pafilis P, Valakos ED, Foufopoulos J. 2005. Comparative postautotomy tail activity in six Mediterranean lacertid lizard species. Physiol. Biochem. Zool. 78, 828–838. ( 10.1086/431192) [DOI] [PubMed] [Google Scholar]
- 17.Traveset A, Riera N. 2005. Disruption of a plant-lizard seed dispersal system and its ecological effects on a threatened endemic plant in the Balearic Islands. Conserv. Biol. 19, 421–431. ( 10.1111/j.1523-1739.2005.00019.x) [DOI] [Google Scholar]
- 18.Perissoratis C, Conispoliatis N. 2003. The impacts of sea-level changes during latest Pleistocene and Holocene times on the morphology of the Ionian and Aegean seas (SE Alpine Europe). Mar. Geol. 196, 145–156. ( 10.1016/S0025-3227(03)00047-1) [DOI] [Google Scholar]
- 19.Foufopoulos J, Kilpatrick AM, Ives AR. 2011. Climate change and elevated extinction rates of reptiles from Mediterranean islands. Am. Nat. 177, 119–129. ( 10.1086/657624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Read J, Bowen Z. 2001. Population dynamics, diet and aspects of the biology of feral cats and foxes in arid South Australia. Wildl. Res. 28, 195–203. ( 10.1071/WR99065) [DOI] [Google Scholar]
- 21.Simou C, Pafilis P, Skella A, Kourkouli A, Valakos E. 2008. Physiology of original and regenerated tails in Aegean Wall Lizard (Podarcis erhardii). Copeia 3, 504–509. ( 10.1643/CP-06-191) [DOI] [Google Scholar]
- 22.Konecny MJ. 1987. Food habits and energetics of feral house cats in the Galapagos Islands. Oikos 50, 24–32. ( 10.2307/3565398) [DOI] [Google Scholar]
- 23.Page RJC, Ross J, Bennet DH. 1992. A study of the home ranges, movements and behaviour of the feral cat population at Avonmouth Docks. Wildl. Res. 19, 263–277. ( 10.1071/WR9920263) [DOI] [Google Scholar]
- 24.Medina FM, Nogales M. 2009. A review on the impacts of feral cats (Felis silvestris catus) in the Canary Islands: implications for the conservation of its endangered fauna. Biodivers. Conserv. 18, 829–846. ( 10.1007/s10531-008-9503-4) [DOI] [Google Scholar]
- 25.Peck D, Faulquier L, Pinet P, Jaquemet S, Le Corre M. 2008. Feral cat diet and impact on sooty terns at Juan de Nova Island, Mozambique Channel. Anim. Conserv. 11, 65–74. ( 10.1111/j.1469-1795.2007.00153.x) [DOI] [Google Scholar]
- 26.Newsome AE, Catling PC, Corbett LK. 2006. The feeding ecology of the dingo II. Dietary and numerical relationships with fluctuating prey populations in south-eastern Australia. Aust. J. Ecol. 8, 345–366. ( 10.1111/j.1442-9993.1983.tb01332.x) [DOI] [Google Scholar]
- 27.Cooper WE, Jr, Pérez-Mellado V, Hawlena D. 2006. Magnitude of food reward affects escape behavior and acceptable risk in Balearic lizards, Podarcis lilfordi. Behav. Ecol. 17, 554–559. ( 10.1093/beheco/arj066) [DOI] [Google Scholar]
- 28.Castilla AM, Herrel A, Gosá A. 2008. Mainland versus island differences in behaviour of Podarcis lizards confronted with dangerous prey: the scorpion Buthus occitanus. J. Nat. Hist. 42, 2331–2342. ( 10.1080/00222930802254763) [DOI] [Google Scholar]
- 29.Maloney RF, McLean IG. 1995. Historical and experimental learned predator recognition in free-living New-Zealand robins. Anim. Behav. 50, 1193–1201. ( 10.1016/0003-3472(95)80036-0) [DOI] [Google Scholar]
- 30.Beauchamp G. 2004. Reduced flocking by birds on islands with relaxed predation. Proc. R. Soc. Lond. B 271, 1039–1042. ( 10.1098/rspb.2004.2703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pafilis P, Pérez-Mellado V, Valakos ED. 2008. Post autotomy tail activity in Balearic wall lizard, Podarcis lilfordi. Naturwissenschaften 95, 217–221. ( 10.1007/s00114-007-0320-5) [DOI] [PubMed] [Google Scholar]
- 32.Cooper WE, Pérez-Mellado V, Vitt LJ. 2004. Ease and effectiveness of costly autotomy vary with predation intensity among lizard populations. J. Zool. 262, 243–255. ( 10.1017/S095283690300462X) [DOI] [Google Scholar]
- 33.Miller B, Biggins D, Hanebury L, Vargas A. 1994. Reintroduction of the black-footed ferret (Mustela nigripes). In Creative conservation: interactive management of wild and captive animals (eds Olney PJS, Mace GM, Feistner ATC.), pp. 455–464. London, UK: Springer. [Google Scholar]
- 34.McLean IG, Lundie-Jenkins G, Jarman PJ. 1996. Teaching an endangered mammal to recognise predators. Biol. Conserv. 75, 51–62. ( 10.1016/0006-3207(95)00038-0) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets can be accessed through Dryad by doi:10.5061/dryad.fm146.