Abstract
Menopause remains an evolutionary puzzle, as humans are unique among primates in having a long post-fertile lifespan. One model proposes that intergenerational conflict in patrilocal populations favours female reproductive cessation. This model predicts that women should experience menopause earlier in groups with an evolutionary history of patrilocality compared with matrilocal groups. Using data from the Indonesia Family Life Survey, we test this model at multiple timescales: deep historical time, comparing age at menopause in ancestrally patrilocal Chinese Indonesians with ancestrally matrilocal Austronesian Indonesians; more recent historical time, comparing age at menopause in ethnic groups with differing postmarital residence within Indonesia and finally, analysing age at menopause at an individual-level, assuming a woman facultatively adjusts her age at menopause based on her postmarital residence. We find a significant effect only at the intermediate timescale where, contrary to predictions, ethnic groups with a history of multilocal postnuptial residence (where couples choose where to live) have the slowest progression to menopause, whereas matrilocal and patrilocal ethnic groups have similar progression rates. Multilocal residence may reduce intergenerational conflicts between women, thus influencing reproductive behaviour, but our results provide no support for the female-dispersal model of intergenerational conflict as an explanation of menopause.
Keywords: menopause, intergenerational conflict, ethnicity, Indonesia, postmarital residence
1. Introduction
We still do not fully understand why human females' reproductive systems senesce well before other somatic systems, as we expect an extension of one's reproductive career to be evolutionarily advantageous [1–10]. This pattern is unique among primates [11,12]. In an evolutionary model (hereafter referred to as the female intergenerational conflict model), Cant & Johnstone [13] demonstrated that intergenerational conflict in populations with female-biased dispersal can lead to fertility cessation in females. Female-biased dispersal—where women join their husband and his kin group at marriage—results in patrilocal communities where mothers-in-law and daughters-in-law co-reside and compete over reproductive resources. The daughter-in-law is unrelated to the mother-in-law's future children and should be unwilling to help her mother-in-law reproduce. By contrast, the mother-in-law will be related to the daughter-in-law's children by as much as 0.25. Given this asymmetry in the cost of forgoing reproduction, the daughter-in-law will have an advantage in negotiating this reproductive conflict with her mother-in-law and should be more likely to reproduce. The fact that women undergo menopause around the age when their children begin reproducing supports the idea that reproductive overlaps are net costly [13].
Selection for menopause in this model relies on several assumptions including that females disperse at marriage, that female competition is more important than male competition and that females control reproductive decision-making. The opposite resolution to the intergenerational conflict is expected when children are in conflict with their own parents [14]. In matrilocal contexts for example, the mother should be the one to reproduce instead of the daughter if the mother's child will be a full sibling to the daughter, because both individuals will be related to the mother's child by 0.5, but the mother will only be related to the daughter's child by 0.25. Additionally, if men have control of reproductive decision-making, then the opposite resolution of the conflict is expected in patrilocal contexts, where fathers should win reproductive conflict over sons [15].
Previous empirical research has shown mixed results when testing assumptions of the female intergenerational conflict model. Using data from pre-industrial Finland, Lahdenperä et al. [16] found that when mothers-in-law and daughters-in-law reproduced concurrently, the offspring experienced significantly higher mortality, but when mothers and daughters reproduced at the same time, there was no significant increase in child mortality, suggesting intergenerational conflict among affines only. However, Mace & Alvergne [17] find that the resolution to conflict over reproduction did not lead to younger women reproducing at a higher rate compared with older women within (typically patrilocal) compounds in rural Gambia. Furthermore, daughters did not experience a reduced rate of reproduction when their own mother reproduced, whereas the mother was less likely to reproduce once the daughter reproduced, suggesting that mother–daughter conflict may also be important in shaping reproduction at older ages. Finally, using historical Norwegian data, Skjærvø & Røskaft [18] find no evidence that reproductive overlap—generously defined as a birth within 15 years—resulted in fewer grandchildren for either generation of women.
Such individual-level studies on the consequences of reproductive overlap between generations speak to the extent of intergenerational reproductive conflict in modern populations, and thus the plausibility of the model's assumptions. However, a more direct test of the Cant and Johnstone model would be to investigate whether age at menopause differs between groups with differing histories of philopatry. One testable prediction from the female intergenerational conflict model is that women should experience menopause earlier in groups with a history of female-biased dispersal compared with groups with a history of male-biased dispersal, because reproductive overlap of mothers-in-law and daughters-in-law should result in daughters-in-law winning the conflict, but the reproductive overlap of mothers and daughters should result in mothers winning the conflict. Several anthropologists have proposed that female-biased dispersal is the ancestral migration pattern for humans [19,20] though this has been disputed [21]. Additionally, the ethnographic record shows that there is much cross-cultural variation in this regard [22] leaving open the possibility that gene–culture coevolutionary processes [23] have led to different selection pressures in societies with histories of matrilocality compared with those with histories of patrilocality.
Humans are the only primate species in which females experience menopause well before other systems of the body senescence [11,12] which suggests that either menopause evolved since the human and chimpanzee lineages split or that hominids subsequently evolved greater longevity. Some researchers debate whether the derived trait we need to explain is early termination of fertility or extension of longevity [24]; however, here we test a model that attempts to explain termination of fertility.
Several lines of evidence suggest the plausibility of changes to age at menopause within human populations. Menopause age is variable among modern humans, ranging from age 40 to 60, and its heritability is estimated between 0.31 and 0.72, suggesting a moderate-to-large genetic effect [25]. Environmental and individual life-history traits, including smoking exposure, parity, use of hormonal contraception, socio-economic status and nutrition also significantly affect age at menopause [26]. Studies in postindustrial countries find conflicting results regarding the effect of ethno-racial group membership on age at menopause in large part because of socio-economic and lifestyle confounds [27,28]. To the best of our knowledge, this study represents the first attempt to test whether ethnic-group membership affects age at menopause in a transitioning society and among ethno-linguistically related groups.
2. Methods
To test the prediction that women should experience menopause earlier in groups with a history of female-biased dispersal compared with groups with a history of male-biased dispersal, we compare ethnic groups in Indonesia. Indonesia is a country with extensive ethnic and linguistic diversity, including over 300 different ethnic groups. Molecular data suggest that the original migration into Indonesia occurred approximately 45 000 years ago, with more recent migrations occurring at the end of the last glacial period between 8 and 35 ka, and with the spread of Hinduism and Islam in the past millennium [29]. Analysis of linguistic and cultural phylogenetic trees of Austronesian societies shows that matrilocal residence was probably the ancestral residence pattern, which predominated approximately 5000 years ago [30].
Given this information, we can test whether female intergenerational conflict is resolved at various timescales. It is possible that there is a deep history of gene–culture coevolution, where the Austronesian language family (with matrilocality as the ancestral residence pattern) has evolved an older age at menopause compared with groups that are ancestrally patrilocal, such as the Chinese [31]. An alternative hypothesis is that intergenerational conflict has been resolved more recently (within the past 5000 years) and that we can detect differences in progression to menopause across different Indonesian ethnic groups, as those groups who have adopted patrilocality in the past several thousand years may have evolved earlier progression to menopause. Finally, it is possible that there has not been any genetic change within humans, but that people use cues about locality from their individual family experience and facultatively terminate fertility earlier when they engage in patrilocal postnuptial residence. In this case, we might expect facultative change in age at menopause depending on a woman's postnuptial residence. This is predicated on the assumption that a woman's own postmarital residence is indicative of her children's postnuptial residence and thus her expected future conflict with younger individuals in the household.
We use Indonesia Family Life Survey (IFLS) data collected in 1993, 1997, 2000 and 2007 on fertility, health and socio-economic outcomes (http://www.rand.org/labor/FLS/IFLS) [32–34]. This includes 2678 women between the ages of 40 and 62 who reported information on whether they still menstruated and their age at last menstrual cycle (age at last menstrual cycle had to be at least 1 year prior to survey). We excluded 270 women who reported that they do not menstruate because of a reason other than menopause, including medication or contraception. Approximately 890 women had experienced menopause, defined as the age the women reported having her last menstrual period.
Ethnic group information was collected in 2007, and we have information on individuals from 16 ethnic groups (we excluded groups that were references to urban centres rather than historically distinct ethnic groups and those with fewer than 15 respondents). We used postmarital residence categorization as a proxy for whether a group practiced female- or male-biased dispersal. The categorization for each ethnic group was based on previous literature, including the Ethnographic Atlas [31] (for a list of each ethnic group, its categorization and reference, see the electronic supplementary material, table S1). Each group was coded as matrilocal (male-biased dispersal), patrilocal (female-biased dispersal) or multilocal (including ambilocal—living near or with either set of parents and neolocal—living separate from both sets of parents). As a check on these categorizations, we examined whether this ethnic-group postmarital residence corresponded to actual postmarital residence for women in this sample (see the electronic supplementary material, table S1). Across all ethnic groups, more women reported the postmarital residence of their own ethnic group than any other postmarital residence pattern, with the exception of Malays. This ethnic group is categorized as patrilocal, but Malay women in our sample are more likely to report living matrilocally after marriage (we will return to this point in the Discussion).
(a). Phylogenetic non-independence
When making comparisons across different ethnic groups, it is important to test whether patterns of menopausal age are driven by phylogenetic relationships [35,36]. In order to account for this, we tested for phylogenetic independence using the test for serial independence [37]. Using a linguistic phylogenetic tree of Austronesian languages [30], we estimated whether (i) median age at menopause, or (ii) the percentage of women who achieved menopause by age 49 was associated with phylogenetic history for the eight ethno-linguistic groups in the sample whose phylogenetic relations had been assessed (Chinese, Bugis, Minang, Melayu, Sundanese, Madurese, Javanese and Balinese). The test for serial independence shows that there is no evidence of phylogenetic dependence of menopausal age in our data (p > 0.30). This is the case for both measurements of menopause and is robust to the removal of ethnic groups with fewer than 20 individuals progressing to menopause. We therefore ignored phylogeny in subsequent analyses.
(b). Analysis
In all analyses, we control for variables that have a known impact on age at menopause, including education, smoking status, marital status, age at menarche, socio-economic status and nulliparity [38–40], except when stated otherwise. While we do not have information on hormone replacement therapy for our respondents, evidence suggests that few Indonesian women have ever used hormone therapy for their menopausal symptoms [41].
(i). Does variation in postnuptial residence at the ethnic-group level predict age at menopause?
We hypothesized that female intergenerational conflict may have been resolved through gene–culture coevolutionary change on two timescales: deep history, which would predict that the ancestral history of matrilocality in Austronesia results in relatively late menopause in these groups compared with ancestrally patrilocal Chinese Indonesian groups; and more shallow history, which would predict that the Indonesian groups in our sample would have evolved different ages at menopause according to whether their more recent history was matrilocal or patrilocal. We test both models by conducting discrete-time event history analyses of progression to menopause. Event history analysis models the time until a particular event (here, menopause) and has the advantage of allowing the inclusion of censored cases, i.e. those women who have not yet reached menopause. Women enter the analysis at age 40 and time is measured in years. Interactions between our time variable and other covariates were included in initial models to test the proportional hazards assumptions of the model. Interactions were included in the final full model when significant. For ease of presentation of results, we discuss the test of more recent within-Indonesian gene–culture coevolution first.
Within Indonesia
To test whether women from Indonesian ethnic groups with a history of patrilocality have earlier ages at menopause compared with women from historically matrilocal ethnic groups, we conducted a discrete-time event history analysis of progression to menopause with a random effect for ethnic group. We test for interactions between ethnic-level postmarital residence and other covariates to determine whether postmarital residence effects change across values of other control variables and find no such effects. We compare two models, a full model with postmarital residence as an ethnic-group-level predictor and a null model without postmarital residence as a predictor. The null model lets us check visually whether ethnic groups with similar postnuptial residence patterns clump together in predicted age of menopause.
Indonesian versus Chinese comparison
One ethnic group currently living in this region that was not ancestrally Austronesian, nor ancestrally matrilocal, are ethnic Chinese who have predominantly migrated in the past 150 years [42]. To test whether there are differences in progression to menopause between ancestrally Austronesian and the ancestrally patrilocal Chinese ethnic groups, we conducted a case–control analysis. We created a matched sample of individuals with a non-Chinese Austronesian background for each Chinese participant aged 40–62 in the database (there are only 28 Chinese women aged 40–62 in the sample). Individuals were matched on age, education, urban/rural residence and region (but the non-Chinese sample could come from matrilocal, patrilocal or multilocal ethnic groups). We conducted a discrete-time event history analysis on progression to menopause, including a dichotomous variable for Chinese/non-Chinese ethnicity. We do not include urban/rural residence, nulliparity or smoking status as controls in this analysis, because the participants were almost identical in these confounders (all but two subjects lived in urban areas, all had given birth and only one had ever smoked).
As an alternative method of analysis, we used a resampling method where we selected 1000 samples of 28 unmatched non-Chinese women and compared their progression to menopause with the progression for Chinese women in a discrete-time event history model controlling for the same covariates as in the matched analysis (we used an unmatched sample, because there were few potential sets of women who fully matched our Chinese sample). This bootstrapping method results in a distribution of differences between Chinese and non-Chinese Indonesians' progression to menopause, allowing us to estimate the effect size and confidence interval.
(ii). Does individual-level variation in postnuptial residence predict age at menopause?
It is possible that ethnic-level postnuptial residence does not predict age at menopause, but that individual-level postnuptial residence does if women are able to facultatively shift their age at menopause based on their personal family circumstances. While we are not suggesting that women can consciously manipulate their age at menopause, there is evidence that exposure to different environmental factors can influence age at menopause [24], so it is possible that environmental exposure to closely related reproductive women or household conflict can influence menopausal age. To explore this possibility, we tested whether individual-level postnuptial residence predicts progression to menopause. We use a woman's own postnuptial residence status to predict her progression to menopause using a discrete-time event history analysis model with a random effect for ethnic group, because the dataset had too much missing data on participants' children's postmarital residence.
3. Results
(a). Does variation in postnuptial residence at the ethnic-group level predict age at menopause?
(i). Within Indonesia
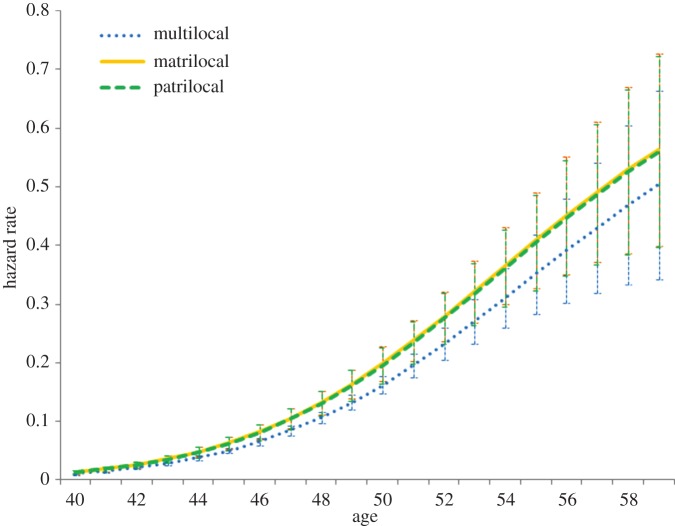
Figure 1 displays the hazard rates for the progression to menopause by postmarital residence at the ethnic group-level (for full model, see the electronic supplementary material, table S2). There is no significant difference between matrilocal and patrilocal ethnic groups in their progression to menopause, although women in multilocal groups progress significantly more slowly (compared with patrilocal, log odds ratio = −0.227, s.e. = 0.102). In this model, significant predictors of menopause at the individual-level include marital status, where widowed women progress to menopause at a faster rate than married or divorced women, and education level, where higher levels of education result in delays to menopause. Contrary to much of the literature from Western societies, whether the woman ever smoked, her age at menarche and nulliparity are not significant predictors of menopause, perhaps because there is little variation in some of these variables (fewer than 5% of women ever smoked and more than 97% of women gave birth).
Figure 1.
Predicted hazard rate of progression to last menstruation based on random effects discrete-time event history analysis by ethnic-level postmarital residence, plotted for the mean group at the means of covariates (see the electronic supplementary material, table S2 for full model). Error bars represent 95% confidence intervals (CIs). (Online version in colour.)
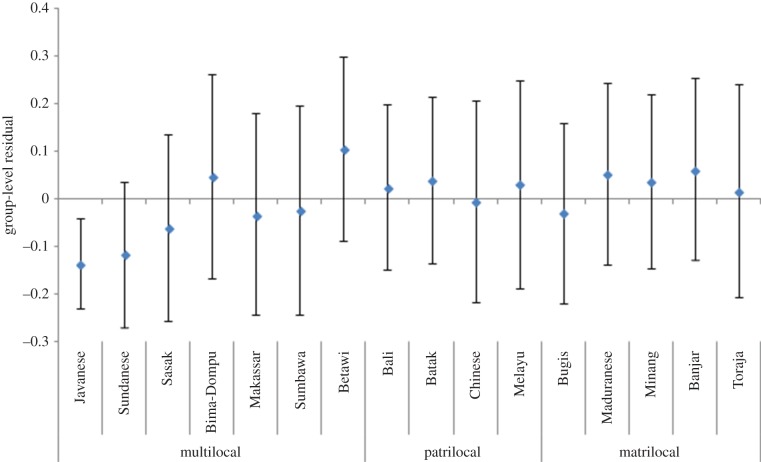
Figure 2 displays the residuals of each ethnic group from the discrete-time event history analysis with ethnicity as a random effect, but no group-level predictors. These are shown grouped by ethnic-level postmarital residence to assess whether there are trends by ethnic postmarital residence. We see no trend of patrilocal groups having earlier age at menopause. Multilocal groups have the three slowest (negative residuals) and the fastest (positive residual) progression to menopause. None of the patrilocal or matrilocal groups have residuals significantly different from zero. We do not see a tendency for groups of a particular postnuptial residence to clump together in the figure. This figure shows that the slower progression to menopause by the multi-local groups is mainly driven by Javanese and Sundanese individuals, although five of the seven multilocal societies show negative residuals.
Figure 2.
Group-level residuals from the null discrete-time event history model with ethnicity as a random effect for each ethnic group. Negative residuals indicate a slower progression to menopause than the mean group. Error bars represent 95% CIs. (Online version in colour.)
(ii). Indonesian versus Chinese comparison
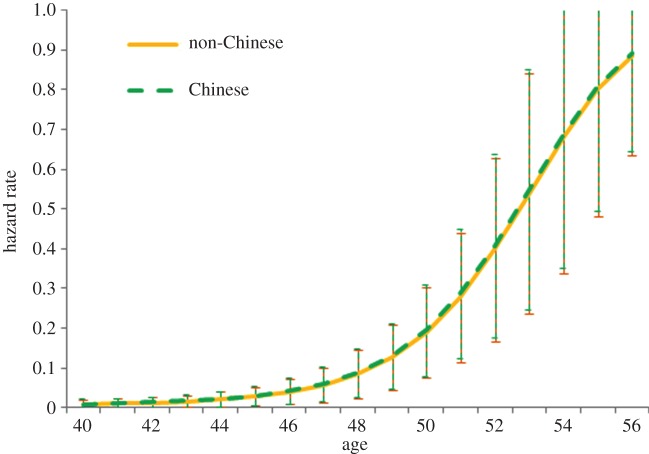
When we compare the Chinese ethnic group residual (from the analysis above) with other ethnic groups in our sample (figure 2), progression to menopause for the Chinese ethnic group has a residual very close to zero (−0.006), suggesting that it is not faster than the ancestrally matrilocal Austronesian groups in our sample. Figure 3 displays the hazard rates of the progression to menopause for our matched set of Chinese Indonesians and non-Chinese Indonesians from the discrete-time event history analysis. There is no significant difference between these groups, although the results are in the predicted direction (log odds ratio = 0.041, s.e. = 0.491), with the Chinese individuals progressing to menopause slightly faster than the non-Chinese comparison group (for full model results, see the electronic supplementary material, table S3). A power analysis demonstrates that to observe a statistically significant difference between these groups given this small effect size we would need a sample of over 10 000 individuals [43,44].
Figure 3.
Predicted hazard rate of progression to last menstruation based on discrete-time event history analysis for matched set of Chinese Indonesians and non-Chinese Indonesians, plotted at the means of covariates (see the electronic supplementary material, table S3 for full model). Error bars represent 95% CIs. (Online version in colour.)
Our resampling analysis finds comparable results. The average effect size (measured as log odds) for the Chinese Indonesians' progression to menopause is 0.001 compared with non-Chinese Indonesians with a 95% confidence interval between −1.05 and 1.22. Again, there is no significant difference between Chinese and non-Chinese Indonesian's progression to menopause.
(b). Does individual-level variation in postnuptial residence predict age at menopause?
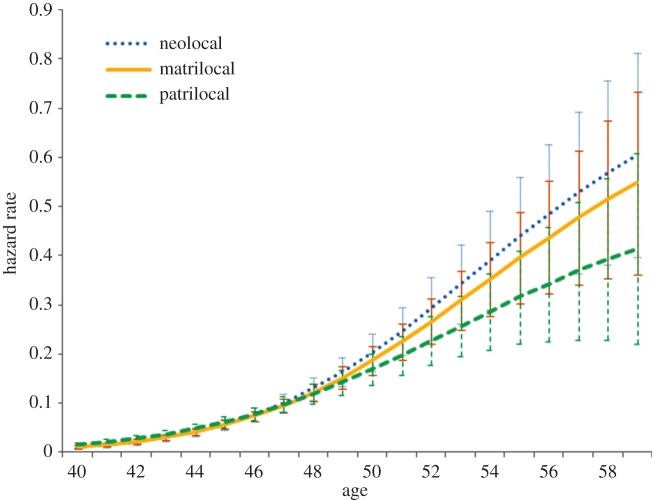
Figure 4 displays the hazard rates based on the random effects discrete-time event history analysis of the effect of individual-level postnuptial residence on progression to menopause (full model shown in the electronic supplementary material, table S4). The graph suggests that women who lived patrilocally postnuptially progress to menopause slightly more quickly at younger ages (before age 47), but progress more slowly afterwards, although at no age are women who lived patrilocally progressing to menopause at a significantly faster rate than either of the other postmarital residence patterns.
Figure 4.
Predicted hazard rate of progression to last menstruation based on random effects discrete-time event history analysis of individual-level postnuptial residence, with a random effect for ethnicity, plotted at the means of covariates (see the electronic supplementary material, table S4 for full model). Error bars represent 95% CIs. (Online version in colour.)
In summary, regardless of the timescale in which we test for female intergenerational conflict effects on menopause, we never find a significantly faster progression for women living patrilocally.
4. Discussion
Our results show no evidence for an earlier age at menopause for ancestrally patrilocal groups compared with matrilocal groups. These findings do not support the female intergenerational conflict model wherein cultural institutions of postnuptial residence select for different ages at menopause. Nor does it support a more recent model of intragenomic conflict suggesting that age at menopause should be earlier in ancestrally matrilocal populations than ancestrally patrilocal ones [45]. However, the result is still consistent with one interpretation of the female intergenerational conflict model: that all humans share genes for similar ages at menopause because they all evolved in ancestrally patrilocal communities.
Even if there have not been genetic changes across human populations affecting age at menopause, it is possible that women are able to facultatively adjust age at last birth more easily than age at menopause based on the degree of intergenerational conflict they face. To test this, we ran a discrete-time event history analysis on the progression to last birth by both ethnic-group-level and individual-level postmarital residence (see the electronic supplementary material). However, we find no evidence that age at last birth is earlier for women who either live patrilocally or are from patrilocal ethnic groups.
It is possible that natural selection has not had enough time to allow age at menopause to evolve by ethnic group. However, recent evidence suggests that humans have experienced rapid recent genetic evolution within the past several thousand years [46] and age at menopause is projected to increase by one month per generation [47], arguably owing to selection pressures (although intergenerational inheritance of lifestyle factors may also account for these changes), making plausible the assumption that age at menopause could have changed in our recent evolutionary history had there been strong enough selection pressures for it to do so. An additional concern is that ethnic groups in our sample may not have been consistently matrilocal or patrilocal throughout their evolutionary past, giving natural selection little opportunity to act. However, cultural phylogenetic analyses from Austronesia suggests that changes from ancestral matrilocality to patrilocality were probably maintained (as opposed to switching back and forth through time) [30]. Finally, high rates of inter-ethnic migration, especially between neighbouring Indonesian groups [48], may result in little associations between cultural institutions and genetic variants, but this should be of less concern for our deep evolutionary comparison of Chinese and non-Chinese Indonesians.
A second possible reason why we failed to find an effect is the necessary simplification in coding postnuptial residence. While we have defined groups as either female-dispersing or male-dispersing, these categories are not always clear cut. In some contexts, many couples may originate from the same village, so that neither individual is dispersing to a new community. In some patrilocal or neolocal contexts, females may not disperse for several years after marriage or the birth of one or more children. In our sample, three ethnic groups have an alternative postnuptial residence immediately following marriage. Javanese, Sumbawanese and Melayu ethnic groups practice matrilocality immediately following marriage. Re-running the ethnic-group-level progression to menopause with these groups coded as matrilocal shows that postnuptial residence is not a significant predictor of progression to menopause (see the electronic supplementary material, table S5), in contrast to our previous result where multilocal ethnic groups progressed significantly more slowly than matrilocal or patrilocal groups (Javanese and Sumbawense were coded as multilocal in our main analysis).
Third, culture may alter the parameters of reproductive conflict between generations. The model of female intergenerational conflict predicts that mothers-in-law may terminate reproduction because of conflict with daughters-in-law. However, in contexts with extra-somatic wealth, parents may have considerable control over the reproductive behaviour of their offspring when the older generation controls the resources necessary for reproduction. In such contexts, intergenerational conflicts may be resolved in a coercive manner favourable to parents, because their investments are necessary for children to marry, for example, either because they arrange the marriage or provide resources for dowry or bride wealth. In such cases, we would expect mothers-in-law to prevent their sons from marrying if they wish to continue reproducing and can foresee a reproductive conflict with future daughters-in-law. This may mean that mothers-in-law actually win the conflict with a potential future daughter-in-law. Additionally, cultural institutions and norms may be used to resolve these conflicts, as is suggested by ethnographic reports that many women terminate reproduction once a grandchild is born, although this varies by context [49]. Ultimately, cultural adaptations may obviate the need for genetic solutions [50,51]. If women are adaptively terminating reproduction through cultural rather than through biological means, we would expect age at last birth to be earlier in traditionally patrilocal communities and find no such effect (see the electronic supplementary material). However, given that cultural norms can become decoupled from genetic fitness, they may no longer serve their original purpose and result in non-fitness maximizing behaviour. The early age at which women have their last birth in this population (well before the biologically possible age at last reproduction) may be maladaptive and could explain why we do not find any association of age at last birth and postnuptial residence.
Fourth, our analysis of whether age at menopause was correlated with individual-level (rather than with group-level) postmarital residence used a woman's own postnuptial residence to predict her age at menopause. A more appropriate test of the intergenerational conflict model would be to use her children's postmarital residence to predict her age at menopause; unfortunately, we do not have enough data on children's postnuptial residence to do this, and assumed instead that the woman's own postmarital residence decisions would be correlated with those of her children. This may not be an accurate assumption, and further analyses should be conducted to investigate whether children's postnuptial residence influences progression to menopause.
Finally, the female intergenerational conflict model does not account for male intergenerational conflict. If we view female-dispersing groups as experiencing conflicts between fathers and sons instead of mothers-in-law and daughters-in-law, then we would expect fathers to win conflicts over sons (if the father's future children will be full siblings to the son), which complicates how couples resolve the conflict [15]. It is possible that the gender which controls reproductive decision-making decides the resolution of the conflict. Alternatively, couples may make reproductive decisions together. In that case there may not be a clear winner to the conflict as both couples lose the same fitness on average if the other couple reproduces (again assuming that the man's parents are producing a full sibling).
In summary, we find no evidence of earlier termination of reproduction in patrilocal communities compared with matrilocal ones. This may suggest that the female intergenerational conflict model should be re-examined as an explanation for the evolution of menopause. We are wary of using our analysis to be too critical of this model, as our results may be affected by data limitations or by cultural changes that affect our ability to detect intergenerational conflict, but we do note that this is the most direct empirical test of the female intergenerational conflict model to date. Additionally, there are theoretical concerns with the model given that it does not adequately incorporate intergenerational conflict among other individuals of the family, suggesting that a re-examination of the details of this model could be beneficial. Despite finding no support for the specific predictions derived from the Cant and Johnstone's model [11], our results do not imply that intergenerational conflict is unimportant. We find that women living in ancestrally multilocal groups progress to menopause at a slower rate than those from matrilocal and patrilocal groups (although these results are sensitive to when in the couple's married life postnuptial residence codes for ethnic groups are assessed). While this may result from gene–culture coevolutionary processes, it is also possible that individuals facultatively adjust age at menopause based on perceptions of their ethnic group's postmarital residence norms. Additionally, the causal direction may be reversed—between-group genetic differences in menopause may have influenced postmarital residence norms. Regardless, this result can be interpreted in light of intergenerational conflict. When couples have a choice of where to live (as is the case with ambilocal postmarital residence), they may choose to live where there are more resources, which may result in reduced intergenerational conflict. Living separate from kin (as is the case with neolocal postmarital residence) may similarly reduce reproductive conflict. The reduction of reproductive conflict may result in a later age of fertility cessation. Further work could be conducted to test whether a later age at menopause in multilocal groups is a consistent finding in other parts of the world, and to explore its possible explanations.
Supplementary Material
Acknowledgements
Thanks to Heidi Colleran, Susie Schaffnit, Paula Sheppard, Peter Sozou, Sandra Virgo and two anonymous reviewers for helpful comments.
Data accessibility
Data are publicly available from http://www.rand.org/labor/FLS/IFLS.
Funding statement
This research was supported by the European Research Council.
References
- 1.Williams GC. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution (N.Y.) 11, 398–411. [Google Scholar]
- 2.Peccei JS. 2001. Menopause: adaptation or epiphenomenon? Evol. Anthropol. 10, 43–57. ( 10.1002/evan.1013) [DOI] [Google Scholar]
- 3.Shanley DP, Kirkwood TB. 2001. Evolution of the human menopause. Bioessays 23, 282–287. () [DOI] [PubMed] [Google Scholar]
- 4.Hawkes K. 2003. Grandmothers and the evolution of longevity. Am. J. Hum. Biol. 15, 380–400. ( 10.1002/ajhb.10156) [DOI] [PubMed] [Google Scholar]
- 5.Lee RD. 2003. Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. Proc. Natl Acad. Sci. USA 100, 9637–9642. ( 10.1073/pnas.1530303100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanley DP, Sear R, Mace R, Kirkwood TBL. 2007. Testing evolutionary theories of menopause. Proc. R. Soc. B 274, 2943–2949. ( 10.1098/rspb.2007.1028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill K, Hurtado A. 1991. The evolution of premature reproductive senescence and menopause in human females. Hum. Nat. 2, 313–350. ( 10.1007/BF02692196) [DOI] [PubMed] [Google Scholar]
- 8.Marlowe F. 2000. An alternative explanation of menopause. Hum. Nat. 11, 27–42. ( 10.1007/s12110-000-1001-7) [DOI] [PubMed] [Google Scholar]
- 9.Levitis DA, Burger O, Lackey LB. 2013. The human post-fertile lifespan in comparative evolutionary context. Evol. Anthropol. 22, 66–79. ( 10.1002/evan.21332) [DOI] [PubMed] [Google Scholar]
- 10.Alberts SC, et al. 2013. Reproductive aging patterns in primates reveal that humans are distinct. Proc. Natl Acad. Sci. USA 110, 13 440–13 445. ( 10.1073/pnas.1311857110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavelka MSM, Fedigan LM. 1991. Menopause: a comparative life history perspective. Yearb. Phys. Anthropol. 34, 13–38. ( 10.1002/ajpa.1330340604) [DOI] [Google Scholar]
- 12.Emery Thompson M, et al. 2007. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr. Biol. 17, 2150–2156. ( 10.1016/j.cub.2007.11.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cant MA, Johnstone RA. 2008. Reproductive conflict and the separation of reproductive generations in humans. Proc. Natl Acad. Sci. USA 105, 5332–5336. ( 10.1073/pnas.0711911105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moya C, Sear R. 2014. An intergenerational conflict model of age at first birth in humans. Peer J. Prepr. 2, e345v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji T, Xu JJ, Mace R. 2013. Intergenerational and sibling conflict under patrilocality: a model of reproductive skew applied to kinship. Hum. Nat. 25, 66–79. ( 10.1007/s12110-013-9188-6) [DOI] [PubMed] [Google Scholar]
- 16.Lahdenperä M, Gillespie DOS, Lummaa V, Russell AF. 2012. Severe intergenerational reproductive conflict and the evolution of menopause. Ecol. Lett. 15, 1283–1290. ( 10.1111/j.1461-0248.2012.01851.x) [DOI] [PubMed] [Google Scholar]
- 17.Mace R, Alvergne A. 2012. Female reproductive competition within families in rural Gambia. Proc. R. Soc. B 279, 2219–2227. ( 10.1098/rspb.2011.2424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skjærvø GR, Røskaft E. 2013. Menopause: no support for an evolutionary explanation among historical Norwegians. Exp. Gerontol. 48, 408–413. ( 10.1016/j.exger.2013.02.001) [DOI] [PubMed] [Google Scholar]
- 19.Chapais B. 2008. Primeval kinship: how pair-bonding gave birth to human society. Cambridge, MA: Harvard University Press. [Google Scholar]
- 20.Ember CR. 1975. Residential variation among hunter–gatherers. Cross-Cultural Res. 10, 199–227. ( 10.1177/106939717501000302) [DOI] [Google Scholar]
- 21.Marlowe FW. 2004. Marital residence among foragers. Curr. Anthropol. 45, 277–284. ( 10.1086/382256) [DOI] [Google Scholar]
- 22.Hill K, et al. 2011. Co-residence patterns in hunter–gatherer societies show unique human social structure. Science 331, 1286–1289. ( 10.1126/science.1199071) [DOI] [PubMed] [Google Scholar]
- 23.Feldman MW, Laland KN. 1996. Gene–culture coevolutionary theory. Trends Ecol. Evol. 5347, 453–457. ( 10.1016/0169-5347(96)10052-5) [DOI] [PubMed] [Google Scholar]
- 24.Hawkes K, Smith KR. 2010. Do women stop early? Similarities in fertility decline in humans and chimpanzees. Ann. NY Acad. Sci. 1204, 43–53. ( 10.1111/j.1749-6632.2010.05527.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Bruin JP, Bovenhuis H, van Noord PA, Pearson PL, van Arendonk JA, te Velde ER, Kuurman WW, Dorland M. 2001. The role of genetic factors in age at natural menopause. Hum. Reprod. 16, 2014–2018. ( 10.1093/humrep/16.9.2014) [DOI] [PubMed] [Google Scholar]
- 26.Te Velde ER, Dorland M, Broekmans FJ. 1998. Age at menopause as a marker of reproductive ageing. Maturitas 30, 119–125. ( 10.1016/S0378-5122(98)00067-X) [DOI] [PubMed] [Google Scholar]
- 27.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. 2001. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am. J. Epidemiol. 153, 865–874. ( 10.1093/aje/153.9.865) [DOI] [PubMed] [Google Scholar]
- 28.Gold EB, et al. 2013. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am. J. Epidemiol. 178, 70–83. ( 10.1093/aje/kws421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karafet TM, Hallmark B, Cox MP, Sudoyo H, Downey S, Lansing JS, Hammer MF. 2010. Major east-west division underlies Y chromosome stratification across Indonesia. Mol. Biol. Evol. 27, 1833–1844. ( 10.1093/molbev/msq063) [DOI] [PubMed] [Google Scholar]
- 30.Jordan FM, Gray RD, Greenhill SJ, Mace R. 2009. Matrilocal residence is ancestral in Austronesian societies. Proc. R. Soc. B 276, 1957–1964. ( 10.1098/rspb.2009.0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murdock G. 1967. Ethnographic atlas. Pittsburgh, PA: University of Pittsburgh Press. [Google Scholar]
- 32.Strauss J, Beegle K, Sikoki B, Dwiyanto A, Herawati Y, Witoelar F. 2004. The third wave of the Indonesia family life survey (IFLS): overview and field report. WR-144/1-NIA/NICHD.
- 33.Strauss J, Witoelar F, Sikoki B, Wattie AM. 2009. The fourth wave of the Indonesian family life survey (IFLS4): overview and field report. WR-675/1-NIA/NICHD.
- 34.Frankenberg E, Thomas D. 2000. The Indonesia family life survey (IFLS): study design and results from waves 1 and 2. Santa Monica, CA: RAND. DRU-2238/1-NIA/NICHD. [Google Scholar]
- 35.Abouheif E. 1999. A method for testing the assumption of phylogenetic independence in comparative data. Evol. Ecol. Res. 1, 895–909. [Google Scholar]
- 36.Nunn C. 2011. The comparative approach in evolutionary anthropology and biology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 37.Reeve JP, Abouheif E. 2003. Phylogenetic independence See http://biology.mcgill.ca/faculty/abouheif/programs_pi.htm.
- 38.Sievert L, Hautaniemi S. 2003. Age at menopause in Puebla, Mexico. Hum. Biol. 75, 205–226. ( 10.1353/hub.2003.0037) [DOI] [PubMed] [Google Scholar]
- 39.Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. 2010. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 13, 419–428. ( 10.3109/13697137.2010.507886) [DOI] [PubMed] [Google Scholar]
- 40.Cramer DW, Xu H. 1996. Predicting age at menopause. Maturitas 23, 319–326. ( 10.1016/0378-5122(96)00992-9) [DOI] [PubMed] [Google Scholar]
- 41.Heinemann K, Rübig A, Strothmann A, Nahum GG, Heinemann LAJ. 2008. Prevalence and opinions of hormone therapy prior to the Women's Health Initiative: a multinational survey on four continents. J. Women's Health 17, 1151–1166. ( 10.1089/jwh.2007.0584) [DOI] [PubMed] [Google Scholar]
- 42.Suryadinata L. 1972. Indonesian Chinese education: past and present. Indonesia 14, 49–71. ( 10.2307/3350732) [DOI] [Google Scholar]
- 43.Schoenfeld DA, Borenstelntt M. 2005. Calculating the power or sample size for the logistic and proportional hazards models. J. Stat. Comput. Simul. 75, 771–785. ( 10.1080/00949650410001729445) [DOI] [Google Scholar]
- 44.Schoenfeld DA. 1983. Sample-size formula for the proportional-hazards regression model. Biometrics 39, 499–503. ( 10.2307/2531021) [DOI] [PubMed] [Google Scholar]
- 45.Úbeda F, Ohtsuki H, Gardner A. 2014. Ecology drives intragenomic conflict over menopause. Ecol. Lett. 17, 165–174. ( 10.1111/ele.12208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawks J, Wang ET, Cochran GM, Harpending HC, Moyzis RK. 2007. Recent acceleration of human adaptive evolution. Proc. Natl Acad. Sci. USA 104, 20 753–20 758. ( 10.1073/pnas.0707650104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byars SG, Ewbank D, Govindaraju DR, Stearns SC. 2009. Natural selection in a contemporary human population. Proc. Natl Acad. Sci. USA 107, 1787–1792. ( 10.1073/pnas.0906199106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinhauer H. 1994. The Indonesian language situation and linguistics: prospects and possibilities. J. Hum. Soc. Sci. Southeast Asia 150, 755–784. [Google Scholar]
- 49.Ware H. 1979. Social influences on fertility at later ages of reproduction. J. Biosoc. Sci. 11, 75–96. ( 10.1017/S0021932000024317) [DOI] [PubMed] [Google Scholar]
- 50.Boyd R, Richerson PJ, Henrich J. 2011. The cultural niche: why social learning is essential for human adaptation. Proc. Natl Acad. Sci. USA 108, 10 918–10 925. ( 10.1073/pnas.1100290108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richerson PJ, Boyd R. 2008. Not by genes alone: how culture transformed human evolution. Chicago, IL: Chicago University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are publicly available from http://www.rand.org/labor/FLS/IFLS.