Abstract
The zygodactyl orientation of toes (digits II and III pointing forwards, digits I and IV pointing backwards) evolved independently in different extant bird taxa. To understand the origin of this trait in modern birds, we investigated the development of the zygodactyl foot of the budgerigar (Psittaciformes). We compared its muscular development with that of the anisodactyl quail (Galliformes) and show that while the musculus abductor digiti IV (ABDIV) becomes strongly developed at HH36 in both species, the musculus extensor brevis digiti IV (EBDIV) degenerates and almost disappears only in the budgerigar. The asymmetric action of those muscles early in the development of the budgerigar foot causes retroversion of digit IV (dIV). Paralysed budgerigar embryos do not revert dIV and are anisodactyl. Both molecular phylogenetic analysis and palaeontological information suggest that the ancestor of passerines could have been zygodactyl. We followed the development of the zebra finch (Passeriformes) foot muscles and found that in this species, both the primordia of the ABDIV and of the EBDIV fail to develop. These data suggest that loss of asymmetric forces of muscular activity exerted on dIV, caused by the absence of the ABDIV, could have resulted in secondary anisodactyly in Passeriformes.
Keywords: anisodactyly, myogenesis, Passeriformes, Psittaciformes, zygodactily
1. Introduction
Differences in the morphology of the foot are among the main factors that allowed the specialization of the avian leg (for a review, see [1]). The ancestral condition to extant birds was a four-toed cursorial foot (but functionally tridactyl), as observed in non-avian theropods [2,3]. The anisodactyl foot—where the digits II, III and IV are oriented forwards, while digit I is oriented backwards—evolved in early Avialae by the retroversion of the hallux [4,5]. In extant birds, modifications of the anisodactyl foot produced similar morphologies independently in different taxa. Several families reduced or lost the hallux, among them some palaeognathous (ostrich and allies), Rallidae (rails), Mirandornithes (flamingos and grebes) and Charadrii (plovers, sandpipers and allies). Palmated feet with webs between the digits evolved in many species of aquatic birds. Further anatomical variation was generated by changes in the orientation of the digits. Zygodactyl foot have digits I and IV oriented backwards and digits II and III oriented forwards, and have evolved independently by the backward orientation of digit IV (dIV) in at least three extant clades: Cuculidae (cuckoos), Psittaciformes (parrots) and Piciformes (woodpeckers and allies). Some birds are semi-zygodactyl and can facultatively change the orientation of dIV, like Musophagidae (turacos), Pandionidae (ospreys), Strigiformes (owls) and Coliidae (mousebirds). An arrangement that is functionally similar to zygodactyly, named heterodactyly, has evolved in Trogoniformes (trogons), where digits I and II are oriented backwards, and digits III and IV are oriented forwards. Finally, swifts (Apodidae) have a pamprodactyl foot, a condition where all four digits can be oriented forwards.
It is remarkable that almost every variation of the anisodactyl foot has evolved independently in different lineages. This suggests that not only ecological demands, but also developmental factors could have driven the direction of evolutionary changes [6,7]. To understand the nature of those factors, we explored one of these transformations—the origin of the zygodactyl feet—from a developmental perspective.
Previous studies about the origin of zygodactyly have focused on its functionality and on its underlying musculoskeletal organization. Zygodactyly has often been considered as an adaptation for climbing, perching or manipulation [1,8–10]. However, taking into account the diversity of niches occupied by extant zygodactyl species, and also the fact that those same niches are occupied by non-zygodactyl birds, it is not straightforward to find a common evolutionary scenario to explain its origin. Moreover, anatomical comparisons show that there is not a common muscular anatomy to all zygodactyl feet [11–14], making it difficult to recognize which muscular topology is specifically related to that arrangement. The digits are controlled by a complex combination of individual muscles. Flexor muscles, responsible for grasping movements, are found in the ventral side, while extensor muscles, responsible for releasing, are found in the dorsal side. Adductor and abductor muscles are defined in relation to the limb axis. Therefore, the abductor of dIV is the muscle inserted on the lateral side of dIV, while the abductor of digit II (dII) is inserted on the medial side of dII. The muscles that control the digits can be further classified according to their location into intrinsic and extrinsic muscles. The extrinsic muscles are situated in the crus, while the intrinsic muscles are situated in the foot. Each zygodactyl taxon differs in the number and the organization of those muscles.
In this work, we investigate the development of the foot musculoskeletal system of a zygodactyl bird—the budgerigar (Melopsittacus undulatus)—to discern which factors are causally related to its development and to understand the possible causes of its convergent evolution. For appropriate inference, we also examined the development of the foot of the Japanese quail (Coturnix japonica) and zebra finch (Taeniopygia guttata), two anisodactyl birds with radically different evolutionary histories.
2. Material and methods
(a). Animals
Fertilized eggs of Japanese quail (C. japonica), zebra finch (T. guttata) and budgerigar (M. undulatus) were obtained from colonies at the University of Chile. The eggs were removed from nests and incubated in an incubator with automatic rotating shelves at 37.5°C and 60% humidity. The nomenclature employed for the skeleton, muscles and tendons follow Nomina Anatomica Avium [15]. The embryos were staged using the normal table for Gallus gallus [16].
(b). Cartilage and bone stain
Developmental series for each species investigated were prepared for skeletal staining. Embryos were fixed in 100% methanol for at least 2 days. Cartilage was stained with a solution of 0.02% Alcian Blue (Sigma-Aldrich) diluted in 5 : 1 ethanol/acetic acid for 24–48 h. Bone was stained with a solution of 0.02% Alizarin Red (Sigma-Aldrich) diluted in water with 0.5% of KOH for 2 h. The excess of dye was washed with water, and the muscles were macerated with 2% KOH. The embryos were cleared in a series of glycerol.
(c). Immunohistochemistry and immunofluorescence
Four embryos for each stage were used for immunofluorescence against myosin (C. japonica, M. undulatus and T. guttata) and tenascin (M. undulatus). Embryos were fixed in Dent's fix (4 : 1 methanol : DMSO) for 2 h, dehydrated in a series of methanol and left for at least 12 h at −80°C. Then, the specimens were bleached in Dent's bleaching (4 : 1 : 1 methanol : DMSO : H2O2) for 24 h at room temperature. They were rehydrated in phosphate buffer with 1% triton X-100 (Sigma) (PBST). Primary antibodies against myosin (MF-20 from DSHB, Iowa) and tenascin (M1-B4 from DSHB, Iowa) were diluted 1 : 20 in PBST, 5% horse serum and 5% DMSO. Embryos were kept in primary antibody for 48 h at 4°C in an orbital shaker. Embryos were washed in PBST six times for 1 h. Secondary antibodies anti-mouse made in donkey coupled to horseradish peroxidase (715-035-150, Jackson ImmunoResearch) or Alexa-Fluor 594 (715-585-150, Jackson ImmunoResearch) were diluted 1 : 300 in PBST, 2% horse serum and 5% DMSO. Embryos were kept in secondary antibody for 24 h at 4°C in an orbital shaker. They were washed again in PBST six times for 1 h. Embryos labelled with horseradish peroxidase were revealed with Diaminobenzidine substrate (11718096001, Roche Applied Bioscience). To avoid background, the first 10 min of reactions were carried out in ice.
(d). Paralysis
Fourteen budgerigar embryos were pharmacologically paralysed; eight survived. After candling the egg, a small hole was opened with a needle over the air sac. A single dose of 20 μl of a solution containing 2 mg ml−1 of decamethonium bromide diluted in phosphate (modified from [17]) was then delivered with a micropipette to embryos at HH31 [16]. The egg was sealed with a glue gun and incubated without movement for 4 days.
3. Results
(a). Rotation of digit IV in budgerigar development (Psittaciformes)
The first step to understand the development of the zygodactyl foot was to determine when and how dIV changes its orientation, departing from the development of the anisodactyl foot. Limb development is well known in chicken and quail, and thus these animals yield good anisodactyl models to compare with the budgerigar. In these taxa, the toes develop from cartilages originated in a pad-like flattening of the distal limb bud. Chicken limbs exhibit the cartilages of the four digits at HH31, except for the distal-most phalanges [16]. At this stage, toes are united by mesenchymal tissue and oriented in the same plane. Posteriorly, the tissue between the toes becomes a thin web, the digits elongate conspicuously, and a collar of bone begins to grow at the centre of each metatarsus (HH34). Eventually, the digits separate from each other (HH35), the hallux rotates ventrally and the primordia of the claws appear (HH36).
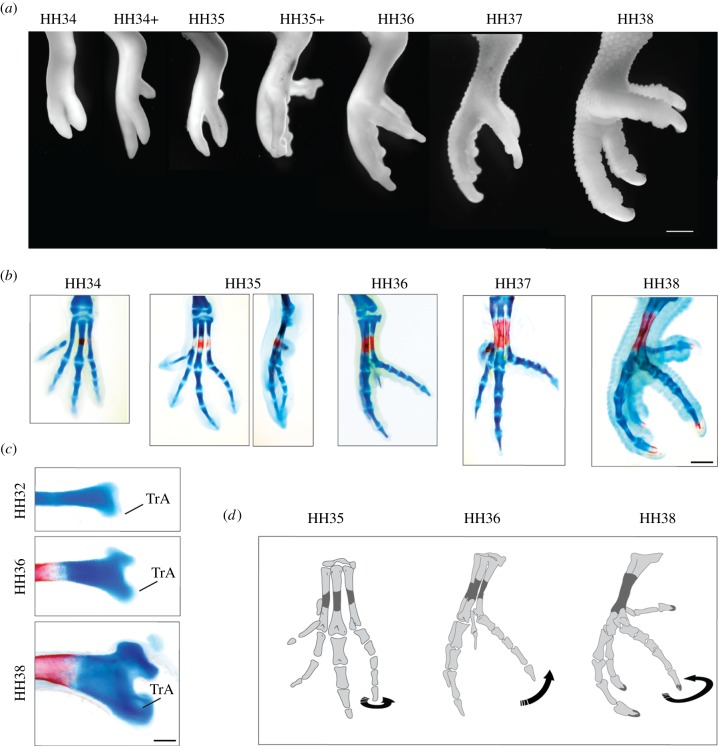
We produced a developmental series for the budgerigar stained with Alcian Blue and Alizarin Red. At early stages, the development of the foot of the budgerigar does not differ from what has been described for the domestic chicken. The first sign of zygodactyly occurs around HH35, when dIV rotates medio-laterally at the level of the articulation between the metatarsus and the proximal phalanx (figure 1a,b). Consequently, the medial side of the phalanges of dIV comes to face dorsal and the lateral side to face ventral. The dIV is then flexed and acquires a right angle in relation to the main axis of the metatarsus (HH36). The morphology at this stage is similar to that of adult semi-zygodactyl birds like owls and touracos. Eventually, dIV rotates further towards medial and the foot becomes fully zygodactyl (HH37). The rotation and flexion of dIV is followed by a ventral outgrowth of the distal end of metatarsal IV. First, it develops a wing-like flange similar to the trochlea accessoria (TrA) observed in semi-zygodactyl birds (HH36); later it acquires the hooked shape (or sehnenhalter) characteristic of the TrA of fully zygodactyl birds like Psittaciformes and Piciformes (figure 1c). In summary, during the development of the budgerigar foot, dIV rotates at HH35, flexes at HH36 and further rotates at HH37 (figure 1d).
Figure 1.
(a) The anatomy of budgerigar early foot development. (b) The anatomy of budgerigar early hindlimb skeletal development. Cartilages stained with Alcian Blue, bones stained with Alizarin Red. (c) The trochlea accessoria (TrA) at different stages. MtIV has been isolated and photographed in lateral view. (d) Diagram picturing the change of dIV orientation during development. Scale bars: (a,b) 1 mm; (c) 200 μm. (Online version in colour.)
(b). Extensor brevis digiti IV is lost in the development of the budgerigar
The time and mode of change in orientation of dIV suggest that early muscular activity could be acting in the transformation of the foot. The early development of avian hindlimb muscles has been studied in the domestic chicken [18–21]. Muscle cell precursors that originated in the lateral somite migrate to the limb bud around HH22 [22]. The precursor cells generate a dorsal and a ventral mass of differentiating muscle fibres. Each mass divides, generating successively smaller masses, and eventually forming each individual muscle around HH35. Movements of the ankle and digits begin at HH32 and at HH35, respectively [23].
The complex combination of muscles and tendons controlling bird toes is highly variable among taxa. There is a large amount of literature concerning this variation, as it has been employed to investigate the high-order phylogeny of birds [12,24,25]. Those studies suggest that for Neornithes, the ancestral condition for the muscles controlling dIV is similar to the condition observed in Galliformes, like the chicken and the quail. In those birds, the extension of dIV is controlled by one extrinsic muscle attached to the dorsal side of the distal phalanx (M. extensor digitorum longus) and one intrinsic muscle attached to the medial side of the proximal phalanx (M. extensor brevis digiti IV—EBDIV). The medial insertion of EBDIV probably enables it to act also as an adductor. The flexion of dIV is controlled by two extrinsic muscles attached to the distal phalanx (M. flexor digitorium longus and flexor perforatus digiti IV), while its abduction is produced by a ventral intrinsic muscle inserted in the lateral face of the proximal phalanx (M. abductor digiti IV—ABDIV) (figure 3d).
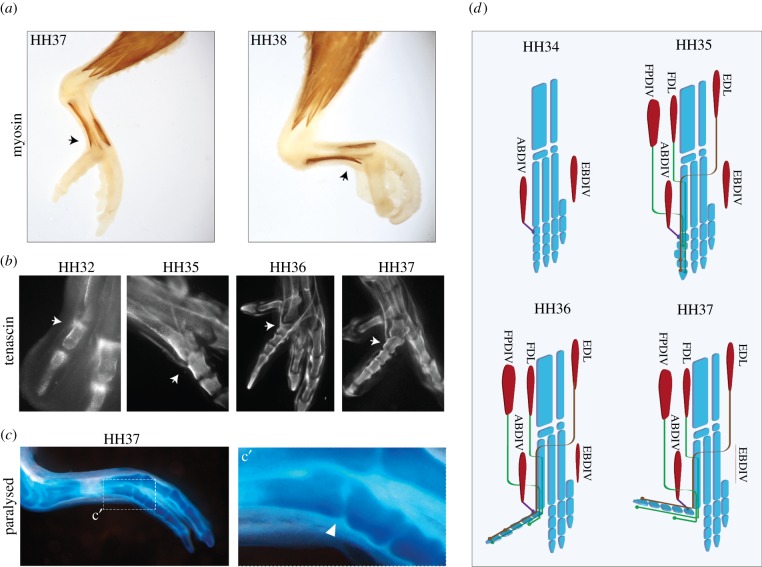
Figure 3.
(a) Immunohistochemical reaction against myosin 2 showing the late disposition of the ABDIV (black arrows) in relation to changes in the orientation of dIV in budgerigars. (b) Immunohistochemical reaction against tenascin showing the insertion of the tendon of the ABDIV (white arrows) during budgerigar development. (c) Morphology of dIV in paralysed embryos; (c′) detail of the trochlea accessoria. (d) Diagram illustrating the development of the musculoskeletal system in the budgerigar. FDL, flexor digitorum longus; EDL, extensor digitorum longus; FPDIV, flexor perforatus digiti IV. (Online version in colour. In (d), muscles are shown in red, skeleton in blue.)
To compare the development of the muscles controlling dIV in the anisodactyl quail and the zygodactyl budgerigar, we examined embryos immunostained for myosin type 2. We found that the development of the extrinsic muscles is very similar in both species. However, the two species differ in the intricate set of fusions, changes in size and displacement of the muscle belly in relation to the metatarsus that are undergone by the intrinsic muscles.
Initially, the feet of both species exhibit the primordia of four dorsal and four ventral muscles (figure 2a,b). The most lateral primordia of the dorsal and ventral sides of the foot generate the EBDIV and ABDIV, respectively. Both muscles become well developed by HH36 in quail embryos. Nevertheless, in budgerigar embryos, EBDIV degenerates soon after it separates from the other muscles and is reduced to a thread at HH37 (the presence of a small EBDIV has been reported for one species of parrot [13], but the muscle is absent in other described Psittaciformes [11,26]). On the other hand, the ABDIV becomes strongly developed and its muscle belly extends for four-fifths of the metatarsal length (figure 3a).
Figure 2.
Immunohistochemical reaction against myosin 2 showing the early development of dorsal and ventral foot muscles in (a) quails and (b) budgerigars. Black arrows indicate the EBDIV. (Online version in colour.)
These differences in muscle size could generate an asymmetric relation of forces, which is coherent with the rotation of dIV observed at HH35. The absence of a muscle inserted in the medial side and a stout muscle in the lateral side could account for the rotation of dIV from medial to lateral. Then, as the lateral side of the phalanges passes to face ventral, the action of ABDIV results in flexion of the digit, which acquires a right angle in relation to the limb main axis (HH37; figure 3b,d). Further flexion of dIV by action of ABDIV, while being constrained by the extensor muscles, would lead to the final zygodactyl orientation, attained at HH38.
(c). Muscular paralysis in budgerigar results in anisodactyl feet
To test the hypothesis of the influence of early muscular activity on the development of zygodactyly, we pharmacologically immobilized budgerigar embryos and examined the resulting foot phenotype. The in ovo injection of the cholinergic agonist and neuromuscular blocker decamethonium bromide (n = 8) produced paralysis in budgerigar embryos. Treated embryos were anisodactyl at HH37 and HH38, and did not develop the TrA (figure 3c). Some other typical deformities observed in paralysed chickens [27] were also observed in paralysed budgerigars, such as reduced body size, incomplete fusion of the sternum and non-fused mandibular symphysis. Nevertheless, paralysed budgerigars did not exhibit any major general deformities that could justify the lack of rotation of dIV.
(d). Both extensor brevis digiti IV and abductor digiti IV are lost in the development of the zebrafinch (Passeriformes)
Most foot muscles are known to have been lost or vestigialized in Passeriformes [25,28], but their early development is still unknown. Taking into account the influence of muscle action on the development of zygodactyly in the budgerigar, we investigated the development of hindlimb muscles in the anisodactyl foot of the zebra finch (T. guttata). We observed that, similar to quails and budgerigars, the primordia of four ventral and four dorsal muscles are present at early stages (figure 4). However, most intrinsic muscles become vestigial during development and are almost indiscernible at HH37 (figure 4). The absence of both EBDIV and ABDIV acquires special significance since phylogenetic analyses of fossil and molecular data suggest that Passeriformes could have evolved from zygodactyl ancestors, and are thus secondarily anisodactyl (see Discussion).
Figure 4.
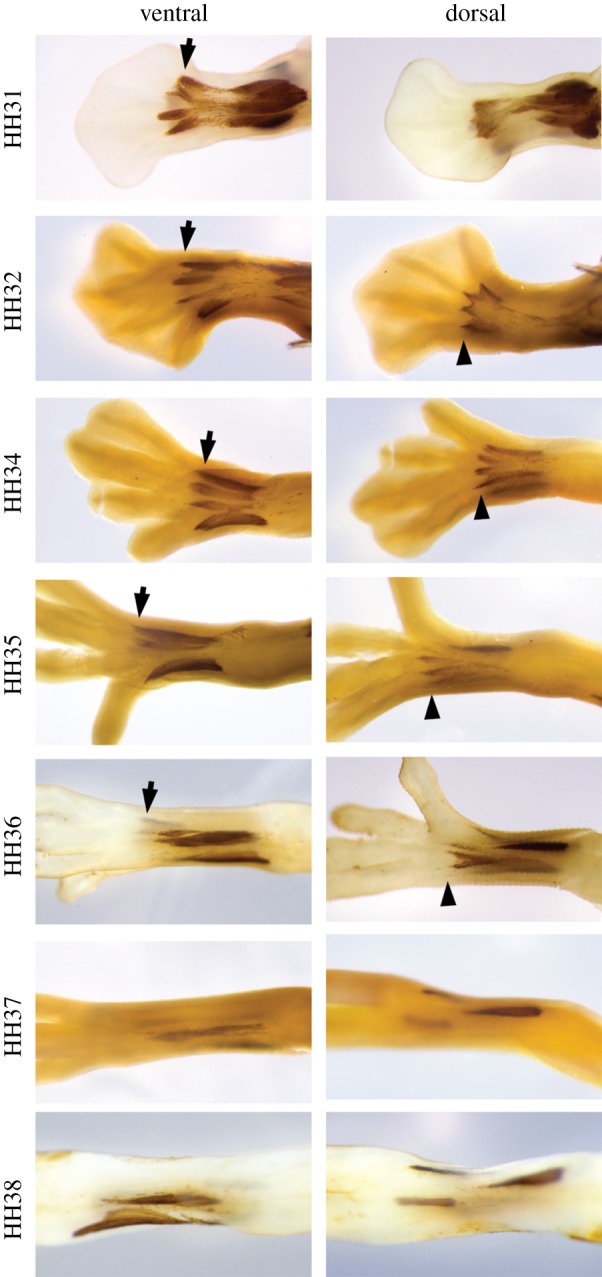
Immunohistochemical reaction against myosin 2 showing the early development of dorsal and ventral foot muscles in zebra finch. Most muscles disappear at HH37. Arrows, EBDIV; arrow heads, ABDIV. (Online version in colour.)
4. Discussion
Whole-mount immunostaining of the small developing muscles and tendons allowed us to compare the embryonic musculoskeletal system of anisodactyl and zygodactyl birds. These observations revealed that changes in the orientation of budgerigar dIV during ontogenesis occur concomitant to the modifications undergone by the intrinsic muscles controlling it. While the anisodactyl quail develops fully functional ABDIV and EBDIV, the zygodactyl budgerigar fails to develop an EBDIV. In the absence of the EBDIV restricting dIV movements, the abduction of dIV could cause the ontogenetic transition from anisodactyly to zygodactyly. Consistently, paralysed budgerigar embryos develop an anisodactyl foot, which confirms that muscle activity is necessary for the ontogenetic reorientation of dIV. In summary, our new data provide compelling evidence that the development of the zygodactyl foot in the budgerigar is caused by the asymmetric action of intrinsic muscles controlling dIV.
As the strong extrinsic flexor and extensor muscles can control more than one digit, most studies of the zygodactyl foot have looked for a common muscular organization of the extrinsic muscles controlling both retroverted digits dI and dIV in zygodactyl birds [1,8]. Our investigation of the ontogenesis of the musculoskeletal system identifies the reduction of small intrinsic muscles simultaneous to digit retroversion as the most likely cause for the development of zygodactyly in the budgerigar. The identification of the importance of intrinsic muscles brings an important new aspect to consider in the evolution of specializations in the avian foot.
(a). Epigenesis and convergence
The development of the musculoskeletal system depends on the functional interlocking of initially independent processes. Muscles, tendons and bones have different embryological origins. When brought together, their interactions have reciprocal morphogenetic effects [29]. Consequently, those interactions are potential sources of variation. Modifications in the early muscle precursor tissue can cause variations in the skeleton, and vice versa. The developmental mechanism here proposed for the origin of zygodactyly provides an example of the power of those embryonic interactions to generate and drive evolutionary transformations [7,30].
The avian tarsometatarsus originates from the fusion among the diaphyses of metatarsals II, III and IV, and the distal tarsal cartilage [31]. This fusion initially produces a common ossified diaphysis with three independent cartilaginous epiphyses in each side. At the proximal end, the interaction with tendons models the hypotarsus. At the distal end, the complex combination of muscles controlling the digits results in an intricate set of epigenetic influences over each of the individual epiphyses. For example, birds that lack intrinsic muscles of the foot usually present small incisuras intertrochlearis, narrow and parallel trochleae, etc. We propose that zygodactyly and the associated TrA are produced by this same kind of process. They result from the epigenetic influence of muscular forces over the skeleton.
The reoccurrence of a similar set of influences over the skeleton of related lineages could cause convergent evolution [32–34]. The early presence of the primordia of intrinsic muscles of the foot and its variable posterior disappearance or reduction yield a drive for the repetition of similar influences and, consequently, for the transformation of the avian foot skeleton. These two factors—the effect of muscle over the form of digits plus the bias for the transformation of intrinsic muscles—provide a mechanistic explanation for the convergent evolution of zygodactyly. The convergence would have been facilitated by the flexibility of the muscular system added to its influence over the skeleton. As muscles were reduced, lost or reacquired, similar skeletal morphologies evolved in parallel, including zygodactyly.
(b). Zygodactyly in extant and fossil birds
Zygodactyly has originated at least three times in extant birds: in Piciformes (Pici + Galbulae), Psittaciformes and Cuculidae (figure 5). The cuckoo-roller (Leptosomus discolor) may represent a fourth independent origin [41–43]. The developmental mechanism proposed for the origin of zygodactyly—an imbalance of forces in the lateral and medial sides of the proximal phalanx—may also explain the evolution of zygodactyly in Pici (barbets, toucans, woodpeckers and allies). They are the only other Telluraves (clade comprising most arboreal birds)—besides parrots—that have lost EBDIV but conserve ABDIV (figure 5) [14,15].
Figure 5.
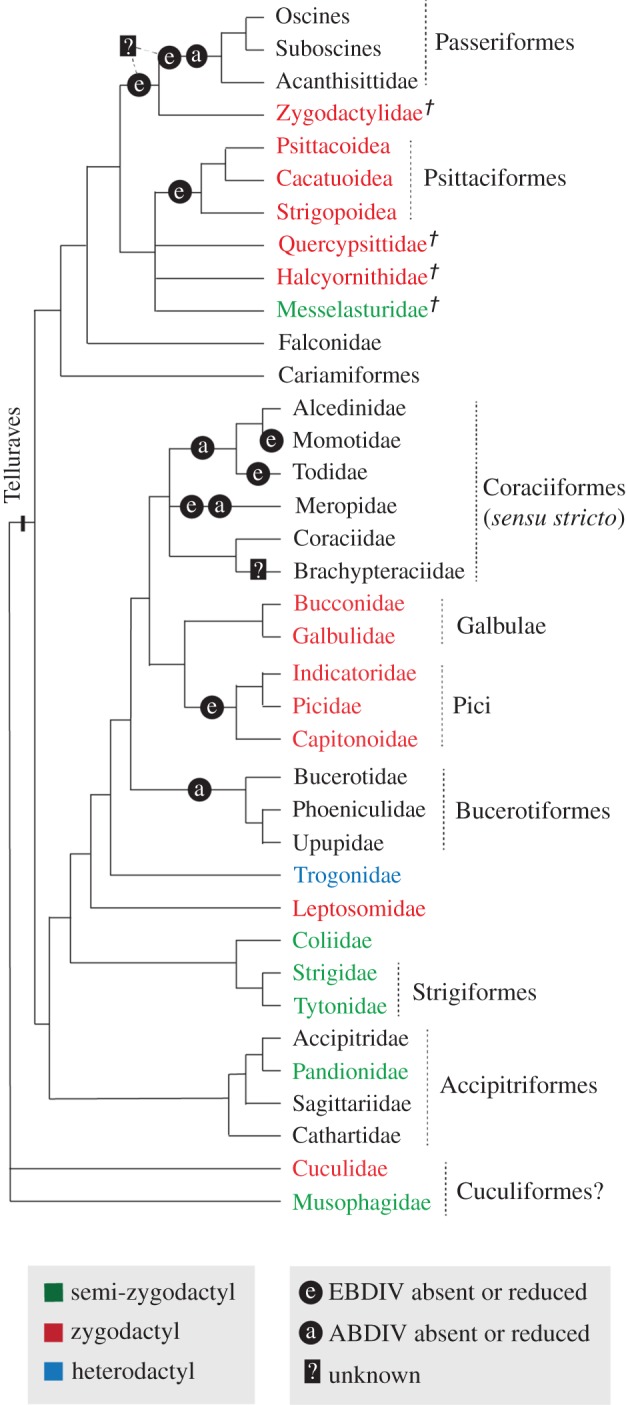
Phylogeny of Telluraves plus Cuculidae and Musophagidae, depicting the position of Zygodactylidae and the loss of the intrinsic muscles of dIV. Clades depicted were congruently obtained by the earlier studies [35–37]. The position of Zygodactylidae and stem Psittaciformes follow [38–40]. (Online version in colour.)
Extant Galbulae [12] and Cuculidae [14,44,45] exhibit the EBDIV, and a furrow (sulcus extensorius) on the dorsal tarsometatarsus indicates that EBDIV is also present in stem Cuculidae [46,47], stem Psittaciformes [38,48–50] and stem Piciformes [10,51]. Those taxa have two characteristics in common: a TrA smaller than the one present in crown Psitacciformes and crown Pici [10,41,46–48,51,52], and an unusual trajectory of the EBDIV tendon: it does not pass through a foramen between mtIII and mtIV—the canalis interosseus distalis—as in most anisodactyl birds; it goes over the incisura intertrochlearis lateralis and backwards to its insertion on the medial side of the proximal phalanx (figure 6) [11,47,50,51,53].
Figure 6.
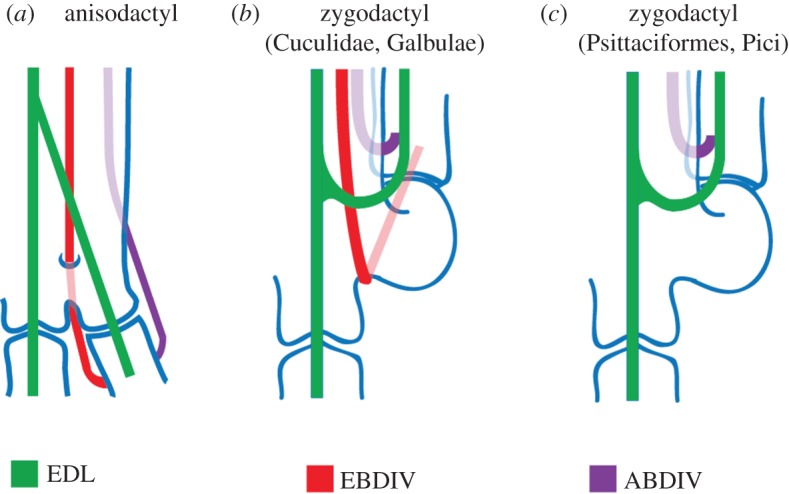
The topology of dIV tendons. (a) Anisodactyl, (b) zygodactyl with EBDIV and (c) zygodactyl lacking the EBDIV. In (b), the EBDIV does not pass through the canalis interosseus distalis. In (c), the tendon is absent. Modified from [11]. (Online version in colour.)
If a mechanism similar to the one proposed here for crown Psittaciformes and crown Pici is responsible for the development of zygodactyly in other taxa, an imbalance of forces acting on the proximal phalanx of dIV may be caused by subtler dissimilarities, like differences in the size of the muscles or differences in the time and position of insertion of the tendons, as suggested by the unusual trajectory of the EBDIV tendon over the incisura intertrochlearis lateralis (not through the canalis interosseus distalis).
The fossil record also shows that the condition observed in crown Psittaciformes and crown Pici (absence of the EBDIV, and large TrA) is convergently derived from zygodactyl ancestors having a smaller TrA and the tendon of EBDIV passing over the incisura intertrochlearis distalis. This morphological pattern suggests that the form and size of the TrA are related to the degree of imbalance of the forces acting over the metatarsus during development: those birds who lost or vestigialized EBDIV—like crown Pici, crown Psittaciformes and, probably, Zygodactylidae (see below)—exhibit larger TrA than those taxa that keep an EBDIV, like Galbulae and Cuculidae.
(c). Zygodactylidae and the loss of abductor digiti IV in Passeriformes
The presence of a TrA in the distal metatarsal of dIV allows the identification of zygodactyly even in non-articulated fossil skeletons. A group of those fossils has been recognized as a family of extinct birds appropriately called Zygodactylidae [10,39]. Even though they exhibit a well-developed TrA in the metatarsus IV, similar to that found in Pici and Psitaciformes, cladistic analysis suggests that Zygodactylidae is the sister taxon of passerines (Passeriformes) (figure 5) [40]. On the other hand, one of the most robust data produced by the new avian molecular phylogenies is the sister relationship between Passeriformes and Psittaciformes [35–37,54–57]. Therefore, both the extinct and extant outgroups to Passeriformes are zygodactyl, suggesting that Passeriformes had zygodactyl ancestors [10].
Considering our hypothesis on the role of ABDIV in the re-orientation of dIV in Psittaciformes, this muscle would be expected to have been present in Zygodactylidae. Therefore, the inferred ancestral state for the hypothetical clade (Psittaciformes (Zygodactylidae + Passeriformes)) would be the presence of ABDIV (figure 5). In this scenario, the further reduction of ABDIV in the lineage of passerine birds could have caused the loss of zygodactyly, making them secondarily anisodactyl. Passeriformes would be anisodactyl not by an equilibrium of forces, as in primarily anisodactyl birds, but by the absence of any force acting on the lateral and medial sides of the proximal phalanx (figure 7), similar to paralysed budgerigars. The presence of the primordia of the intrinsic muscles of the foot and their differential loss during the development of each taxon indicate the phylogenetic flexibility of the trait and support this possibility.
Figure 7.
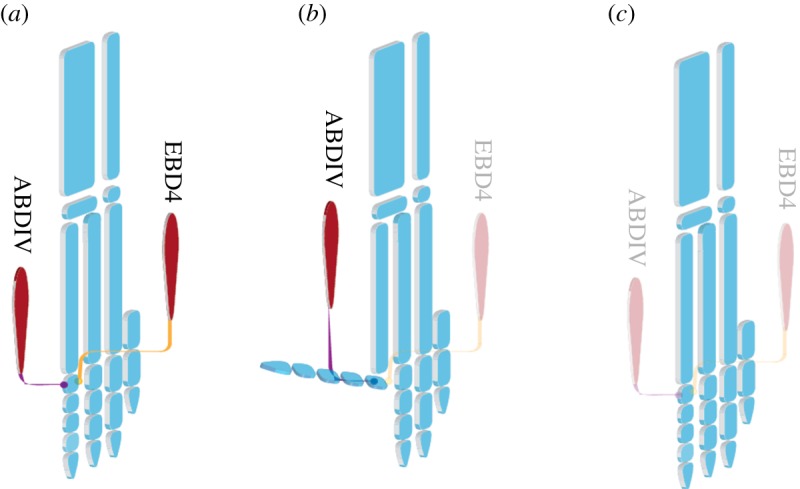
The distribution of dIV intrinsic muscles in (a) the primary anisodactyl quail, (b) the zygodactyl budgerigar and (c) the putative secondary anisodactyl zebra finch. Budgerigar lost the EBDIV but conserves the ABDIV. Both muscles are absent in the zebra finch. (Online version in colour. Muscles are shown in red, skeleton in blue.)
(d). Heterodactyly
The singular arrangement known as heterodactyly is unique to birds of the family Trogonidae. Those birds have digits I and II reverted, and digits III and IV pointing forwards. Molecular phylogenies include trogons in the Telluraves assemblage [35], but its specific position is unresolved. If a similar mechanism of asymmetrical forces at early development is responsible for the unique change of digit II orientation found in trogons, the loss of muscles ought to have occurred in the opposite sides to that found in zygodactlyl birds. The dorsal musculus abductor digiti II (ABDII) is inserted in the medial side of proximal phalange of the dII and the ventral musculus adductor digiti II (ADDII) is inserted in the lateral side. As the toe must rotate in the opposite direction in relation to the zygodactyl feet, the rotation would demand the loss of the ventral intrinsic muscle (ADDII). The hindlimb muscles of two species of the genus Trogon have been described by Maurer & Raikow [58]. The species described present the ABDII but do not have the ADDII, suggesting that a similar mechanism of asymmetric muscular action could be responsible for the development of heterodactyly. Furthermore, they are the only Telluraves clade that have lost ADDII but not ABDII, since other clades that do not have ADDII also lost ABDII (some Coraciiformes, Upupiformes, Pici, Psitaciformes and Passeriformes) [12,28,58].
5. Conclusion
Extant birds exhibit a diversity of foot forms, which is absent in any other archosaur group. The mechanism here proposed for the origin of zygodactyly is based on transformations that indirectly allowed that diversification in Aves. While the intrinsic muscles of the foot are relatively small and homogeneous in crocodilians and theropod dinosaurs, birds have larger, more variable and individualized muscles [59,60], which allowed their differential influence on the skeleton. Furthermore, zygodactyl birds are very altricial and maintain the skeleton cartilaginous for most of the embryonic period. The action of the musculature on the avian embryonic skeleton has been recognized by different authors as an evolutionary mechanism [27,61,62]. We propose that foot muscle diversity and their action over the flexible embryonic skeleton caused the appearance of new foot configurations in birds, like zygodactyly. New fossil discoveries and further investigations on the anatomy and, especially, the development of the foot of other zygodactyl birds will contribute to test this hypothesis and further clarify the relationship between foot morphology and muscle development.
Acknowledgement
We are thankful to Macarena Faunes, Jorge Mpodozis and Gonzalo Marín for the useful commentaries on the manuscript. We also thank to Solano Henríquez, Daniel Opazo and all those who contributed to maintaining the animal facilities. We are grateful to Magno Rompiatto for the inspiration.
All procedures were approved by the ethical committee of the University of Chile.
Funding statement
This work was funded by Fondecyt grant no. 1120424 (A.V.) and Conicyt grant no. 24100058 (J.F.B.).
References
- 1.Raikow RJ. 1985. Locomotor system. In Form and function in birds (eds King AS, McLelland J.), pp. 57–147. London, UK: Academic Press. [Google Scholar]
- 2.Ostrom JH. 1976. Archaeopteryx and the origin of birds. Biol. J. Linn. Soc. 8, 91–182. ( 10.1111/j.1095-8312.1976.tb00244.x) [DOI] [Google Scholar]
- 3.Padian K, Chiappe L. 1998. The origin and early evolution of birds. Biol. Rev. 73, 1–42. ( 10.1111/j.1469-185X.1997.tb00024.x) [DOI] [Google Scholar]
- 4.Mayr G, Pohl B, Peters DS. 2005. A well-preserved Archaeopteryx specimen with theropod features. Science 310, 1483–1486. ( 10.1126/science.1120331) [DOI] [PubMed] [Google Scholar]
- 5.Middleton KM. 2001. The morphological basis of hallucal orientation in extant birds. J. Morphol. 250, 51–60. ( 10.1002/jmor.1058) [DOI] [PubMed] [Google Scholar]
- 6.Arthur W. 2004. Biased embryos and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 7.Newman SA, Muller GB. 2005. Origination and innovation in the vertebrate limb skeleton: an epigenetic perspective. J. Exp. Zool. B Mol. Dev. Evol. 304, 593–609. ( 10.1002/jez.b.21066) [DOI] [PubMed] [Google Scholar]
- 8.Bock WJ, Miller WD. 1959. The scansorial foot of the woodpeckers, with comments on the evolution of perching and climbing feet in birds. Am. Mus. Novit 1931, 1–45. [Google Scholar]
- 9.Zelenkov NV. 2007. The structure and probable mechanism of evolutionary formation of the foot in piciform birds (Aves: Piciformes). Paleontol. J. 41, 290–297. ( 10.1134/s0031030107030082) [DOI] [Google Scholar]
- 10.Mayr G. 2009. Paleogene fossil birds. Berlin, Germany: Springer. [Google Scholar]
- 11.Steinbacher G. 1935. Funktionell-anatomische Untersuchungen an Vogelfüßen mit Wendezehen und Rückzehen. J. Ornithol. 83, 214–282. ( 10.1007/bf01905356) [DOI] [Google Scholar]
- 12.Swierczewski EV, Raikow RJ. 1981. Hind limb morphology, phylogeny, and classification of the Piciformes. The Auk 98, 466–480. [Google Scholar]
- 13.Berman SL. 1984. The hindlimb musculature of the white-fronted amazon (Amazona albifrons, Psittaciformes). The Auk 101, 74–92. [Google Scholar]
- 14.Berger AJ. 1952. The comparative functional morphology of the pelvic appendage in three genera of Cuculidae. Am. Midl. Nat. 47, 513–605. ( 10.2307/2422033) [DOI] [Google Scholar]
- 15.Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC. 1993. Handbook of avian anatomy: nomina anatomica avium. Cambridge, MA: Publications of the Nuttall Ornithological Club. [Google Scholar]
- 16.Hamburger V, Hamilton HL. 1951. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92. ( 10.1002/jmor.1050880104) [DOI] [PubMed] [Google Scholar]
- 17.Hall BK. 1975. A simple, single-injection method for inducing long-term paralysis in embryonic chicks, and preliminary observations on growth of the tibia. Anat. Rec. 181, 767–777. ( 10.1002/ar.1091810408) [DOI] [PubMed] [Google Scholar]
- 18.Wortham RA. 1948. The development of the muscles and tendons in the lower leg and foot of chick embryos. J. Morphol. 83, 105–148. ( 10.1002/jmor.1050830106) [DOI] [PubMed] [Google Scholar]
- 19.Kieny M, Mauger A, Chevallier A, Pautou MP. 1988. Origin and development of avian skeletal musculature. Reprod. Nutr. Dev. 28, 673–686. ( 10.1051/rnd:19880501) [DOI] [PubMed] [Google Scholar]
- 20.Kardon G. 1998. Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019–4032. [DOI] [PubMed] [Google Scholar]
- 21.Chevallier A, Kieny M, Mauger A. 1977. Limb–somite relationship: origin of the limb musculature. J. Embryol. Exp. Morphol. 41, 245–258. [PubMed] [Google Scholar]
- 22.Hayashi K, Ozawa E. 1991. Vital labelling of somite-derived myogenic cells in the chicken limb bud. Roux‘s Arch. Dev. Biol. 200, 188–192. ( 10.1007/BF00361336) [DOI] [PubMed] [Google Scholar]
- 23.Kuo ZY. 1932. Ontogeny of embryonic behavior in aves. I. The chronology and general nature of the behavior of the chick embryo. J. Exp. Zool. 61, 395–430. ( 10.1002/jez.1400610304) [DOI] [Google Scholar]
- 24.Gadow H. 1882. Observations in comparative myology. J. Anat. 16, 493–514. [PMC free article] [PubMed] [Google Scholar]
- 25.Hudson GE. 1937. Studies on the muscles of the pelvic appendage in birds. Am. Midl. Nat. 18, 1–108. ( 10.2307/2420619) [DOI] [Google Scholar]
- 26.Carril J, Mosto MC, Picasso MBJ, Tambussi CP. 2014. Hindlimb myology of the monk parakeet (Aves, Psittaciformes). J. Morphol . 275, 732–744. ( 10.1002/jmor.20253) [DOI] [PubMed] [Google Scholar]
- 27.Hall BK, Herring SW. 1990. Paralysis and growth of the musculoskeletal system in the embryonic chick. J. Morphol. 206, 45–56. ( 10.1002/jmor.1052060105). [DOI] [PubMed] [Google Scholar]
- 28.Raikow RJ. 1987. Hindlimb myology and evolution of the old world suboscine passerine birds (Acanthisittidae, Pittidae, Philepittidae, Eurylaimidae). Ornithol. Monogr. 41, vii–81. [Google Scholar]
- 29.Schweitzer R, Zelzer E, Volk T. 2010. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137, 2807–2817. ( 10.1242/dev.047498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller GB. 2003. Embryonic motility: environmental influences and evolutionary innovation. Evol. Dev. 5, 56–60. ( 10.1046/j.1525-142X.2003.03009.x) [DOI] [PubMed] [Google Scholar]
- 31.Namba Y, Yamazaki Y, Yuguchi M, Kameoka S, Usami S, Honda K, Isokawa K. 2010. Development of the Tarsometatarsal skeleton by the lateral fusion of three cylindrical periosteal bones in the chick embryo (Gallus gallus). Anat. Rec. 293, 1527–1535. ( 10.1002/ar.21179) [DOI] [PubMed] [Google Scholar]
- 32.Losos JB, Creer DA, Glossip D, Goellner R, Hampton A, Roberts G, Haskell N, Taylor P, Ettling J. 2000. Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54, 301–305. ( 10.1111/j.0014-3820.2000.tb00032.x) [DOI] [PubMed] [Google Scholar]
- 33.Machado-Schiaffino G, Henning F, Meyer A. In press. Species-specific differences in adaptive phenotypic plasticity in an ecologically relevant trophic trait: hypertrophic lips in Midas cichlid fishes. Evolution. ( 10.1111/evo.12367) [DOI] [PubMed] [Google Scholar]
- 34.Huang AH, Riordan TJ, Wang L, Eyal S, Zelzer E, Brigande JV, Schweitzer R. 2013. Repositioning forelimb superficialis muscles: tendon attachment and muscle activity enable active relocation of functional myofibers. Dev. Cell 26, 544–551. ( 10.1016/j.devcel.2013.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hackett SJ, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768. ( 10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 36.McCormack JE, Harvey MG, Faircloth BC, Crawford NG, Glenn TC, Brumfield RT. 2013. A phylogeny of birds based on over 1,500 loci collected by target enrichment and high-throughput sequencing. PLoS ONE 8, e54848 ( 10.1371/journal.pone.0054848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimball RT, Wang N, Heimer-McGinn V, Ferguson C, Braun EL. 2013. Identifying localized biases in large datasets: a case study using the avian tree of life. Mol. Phylogenet. Evol. 69, 1021–1032. ( 10.1016/j.ympev.2013.05.029) [DOI] [PubMed] [Google Scholar]
- 38.Mayr G, Rana R, Rose K, Sahni A, Kumar K, Smith T. 2013. New specimens of the early Eocene bird Vastanavis and the interrelationships of stem group Psittaciformes. Paleontol. J. 47, 1308–1314. ( 10.1134/S0031030113110105) [DOI] [Google Scholar]
- 39.DeBee AM. 2012. A taxonomic and anatomic assessment of the extinct Zygodactylidae (Aves) from the Green River Formation of Wyoming and placement of Zygodactylidae within Aves. Austin, TX: University of Texas. [Google Scholar]
- 40.Mayr G. 2008. Phylogenetic affinities of the enigmatic avian taxon zygodactylus based on new material from the early oligocene of France. J. Syst. Palaeontol. 6, 333–344. ( 10.1017/s1477201907002398) [DOI] [Google Scholar]
- 41.Kirchman JJ, Hackett SJ, Goodman SM, Bates JM. 2001. Phylogeny and systematics of ground rollers (Brachypteraciidae) of Madagascar. The Auk 118, 849–863. ( 10.1642/0004-8038(2001)118[0849:PASOGR]2.0.CO;2) [DOI] [Google Scholar]
- 42.Goodman S. 2001. Family Leptosomatidae (cuckoo-roller). In Handbook of the birds of the world. Volume 6: mousebirds to hornbills (eds Hoyo J, Elliot A, Sargatal J.), pp. 390–395. Barcelona, Spain: Lynx Edicions. [Google Scholar]
- 43.Forbes-Watson A. 1967. Observations at a nest of the cuckoo-roller Leptosomus discolor. Ibis 109, 425–430. ( 10.1111/j.1474-919X.1967.tb04015.x) [DOI] [Google Scholar]
- 44.Berger AJ. 1953. On the locomotor anatomy of the blue coua, Coua caerulea. The Auk 70, 49–83. ( 10.2307/4081058) [DOI] [Google Scholar]
- 45.Berger AJ. 1960. Some anatomical characters of the Cuculidae and the Musophagidae. Wilson Bull. 72, 60–104. [Google Scholar]
- 46.Mourer-Chauviré C, Tabuce R, Essid EM, Marivaux L, Khayati H, Vianey-Liaud M, Ali MBH. 2013. A new taxon of stem group Galliformes and the earliest record for stem group Cuculidae from the Eocene of Djebel Chambi, Tunisia. In Proceedings of the 8th international meeting of the Society of Avian Paleontology and Evolution (eds Göhlich UB, Kroh A.). Vienna, Austria: Verlag Naturhistorisches Museum Wien. [Google Scholar]
- 47.Mayr G. 2006. A specimen of Eocuculus Chandler, 1999 (Aves, ? Cuculidae) from the early Oligocene of France. Geobios 39, 865–872. ( 10.1016/j.geobios.2005.10.007) [DOI] [Google Scholar]
- 48.Mayr G. 2011. Well-preserved new skeleton of the Middle Eocene Messelastur substantiates sister group relationship between Messelasturidae and Halcyornithidae (Aves, ?Pan-Psittaciformes). J. Syst. Palaeontol. 9, 159–171. ( 10.1080/14772019.2010.505252) [DOI] [Google Scholar]
- 49.Ksepka DT, Clarke JA. 2012. A new stem parrot from the Green River Formation and the complex evolution of the grasping foot in Pan-Psittaciformes. J. Vertebr. Paleontol. 32, 395–406. ( 10.1080/02724634.2012.641704) [DOI] [Google Scholar]
- 50.Mayr G. 2002. On the osteology and phylogenetic affinities of the Pseudasturidae–Lower Eocene stem-group representatives of parrots (Aves, Psittaciformes). Zool. J. Linn. Soc. 136, 715–729. ( 10.1046/j.1096-3642.2002.00042.x) [DOI] [Google Scholar]
- 51.Mayr G, Gregorová R. 2012. A tiny stem group representative of Pici (Aves, Piciformes) from the early Oligocene of the Czech Republic. Paläontol. Zeitschrift 86, 333–343. ( 10.1007/s12542-012-0133-5) [DOI] [Google Scholar]
- 52.Olson SL. 1983. Evidence for a polyphyletic origin of the Piciformes. The Auk 100, 126–133. [Google Scholar]
- 53.Mayr G. 2003. Monophyletic groups within ‘higher land birds’—comparison of morphological and molecular data. J. Zool. Syst. Evol. Res. 41, 233–248. ( 10.1046/j.1439-0469.2003.00230.x) [DOI] [Google Scholar]
- 54.Ericson PG, et al. 2006. Diversification of Neoaves: integration of molecular sequence data and fossils. Biol. Lett. 2, 543–547. ( 10.1098/rsbl.2006.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suh A, Paus M, Kiefmann M, Churakov G, Franke FA, Brosius J, Kriegs JO, Schmitz J. 2011. Mesozoic retroposons reveal parrots as the closest living relatives of passerine birds. Nat. Commun. 2, 443 ( 10.1038/ncomms1448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang N, Braun EL, Kimball RT. 2012. Testing hypotheses about the sister group of the passeriformes using an independent 30-locus data set. Mol. Biol. Evol. 29, 737–750. ( 10.1093/molbev/msr230) [DOI] [PubMed] [Google Scholar]
- 57.Yuri T, et al. 2013. Parsimony and model-based analyses of indels in avian nuclear genes reveal congruent and incongruent phylogenetic signals. Biology 2, 419–444. ( 10.3390/biology2010419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maurer DR, Raikow RJ. 1981. Appendicular myology, phylogeny, and classification of the avian order Coraciiformes (including Trogoniformes). Ann. Carnegie Mus. 50, 417–434. [Google Scholar]
- 59.Hutchinson JR. 2002. The evolution of hindlimb tendons and muscles on the line to crown-group birds. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 1051–1086. ( 10.1016/S1095-6433(02)00158-7) [DOI] [PubMed] [Google Scholar]
- 60.Newman SA, Mezentseva NV, Badyaev AV. 2013. Gene loss, thermogenesis, and the origin of birds. Ann. NY Acad. Sci. 1289, 36–47. ( 10.1111/nyas.12090) [DOI] [PubMed] [Google Scholar]
- 61.Streicher J, Muller G. 1992. Natural and experimental reduction of the avian fibula: developmental thresholds and evolutionary constraint. J. Morphol. 214, 260–285. ( 10.1002/jmor.1052140304) [DOI] [PubMed] [Google Scholar]
- 62.Nowlan NC, Sharpe J, Roddy KA, Prendergast PJ, Murphy P. 2010. Mechanobiology of embryonic skeletal development: insights from animal models. Birth Defects Res. C 90, 203–213. ( 10.1002/bdrc.20184) [DOI] [PMC free article] [PubMed] [Google Scholar]