Abstract
The broad palette of feather colours displayed by birds serves diverse biological functions, including communication and camouflage. Fossil feathers provide evidence that some avian colours, like black and brown melanins, have existed for at least 160 million years (Myr), but no traces of bright carotenoid pigments in ancient feathers have been reported. Insight into the evolutionary history of plumage carotenoids may instead be gained from living species. We visually surveyed modern birds for carotenoid-consistent plumage colours (present in 2956 of 9993 species). We then used high-performance liquid chromatography and Raman spectroscopy to chemically assess the family-level distribution of plumage carotenoids, confirming their presence in 95 of 236 extant bird families (only 36 family-level occurrences had been confirmed previously). Using our data for all modern birds, we modelled the evolutionary history of carotenoid-consistent plumage colours on recent supertrees. Results support multiple independent origins of carotenoid plumage pigmentation in 13 orders, including six orders without previous reports of plumage carotenoids. Based on time calibrations from the supertree, the number of avian families displaying plumage carotenoids increased throughout the Cenozoic, and most plumage carotenoid originations occurred after the Miocene Epoch (23 Myr). The earliest origination of plumage carotenoids was reconstructed within Passeriformes, during the Palaeocene Epoch (66–56 Myr), and not at the base of crown-lineage birds.
Keywords: ancestral state reconstruction, birds, coloration, pigmentation, plumage, supertree
1. Introduction
Feather colour plays an important role in the communication or camouflage of many modern bird species (Neornithes). For some mechanisms of coloration, the evolutionary history is well known [1]. Black and brown melanin colours, for example, are displayed by practically all modern birds and have been displayed as plumage pigments for many millions of years [2,3]. Histological evidence for melanin pigmentation has been reported from Cenozoic and Mesozoic fossil feathers, including from the non-avian dinosaur Anchiornis huxleyi (160 Myr) [2]. Birds apparently also inherited melanin-based structural feather colours from dinosaurian ancestors (e.g. iridescent blue-black) [4]. However, today many of the most vibrant feather colours are conferred by carotenoid pigments (red, orange, yellow, and rarely, pink and purple) [5,6], and the evolutionary history of carotenoid-based plumage pigmentation has yet to be resolved.
Previously, analytical chemistry has been used to confirm the presence of carotenoids in feathers from 197 species spanning 36 out of 236 modern neornithine families and seven of 39 orders (see the electronic supplementary material) and has shown that non-carotenoid pigments produce colourful plumage displays in penguins, parrots and turacos [7]. Stoddard & Prum [1] surveyed the plumage of 111 avian species, both visually and with spectrophotometry and proposed that plumage carotenoids were present in 10 avian orders. Here, our aim is to develop and test a comprehensive hypothesis for the taxonomic distribution of carotenoids in plumage across all modern birds, and use this distribution to reconstruct the evolutionary history of colourful carotenoid-based plumage displays in Neornithes.
As background, colourful plumage has a patchy taxonomic distribution in birds and its phenotypic expression can vary dramatically within species (e.g. between sexes) and even within individual birds (e.g. in the case of alternate plumages). This is perhaps not surprising considering that birds can extract carotenoids from food and circulate the pigments in blood using nutrient-uptake and -delivery mechanisms (e.g. lipoproteins) [8–10] that are conserved across vertebrates [11]. Hence, the dietary uptake of carotenoids is plesiomorphic to all birds, dinosaurs and reptiles (see [10] for further discussion). The carotenoids that circulate in the blood of many birds, snakes and lizards can accumulate in keratinous scales [12,13]. This suggests that the biochemical mechanisms for accumulating carotenoids in keratinous feathers may be also taxonomically widespread despite the current absence of chemical evidence for plumage carotenoids in most families of birds.
Prior studies of plumage carotenoids have placed heavy emphasis on perching birds (Passeriformes; see the electronic supplementary material), a diverse group with an evolutionary history that apparently tracks at least to the Eocene Epoch (56–34 Myr) [14]. Research has focused on the proximal causes for colour/pigment variation within a family, genus or species. Gains and losses of carotenoid-modifying pathways have been studied in select taxa, as have the relative contributions of environment and heredity to feather coloration [15–17]. Our data instead address the broad taxonomic distribution of carotenoid-pigmented feathers across all modern birds.
Using as a reference, the set of species for which the presence of plumage carotenoids has been chemically confirmed, we visually surveyed the plumage of all other neornithine species and hypothesized whether carotenoids were present or absent in each one. We then tested as many of these hypotheses as practical using high-performance liquid chromatography (HPLC) and Raman spectroscopy [18] aiming to confirm the distribution of plumage carotenoids at least at the taxonomic level of the family, or lower. Our chemically supported visual observations provided a character state matrix with which we could model trait evolution [19,20] on recently published supertrees for all modern birds [21], enabling insights into the broad pattern of colourful plumage evolution in birds.
2. Material and methods
(a). Carotenoid character state coding
To date, carotenoids have been chemically identified as feather pigments in 197 extant bird species (see the electronic supplementary material). We treated these species as a reference group for inspecting all other modern birds for the presence or the absence of plumage with carotenoid-consistent colours. Here, we define a ‘carotenoid colour’ as a colour that has been shown to derive from a carotenoid pigment in a previous chemical study. A ‘carotenoid-consistent colour’ is thus any feather colour that is visually consistent with a ‘carotenoid colour’. Working from the 9993 neornithine species listed by Jetz et al. [21], a single observer (D.B.T.) visually compared the feather colours of the remaining 9796 species to those in the reference group and coded the plumage of each species as either carotenoid-consistent (1) or carotenoid-absent (0) (similar to [22]). Primary resources for plumage comparisons included the Handbook of the Birds of the World [23] (primarily illustrations) and study skins curated by the Division of Birds, Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution. Photographs published on two reliable websites were consulted as secondary resources (The Internet Bird Collection http://ibc.lynxeds.com/; Oriental Bird Image http://orientalbirdimages.org/). Each coding represents a testable hypothesis; just as we biochemically validated our coding in 357 species (see below), subsequent studies may develop alternate hypotheses based on additional chemical data (colour states are provided in the electronic supplementary material). Note that birds with confirmed non-carotenoid red, orange or yellow feathers (e.g. turacos, parrots, penguins) were coded ‘carotenoid-absent’ [7].
Our rates of type I error (carotenoids present but coded absent) are probably lower than our rates of type II error (carotenoids absent but coded present). Type I errors may occur if comparatively low concentrations of carotenoids were co-deposited with melanin, producing plumage colours that were atypical for carotenoid pigmentation. However, it is noteworthy that our HPLC analyses of feathers from 124 species without ‘carotenoid-consistent’ colours (method described in [24]) did not produce any type I errors. Also, carotenoid-diagnostic spectral bands have not been reported from feathers without carotenoid pigments in previous Raman studies [18,25,26]. A type II error may occur if a plumage is recorded to have carotenoid-consistent colours but does not actually contain carotenoids. Green plumages may result from carotenoid pigments in feathers with structural coloration, or from structural coloration alone, and may be prime candidates for type II errors (e.g. scarlet-thighed dacnis Dacnis venusta, green-and-gold tanager Tangara schrankii and swallow tanager Tersina viridis). Here, we only report one empirical measurement of carotenoids in a yellow-green feather (rifleman Acanthisitta chloris; see the electronic supplementary material). The limited number of results from green feathers reflects the potential (and rarely acceptable) risk of sample damage during analysis. Green feathers may contain melanin, which can absorb laser light during a Raman analysis and cause the feather to burn. Raman analyses that occur during sample destruction rarely furnish useful spectra.
In addition to the potential type II errors of green feathers, parrots, penguins and turacos would have certainly constituted type II errors if we had considered their plumages to be ‘carotenoid-consistent’. Outside of these three groups, we did code four instances of plumages with ‘carotenoid-consistent’ colours that did not produce evidence for carotenoid pigments upon HPLC analysis. For context, 222 feathers with carotenoid-consistent colours were studied with chemical methods (described below). The apparent type II errors included the yellow or pink plumages of Lamprotornis regius, Macrocephalon maleo, Paradisaea apoda and Pelecanus rufescens. Owing to the absence of chemical evidence for carotenoids, the plumages of these four species have been coded as ‘carotenoid-absent’ in our survey. Type II errors will have a substantial impact on our results only if the species have high leverage (i.e. are distantly related to species with chemically confirmed plumage carotenoids). These four species represent four families that otherwise do not have chemically confirmed records for plumage carotenoids, and hence these potential type II errors could have impacted the ancestral state reconstructions within their respective orders. Our research design aimed to remove as many high-leverage codings from the dataset as possible using analytical chemistry.
(b). Chemical measurements
We used chemical measurements of plumage pigments to both evaluate our visual-assessment dataset for potential errors, and to pursue rigorous chemical evidence for carotenoids in the feathers of at least one member of each avian family that had been coded as exhibiting carotenoid-consistent colours (we followed the family nomenclature of the International Ornithological Committee 3.2 [27]). Here, our intention was to limit the phylogenetic distance between a chemically studied species and those species hypothesized to bear carotenoid feather pigments (i.e. to minimize the leverage of any potential type II errors).
Feathers from 205 species (89 families and 33 orders) were analysed with HPLC, a technique that identifies carotenoids based on light-absorption and molecular-polarity properties [28]. The taxonomically broad selection of feathers was chosen to represent a range of colours including buff, yellow, orange, chestnut, rufous, pink and red (see the electronic supplementary material). The feathers were from either male or female birds, had been collected as early as 1865, and were supplied by the American Museum of Natural History (New York, NY, USA), the Field Museum (Chicago, IL, USA) and the Museum of Southwestern Biology, University of New Mexico (Albuquerque, NM, USA). Each pigment extraction was performed with at least 3 mg of feather barb to provide the best opportunity for carotenoid detection (see [24] for pigment extraction and analytical methods).
In addition, feathers from 164 species (76 families and 11 orders) were analysed with Raman spectroscopy (12 of these species were also included in the HPLC study group). As with the HPLC group, the group of feathers analysed with Raman spectroscopy included both sexes and a range of colours (yellow, orange, red and pink); feathers were supplied by the National Museum of Natural History (Washington, DC, USA). Raman spectroscopy diagnoses carotenoid molecules from a pattern of spectral bands with the appropriate energy values [29] (figure 1). Significantly, carotenoids can be unequivocally diagnosed from three Raman spectral bands that exhibit small shifts in band position: 1490–1530 cm−1, 1145–1165 cm−1 and approximately 1003 cm−1 [29]. The 1490–1530 cm−1 and 1145–1165 cm−1 bands dominate the pigment spectrum, and may be the only discernible signal in a spectrum with a low signal-to-noise ratio. Hence, Raman spectra with high signal-to-noise ratios, collected from carotenoid-rich samples, present three illustrative bands and provide a reference for interpreting bands in noisier spectra (electronic supplementary material, figure S1). Bands from feather keratin may be evident when the pigment is at low concentrations (electronic supplementary material, figure S1). Data were collected using one of three Raman instruments sensitive to carotenoid pigments: (i) Nicolet Almega XR spectrometer (Thermo Electron Corporation, Madison, WI, USA), spectra were collected at 780 nm excitation across 100–3500 cm−1 at 3 cm−1 resolution; (ii) portable MiniRam II spectrometer with a fibre-optic probe (B&W Tek, Newark, DE, USA), spectra were collected at 785 nm excitation across 160–3200 cm−1 at 10 cm−1 resolution; (iii) Nomadic Raman microscope (BaySpec, San Jose CA, USA), spectra were collected at 1064 nm excitation across 277–1866 cm−1 at 3.5 cm−1 resolution. Loose feathers and study skins were analysed; specimens were studied at the Museum Conservation Institute, Smithsonian Institution, and at the Division of Birds, Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution. Low-power settings were maintained to avoid burning feathers, producing a spectral library with a broad range of signal-to-noise ratios. Note that 10 families (but no additional orders) were not available for chemical analysis (see the electronic supplementary material).
Figure 1.
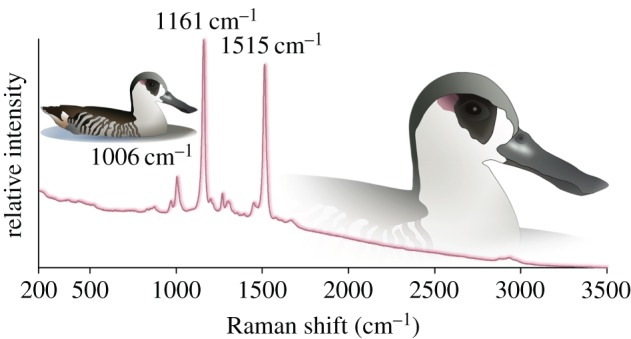
Raman spectrum from the ‘pink-ear’ of a pink-eared duck, Malacorhynchus membranaceus. The Raman spectrum was collected from the tips of pink feather barbs snipped from the right ear patch of specimen USNM 32616 (see the electronic supplementary material). The three carotenoid-informative bands are labelled with band position values. (Online version in colour.)
(c). Reconstructing ancestral states
The history of carotenoid-consistent coloration was reconstructed using supertree phylogenies from Jetz et al. [21] available from A Global Phylogeny of Birds (http://birdtree.org/). Supertrees 1–500 were analysed; each contained 9993 species and used the phylogeny of Hackett et al. [30] as a backbone constraint.
Ancestral states were reconstructed using three models. The first two models were applied to all 500 supertrees; (i) an equal-rates maximum-likelihood model was first applied (one character transition rate) and then (ii) an asymmetric-rates maximum-likelihood model was applied (two character transition rates) [31]. Reconstructions from these two models were calculated with conditional scaled likelihoods [31–33] and model fit was compared with Akaike information criterion (AIC) values (i.e. lowest AIC = best reconstruction, e.g. [33]). The supertree with the best reconstruction was then analysed with the third model, (iii) a ‘hidden’ transition rates model [33]. A single supertree was chosen for the hidden rates model calculations because of computational expense.
The hidden rates model reconstructed ancestral states with an increasing number of transition rates. Up to 20 transition rates in up to four rate categories were necessary for our analysis; model fit was judged from AIC values. For the ‘hidden rates’ models, each theoretical ancestor (i.e. node) was reconstructed with a character state as well as a rate category. For example, an ancestor may have gained carotenoid-rich plumage (character state 1) as the result of a slow transition (slow; 0 → 1 s), an intermediate rate of transition (medium; 0 → 1 m) or a fast transition (fast; 0 → 1 f). Transition rate categories could change between nodes even if the states did not change. For example, the transition rate for the gain of plumage carotenoids may have accelerated between a theoretical ancestor and the theoretical descendent (1 s → 1 f). Ancestral states and rates were modelled with a marginal likelihood method adapted from Yang et al. [33,34]. Calculations were performed in R v. 3.0.2 [35]: asymmetric-rates and equal-rates maximum-likelihood reconstructions were calculated using the ‘ace’ function in the ‘ape’ package [31]; hidden rates reconstructions were calculated using the ‘corHMM’ function in the ‘corHMM’ package [33].
We have defined the presence of carotenoids in plumage (1) as a reversible character (0 ↔ 1), pending biological evidence to the contrary. Furthermore, we assumed that the display of plumage carotenoids may have originated multiple times during the evolutionary history of birds (i.e. 0 → 1). Here we define a principal origination event (POE) as the earliest node in the evolutionary history of an extant species (i.e. shortest distance to the root) that has a proportional likelihood of 0.5 or greater for carotenoids in plumage. The POE method assumes that plumage carotenoids evolved between the node that is immediately ancestral to the POE node and the POE node itself, and that descendants of a POE node inherited proportional likelihoods of greater than 0.5 from the POE node. After a POE, the plumage of descendants may transition between states (i.e. 0 → 1 → 0). Regarding the hidden rates model, proportional likelihoods for (0) and (1) were the sum of proportional likelihoods in each rate category.
(d). Model considerations
Modelling trait evolution on a small taxonomic sampling of birds would give a misleading evolutionary reconstruction. Consider, for example, a species-rich clade of birds where all but one recently diverged species lacks carotenoids. Our likelihood model would reconstruct a relatively recent origination of carotenoids in the clade. However, if we only sampled the carotenoid-pigmented taxon and a few very basal taxa, the model might reconstruct that origination far deeper into the clade. The risk of such errors would be high for key avian orders like Anseriformes and Galliformes, as shown by results below. To avoid such errors of inference, we have provided character states for all 9993 ‘tips’ in the supertree phylogeny, which has meant supplementing our chemical measurements with visual observations. We obtained chemical evidence for carotenoids in feathers from at least one member of 95 families, shortening the taxonomic distance between a chemically studied taxon and a species that has been visually assessed to have carotenoid-consistent feather colours. The combined visual and chemical dataset was then used in the reconstruction of ancestral states.
Ancestral state reconstructions are bound by several caveats. For example, extinction can have a dramatic impact on ancestral state reconstructions that are based entirely on living taxa. Uncertainty due to ghost lineages is stronger towards the base of the tree, and consequently it is wise to interpret our results in ways that are robust to error in character state reconstructions on the more basal nodes. Furthermore, our phylogenetic hypotheses are previously published supertrees [21], and we therefore assume that each supertree is a reasonable approximation of the ‘true’ phylogeny. Our confidence in the age of each hypothetical ancestor varies within the supertree for three reasons: (i) not all nodes have been calibrated with fossil data; (ii) some node positions were arbitrarily assigned in the absence of molecular data (see the supplement of [21]) and (iii) some fossils may not be appropriate for the nodes they have been selected to constrain [21,36]. These concerns are likely to be ameliorated with improvements in phylogenetic resolution and fossil calibration. Importantly for our conclusions, shifts in the node ages [21], which are used here for evolutionary reconstructions, will not diminish the taxonomic breadth of carotenoid pigmentation that has been documented with chemical measurements. Although the construction methods for the supertrees have been contended [37], at present these phylogenetic frameworks remain the best resource for studying large-scale patterns in neornithine evolution.
3. Results
(a). Taxonomic distribution
Carotenoids had previously been identified as plumage pigments in doves (order Columbiformes), flamingos (Phoenicopteriformes), gulls (Charadriiformes), ibises and spoonbills (Pelecaniformes), perching birds (Passeriformes), trogons (Trogoniformes) and woodpeckers (Piciformes) [38–40]. We used HPLC and/or Raman spectroscopy to increase the chemical evidence for carotenoids in plumage from 197 to 415 species, uncovering new evidence in six additional orders and 59 additional families (see the electronic supplementary material). Here, we report that carotenoids occur in the plumages of cuckoos (two species; Cuculiformes), ducks (one species; Anseriformes), bee-eaters, kingfishers and todies (10 species; Coraciiformes), pheasants (three species; Galliformes), storks (one species; Ciconiiformes) and tropic birds (two species; Phaethontiformes). We noted some unusual appearances of carotenoid feather pigments, including the ‘pink ears’ of the pink-eared duck Malacorhynchus membranaceus (figure 1), the ‘bloody feathers’ of the blood pheasant Ithaginis cruentus, the ‘bleeding heart’ of the Luzon bleeding-heart Gallicolumba luzonica and the powder down of the green heron Butorides virescens. Carotenoid plumage displays are diverse, ranging from bright and bold exhibition (e.g. Narina trogon Apaloderma narina) to faint or muted coloration (rifleman Acanthisitta chloris). The taxonomic range of carotenoid pigmentation chemically confirmed in feathers has been broadened to include 33% of all extant orders (13/39) and 40% of all extant families (95/236).
In total, 2956 species of modern bird from 105 families have feather colours consistent with carotenoid pigmentation; this represents 40% of perching bird species (Passeriformes) and 13% of non-passerine species. The taxonomic distribution of species with carotenoid-consistent plumage was discontinuous and heterogeneous, where carotenoid-consistent colours might occur in only one species within an order (e.g. Anseriformes), in several species from only one family within an order (e.g. Galliformes), or be distributed across multiple families within an order (e.g. Coraciiformes). The presence of carotenoid pigmentation also varied between the plumages of congeneric species (e.g. rose-collared piha, Lipaugus streptophorus versus rufous piha, Lipaugus unirufus).
(b). Visual-assessment testing
We chemically tested a selection of our visual assessments for errors. Feathers we analysed that lacked ‘carotenoid-consistent’ colours (representing 127 species spanning 25 families and eight orders) did not yield detectable amounts of carotenoid pigments (see the electronic supplementary material). These feathers were, for example, yellow, buff, brown, tan and pink, or were iridescent (e.g. the buff coloured primary feather of a sunbittern (Eurypyga helias) and the yellow-orange gorget of a fiery-throated hummingbird (Panterpe insignis), respectively). We considered these feathers to have the highest potential for type I errors. Dual analyses with Raman spectra and HPLC only produced a single conflicting result: a yellow feather from a golden pheasant (Chrysolophus pictus) provided Raman spectral evidence for carotenoids, but HPLC analyses did not yield detectable amounts of carotenoid pigments. This instrument discrepancy may relate to the binding of pigments into the feather, which is an important consideration for chemical extraction (i.e. HPLC) and less crucial for Raman analyses in which the pigment is analysed in situ and does not need to be extracted from the feather.
(c). Ancestral state reconstruction
The minimum AIC value for the one-parameter equal-rates model was 5043.0 for the 500 supertrees. The minimum AIC value for the two-parameter asymmetric-rates model was lower (4732.4, n = 500). Supertree 103 had the lowest AIC from these two models and was subsequently analysed for hidden rates. Analyses with a single rate category produced a minimum AIC value of 4732.8 (n = 50). The minimum AIC values for two-, three- and four-rate category models were 4164.6, 4002.5 and 4009.4, respectively (all n = 50). Increasing the number of rate categories from three to four did not decrease the AIC value. Hence, the best model [33] that we found after 50 repeats had three rate categories and is the focus of the following results and discussion (figure 2).
Figure 2.
A reconstruction of the evolutionary history of carotenoid pigmentation in feathers. The likelihood that ancestors could display carotenoid feather pigments has been reconstructed using ‘hidden’ transition rates in three rate categories (AIC = 4002.5, 11 transition rates) [33]. The POEs (defined in Material and methods) for carotenoid feather pigmentation are identified by red circles. Branches are coloured according to the proportional likelihood of carotenoid-consistent colours at the preceding node. Solid purple points indicate species for which carotenoid feather pigments were confirmed present from chemical analysis; open black points represent those for which where carotenoids were not detected in feathers after chemical analysis. Supertree phylogeny from [21]. (Online version in colour.)
Ancestral states were reconstructed with 11 transition rates in three categories; slow (s), medium (m) and fast (f) [33]. The 11 rates, where 0 and 1 represent absence and presence of carotenoid-consistent colours, respectively, were: 0 s → 0 m, 0.004 transitions per million years (tr.Myr−1); 1 s → 1 m, 0.002 tr.Myr−1; 0 m → 0 s, 0.061 tr.Myr−1; 0 m → 1 m, 0.002 tr.Myr−1; 0 m → 0 f, 0.022 tr.Myr−1; 1 m → 1 s, 0.028 tr.Myr−1; 1 m → 1 f, 0.028 tr.Myr−1; 0 f → 0 m, 0.089 tr.Myr−1; 0 f → 1 f, 35.801 tr.Myr−1; 1 f → 1 m, 0.124 tr.Myr−1; 1 f → 0 f, 58.401 tr.Myr−1. A proportional likelihood for each of the six rate and state combinations was calculated for each node. Only five of the combinations were represented: plumage carotenoids were never present as the result of a fast transition (1 f). Most theoretical ancestors were reconstructed with the absence of plumage carotenoids as the result of a slow transition (0 s; 5158 of 9992 nodes).
This 11-rate model (i.e. ‘hidden rates’ model) identified 72 POEs (see Material and methods) for carotenoids as plumage pigments (AIC = 4002.5). By contrast, the equal-rates maximum-likelihood model identified 42 POEs (one transition rate; AIC = 5043.0) and the asymmetric-rates maximum-likelihood model identified five POEs (two transition rates, AIC = 4732.4). The following clade and node ages are based on calibrations by Jetz et al. [21]. The earliest POE in the 11-rates model occurred before 59 Ma, immediately preceding the clade bracketed by red-bellied pitta (Pitta erythrogaster) and Chotoy spinetail (Schoeniophylax phryganophilus) (figure 2). In this suboscine clade, carotenoid-consistent colours are coded for 17% of species (496/2956 species). Carotenoid-consistent plumage colours in another 37% of the 2956 extant species were explained by a POE during the Eocene (approx. 42 Myr). These 1079 species are also passerines and are descendants of the most recent common ancestor (MRCA) shared by cedar waxwing (Bombycilla cedrorum) and summer tanager (Piranga rubra). Three other Palaeogene POEs explained the existence of plumage carotenoids in another 22% of modern bird species. Carotenoid plumage pigments were basal to: crown-lineage Piciformes (347/2956 species with carotenoid-consistent colours; POE before 38 Myr); the clade bracketed by red-vented bulbul (Pycnonotus cafer) and red-billed leiothrix (Leiothrix lutea) (300/2956 species, POE before 33 Myr); and the clade bracketed by slaty-headed longbill (Toxorhamphus poliopterus) and golden monarch (Carterornis chrysomela) (181/2956 species, POE before 53 Myr). The remaining 67 POEs explained the presence of plumage carotenoids in only 19% of the 2956 colourful species. Most POEs are estimated to have occurred since the start of the Neogene Period (23.0 Myr; 53 of 72 POEs), with 17% of POEs having occurred since the start of the Quaternary Period (2.6 Myr; 12 of 72 POEs; figure 2).
The most basal node (i.e. root) of the avian supertree was not reconstructed with carotenoid-consistent plumage colours. The supertree root has elsewhere [21] been calibrated as a theoretical ancestor of modern birds that lived during the Cretaceous Period (145–66 Myr), and here we do not reconstruct any Cretaceous-aged nodes (n = 46) with high proportional likelihoods for carotenoid-consistent plumage colours (figure 3). One of the 37 nodes from the Palaeocene Epoch (66–56 Myr) had a high proportional likelihood for carotenoid-consistent plumage colours (i.e. proportional likelihood more than 0.5). By contrast, 40 of the 213 Eocene Epoch (56–34 Myr) nodes and 105 of the 465 Oligocene Epoch (34–23 Myr) nodes had proportional likelihood values that were more than 0.5. Nodes with high proportional likelihoods for carotenoid-consistent colours were similarly represented during the Miocene Epoch (23.0–5.3 Myr), Pliocene Epoch (5.3–2.6 Myr) and Quaternary Period: 1291/4565 nodes, 590/1987 nodes and 739/2679 nodes, respectively. If we assume that extinction has occurred at similar rates in lineages with and without carotenoid-consistent colours, then the evolutionary reconstruction based entirely on modern taxa suggests that the proportion of the global bird fauna that displays carotenoid-consistent plumage colours has steadily risen since the Palaeocene Epoch (i.e. ‘Gauguin’ hypothesis; figure 3).
Figure 3.
(a) Proportion of supertree nodes with a proportional likelihood of more than 0.5 for displaying carotenoid-consistent plumage colours. If extinction has occurred at similar rates in lineages with and without carotenoid-consistent colours, then the evolutionary reconstruction based on the Jetz et al. supertree [21] predicts a relative increase in colourful lineages since the Palaeocene Epoch (66–56 Myr). Node proportion was calculated with two types of time bin, Cenozoic Epoch midpoint and every 10 Myr since the Palaeocene. The likelihood that ancestors could display carotenoid feather pigments was reconstructed using three rate categories (AIC = 4002.5, 11 transition rates). (b) The relative increase in the number of colourful lineages can also be described without an absolute time framework. This curve shows the proportion of coeval avian families with carotenoid-consistent feather colours, tracked as the 236 families originate through time. The MRCA of each extant avian family has been ordered according to the origination sequence of Jetz et al. [21]. The proportion of families with carotenoid-consistent colours is tracked as the origination sequence unfolds and the number of coeval crown-lineage families accumulates (i.e. in relative and not absolute time). As above, the likelihood that a family MRCA could display carotenoid feather pigments was reconstructed using three transition rate categories. (Online version in colour.)
(d). Temporal framework
Our ancestral state reconstruction is firmly tied to the temporal framework of the Jetz et al. supertree [21]. The temporal framework of the supertree will likely be revised, however, and the age assignments of several key nodes will change [36,37]. Consequently, our epoch by epoch description of POEs may shift with the temporal revision. Our ‘Gauguin’ hypothesis, where more avian species are reconstructed with carotenoid plumage pigmentation over time, may be robust to changes in key node ages. The robustness of ‘Gauguin’ can be examined by considering the radiation of birds from a relative time perspective.
To establish a relative time framework, we assume that the Jetz et al. supertree [21] truly represents the radiation of Neornithes. Avian families evolved in a temporal sequence, and here we assume that the relative position of each family in the supertree truly reflects that sequence (i.e. Struthionidae originated before Acanthisittidae, which originated before Cardinalidae). From this assumption, we can establish the relative timing for the recruitment of each extant family into the global avifauna. Each family in the relative time framework is represented by its family MRCA. Furthermore, each family MRCA has a proportional likelihood for plumage carotenoids, although we note that these likelihood values are based on the supertree topology, and therefore reflect absolute time (this is an unavoidable constraint at present). Thus, as each family is recruited into the global avifauna, the proportion of contemporaneous families that display plumage carotenoids changes (figure 3b). Significantly, as each of the 236 extant families is recruited over time, we observe an increasing prevalence of carotenoid plumage pigmentation among Neornithes.
4. Discussion
(a). Phylogenetic mapping
The MRCA of all living birds was not predicted to have carotenoids as plumage pigments in the 11-rates reconstruction model. Instead, carotenoid deposition in plumage apparently evolved within the radiation of neornithine birds. The distribution of plumage carotenoids in modern species can be explained by 72 POEs. The origination timing discussed here is based on supertree node ages, which had previously been calibrated by Jetz et al. [21]. The earliest POE for carotenoid-based plumage was predicted within a perching bird (Passeriformes) lineage calibrated to have appeared during the Palaeocene, up to 6 Myr after the demise of non-avian dinosaurs. Originations of plumage carotenoids continued throughout the Cenozoic. The POEs for Coraciiformes, Piciformes and Trogoniformes occurred up to 21 Myr after the POE in Passeriformes. By 2 million years ago, carotenoids as plumage pigments had evolved in 13 orders of birds. Gains and losses of carotenoid-coloured plumage often continued in a lineage after the POE. Carotenoid plumage colours are thus an ancient and labile trait within Neornithes. Significantly, the multiple appearances of plumage carotenoids throughout the modern radiation of birds prompts an important question: are originations the result of de novo evolution or parallelism? [41]
Each origination may represent an independent evolution of a biochemical and/or cellular pathway that permits carotenoid deposition into feathers (i.e. de novo evolution). Alternatively, each origination may result from activation of a dormant but conserved pathway for uptake of carotenoids at the level of the feather follicle (i.e. parallelism). Such mechanisms are presumably responsible for seasonal plumages of modern birds that vary in carotenoid pigmentation (e.g. colourful breeding plumage followed by a drab non-breeding plumage) [10] and, though such pathways have not been molecularly described to date, carotenoid-pigmented feathers across all birds may be similarly determined by the expression of a common physiological mechanism. Support for de novo evolution versus parallelism could come from identifying the enzymes or proteins in the feather follicles of distantly related, colourful birds. If the plumage carotenoids in all modern birds results from parallelism, then the number of POEs we have reconstructed may be an over-estimate. However, the over-estimate of POEs would not alter the conclusion that carotenoid plumage displays are taxonomically widespread and evolutionarily labile in birds.
(b). Future considerations
Our character state reconstructions generate many opportunities for further research beyond the scope of our current study. For example, the documented extent of plumage carotenoids has been increased from 36 to 95 of the 236 modern bird families. Each of these new reports (i.e. 59 families) presents an opportunity to study the physiology and behavioural significance of displaying these colourful pigments, and to explore the chemical identities of the carotenoids in each species [18].
Furthermore, we identified proportionally more POEs in passerines than in non-passerines. Passeriformes is the most species-rich order of birds and sexual selection can be an important driver of speciation [42]. Hence, the evolutionary history of carotenoid pigmentation may be informative about the diversification rates of some avian taxa. Deeper insight into the correlation between diversification rate and plumage coloration may be gained from a binary state speciation and extinction (BiSSE) model [43]. We chose not to use a BiSSE model for this study because we are not confident that a priori extinction rates would be realistic for the supertrees. Specifically, we doubt that there is enough information available to develop accurate extinction rates for every neornithine lineage. However, future studies of selected lineages using a BiSSE model could explore the association between diversification and carotenoid pigments in plumage.
We report a ‘Gauguin’ hypothesis, where relatively more species display plumage carotenoids over time. This pattern was observed using either an absolute-time scale or a relative-time scale, which is unsurprising, given that the same supertree framework underlies both patterns. However, the relative-time perspective did suggest that the ‘Gauguin’ hypothesis may be robust to revisions of key node ages. We anticipate that future revisions to the temporal framework of the Jetz et al. supertree [21] will not eliminate the ‘Gauguin’ hypothesis, or trait lability pattern, that we describe here.
The presence of plumage carotenoids in many genera and species has been hypothesized in the absence of chemical evidence. We acknowledge that other, non-carotenoid pigments can be responsible for colourful feathers (e.g. in parrots, penguins and tauracos). Interestingly, each of these other pigments may have evolved within the radiation of modern birds and are now displayed by multiple confamilial genera. Hence, there is no precedent for a chemical class of plumage pigment that is displayed exclusively by a single genus or species; we do not anticipate that our codings of carotenoid-consistent colours have been misapplied to non-carotenoid pigments. However, we encourage other researchers to recall that the plumages of most bird species have not been chemically studied and new discoveries are possible. While our current research reconstructs plumage carotenoids into deep time (figure 3), future discoveries may revise that pattern, perhaps extending the origins of bright feather colours closer to the origins of Neornithes. Our research has focused on confirming avian plumage carotenoids at the family taxonomic level, and we trust that this study will serve as a useful guide for future research.
(c). Summary
The two objectives of this study were to establish the distribution of carotenoid pigmentation in feathers across 236 modern bird families and to investigate the evolutionary history of carotenoid deposition in neornithine plumage. To accomplish the latter objective, we hypothesized the distribution of carotenoids in feathers across all 9993 modern bird species. The confirmed taxonomic distribution of carotenoid feather pigments was revealed to be broad and patchy: 13 orders of birds including ducks, storks and cuckoos (Anseriformes, Ciconiiformes and Cuculiformes, respectively) display carotenoids in feathers. Our observations confirm an earlier prediction by Stoddard & Prum [1] that plumage carotenoids are displayed by several coraciiform and galliform species. We observed considerable variation among orders with respect to the percentage of species that display plumage carotenoids or the associated colours. For example, 100% of extant flamingo species (Phoenicopteriformes) accumulate carotenoids in their plumage, whereas only 41% of perching bird species (Passeriformes) and less than 1% of duck species (Anseriformes) have carotenoid-consistent plumage colours. Carotenoids as feather pigments are a labile trait that has originated multiple times during the radiation of modern birds. The earliest origination of carotenoid plumage pigments was traced to a theoretical ancestor of living perching birds (Passeriformes) that lived at least 56 million years ago, as calibrated by a recent supertree for practically all extant birds [21]. Apparently, these red, yellow, orange, pink or purple feather colours have likely been displayed by perching birds for most of the Cenozoic. We also found surprising evidence for relatively recent appearances of carotenoid pigmented plumages in several orders, including Anseriformes and Galliformes. The evolutionary reconstructions based on our character trait data strongly suggest that ancient Cenozoic plumages exhibited the full colour range that we now see in modern birds.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Christina Gebhard, Brian Schmidt and Carla Dove for specimens (Division of Birds, National Museum of Natural History); Andrew Stewart for help and access to the Hydra cluster (Smithsonian Institution); Andrew Punnett, Joshua Bodyfelt and Peter Schwerdtfeger for help and access to the Marvin cluster (Massey University Albany); Gary Graves and Cushla McGoverin for helpful discussion. We also thank Editor Innes Cuthill, Associate Editor Irby Lovette and two anonymous referees for their very helpful comments. The MCI thanks Judith Cherwinka for the purchase of the B&W Tek MiniRam II instrument.
Funding statement
D.B.T. was funded by a Peter Buck Postdoctoral Fellowship from the National Museum of Natural History, Smithsonian Institution.
References
- 1.Stoddard MC, Prum RO. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052. ( 10.1093/beheco/arr088) [DOI] [Google Scholar]
- 2.Li Q, Gao K-Q, Vinther J, Shawkey MD, Clarke JA, D'Alba L, Meng Q, Briggs DEG, Prum RO. 2010. Plumage color patterns of an extinct dinosaur. Science 327, 1369–1372. ( 10.1126/science.1186290) [DOI] [PubMed] [Google Scholar]
- 3.Zhang F, Kearns SL, Orr PJ, Benton MJ, Zhou Z, Johnson D, Xu X, Wang X. 2010. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463, 1075–1078. ( 10.1038/nature08740) [DOI] [PubMed] [Google Scholar]
- 4.Li Q, et al. 2012. Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335, 1215–1219. ( 10.1126/science.1213780) [DOI] [PubMed] [Google Scholar]
- 5.McGraw KJ. 2006. The mechanics of carotenoid coloration in birds. In Bird coloration. I. Mechanisms and measurements (eds Hill GE, McGraw KJ.), pp. 177–242. Cambridge, MA: Harvard University Press. [Google Scholar]
- 6.Mendes-Pinto MM, LaFountain AM, Stoddard MC, Prum RO, Franks HA, Robert B. 2012. Variation in carotenoid–protein interaction in bird feathers produces novel plumage coloration. J. R. Soc. Interface 9, 3338–3350. ( 10.1098/rsif.2012.0471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGraw KJ. 2006. Mechanics of uncommon colours: pterins, porphyrins, and psittacofulvins. In Bird coloration. I. Mechanisms and measurements (eds Hill GE, McGraw KJ.), pp. 354–398. Cambridge, MA: Harvard University Press. [Google Scholar]
- 8.During A, Dawson HD, Harrison EH. 2005. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1 and ABCA1 is downregulated in Caco-2 cells treated with Ezetimibe. J. Nutr. 135, 2305–2312. [DOI] [PubMed] [Google Scholar]
- 9.Duggan AE, Marie RS, Jr, Callard IP. 2002. Expression of SR-BI (scavenger receptor class B type I) in turtle (Chrysemys picta) tissues and other nonmammalian vertebrates. J. Exp. Zool. 292, 430–434. ( 10.1002/jez.10067) [DOI] [PubMed] [Google Scholar]
- 10.Walsh N, Dale J, McGraw KJ, Pointer MA, Mundy NI. 2012. Candidate genes for carotenoid coloration in vertebrates and their expression profiles in the carotenoid-containing plumage and bill of a wild bird. Proc. R. Soc. B 279, 58–66. ( 10.1098/rspb.2011.0765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes RS, Cox LA. 2002. Comparative studies of vertebrate scavenger receptor class B type I: a high-density lipoprotein biding protein. Res. Rep. Biochem. 2, 9–24. [Google Scholar]
- 12.Steffen JE, McGraw KJ. 2007. Contributions of pterin and carotenoid pigments to dewlap coloration in two anole species. Comp. Biochem. Physiol. B 146, 42–46. ( 10.1016/j.cbpb.2006.08.017) [DOI] [PubMed] [Google Scholar]
- 13.Eriksson J, et al. 2008. Identification of the Yellow Skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 4, e1000010 ( 10.1371/journal.pgen.1000010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boles WE. 1995. The world's oldest songbird. Nature 374, 21–22. ( 10.1038/374021b0) [DOI] [Google Scholar]
- 15.Hadfield JD, Owens IPF. 2006. Strong environmental determination of a carotenoid-based plumage trait is not mediated by carotenoid availability. J. Evol. Biol. 19, 1104–1114. ( 10.1111/j.1420-9101.2006.01095.x) [DOI] [PubMed] [Google Scholar]
- 16.McGraw KJ, Schuetz JG. 2004. The evolution of carotenoid coloration in estrildid finches: a biochemical analysis. Comp. Biochem. Physiol. B 139, 45–51. ( 10.1016/j.cbpc.2004.06.006) [DOI] [PubMed] [Google Scholar]
- 17.Prum RO, LaFountain AM, Berro J, Stoddard MC, Frank HA. 2012. Molecular diversity, metabolic transformation, and evolution of carotenoid feather pigments in cotingas (Aves: Cotingidae). J. Comp. Physiol. B. 182, 1095–1116. ( 10.1007/s00360-012-0677-4) [DOI] [PubMed] [Google Scholar]
- 18.Thomas DB, McGraw KJ, James HF, Madden O. 2014. Non-destructive descriptions of carotenoids in feathers using Raman spectroscopy. Anal. Methods 6, 1301–1308. ( 10.1039/C3AY41870G) [DOI] [Google Scholar]
- 19.Witmer LM. 1995. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In Functional morphology in vertebrate paleontology (ed. Thomason JJ.), pp. 19–33. New York, NY: Cambridge University Press. [Google Scholar]
- 20.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 22, 37–45. ( 10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 21.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 22.Olson VA, Owens IPF. 2005. Interspecific variation in the use of carotenoid-based coloration in birds: diet, life history and phylogeny. J. Evol. Biol. 18, 1534–1546. ( 10.1111/j.1420-9101.2005.00940.x) [DOI] [PubMed] [Google Scholar]
- 23.del Hoyo J, Elliott A, Sargatal J, Christie DA. (eds) 1992–2011. Handbook of the birds of the world, vol. 1–16 Barcelona, Spain: Lynx Edicions. [Google Scholar]
- 24.McGraw KJ, HIll GE, Parker RS. 2003. Carotenoid pigments in a mutant cardinal: implications for the genetic and enzymatic control mechanisms of carotenoid metabolism in birds. Condor 105, 587–592. ( 10.1650/7281) [DOI] [Google Scholar]
- 25.Thomas DB, McGoverin CM, McGraw KJ, James HF, Madden O. 2013. Vibrational spectroscopic analyses of unique yellow feather pigments (spheniscins) in penguins. J. R. Soc. Interface 10, 20121065 ( 10.1098/rsif.2012.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galvan I, Jorge A, Solano F, Wakamatsu K. 2013. Vibrational characterization of pheomelanin and trichochrome F by Raman spectroscopy. Spectrochim. Acta A 110, 55–59. ( 10.1016/j.saa.2013.03.027) [DOI] [PubMed] [Google Scholar]
- 27.Gill F, Donsker D (eds) 2013. IOC World Bird Names v 3.2 See http://www.worldbirdnames.org (accessed 7 March 2013).
- 28.Stradi R, Celentano G, Nava D. 1995. Separation and identification of carotenoids in bird's plumage by high-performance liquid chromatography—diode array detection. J. Chromatogr. B 670, 337–348. ( 10.1016/0378-4347(95)00173-5) [DOI] [PubMed] [Google Scholar]
- 29.Veronelli M, Zerbi G, Stradi R. 1995. In situ resonance Raman spectra of carotenoids in bird's feathers. J. Raman Spectrosc. 26, 683–692. ( 10.1002/jrs.1250260815) [DOI] [Google Scholar]
- 30.Hackett SJ, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768. ( 10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 31.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 32.Felsenstein J. 1981. A likelihood approach to character weighting and what it tells us about parsimony and compatibility. Biol. J. Linnean Soc. 16, 183–196. ( 10.1111/j.1095-8312.1981.tb01847.x) [DOI] [Google Scholar]
- 33.Beaulieu JM, O'Meara BC, Donoghue MJ. 2013. Identifying hidden rate changes in the evolution of a binary morphological character: the evolution of plant habit in campanulid angiosperms. Syst. Biol. 62, 725–737. ( 10.1093/sysbio/syt034) [DOI] [PubMed] [Google Scholar]
- 34.Yang ZH, Kumar S, Nei M. 1995. A new method of inference of ancestral nucleotide and amino-acid sequences. Genetics 141, 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team. 2012. R: a language and environment for statistical computing. See http://www.R-project.org/ Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 36.Mayr G. 2013. The age of the crown group of passerine birds and its evolutionary significance—molecular calibrations versus the fossil record. Syst. Biodivers. 11, 1–6. ( 10.1080/14772000.2013.765521) [DOI] [Google Scholar]
- 37.Ricklefs RE, Pagel M. 2012. Evolutionary biology: birds of a feather. Nature 491, 336–337. ( 10.1038/nature11642) [DOI] [PubMed] [Google Scholar]
- 38.Brockman H, Völker O. 1934. Der gelbe Federfarbstoff des Kanarienvogels (Serinus canaria canaria [L.]) und das vorkommen von Carotinoiden bei Vögeln. Z. Physiol. Chem. 224, 193–215. ( 10.1515/bchm2.1934.224.5-6.193) [DOI] [Google Scholar]
- 39.Völker O. 1961. The chemical characteristic of red lipochrome in bird feathers. J. Ornithol. 102, 430–438. ( 10.1007/BF01671012) [DOI] [Google Scholar]
- 40.McGraw KJ, Hardy LS. 2006. Astaxanthin is responsible for the pink plumage flush in Franklin's and ring-billed gulls. J. Field Ornithol. 77, 29–33. ( 10.1111/j.1557-9263.2006.00008.x) [DOI] [Google Scholar]
- 41.Hall BK. 2003. Descent with modification: the unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biol. Rev. 78, 409–433. ( 10.1017/S1464793102006097) [DOI] [PubMed] [Google Scholar]
- 42.Price T. 1998. Sexual selection and natural selection in bird speciation. Phil. Trans. R. Soc. Lond. B 353, 251–260. (10.1098/rstb.1998.0207) [Google Scholar]
- 43.Maddison WP, Midford PE, Otto SP. 2007. Estimating a binary character's effect on speciation and extinction. Syst. Biol. 56, 701–710. ( 10.1080/10635150701607033) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




