Abstract
Ecological immunology examines the adaptive responses of animals to pathogens in relation to other environmental factors and explores the consequences of trade-offs between investment in immune function and other life-history traits. Among species of herbivorous insects, diet breadth may vary greatly, with generalists consuming a wide variety of plant families and specialists restricted to a few species. Generalists may thus be exposed to a wider range of pathogens exerting stronger selection on the innate immune system. To examine whether this produces an increase in the robustness of the immune response, we compared larvae of the generalist herbivore Heliothis virescens and the specialist Heliothis subflexa challenged by entomopathogenic and non-pathogenic bacteria. Heliothis virescens larvae showed lower mortality, a lower number of recoverable bacteria, lower proliferation of haemocytes and higher phagocytic activity. These results indicate a higher tolerance to entomopathogenic bacteria by the generalist, which is associated with a more efficient cell-mediated immune response by mechanisms that differ between these closely related species. Our findings provide novel insights into the consequences of diet breadth and related environmental factors, which may be significant in further studies to understand the ecological forces and investment trade-offs that shape the evolution of innate immunity.
Keywords: ecological immunology, diet breadth, entomopathogenic bacteria, haemocytes
1. Introduction
Ecological immunology is an emerging field that seeks to understand the causes and consequences of variation in immune system responses in relation to evolution and ecology [1,2]. The general importance of the influence of environmental factors, e.g. interactions with pathogens and diet breadth, on host immune system evolution has been highlighted in several theoretical articles [2]. Interactions with pathogens will influence the evolution of immune system responses to protect the host from tissue damage by pathogenic infections. The unpredictable assembly of pathogens in the environment of herbivorous insects requires a high diversity and flexibility in host recognition and immune effector mechanisms [2]. As an example of the influence of environmental factors on the evolution of the immune system, it has been shown that invasive species can display variation in their immune defences, such as expansion of the antimicrobial peptide repertoire, and are thus better defended against potential pathogens compared with closely related non-invasive species [3,4]. Immune defence strategies among species with different diet breadth might vary owing to the fact that these species compensate costs and benefits differently depending on environmental factors related to their life history [5,6]. Here, we posed the question of whether generalist herbivores have a more effective immune system than closely related specialist herbivores. To address this question, we selected the generalist Heliothis virescens (Hv) and the specialist Heliothis subflexa (Hs), two closely related heliothine moths co-occurring in North and South America. Hv is a major pest in many crops and can feed on at least 14 different plant families [7]. The broad host plant range provides great resource availability, but also leads to an elevated level of exposure to stress factors (e.g. diverse plant defensive compounds) and a large variety of potential pathogens encountered in this variable environment. In contrast to the broad generalist Hv, Hs has a narrow nutritional niche, it is specialized on Physalis plants, and cannot develop on plants outside this genus [8]. Larvae of Hs could theoretically profit in two ways from the Physalis fruits they are feeding on: first, the Physalis fruit is covered by a calyx that creates a so-called enemy-free space [9,10]; second, withanolides, secondary metabolites of Physalis species, display antibacterial activity [11–13], which may protect the feeding larvae from environmental pathogens.
The diversity of potential pathogens on a single plant species is likely to be much lower than on the high number of plant species that Hv uses. Although the large majority of bacteria in the environment of herbivores may be harmless or sometimes even beneficial, a number of pathogenic bacteria can cause insect-specific infectious diseases. For instance, the Gram-positive bacterium Bacillus thuringiensis (Bt) and the Gram-negative bacterium Serratia entomophila are pathogenic to insects [14–16]. Although in Bt, the endotoxin that is produced during the process of spore formation is the major virulence factor [17], vegetative cells of this bacterium can kill insects in vitro and in vivo [18,19]. As Bt generates toxins against various pests, it is not only frequently used for insect pest management, but is also a common entomopathogenic bacterium in the environment of herbivorous insects [20]. Serratia entomophila has been mostly studied in relation to the scarab beetle Costelytra zealandica and only a few studies focused their attention on their pathogenicity to lepidopteran larvae [15]. As both entomopathogenic bacteria occur on plants, caterpillars may acquire these bacteria either by ingestion, with bacteria colonizing the digestive tract, or through wounding, such as a bite or cut.
In general, ingestion of pathogens is probably the main route of infection in insects. However, pathogens may also access the haemocoel directly, either through a breaching of the cuticle, or by assisted transport by entomophagous parasites [21]. Once entomopathogenic bacteria reach the haemocoel of insects, they must have the ability to attack or manipulate host immunity to colonize the host. Many bacteria are mediators of apoptosis to host cells during their pathogenesis and killing host haemocytes might be an important tool to successfully multiply within the host organism [22–24]. Subsequently, pathogenic bacteria are able to grow unrestricted by the host immune system [25] and by producing toxins they are able to kill the host [26]. In addition, non-pathogenic or opportunistic bacteria, like the Gram-positive Bacillus subtilis, have the potential to cause damage in insects with impaired immunity but not in healthy insects [27].
The very different probabilities of exposure to pathogens or at least pathogen diversity of these closely related species inspired us to study differences in their immune defence responses. As the immune response results in a competition of limited metabolic resources, the final outcome of the competition between host and pathogen depends largely on the effectiveness of co-occurring physiological, biochemical and behavioural mechanisms of the host. One would expect highly polyphagous insects to differ from specialist herbivores in requiring a more efficient and diverse immune defence response to deal with the high variety of microorganisms. However, so far, to our knowledge, no studies have been conducted in herbivorous insects to address the influence of environmental factors (i.e. likelihood of pathogen exposure) on immunity. We chose to expose both Hv and Hs to the non-pathogenic bacterium B. subtilis and the entomopathogenic bacteria Bt and S. entomophila to compare the immune defence responses between a generalist and a specialist. Our data suggest that the specialist Hs is more sensitive to pathogenic bacteria and possesses a weaker cell-mediated immune response when compared with the closely related generalist Hv.
2. Material and methods
(a). Insects
Hs and Hv were provided from North Carolina State University (NCSU) laboratory colonies. Hv originates from larvae collected in Clayton, North Carolina in 1988 (JEN2 strain), while Hs larvae were collected in 1985 near Gainesville, FL, and reared at the US Department of Agriculture Insect Attractants, Behavior and Basic Biology Research Laboratory until 1989, after which the rearing was continued at NCSU [28] (see the electronic supplementary material for rearing details).
(b). Bacterial strains
Bacterial strains used in this study were the non-pathogenic B. subtilis, and two entomopathogenic species Bt subsp. kurstaki strain HD73 and S. entomophila. The preparation of vegetative cells of these bacteria is described in the electronic supplementary material. Vegetative cells of S. entomophila, Bt and B. subtilis were used for larval survival analysis (figure 2), bacterial survival analysis (figure 3), phagocytosis assay (figure 4), migration assay (electronic supplementary material, figure S3), apoptosis assay (figure 5), lytic zone assay and phenoloxidase (PO) assay (electronic supplementary material, figure S4).
Figure 2.
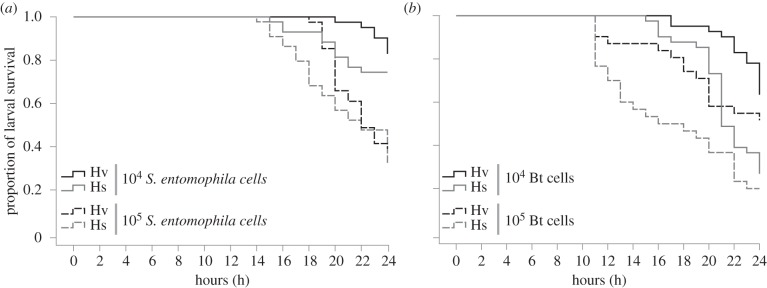
Larval survival of infected H. virescens (Hv) and H. subflexa (Hs) upon injection with different entomopathogenic bacterial doses. Kaplan–Meier survival plot of Hv (black) and Hs (grey) lavae injected with (a) 104 (Hv: n = 41; Hs: n = 43) or 105 (Hv: n = 44; Hs: n = 41) cells of S. entomophila and (b) 104 (Hv: n = 41; Hs: n = 31) or 105 (Hv: n = 41; Hs: n = 30) cells of Bt.
Figure 3.
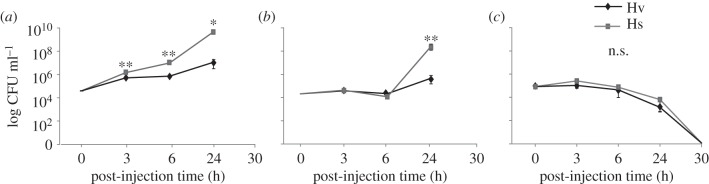
CFU of S. entomophila, Bt and B. subtilis in infected Hv and Hs larvae. Number of bacteria over specific time points after injection of Hv and Hs with (a) 1 × 104 cells of S. entomophila, (b) 1 × 104 cells of Bt and (c) 4 × 104 cells of B. subtilis. Each time point represents the mean of six insects and their associated standard error. Differences between the two species for each time point within one treatment were assessed with a Student's t-test after normalization by log-transformation (Kolmogorov–Smirnov test: p > 0.05). Significant differences are indicated by **p < 0.01; *p < 0.05; n.s., not significant.
Figure 4.
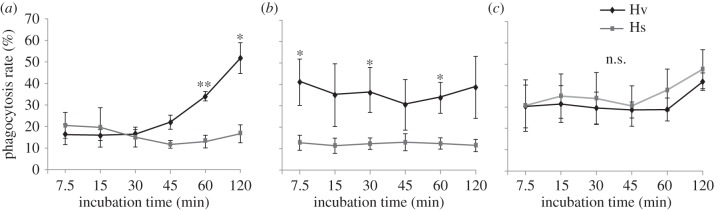
Time kinetics of phagocytosis of S. entomophila, Bt and B. subtilis by Hv and Hs haemocytes in vitro. Haemocytes were stimulated with (a) S. entomophila, (b) Bt and (c) B. subtilis. Error bars represent the s.e.m. of at least three independent experiments. Differences between the two species for each time point within one treatment were assessed with a Student's t-test after normalization by arcsine-square root transformation (Kolmogorov–Smirnov test: p > 0.05). Significant differences are indicated by **p < 0.01; *p < 0.05; n.s., not significant.
Figure 5.
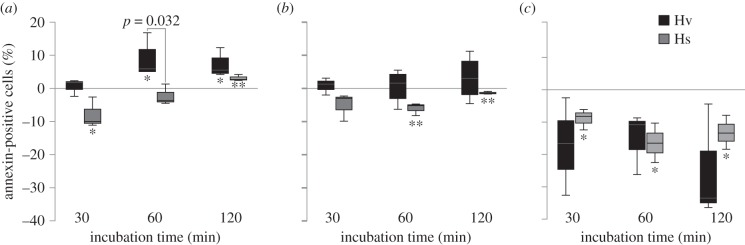
Percentage of annexin-positive haemocytes exposed to S. entomophila, Bt and B. subtilis. Haemocytes were incubated with (a) S. entomophila, (b) Bt and (c) B. subtilis for 30, 60 and 120 min. Values on the y-axis represent percentages of annexin-positive haemocytes, as proportions of total haemocytes relative to the control (base line 0% apoptosis). Each time point represents the mean of three to four biological replicates and their corresponding s.e.m. Significant differences were determined between the two species for each time point within one treatment (p-value above the bars) and within one species compared to their corresponding control (asterisks below the bars) using a Student t-test of arcsine-square root transformed data. Significant differences are indicated by **p < 0.01; *p < 0.05.
(c). Effect of Bacillus thuringiensis spores and Cry1Ac on larval growth and survivorship
Feeding experiments using Bt HD73 spores were conducted to investigate effects on Hv and Hs larval growth and survival. Early third instar larvae were exposed to diets containing 5 × 104, 7.5 × 104, 105 and 5 × 105 Bt spores per millilitre diet, whereby the respective specific larval diet for Hv and Hs was used. For each treatment, the spore solution of Bt HD73 (see the electronic supplementary material) was applied to 1 ml diet and larval growth and survival of third instar larvae was recorded daily for 7 days. In the control treatment, larvae were reared on pure artificial diet. The larval growth rate is given as average larval growth rate over 7 days. Further, to investigate the possibility that Hv and Hs differ in their susceptibility to Cry1Ac toxin, we determined the efficacy of Cry1Ac apart from spore effects in a feeding experiment with both species (see the electronic supplementary material).
(d). Effect of vegetative bacterial cells on larval survival and bacterial recovery in vivo
Direct injection of bacterial cells into the body cavity mimic bacteria that directly breach the cuticle and has been used to decipher molecules that are involved in the immune response [29]. Using injection, we achieved a defined, equal and straightforward immune response in haemocytes of both larvae. For all in vivo experiments, fourth instar larvae of Hs and Hv were injected with 2 µl 1× phosphate buffered saline (PBS) containing vegetative cells of B. subtilis, Bt or S. entomophila into the larval haemocoel using a 10 µl Hamilton syringe. See the electronic supplementary material for details about larval survival and bacterial recovery analysis.
(e). In vitro assays to evaluate cell-mediated immunity
To determine whether Hv and Hs caterpillars showed a differential response to vegetative cells of entomopathogenic or non-pathogenic bacteria in vitro, we assessed the rate of phagocytosis, haemocyte migration and cell apoptosis by haemocytes of both species. For these assays, we needed to prepare a primary haemocyte culture (see the electronic supplementary material for more details).
See the electronic supplementary material for details about phagocytosis, migration and apoptosis assay.
(f). Enzyme activity in the haemolymph in vivo
To estimate the haemocytic lysozyme-like and PO activity in response to injection with PBS and vegetative cells of different bacterial strains in both species, haemolymph was directly collected from larvae. Twenty larvae of each strain were sampled from each treatment, 60 and 120 min post injection. Haemolymph was collected by puncturing the larvae with a sterile hypodermic needle. The freely flowing haemolymph was collected on a Parafilm. See the electronic supplementary material for details on the lysozyme-like and PO assay.
(g). Data analysis
All statistical tests were run with the computer program SPSS v. 17.0 and are described in the electronic supplementary material.
3. Results
(a). Effect of Bacillus thuringiensis spores and Cry1Ac toxin on larval growth and survivorship
In general, growth rate of untreated Hv larvae was higher compared with Hs on their respective artificial diets (figure 1a). A gradual decrease in larval growth rate was correlated with an increase in Bt spore concentration in both larvae. Already the lowest concentration of 5 × 104 Bt spores had a significant negative impact on the larval growth rate of both species. With the highest concentration of 5 × 105 Bt spores, larval growth rate turned negative due to a high weight loss and mortality of both larvae.
Figure 1.

Larval growth rate and survival for H. virescens (Hv) and H. subflexa (Hs) on different concentration of Bt spores. (a) Average growth rates of Hv (black) and Hs (grey) larvae on different Bt spore concentrations after 7 days. Bars indicate means and standard errors (n = 24). A Mann–Whitney U-test was used for the paired comparisons between the treatments within each species. Different letters above the bars represent significant differences. (b) Kaplan–Meier survival plot of Hv (black) and Hs (grey) lavae fed on 7.5 × 104 Bt spores (n = 24). Statistical significance was determined using Cox regression survival analysis.
In the 7-day growth experiment, larval mortality was similar in both species (electronic supplementary material, figure S1) except in treatments with concentrations of 7.5 × 104 Bt spores, where Hs larvae showed a significantly higher mortality than Hv larvae (figure 1b). In addition, there was an obvious lower susceptibility in Hv larvae when exposed to 7.5 × 104 Bt spores: growth rate of Hv larvae was not significantly different between concentrations of 5 × 104 and 7.5 × 104 spores, whereas Hs larvae were significantly more affected by 7.5 × 104 Bt spores compared with 5 × 104 Bt spores (figure 1a).
When we tested the susceptibility of both species to Cry1Ac (MVP) apart from spores, we found the opposite result. Hs appeared to have a lower susceptibility than Hv when exposed to Cry1Ac (electronic supplementary material, figure S2). This was evident in a significantly higher larval growth rate of Hs compared with Hv, when larvae fed on diet containing 0.1 µg Cry1Ac (electronic supplementary material, figure S2a). With the highest concentration of 1 and 10 µg Cry1Ac, we did not find any significant differences in the larval growth rate between both species. Furthermore, Hv larvae showed a significantly higher mortality than Hs larvae at all Cry1Ac concentrations tested (electronic supplementary material, figure S2b–d).
Finding a lower susceptibility of Hv larvae compared with Hs larvae to Bt spores in a specific concentration range, we assessed whether there are additional, immune-related differences between both species. To directly compare defence strategies of both species, we continued with in vivo (injections) and in vitro (primary cell cultures) experiments to target haemocyte-mediated immune responses using vegetative cells of all three bacterial strains.
(b). Effect of vegetative bacterial cells on larval survival and bacterial recovery in vivo
We found that vegetative cells of both entomopathogenic bacteria, S. entomophila (figure 2a) and Bt (figure 2b) were indeed pathogenic to Hv and Hs larvae. Overall, 105 cells of either bacterial species significantly increased larval mortality of both insect species compared with 104 cells of these bacteria, thus the toxicity of these bacteria is dose-dependent in both cases (electronic supplementary material, table S1). There was no significant overall difference in mortality between the two insect species when 104 or 105 cells of S. entomophila (figure 2a) were injected. Also, in both insect species larval mortality started sooner and was higher in response to injection with Bt compared with injections with S. entomophila (compare figure 2a to 2b). However, a concentration of 105 cells of Bt (figure 2b) resulted in a significantly higher mortality in the specialist Hs than in the generalist Hv. In addition, all dosages of both pathogenic bacteria induced larval death 4–6 h earlier in Hs than Hv larvae.
Given the high mortality rate of both Hs and Hv larvae in response to the pathogenic bacteria, we investigated the survival rate of these bacteria within infected larvae. Following injection of 5 × 104 vegetative cells of S. entomophila (figure 3a) or Bt (figure 3b), the number of recoverable bacteria increased over time. With S. entomophila, the increase in colony forming units (CFU) was significantly higher at every time point in Hs than in Hv (figure 3a). With Bt, CFU was only significantly higher in the specialist Hs than in the generalist Hv larvae at 24 h post injection (figure 3b). Interestingly, the time frame in which the highest number of recoverable pathogenic cells occurred correlated with the highest mortality rate of both larvae (compare figure 3 to figure 2). By contrast, the non-pathogenic B. subtilis was cleared from the haemolymph of both species with no live bacteria being recoverable after 30 h (figure 3c).
(c). Haemocyte phagocytosis in vitro
In their ability to phagocytize either vegetative cells of S. entomophila or Bt in vitro, Hv and Hs did not differ within the first 30 min after incubation with S. entomophila (figure 4a): Hv and Hs haemocytes showed the same phagocytic activity against this entomopathogenic bacterium. However, after an incubation of 60 and 120 min, there was a significant difference in phagocytosis between both haemocytes: Hv haemocytes showed a higher phagocytic activity against S. entomophila compared with Hs haemocytes. Similarly, the rate of phagocytosis of Bt by Hv haemocytes was greater at all time-points than that of Hs haemocytes and significantly greater after 7.5, 30 and 60 min (figure 4b). When the non-pathogenic bacterium B. subtilis was incubated with either haemocytes, we found no significant difference in phagocytosis between Hv and Hs haemocytes (figure 4c). Thus, the higher number of recoverable pathogenic bacteria in the specialist Hs larvae is based on a lower Hs haemocyte phagocytosis of pathogenic bacteria compared with the generalist Hv haemocytes. However, we found the overall phagocytosis rate of bacterial cells by haemocytes never to reach 100%, which is because the assay was in vitro, where bacterial cells only have to deal with haemocytes and are in a perfect buffered solution which allows multiplication of bacterial cells. Owing to the limited lifetime of haemocytes in vitro, we only observed phagocytosis for up to 120 min.
(d). Haemocyte migration in vitro
We found no significant difference between Hv and Hs haemocyte migration rates towards the pathogenic Bt and the non-pathogenic B. subtilis, and these migration rates were not different compared with untreated haemocytes either. However, Hv and Hs haemocyte migration was significantly higher towards S. entomophila when compared with untreated haemocytes (electronic supplementary material, figure S3). Thus, the higher phagocytosis rate in response to pathogenic bacteria in Hv larvae was not based on a higher Hv haemocyte migration towards pathogenic bacteria compared with Hs haemocytes.
(e). Haemocyte apoptosis in vitro
When Hv haemocytes were incubated with vegetative cells of S. entomophila, a time dependent increase in annexin-positive cells was evident, with a significant increase in apoptotic cells after 60 and 120 min compared with the base line (figure 5a). By contrast, Hs larvae showed a significantly induced proliferation of haemocytes (negative percentage of annexin-positive cells) in response to S. entomophila after 30 min, but after 120 min of incubation annexin-positive haemocytes significantly increased compared with the base line (figure 5a). While there was a significant difference between Hv and Hs haemocytes in apoptosis rate after 60 min incubation with S. entomophila, after 120 min S. entomophila induced apoptosis of haemocytes in both species.
Vegetative cells of Bt and B. subtilis had no significant effect on Hv haemocytes, neither on apoptosis nor on proliferation of haemocytes (figure 5b,c). By contrast, there was a significant proliferation of Hs haemocytes when incubated with B. subtilis at all time-points and with Bt after 60 and 120 min of incubation (figure 5b,c). Overall, Hs haemocytes showed an induced proliferation in response to all bacterial cells, however at different time points, than Hv haemocytes (figure 5).
(f). Enzyme activity in the haemolymph in vivo
When Hv larvae were injected with vegetative cells of the entomopathogenic bacteria S. entomophila or Bt, lysozyme activity significantly increased after 60 min compared with the control, but this increase in activity was not significantly different from wounded larvae. However, 120 min post injection of S. entomophila, lysozyme activity significantly increased in Hv larvae compared with untreated and wounded larvae (electronic supplementary material, figure S4a). By contrast, injection of S. entomophila and Bt in Hs larvae led to a significant decrease in lysozyme activity compared with uninfected and wounded control larvae at both time points (electronic supplementary material, figure S4b,d).
No upregulation was found for PO activity in Hv larvae following 60 min post injection of S. entomophila or Bt compared with untreated control larvae. However, after 120 min, the PO response against Bt was enhanced in Hv larvae, although this was not significantly different from wounded Hv larvae, whereas no changes in PO activity was observed in Hs larvae after 60 and 120 min. Following injection with the non-pathogenic bacterium, B. subtilis, Hv larvae showed no significant response in their lysozyme and PO activity, while Hs larvae had a significantly lower lysozyme after 120 min compared with wounded and untreated larvae. Overall, Hv larvae showed an increase in both lysozyme and PO activity, while Hs larvae showed a decrease in lysozyme activity and no change in PO activity in response to both pathogenic and non-pathogenic bacterial cells.
4. Discussion
The data presented here demonstrates the fundamental role of diet breadth and pathogen exposure on the evolution of immune defence strategies in two very closely related species, the generalist Hv and the specialist Hs. Our results show that the closely related Hv and Hs, with overlapping geographical ranges but non-overlapping host plant ranges, combat bacterial infections in remarkably different ways.
(a). Lower susceptibility of Heliothis virescens larvae fed on Bacillus thuringiensis spores
Our findings that the generalist Hv can withstand higher Bt spore concentrations than the specialist Hs may reflect a higher likelihood of Hv encountering Bt spores on diverse host plant surfaces. Our results are consistent with previous studies, showing that Bt spores are acutely toxic in high amounts and growth-inhibiting at low concentrations [30,31]. One important virulence factor is the parasporal crystalline inclusion of the pore-forming Cry-type protein toxin, which lyses the insect's midgut epithelial cells and allows vegetative bacterial cells to enter the body cavity and proliferate [32]. Spore toxicity is greatly reduced or absent in Bt strains that do not produce Cry toxins [33]. However, a toxin-spore mixture causes higher mortality than toxin alone [34]. The JEN2 strain of Hv we studied does not possess toxin-resistance mechanisms found in other Hv strains, such as altered proteolytic activity affecting protoxin processing and maturation [35] or modification of toxin receptors [36,37]. In fact, we found that the JEN2 strain of Hv was more sensitive to Cry1Ac toxin than was Hs, thus the lower sensitivity of Hv to spores must be due to factors other than the direct response to toxin. Previously, it has been shown that an elevated immune status of Ephestia kuehniella was associated with a higher tolerance against a mixture of endotoxins and spores of Bt [38]. In our studies, the lower susceptibility of Hv to Bt spores suggests the presence of efficient immune defence mechanisms which combat newly germinated vegetative bacteria resulting in a retarded or reduced sepsis in the haemocoel. Further, the higher efficacy of Bt spores in the specialist Hs might indicate that the bacteria can cope better with cellular and humoural immune effectors of Hs, by modifications of their surface proteins or secreted proteases that degrade antimicrobial proteins. Because of the importance of Bt toxins in pest control, most studies have focused their attention on purified Bt endotoxins or transgenic Bt plants [39,40] and only a few have tested Bt spores in feeding experiments [30,31,41]. Therefore, little is known about the infection strategy of entire Bt spores and how this could be combated by insect innate immunity.
(b). Higher larval mortality and colony forming units in Heliothis subflexa than in Heliothis virescens larvae
To date only a few studies exist where insecticidal activities of vegetative cells of Bt to lepidopteran larvae were tested [19,42,43], and even fewer studies focused their attention on the toxicity of S. entomophila to lepidopteran larvae [15]. Similar to what has been documented for pathogenic bacteria in Manduca sexta larvae [44], the high mortality rate in response to entomopathogenic bacteria correlated with high bacterial titres in the haemolymph of Hv and Hs larvae. The higher pathogenicity and the unhindered growth of both pathogenic bacteria in larvae of the specialist Hs compared with Hv may be owing to a relative resistance of those bacteria to phagocytosis by Hs haemocytes, resulting in multiplication of bacterial cells, while a significantly lower bacterial multiplication in Hv larvae may reflect efficient phagocytosis by Hv haemocytes. This suggests that Hv both recognizes and can resist these bacteria up to a certain number, whereas Hs does not. The fact that we did not find a significant difference in mortality between Hs and Hv in response to S. entomophila may be owing to a lower distribution frequency of S. entomophila in nature compared with Bt.
(c). Heliothis virescens haemocytes showed a higher phagocytic activity against pathogenic bacteria than Heliothis subflexa
Our results suggest a more efficient capability of the generalist Hv to kill pathogenic bacteria, compared with the specialist Hs. The low phagocytic activity of Hs haemocytes might indicate that both pathogenic bacteria resist phagocytosis by Hs haemocytes due to immune suppressive factors. For instance, pathogenic bacteria, such as Photorhabdus, are able to inhibit their own phagocytosis due to immune suppressive factors [26,45]. In addition, it is important to note that distinct haemocyte cell types appear to be involved in phagocytosis in different insect species [46,47]. For instance, in Helicoverpa armigera, granular cells and plasmatocytes appear to be the only phagocytic cells [47,48]. It may thus be possible that Hv and Hs vary in the amount of haemocyte cell types, which ultimately results in different phagocytic capabilities.
(d). Haemocyte migration rate of both species is similar
Our results reveal that: (i) the higher phagocytosis rate of Hv haemocytes is not based on a higher migration capability of haemocytes, and (ii) haemocytes of both Hv and Hs are attracted only to the Gram-negative bacterium S. entomophila. Similarly, Schneeweiß et al. [49] reported that Serratia marcescens stimulates the migration of mussel haemocytes. Interestingly, Bt was not attractive to Hv and Hs haemocytes, while previous studies showed that invertebrate cells are attracted to Gram-positive bacterial cells [50]. The lack of Heliothis haemocyte migration towards the Gram-positive Bt suggests a pathogenic strategy to avoid (or delay) detection by not displaying immune elicitors on their surfaces [21].
(e). Heliothis subflexa haemocytes induced proliferation instead of apoptosis in response to bacterial challenge
Studies in Bombyx mori have shown that the Gram-negative S. marcescens induces apoptosis via a lipopolysaccharide (LPS)-dependent mechanism [51]. Such an LPS-induced apoptosis may also have occurred in Hv and Hs haemocytes in our study, because we measured apoptosis in response to the Gram-negative S. entomophila in both haemocytes. In vitro studies showed that entomopathogenic Bt (Bt13) can kill insect cells [18]. However, our present study does not confirm that another Bt strain (HD 73) is able to kill Hv and Hs haemocytes within 120 min of incubation. Interestingly, we found exclusively in the specialist Hs an induced proliferation of haemocytes in response to Bt and S. entomophila. The induction of haemocyte proliferation in Hs probably consumes energetic resources. Based on the observed low larval survival rate in Hs, in combination with high growth rate and low phagocytosis of bacterial cells, the immune defence strategy of Hs appears to be disadvantageous, because an optimal immune defence response should be most efficient at the lowest energetic cost. In addition, pathogenic bacteria might be able to kill Hs haemocytes by using mechanisms that suppresses key cellular immune functions such as phagocytosis, and therefore Hs has to induce haemocyte proliferation to try to overcome the infection [52].
(f). Heliothis virescens larvae display a different immune-related enzymatic response than Heliothis subflexa larvae
Different immune response strategies in both species might account for the fact that the immune system is a coherent system within an organism, where energy expenditure in one component of immunity may lead to trade-offs in other components. In this case, increased phagocytic activity in the generalist Hv may lead to the downregulation of further components of immunity, reflected in lower PO or lysozyme levels. However, we found that Hv significantly induced lysozyme activity in response to S. entomophila after 120 min of incubation, where we also detected a significantly induced phagocytosis activity. However, there was a reduced lysozyme activity in the specialist Hs after incubation with both pathogenic bacteria. Thus, a reduced lysozyme and phagocytosis activity during infections in Hs larvae might be owing to a certain level of defencelessness against pathogenic bacteria, which might have evolved in an enemy-free environment within the calyx of Physalis fruits over time.
(g). Differences in the defence strategy against non-pathogenic bacteria between both species
Although B. subtilis is a non-pathogenic bacterium, it was recognized by both Hv and Hs as foreign, resulting in phagocytosis, the most effective mechanism for eliminating foreign material from the haemolymph [53]. However, the time period of clearance in Hs larvae was associated with a significantly induced haemocyte proliferation in vitro, suggesting that exposure to non-pathogenic bacteria leads to the formation of more phagocytes in Hs larvae, whereas Hv does not require induction of proliferation in response to non-pathogenic bacteria. A response to non-pathogenic bacteria may be dependent on the immune status of the organism. Hs larvae may have to invest in protection against any kind of potentially infectious microbes, whereas Hv larvae may differentiate more specifically between pathogenic and non-pathogenic bacteria, and thus invest significant resources only in case of ‘dangerous’ infections.
(h). Impact of environmental factors on the innate immunity in herbivorous insects
Dietary specialization of Hs may seem to be a suboptimal evolutionary strategy, because our laboratory study found no apparent benefit that outweighs the increased susceptibility to pathogens due to the reduced efficiency of immune defence. However, a narrow diet breadth may yield benefits seen only under natural conditions, taking the effects of nutrients and secondary metabolites of the host plant additionally into account. Studies on the relationship between insect immunity and diet breadth have revealed that variation in host plant quality can alter the immune defence response [54], and that secondary metabolites can decrease the immunocompetence and increase parasitism rates in insects [55–57]. By contrast, a recent study demonstrated that the immune system response of a generalist did not vary when fed on three different plant species, suggesting that feeding on various host plants of different nutritional qualities and secondary metabolite content has no detrimental effect on immune system response in this case [58]. Interestingly, phytochemicals consumed by herbivores can also affect entomopathogens infecting them [59,60]. Considering the impact of host plants on the immune status and pathogen susceptibility of insects, our results might have been different in a natural setting. However, it has been predicted that organisms in good environmental conditions have optimal resource amounts to invest in immunity [61]. Owing to the high complexity of secondary metabolites and the various nutritional conditions experienced by generalists in nature, measurements of the immune status of a generalist on a single host plant are unlikely to reveal its overall immune defence ability. Therefore, we used artificial diet to provide both species with their optimal nutritional requirements, to obtain their immune system response under controlled conditions.
Besides diet breadth, pathogen exposure is likely to influence immune defence strategies in insects and might provide explanations for immune system variations. Herbivorous insects with a broad host range are more likely to invest more into developing an efficient and specific immune system, because of a high frequency of challenges by pathogens throughout life. Therefore, a higher likelihood of pathogen exposure might affect the selective advantage of specific immune defence strategies by structuring an enhanced immune response sensitivity and specificity to broad environmental factors in generalist herbivores. An alternate strategy to investing in a highly efficient immune system is to minimize exposure to pathogens by specialization on host plants which provides an enemy-free space. For instance, host plants could possess chemicals on leaf surfaces or an architecture which affects pathogen load or persistence [9,11,62]. Larvae of Hs spend most of their time surrounded by the large calyx enclosing the fruit on which they feed. Even if specialists gain such a benefit from host plant specialization, they may sacrifice some degree of resilience to environmental changes as they become increasingly dependent on a restricted host plant. On the other hand, there might be a trade-off in evolving a more powerful immune response and other fitness-relevant traits [63]; however, this might be required when exploiting a broad host range. Whether these traits coevolved in generalist herbivores, and whether the evolution of less effective immune defence strategies in specialist herbivores is a general pattern, can be judged when additional comparisons of generalists and specialists are carried out in the future.
5. Conclusion
This study confirms that the specialist employs a different strategy to overcome infections with non-pathogenic and entomopathogenic bacteria by investing in haemocyte proliferation-based defence, but sacrifices phagocytosis efficiency and larval survival, compared with the closely related generalist species. Our data are thus consistent with the hypothesis that generalist herbivores have a more efficient immune defence strategy than specialist herbivores. This study is one of the few that has considered the ecological and evolutionary impact of differences in diet breadth on the herbivorous innate immune response in an experimental context. Further immunological studies should take the fundamental role of diet breadth and pathogen exposure on the evolution of innate immunity into account, to enhance our understanding of the ecological and evolutionary forces structuring variation in the immune system of invertebrates.
Supplementary Material
Acknowledgements
We thank Elfi Töpfer, Anna Schwind, Anne Hahn, Stepahnie Ley, Susanne Donnerhacke and Antje Schmalz for their technical assistance and insect rearing. We also thank Yannick Pauchet for his enthusiasm and advice on Bt spores and primary cell cultures, Sandra Paa and Fred Gould from NSCU for providing CSB diet and H. subflexa and Andreas Vilcinskas for his valuable comments on the manuscript.
Funding statement
This research was financially supported by the Max-Planck-Gesellschaft.
References
- 1.Rolff J, Siva-Jothy MT. 2003. Invertebrate ecological immunology. Science 301, 472–475. ( 10.1126/science.1080623) [DOI] [PubMed] [Google Scholar]
- 2.Schulenburg H, Kurtz J, Moret Y, Siva-Jothy MT. 2009. Introduction. Ecological immunology. Phil. Trans. R. Soc. B 364, 3–14. ( 10.1098/rstb.2008.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KA, Klasing KC. 2004. A role for immunology in invasion biology. Trends Ecol. Evol. 19, 523–529. ( 10.1016/j.tree.2004.07.012) [DOI] [PubMed] [Google Scholar]
- 4.Vilcinskas A, Mukherjee K, Vogel H. 2013. Expansion of the antimicrobial peptide repertoire in the invasive ladybird Harmonia axyridis. Proc. R. Soc. B 280, 20122113 ( 10.1098/rspb.2012.2113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KA, Martin LB, II, Hasselquist D, Ricklefs RE, Wikelski M. 2006. Contrasting adaptive immune defenses and blood parasite prevalence in closely related Passer sparrows. Oecologia 150, 383–392. ( 10.1007/s00442-006-0537-6) [DOI] [PubMed] [Google Scholar]
- 6.Moret Y. 2003. Explaining variable costs of the immune response: selection for specific versus non-specific immunity and facultative life history change. Oikos 102, 213–216. ( 10.1034/j.1600-0706.2003.12496.x) [DOI] [Google Scholar]
- 7.Sheck AL, Gould F. 1993. The genetic basis of host range in Heliothis virescens: larval survival and growth. Entomol. Exp. Appl. 69, 157–172. ( 10.1111/j.1570-7458.1993.tb01738.x) [DOI] [Google Scholar]
- 8.Laster ML, Pair SD, Martin DF. 1982. Acceptance and development of Heliothis subflexa and Heliothis virescens (Lepidopters, Noctuidae), and their hybrid and backcross progeny on several plant species. Environ. Entomol. 11, 979–980. [Google Scholar]
- 9.Oppenheim SJ, Gould F. 2002. Behavioral adaptations increase the value of enemy-free space for Heliothis subflexa, a specialist herbivore. Evolution 56, 679–689. ( 10.1111/j.0014-3820.2002.tb01379.x) [DOI] [PubMed] [Google Scholar]
- 10.Puente ME, Kennedy GG, Gould F. 2008. The impact of herbivore-induced plant volatiles on parasitoid foraging success: a general deterministic model. J. Chem. Ecol. 34, 945–958. ( 10.1007/s10886-008-9471-x) [DOI] [PubMed] [Google Scholar]
- 11.Nathiya M, Dorcus D. 2012. Preliminary phytochemical and anti-bacterial studies on Physalis minima Linn. Int. J. Curr. Sci. 2012, 24–30. [Google Scholar]
- 12.Pietro RCLR, Kashima S, Sato DN, Januário AH, França SC. 2000. In vitro antimycobacterial activities of Physalis angulata L. Phytomedicine 7, 335–338. ( 10.1016/S0944-7113(00)80052-5) [DOI] [PubMed] [Google Scholar]
- 13.Januário AH, Rodrigues E, Pietro RCLR, Kashima S, Sato DN, França SC. 2002. Antimycobacterial physalins from Physalis angulata L. (Solanaceae). Phytother. Res. 16, 445–448. ( 10.1002/ptr.939) [DOI] [PubMed] [Google Scholar]
- 14.Jackson TA, Glare TR, O'Callaghan M. 1991. Pathotypic boundaries for Serratia spp. causing amber disease in the New Zealand grass grub, Costelytra zealandica. Bull. SROP 14, 148–152. [Google Scholar]
- 15.Núñez-Valdez ME, Calderón MA, Aranda E, Hernández L, Ramírez-Gama RM, Lina L, Rodríguez-Segura Z, Gutiérrez MDC, Villalobos FJ. 2008. Identification of a putative Mexican strain of Serratia entomophila pathogenic against root-damaging larvae of scarabaeidae (Coleoptera). Appl. Environ. Microbiol. 74, 802–810. ( 10.1128/aem.01074-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macintosh SC, Stone TB, Sims SR, Hunst PL, Greenplate JT, Marrone PG, Perlak FJ, Fischhoff DA, Fuchs RL. 1990. Specificity and efficacy of purified Bacillus thuringiensis proteins against agronomically important insects. J. Invertebr. Pathol. 56, 258–266. ( 10.1016/0022-2011(90)90109-j) [DOI] [PubMed] [Google Scholar]
- 17.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang MY, Lovgren A, Landen R. 1995. Adhesion and cytotoxicity of Bacillus thuringiensis to cultured Spodoptera and Drosophila cells. J. Invertebr. Pathol. 66, 46–51. ( 10.1006/jipa.1995.1059) [DOI] [PubMed] [Google Scholar]
- 19.Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, Koziel MG. 1996. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl Acad. Sci. USA 93, 5389–5394. ( 10.1073/pnas.93.11.5389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betz FS, Hammond BG, Fuchs RL. 2000. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharmacol. 32, 156–173. ( 10.1006/rtph.2000.1426) [DOI] [PubMed] [Google Scholar]
- 21.Vallet-Gely I, Lemaitre B, Boccard F. 2008. Bacterial strategies to overcome insect defences. Nat. Revi. Microbiol. 6, 302–313. ( 10.1038/nrmicro1870) [DOI] [PubMed] [Google Scholar]
- 22.Chen YJ, Zychlinsky A. 1994. Apoptosis induced by bacterial pathogens. Microb. Pathog. 17, 203–212. ( 10.1006/mpat.1994.1066) [DOI] [PubMed] [Google Scholar]
- 23.Zychlinsky A, Sansonetti P. 1997. Apoptosis in bacterial pathogenesis. J. Clin. Invest. 100, 493–496. ( 10.1172/jci119557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zychlinsky A, Sansonetti PJ. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5, 201–204. ( 10.1016/s0966-842x(97)01044-5) [DOI] [PubMed] [Google Scholar]
- 25.Daborn PJ, Waterfield N, Silva CP, Au CPY, Sharma S, Ffrench-Constant RH. 2002. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc. Natl Acad. Sci. USA 99, 10 742–10 747. ( 10.1073/pnas.102068099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ffrench-Constant R, et al. 2003. Photorhabdus: towards a functional genomic analysis of a symbiont and pathogen. FEMS Microbiol. Rev. 26, 433–456. ( 10.1016/s0168-6445(02)00130-4) [DOI] [PubMed] [Google Scholar]
- 27.Pirofski L-A, Casadevall A. 2012. Q&A: what is a pathogen? A question that begs the point. BMC Biol. 10, 6 ( 10.1186/1741-7007-10-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheck AL, Gould F. 1995. Genetic analysis of differences in oviposition preferences of Heliothis virescens and Heliothis subflexa (Lepidoptera: Noctuidae). Environ. Entomol. 24, 341–347. [Google Scholar]
- 29.Shelby KS, Popham HJR. 2008. Cloning and characterization of the secreted hemocytic prophenoloxidases of Heliothis virescens. Arch. Insect Biochem. Physiol. 69, 127–142. ( 10.1002/arch.20274) [DOI] [PubMed] [Google Scholar]
- 30.Johnson DE, McGaughey WH. 1996. Contribution of Bacillus thuringiensis spores to toxicity of purified cry proteins towards Indianmeal moth larvae. Curr. Microbiol. 33, 54–59. ( 10.1007/s002849900074) [DOI] [PubMed] [Google Scholar]
- 31.Gould F, Anderson A, Landis D, Van Mellaert H. 1991. Feeding behavior and growth of Heliothis virescens larvae on diets containing Bacillus thuringiensis formulations or endotoxins. Entomol. Exp. Appl. 58, 199–210. ( 10.1111/j.1570-7458.1991.tb01469.x) [DOI] [Google Scholar]
- 32.Aronson AI. 1993. The 2 faces of Bacillus thuringiensis: insecticidal proteins and postexponential survival. Mol. Microbiol. 7, 489–496. ( 10.1111/j.1365-2958.1993.tb01139.x) [DOI] [PubMed] [Google Scholar]
- 33.Raymond B, West SA, Griffin AS, Bonsall MB. 2012. The dynamics of cooperative bacterial virulence in the field. Science 337, 85–88. ( 10.1126/science.1218196) [DOI] [PubMed] [Google Scholar]
- 34.Li RS, Jarrett P, Burges HD. 1987. Importance of spores, crystals, and delta-endotoxins in the pathogenicity of different varieties of Bacillus thuringiensis in Galleria mellonella and Pieris brassicae. J. Invertebr. Pathol. 50, 277–284. ( 10.1016/0022-2011(87)90093-0) [DOI] [Google Scholar]
- 35.Oppert B. 1999. Protease interactions with Bacillus thuringiensis insecticidal toxins. Arch. Insect Biochem. Physiol. 42, 1–12. () [DOI] [PubMed] [Google Scholar]
- 36.Gahan LJ, Gould F, Heckel DG. 2001. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 293, 857–860. ( 10.1126/science.1060949) [DOI] [PubMed] [Google Scholar]
- 37.Gahan LJ, Pauchet Y, Vogel H, Heckel DG. 2010. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 6, e1001248 ( 10.1371/journal.pgen.1001248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman MM, Roberts HLS, Sarjan M, Asgari S, Schmidt O. 2004. Induction and transmission of Bacillus thuringiensis tolerance in the flour moth Ephestia kuehniella. Proc. Natl Acad. Sci. USA 101, 2696–2699. ( 10.1073/pnas.0306669101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shelton AM, Zhao JZ, Roush RT. 2002. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 47, 845–881. ( 10.1146/annurev.ento.47.091201.145309) [DOI] [PubMed] [Google Scholar]
- 40.Zhao JZ, Cao J, Li YX, Collins HL, Roush RT, Earle ED, Shelton AM. 2003. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotechnol. 21, 1493–1497. ( 10.1038/nbt907) [DOI] [PubMed] [Google Scholar]
- 41.McGaughey WH. 1978. Response of Plodia interpunctella and Ephestia cautella larvae to spores and parasporal crystals of Bacillus thuringiensis. J. Econ. Entomol. 71, 687–688. [Google Scholar]
- 42.Lövgren A, Zhang M, Engström A, Dalhammar G, Landén R. 1990. Molecular characterization of immune inhibitor A, a secreted virulence protease from Bacillus thuringiensis. Mol. Microbiol. 4, 2137–2146. ( 10.1111/j.1365-2958.1990.tb00575.x) [DOI] [PubMed] [Google Scholar]
- 43.Salamitou S, Ramisse F, Brehelin M, Bourguet D, Gilois N, Gominet M, Hernandez E, Lereclus D. 2000. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146, 2825–2832. [DOI] [PubMed] [Google Scholar]
- 44.Au C, Dean P, Reynolds SE, ffrench-Constant RH. 2004. Effect of the insect pathogenic bacterium Photorhabdus on insect phagocytes. Cell Microbiol. 6, 89–95. ( 10.1046/j.1462-5822.2003.00345.x) [DOI] [PubMed] [Google Scholar]
- 45.van Sambeek J, Wiesner A. 1999. Successful parasitation of locusts by entomopathogenic nematodes is correlated with inhibition of insect phagocytes. J. Invertebr. Pathol. 73, 154–161. ( 10.1006/jipa.1998.4823) [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro C, Brehelin M. 2006. Insect haemocytes: what type of cell is that? J. Insect Physiol. 52, 417–429. ( 10.1016/j.jinsphys.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 47.Lavine MD, Strand MR. 2002. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 32, 1237–1242. ( 10.1016/S0965-1748(02)00092-9) [DOI] [PubMed] [Google Scholar]
- 48.Mazet I, Pendland J, Boucias D. 1994. Comparative analysis of phagocytosis of fungal cells by insect hemocytes versus horse neutrophils. Dev. Comp. Immunol. 18, 455–466. ( 10.1016/s0145-305x(06)80001-7) [DOI] [PubMed] [Google Scholar]
- 49.Schneeweiß H, Renwrantz L. 1993. Analysis of the attraction of hemocytes from Mytilus edulis by molecules of bacterial origin. Dev. Comp. Immunol. 17, 377–387. ( 10.1016/0145-305x(93)90029-p) [DOI] [PubMed] [Google Scholar]
- 50.Cheng TC, Howland KH. 1979. Chemotactic attraction between hemocytes of the oyster, Crassostrea virginica, and bacteria. J. Invertebr. Pathol. 33, 204–210. ( 10.1016/0022-2011(79)90154-x) [DOI] [Google Scholar]
- 51.Ishii K, Adachi T, Imamura K, Takano S, Usui K, Suzuki K, Hamamoto H, Watanabe T, Sekimizu K. 2012. Serratia marcescens induces apoptotic cell death in host immune cells via a lipopolysaccharide- and flagella-dependent mechanism. J. Biol. Chem. 287, 36 582–36 592. ( 10.1074/jbc.M112.399667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eleftherianos I, Ffrench-Constant RH, Clarke DJ, Dowling AJ, Reynolds SE. 2010. Dissecting the immune response to the entomopathogen Photorhabdus. Trends Microbiol. 18, 552–560. ( 10.1016/j.tim.2010.09.006) [DOI] [PubMed] [Google Scholar]
- 53.Anderson CL, et al. 2005. Molecular mechanisms of phagocytosis. Georgetown, TX: Landes Biosciences/Eurekah.com and Springer Science+Business Media. [Google Scholar]
- 54.Klemola N, Klemola T, Rantala MJ, Ruuhola T. 2007. Natural host–plant quality affects immune defence of an insect herbivore. Entomol. Exp. Appl. 123, 167–176. ( 10.1111/j.1570-7458.2007.00533.x) [DOI] [Google Scholar]
- 55.Haviola S, Kapari L, Ossipov V, Rantala MJ, Ruuhola T, Haukioja E. 2007. Foliar phenolics are differently associated with Epirrita autumnata growth and immunocompetence. J. Chem. Ecol. 33, 1013–1023. ( 10.1007/s10886-007-9271-8) [DOI] [PubMed] [Google Scholar]
- 56.Zvereva EL, Rank NE. 2003. Host plant effects on parasitoid attack on the leaf beetle Chrysomela lapponica. Oecologia 135, 258–267. ( 10.1007/s00442-003-1184-9) [DOI] [PubMed] [Google Scholar]
- 57.Smilanich AM, Dyer LA, Chambers JQ, Bowers MD. 2009. Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol. Lett. 12, 612–621. ( 10.1111/j.1461-0248.2009.01309.x) [DOI] [PubMed] [Google Scholar]
- 58.Smilanich AM, Vargas J, Dyer LA, Bowers MD. 2011. Effects of ingested secondary metabolites on the immune response of a polyphagous caterpillar Grammia incorrupta. J. Chem. Ecol. 37, 239–245. ( 10.1007/s10886-011-9924-5) [DOI] [PubMed] [Google Scholar]
- 59.Keating ST, Yendol WG, Schultz JC. 1988. Relationship between susceptibility of gypsy moth larvae (Lepidoptera, Lymantriidae) to a baculovirus and host plant foliage constituents. Environ. Entomol. 17, 952–958. [Google Scholar]
- 60.Cory JS, Hoover K. 2006. Plant-mediated effects in insect-pathogen interactions. Trends Ecol. Evol. 21, 278–286. ( 10.1016/j.tree.2006.02.005) [DOI] [PubMed] [Google Scholar]
- 61.Triggs A, Knell RJ. 2012. Interactions between environmental variables determine immunity in the Indian meal moth Plodia interpunctella. J. Anim. Ecol. 81, 386–394. ( 10.1111/j.1365-2656.2011.01920.x) [DOI] [PubMed] [Google Scholar]
- 62.Hoover K, Yee JL, Schultz CM, Rocke DM, Hammock BD, Duffey SS. 1998. Effects of plant identity and chemical constituents on the efficacy of a baculovirus against Heliothis virescens. J. Chem. Ecol. 24, 221–252. ( 10.1023/a:1022576207506) [DOI] [Google Scholar]
- 63.Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50, 529–551. ( 10.1146/annurev.ento.50.071803.130420) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


