Abstract
Testosterone (T) can be released by stimuli such as social interactions, and thereby influence future social behaviours. Because the reinforcing effects of T can induce preferences for specific environmental locations, T has the potential to alter behaviour through space use. In a monogamous species, this T pulse may contribute differently to space use in sexually naive (SN) and pair-bonded (PB) males: SN males may be more likely to explore new areas to set up a territory than PB males, which are more likely to defend an existing, established territory. In this study, we test for variation in T-driven space use by examining variation in the formation of conditioned place preferences (CPPs) in SN and PB male California mice. In the three-chambered CPP apparatus, subcutaneous administrations of physiological levels of T were used to repeatedly condition SN and PB males to a side chamber, which is an unfamiliar/neutral environment. The final tests revealed that T-induced CPPs in the side chamber are developed in SN, but not PB males. This study fills a gap in our knowledge about plasticity in the rewarding nature of T pulses, based on past social experience.
Keywords: testosterone, reinforcing effects, conditioned place preference, pair bonding
1. Introduction
Reinforcing effects are elicited by the incentive properties of a stimulus, and may have been evolutionarily important for survival, reproduction and fitness. One mechanism through which reinforcing effects can be measured is the development of a preference for the location in which an animal was exposed to a stimulus that, in turn, activates the internal reward systems. The strength of addicting and rewarding drugs can be measured by the development and strength of conditioned place preferences (CPPs). Testosterone is a natural hormone that elicits CPPs in rats [1,2] and mice [3]. In hamsters, T is voluntarily consumed through oral, intravenous and intracerebroventricular self-administration [4–6]. Together, these studies suggest that T has reinforcing effects. However, the natural functions of T's reinforcing effects are not well understood.
Testosterone release in a male animal usually occurs under two social contexts: male–male aggressive encounters and male–female sexual encounters [7]. Post-encounter T surges modulate many reproduction-related behaviours, such as territorial defence, mate guarding and exploratory behaviours. The modulation of these behaviours can require an animal to adjust the space use by allocating more time to one location and less time to another. We therefore speculate a potential natural function of T's reinforcing effects is to influence an animal's space use. Specifically, the reinforcing effects of T facilitate the association of the environmental context with the stimulus that elicits the T pulses.
The pair bond is a marker for an important life-history stage in monogamous animals, and affects several T-related social behaviours such as aggression and partner preferences [8,9]. Pair-bonded (PB) male California mice (Peromyscus californicus), a monogamous and territorial species, dampen their scent-marking responses towards novel females [10]. While some neural changes (i.e. dopamine system) associated with pair bond formation have been uncovered [11,12], less is known about how the pair-bonding experiences affect the reinforcing properties of hormones or neurochemicals. Formation of a pair bond in male prairie voles (Microtus ochrogaster) decreases the effect of the drug amphetamine on the formation of a CPP [13]. This finding suggests that social experience can alter the reinforcing effects of other external and internal stimuli.
This study investigated the influence of pair-bonding experience on the reinforcing effects of T by testing T's ability to produce CPPs in PB and sexually naive (SN) male California mice. Studies on wild California mice showed that SN males are more likely to explore unfamiliar areas to set up a territory than PB males, which are more likely to defend an existing, established territory [14,15]. We predicted that the reinforcing effects of T pulses in an unfamiliar/neutral environment would be greater in SN males than in PB males, illustrating how pair bonding can alter the reinforcing effects of T.
2. Material and methods
(a). Subjects
We used 48 male and 24 female P. californicus aged 6 to 12 months. They were group-housed (two to three per cage; 48 × 27 × 16 cm) under a 10 L : 14 D cycle. Animals were maintained in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Males were randomly assigned to either the PB group (n = 24) or the SN group (n = 24). For the PB group, each male was paired with a female 10 days before the experiment. The paired male and female mice were huddling side by side after 24 h of pairing, which is a well-accepted indicator of partner preference in monogamous prairie voles (M. ochrogaster) [16–18]. We did not record the mating behaviour of paired animals, but under laboratory conditions, 50% of animals have mated after 10 days of pairing with a range from the first few days after introduction to at least 35 days [19]. Pairs were always observed for compatibility, and if fighting occurred then both the male and female were excluded from the experiment. The other 24 males (SN group) were sexually naive and housed in male–male groups (including two males that were either siblings or males of a similar age) established after weaning (no wounds were observed on any of the males and no aggression was observed).
(b). Testosterone dose
We used 36 μg kg−1 T injections (T-cyclodextrin inclusion complex, Sigma, St Louis, MO) because a previous study found that 36 μg kg−1 subcutaneous T injection produces a transient increase in plasma T levels that is approximately three to five times higher than the baseline, and lasts for about 10 min [20]. While the dose in this study is lower than those used to identify CPPs in rats and mice, it mimics natural changes in T found in intact California mice after winning a male–male encounter [21]. Intraperitoneal T injections of 36 μg kg−1 are enough to elicit an intermediate winner effect and necessary for a full winner effect [7,20,22,23].
(c). Conditioned place preference apparatus and procedure
Conditioning took place in a large polycarbonate testing cage (91 cm long × 46 cm wide × 43 cm high) divided into three equal chambers. The two side chambers were connected to the middle chamber by manually controlled, sliding guillotine doors. The walls of one side chamber were decorated by horizontal black-and-white stripes, whereas vertical black-and-white stripes were used for the other side chamber.
Our CPP procedure was modelled after a previous study [24]. On day 1, each male's initial compartment preference was tested (pre-test). Following a 5 min habituation period in the middle chamber, we raised the doors to allow the male to move throughout all three chambers for 30 min. The side chamber in which the male spent the most time was the initially preferred side chamber, which was defined as CS− chamber during the conditioning phase. Accordingly, the initially non-preferred side chamber was defined as CS+ chamber. From days 2–7, males received a series of 45 min conditioning sessions, one session per day on six consecutive days. Saline or T conditioning sessions occurred on alternating days, beginning with the saline conditioning session on day 2. During the saline conditioning sessions (days 2, 4 and 6), all males were taken from their home cages and placed into CS− chamber, where they were given a subcutaneous saline injection and isolated for 45 min. Animals were randomly assigned to either the T group or the control group. During T conditioning sessions (days 3, 5 and 7), mice in the T group were placed in the CS+ chamber and administered subcutaneous T injections. Control group still received saline injections in CS+ chamber. The CPP apparatus was cleaned thoroughly with 50% ethanol following each conditioning session and test. Twenty-four hours after the last conditioning session, mice were tested for their place preferences using the same procedure as in the pre-test. During all conditioning sessions and tests, the female partner or male cage mate of the focal male was moved out of the testing room.
(d). Data analysis
Two types of scores were used to assess whether the T injection induced a CPP. The preference score was the time spent in the CS+ chamber divided by the sum of the time in the CS+ and CS− chambers. The difference score was the time spent in the CS− chamber subtracted by time in the CS+ chamber. The difference score was used to confirm the production of CPP because the preference score may increase solely due to decreased time spent in the CS− chamber (i.e. more time spent in the middle chamber). Therefore, the formation of a CPP was defined as significant changes in both preference and difference scores. These two scores have been widely used in CPP studies [25–28]. We tested normality using the Kolmogorov–Smirnov test. Groups were compared using paired t-tests to evaluate the changes in pre- and post-conditioning scores. Three mice were excluded from the statistical analysis because they either did not explore all three CPP chambers or were not compatible with the female partners.
3. Results
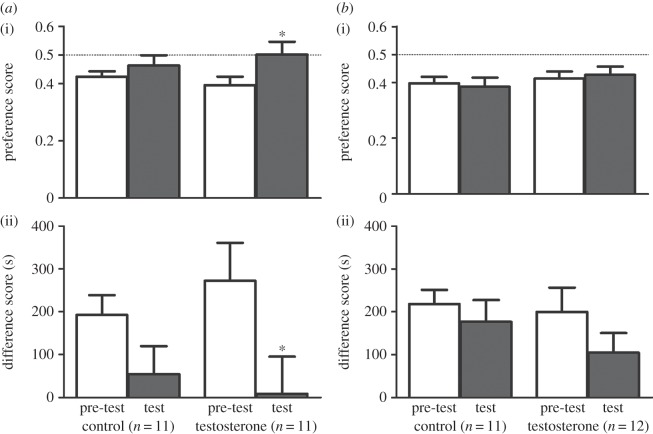
SN male California mice receiving saline injections did not form a CPP, whereas T-treated mice formed a CPP for the side chamber associated with the T injections (figure 1a). Preference scores during pre-tests and tests were not significantly different for the control group (t10 = 0.873, p = 0.40), but increased significantly for the T group (t10 = 2.229, p = 0.049). Likewise, the difference scores of pre-test and test did not change significantly for the control group (t10 = 1.634, p = 0.13), but decreased significantly for the T group (t10 = 2.797, p = 0.02).
Figure 1.
(i) Preference and (ii) difference scores on pre-tests (white bars) and tests (grey bars) for control and testosterone groups of (a) SN and (b) PB male California mice. Asterisks indicate a significant change in preference and difference scores between pre-test and test for the testosterone group (p < 0.05). Data are mean ± s.e.
By contrast, PB males did not show a CPP for the side chamber associated with the T injections (figure 1b). Paired t-tests showed that the preference scores did not change significantly for either the control group (t10 = 0.504, p = 0.63) or the T group (t11 = 0.349, p = 0.73). Likewise, the difference scores did not decrease significantly for either the control (t10 = 0.934, p = 0.37) group or the T group (t11 = 1.386, p = 0.19).
4. Discussion
Using a classical design for examining reinforcing effects of drugs, we demonstrate that subcutaneous administration of T (36 μg kg−1) can produce CPPs to an unfamiliar/neutral environment in male California mice. This finding is consistent with previous studies showing that transient T pulses are reinforcing in rats, mice and hamsters [1–6]. More importantly, we find that the reinforcing effects of T rely on the pair-bonding status; T created CPPs for a previously neutral environment in SN males, but not PB males. To our knowledge, this is the first study demonstrating variation in T-induced CPPs based on social experience. The variation in T-induced CPPs in SN and PB males may reflect plasticity in the strength of the reinforcing effects of T based on social experience. This plasticity may further affect an animal's space use, which is related to different reproductive priorities. Before forming pair bonds, most SN males are motivated to disperse up to 80 m and establish ownership of a territory [15]. The T-induced CPPs observed in SN males may reinforce the allocation of time towards exploration of an unfamiliar environment [29] and/or help to initiate territoriality in SN mice. By contrast, PB males have already established their own territories, where the interactions with the partner and familiarity with the environment may increase the salience of the territory. The importance of the territory to PB California mice has been revealed in other studies; the winner effect (increased ability to win based on previous wins) developed in the home cage is later expressed in the home cage but not in an unfamiliar cage [30,31]. Owing to the higher salience of the territory over a neutral environment, PB males may decrease time spent in a neutral environment but increase the time spent close to their mates, which might be the mechanism of maintaining sexual fidelity and reducing the risk of extra-pair copulations [32]. Moreover, the T pulses induced by the interactions with female partners may also help enhance the proximity between PB animals [33] and further block formation of CPPs in an unfamiliar/neutral environment. The variation in T functions between SN and PB males may underlie the plasticity of behavioural reinforcement in the neutral environment and orient males to allocate more time in the salient environmental cues in which the display of T-related behaviours are important for individual fitness.
The potential of T to influence space use related to reproductive effort appears to be mediated by long-term changes in T as well as short-term T pulsatile changes as described in our study. In birds, the long-term elevation of T increases behaviours associated with reproductive success, such as song rate [34], mate guarding [35], territorial extension [36] and defence [37]. Male dark-eyed juncos (Junco hyemalis) implanted with T tend to increase the allocation of time to locomotion and foraging, and decrease the time allocated to sleeping and preening [38]. In spiny lizards (Sceloporus jarrovi), T-implanted males spend more time in territorial defence during the day [39] and increase frequencies of male–female interactions following a territorial encounter with an introduced male [40]. The change in the above reproductively associated behaviours requires animals to allocate more time to salient locations such as the territory and the nest.
As a complement to previous studies using T implants, our results indicate that a few T pulses may also impact time allocation to different environments. Such pulsatile T releases are hypothesized to help animals cope with the immediate situation that stimulated release [41]. The T pulse that occurs after either male–male antagonistic encounters or male–female sexual encounters may induce males to differentially allocate time based on location of the encounter [7]. Aggressive encounters can induce CPPs per se [42,43], and the T pulses following a fight might mediate the CPPs for the location in which a given encounter occurs [44]. Therefore, the T-induced CPPs might influence territoriality by adjusting site preferences during instances of territory settlement. Moreover, the T pulses after the sexual encounters may drive the male to allocate more time in the environment in which the possibility of encountering a female is higher.
The focus in this study is on pulses of T; however, we cannot rule out the possibility that the baseline T levels are dissimilar between SN and PB males as levels were not measured, but we consider it unlikely because the baseline levels of T are not significantly different between SN and PB male California mice that have not had pups [45]. Also, in California mice, the T levels are characterized by being high during the first 24 h after a pair is introduced and fall back to baseline by three weeks [19].
On a neurophysiological level, pair-bonding experience may affect T's ability to produce CPPs by altering the dopamine system. In monogamous prairie voles, the pair-bonding experience itself elevates nucleus accumbens dopamine receptor binding [9]. Further, such experience decreased the reinforcing properties of amphetamine by decreasing the effect of amphetamine on dopamine-1 receptor binding, but not amphetamine-induced dopamine release or metabolism [13]. The same effects of pair-bond formation on amphetamine may be applied to T, which also activates the dopamine system to induce its reinforcing effects. While it is not possible to identify whether these effects are occurring rapidly via the final T injection or a cumulatively additive effect over the week-long injection, there is evidence that the reinforcing effects of T can be mediated through some of its metabolites that can affect the nucleus accumbens dopamine system through effects on γ-aminobutyric acid (GABA)A/benzodiazepine receptor complexes [41]. Also, a study in rats showed that the mutation of classical androgen receptors does not inhibit the development of dihydrotestosterone self-administration [46], suggesting that the reinforcing effects of T may be mediated through the activation of non-classical receptors. Future research will be needed to examine changes in the expression of androgen receptors and also other neurophysiological alterations that occurred after forming the pair bond, and how these alterations contribute to the decreased reinforcing effects of T in the neutral environment.
Acknowledgements
Thanks to Sarah Jane Alger, Josh Pultorak and Sarah Petruno for reviewing the paper. Also thanks to Lin Pei-Shan and Lin Chia-Te for their assistance.
Data accessibility
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.qd1g6.
Funding statement
This research was supported by NSF grant no. IOB-0620042 and the Wisconsin Alumni Research Foundation.
References
- 1.Alexander GM, Packard MG, Hines M. 1994. Testosterone has rewarding affective properties in male rats: implications for the biological basis of sexual motivation. Behav. Neurosci. 108, 424–428. ( 10.1037/0735-7044.108.2.424) [DOI] [PubMed] [Google Scholar]
- 2.Packard MG, Cornell AH, Alexander GM. 1997. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav. Neurosci. 111, 219–224. ( 10.1037/0735-7044.111.1.219) [DOI] [PubMed] [Google Scholar]
- 3.Arnedo M, Salvador A, Martinez-Sanchis S, Gonzalez-Bono E. 2000. Rewarding properties of testosterone in intact male mice: a pilot study. Pharmacol. Biochem. Behav. 65, 327–332. ( 10.1016/S0091-3057(99)00189-6) [DOI] [PubMed] [Google Scholar]
- 4.Johnson LR, Wood RI. 2001. Oral testosterone self-administration in male hamsters. Neuroendocrinology 73, 285–292. ( 10.1159/000054645) [DOI] [PubMed] [Google Scholar]
- 5.Wood RI, Johnson LR, Chu L, Schad C, Self DW. 2004. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology 171, 298–305. ( 10.1007/s00213-003-1587-7) [DOI] [PubMed] [Google Scholar]
- 6.Wood RI. 2002. Oral testosterone self-administration in male hamsters: dose-response, voluntary exercise, and individual differences. Horm. Behav. 41, 247–258. ( 10.1006/hbeh.2002.1769) [DOI] [PubMed] [Google Scholar]
- 7.Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. 2009. Testosterone release and social context: when it occurs and why. Front. Neuroendocrinol. 30, 460–469. ( 10.1016/j.yfrne.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 8.Insel TR, Preston S, Winslow JT. 1995. Mating in the monogamous male: behavioral consequences. Physiol. Behav. 57, 615–627. ( 10.1016/0031-9384(94)00362-9) [DOI] [PubMed] [Google Scholar]
- 9.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. 2005. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 9, 133–139. ( 10.1038/nn1613) [DOI] [PubMed] [Google Scholar]
- 10.Becker EA, Petruno S, Marler CA. 2012. A comparison of scent marking between a monogamous and promiscuous species of Peromyscus: pair bonded males do not advertise to novel females. PLoS ONE 7, e32002 ( 10.1371/journal.pone.0032002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. 2003. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 23, 3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. 2000. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav. Neurosci. 114, 173–183. ( 10.1037/0735-7044.114.1.173) [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. 2011. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. J. Neurosci. 31, 7960–7966. ( 10.1523/JNEUROSCI.1006-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribble DO. 1991. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 29, 161–166. ( 10.1007/BF00166397) [DOI] [Google Scholar]
- 15.Ribble DO. 1992. Dispersal in a monogamous rodent, Peromyscus californicus. Ecology 73, 859–866. ( 10.2307/1940163) [DOI] [Google Scholar]
- 16.Williams JR, Catania KC, Carter CS. 1992. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 26, 339–349. ( 10.1016/0018-506X(92)90004-F) [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Curtis J, Wang Z. 2001. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav. Neurosci. 115, 910–919. ( 10.1037/0735-7044.115.4.910) [DOI] [PubMed] [Google Scholar]
- 18.Ahern TH, Modi ME, Burkett JP, Young LJ. 2009. Evaluation of two automated metrics for analyzing partner preference tests. J. Neurosci. Methods 182, 180–188. ( 10.1016/j.jneumeth.2009.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleason ED, Marler CA. 2010. Testosterone response to courtship predicts future paternal behavior in the California mouse, Peromyscus californicus. Horm. Behav. 57, 147–154. ( 10.1016/j.yhbeh.2009.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trainor BC, Bird IM, Marler CA. 2004. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm. Behav. 45, 115–121. ( 10.1016/j.yhbeh.2003.09.006) [DOI] [PubMed] [Google Scholar]
- 21.Oyegbile TO, Marler CA. 2005. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 48, 259–267. ( 10.1016/j.yhbeh.2005.04.007) [DOI] [PubMed] [Google Scholar]
- 22.Fuxjager MJ, Oyegbile TO, Marler CA. 2011. Independent and additive contributions of postvictory testosterone and social experience to the development of the winner effect. Endocrinology 152, 3422–3429. ( 10.1210/en.2011-1099) [DOI] [PubMed] [Google Scholar]
- 23.Fuxjager MJ, Montgomery JL, Marler CA. 2011. Species differences in the winner effect disappear in response to post-victory testosterone manipulations. Proc. R. Soc. B 278, 3497–3503. ( 10.1098/rspb.2011.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardo M, Klebaur J, Valone J, Deaton C. 2001. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology 155, 278–284. ( 10.1007/s002130100720) [DOI] [PubMed] [Google Scholar]
- 25.Bell MR, Meerts SH, Sisk CL. 2010. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Horm. Behav. 58, 410–414. ( 10.1016/j.yhbeh.2010.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez I, Paredes RG. 2001. Only self-paced mating is rewarding in rats of both sexes. Horm. Behav. 40, 510–517. ( 10.1006/hbeh.2001.1712) [DOI] [PubMed] [Google Scholar]
- 27.Meerts SH, Clark AS. 2007. Female rats exhibit a conditioned place preference for nonpaced mating. Horm. Behav. 51, 89–94. ( 10.1016/j.yhbeh.2006.08.007) [DOI] [PubMed] [Google Scholar]
- 28.Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. 2009. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm. Behav. 55, 93–97. ( 10.1016/j.yhbeh.2008.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. 2013. Testosterone modulates spatial recognition memory in male rats. Horm. Behav. 63, 559–565. ( 10.1016/j.yhbeh.2013.02.007) [DOI] [PubMed] [Google Scholar]
- 30.Fuxjager MJ, Marler CA. 2010. How and why the winner effect forms: influences of contest environment and species differences. Behav. Ecol. 21, 37–45. ( 10.1093/beheco/arp148) [DOI] [Google Scholar]
- 31.Fuxjager MJ, Mast G, Becker EA, Marler CA. 2009. The ‘home advantage’ is necessary for a full winner effect and changes in post-encounter testosterone. Horm. Behav. 56, 214–219. ( 10.1016/j.yhbeh.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 32.Gubernick DJ, Nordby JC. 1993. Mechanisms of sexual fidelity in the monogamous California mouse, Peromyscus californicus. Behav. Ecol. Sociobiol. 32, 211–219. ( 10.1007/BF00173779) [DOI] [Google Scholar]
- 33.Gleason ED, Marler CA. 2012. A positive link between male testosterone and spacing behavior in pair-bonded California mice. Ethology 118, 1045–1050. ( 10.1111/eth.12005) [DOI] [Google Scholar]
- 34.Silverin B. 1980. Effects of long-acting testosterone treatment on freeliving pied flycatchers, Ficedula hypoleuca, during the breeding period. Anim. Behav. 28, 906–912. ( 10.1016/S0003-3472(80)80152-7) [DOI] [Google Scholar]
- 35.Saino N, Møller A. 1995. Testosterone correlates of mate guarding, singing and aggressive behaviour in male barn swallows, Hirundo rustica. Anim. Behav. 49, 465–472. ( 10.1006/anbe.1995.0060) [DOI] [Google Scholar]
- 36.Chandler C, Ketterson ED, Nolan V, Ziegenfus C. 1994. Effects of testosterone on spatial activity in free-ranging male dark-eyed juncos, Junco hyemalis. Anim. Behav. 47, 1445–1455. ( 10.1006/anbe.1994.1191) [DOI] [Google Scholar]
- 37.Hegner RE, Wingfield JC. 1987. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. The Auk 104, 462–469. ( 10.2307/4087545) [DOI] [Google Scholar]
- 38.Lynn SE, Houtman AM, Weathers WW, Ketterson ED, Nolan V., Jr 2000. Testosterone increases activity but not daily energy expenditure in captive male dark-eyed juncos, Junco hyemalis. Anim. Behav. 60, 581–587. ( 10.1006/anbe.2000.1510) [DOI] [PubMed] [Google Scholar]
- 39.Marler CA, Moore MC. 1989. Time and energy costs of aggression in testosterone-implanted free-living male mountain spiny lizards (Sceloporus jarrovi). Physiol. Zool. 62, 1334–1350. [Google Scholar]
- 40.Marler CA, Moore MC. 1991. Supplementary feeding compensates for testosterone-induced costs of aggression in male mountain spiny lizards, Sceloporus jarrovi. Anim. Behav. 42, 209–219. ( 10.1016/S0003-3472(05)80552-4) [DOI] [Google Scholar]
- 41.Nyby JG. 2008. Reflexive testosterone release: a model system for studying the nongenomic effects of testosterone upon male behavior. Front. Neuroendocrinol. 29, 199–210. ( 10.1016/j.yfrne.2007.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martínez M, Guillén-Salazar F, Salvador A, Simón VM. 1995. Successful intermale aggression and conditioned place preference in mice. Physiol. Behav. 58, 323–328. ( 10.1016/0031-9384(95)00061-M) [DOI] [PubMed] [Google Scholar]
- 43.Farrell WJ, Wilczynski W. 2006. Aggressive experience alters place preference in green anole lizards, Anolis carolinensis. Anim. Behav. 71, 1155–1164. ( 10.1016/j.anbehav.2005.10.006) [DOI] [Google Scholar]
- 44.Marler CA, Oyegbile TO, Plavicki J, Trainor BC. 2005. Response to Wingfield's commentary on ‘A continuing saga: the role of testosterone in aggression’. Horm. Behav. 48, 256–258. ( 10.1016/j.yhbeh.2005.05.010) [DOI] [PubMed] [Google Scholar]
- 45.Gubernick DJ, Nelson RJ. 1989. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm. Behav. 23, 203–210. ( 10.1016/0018-506X(89)90061-5) [DOI] [PubMed] [Google Scholar]
- 46.Sato SM, Johansen JA, Jordan CL, Wood RI. 2010. Membrane androgen receptors may mediate androgen reinforcement. Psychoneuroendocrinology 35, 1063–1073. ( 10.1016/j.psyneuen.2010.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.qd1g6.