Abstract
A critical task in evolutionary genetics is to explain the persistence of heritable variation in fitness-related traits such as immunity. Ecological factors can maintain genetic variation in immunity, but less is known about the role of other factors, such as antagonistic pleiotropy, on immunity. Sexually dimorphic immunity—with females often being more immune-competent—may maintain variation in immunity in dioecious populations. Most eco-immunological studies assess host resistance to parasites rather than the host's ability to maintain fitness during infection (tolerance). Distinguishing between resistance and tolerance is important as they are thought to have markedly different evolutionary and epidemiological outcomes. Few studies have investigated tolerance in animals, and the extent of sexual dimorphism in tolerance is unknown. Using males and females from 50 Drosophila melanogaster genotypes, we investigated possible sources of genetic variation for immunity by assessing both resistance and tolerance to the common bacterial pathogen Pseudomonas aeruginosa. We found evidence of sexual dimorphism and sexual antagonism for resistance and tolerance, and a trade-off between the two traits. Our findings suggest that antagonistic pleiotropy may be a major contributor to variation in immunity, with implications for host–parasite coevolution.
Keywords: sexual antagonism, host–parasite coevolution, sexual dimorphism, immunity, antagonistic pleiotropy
1. Introduction
If there is a cost of maintaining resistance in the absence of parasites [1], then the benefit of being resistant will depend on parasite prevalence. By reducing parasite fitness, resistance is expected to lead to negative frequency-dependent selection and the maintenance of genetic variation in both hosts and parasites [2–4]. By contrast, tolerance, the maintenance of fitness in spite of infection, does not decrease parasite fitness and selects for increased parasite prevalence. The positive feedback between tolerance and prevalence is predicted to result in the fixation of alleles that confer tolerance [4,5, but see 6]. Resistance and tolerance may therefore have different and potentially contrasting effects on host–parasite dynamics and genetic variation in immunity.
Although plant biologists have long recognized tolerance as a mechanism for dealing with stress [7–12], it is only recently that studies of tolerance in animals made their way into medical, evolutionary and ecological discourse. Sex differences in resistance and tolerance have been found in plants [8], and sex-biased immunity is a common finding in animals [13–16]. Several theories have been put forth to explain the prevalence of female-biased immunocompetence [17–19], but one possibility is that the sexes differ in their optimal investment in immunity or resource allocation to different components of the immune response (e.g. resistance and tolerance) [18,19]. Furthermore, the fact that resistance and tolerance have been found to be negatively genetically correlated [20] suggests that greater resistance in one sex may be accompanied by greater tolerance in the other. Sexually dimorphic immunity suggests that some alleles could have sexually antagonistic effects on immunity [21], and a significant fraction of standing genetic variation in immunity may result from sexually antagonistic selection.
Genetic variation in immunity is particularly likely to arise if immune traits present large mutational ‘targets’ [22]. In the absence of pleiotropy, immunity will be positively related to fitness, such that new mutations will have a negative effect on immunity, if any. However, mutational effects could be bidirectional if immunity is under balancing or stabilizing selection [23]. We investigated sex differences and the effect of spontaneous mutations on resistance and tolerance to Pseudomonas aeruginosa infection in the fruit fly Drosophila melanogaster. P. aeruginosa is a common insect pathogen, and Pseudomonas species have been identified in wild D. melanogaster populations [24]. We used 25 mutation accumulation (MA) lines and their corresponding control (25 non-MA) isogenic lines of D. melanogaster to test for sexual and genetic variation in resistance and tolerance. The MA genotypes studied are known to have reduced mean viability and reproductive success relative to their controls [25,26]. All genotypes were homozygous, and derived from the same ancestral genotype.
2. Methods
Fly stocks were maintained on yeast–sugar–agar medium at 25°C, 50% relative humidity, with a 12 L : 12 D cycle. Experimental flies were collected as virgins and housed in separate-sex vials of no more than 25 flies each. Flies were anaesthetized using CO2 to conduct manipulations and crosses. We studied lines that accumulated mutations on the second chromosome for 62 generations, and corresponding controls; the MA procedure is described elsewhere [25], and shown in the electronic supplementary material, figure S1. Following MA, crosses were performed to situate 25 MA chromosomes and 25 control chromosomes on a common isogenic background derived from an outbred laboratory population. These crosses served to eliminate all genetic variation within, as well as among, lines that was not owing to mutations that accumulated in MA lines or were segregating in control populations, and any variation on the tiny fourth chromosome, which was not manipulated (see the electronic supplementary material, figure S1 for details).
Flies were infected using the injector pumping method [27]. A single colony of P. aeruginosa (PA01) was grown overnight in LB broth at 37°C. The overnight culture was diluted so that the optical density at 600 nm (OD600 nm) was less than 0.05 and allowed to grow for approximately 5 h, corresponding to the log phase of growth (C. Vincent 2011, unpublished data). Culture (1 ml) was centrifuged and resuspended in 10 mM MgSO4, and the desired final concentration was obtained through serial dilution. Two doses were selected based on preliminary assays of fly survivorship, corresponding to OD600 nm of 0.001 and 0.002. Each focal fly was injected with one of three inoculants: a ‘sham’ of sterile MgSO4 solution, or one of the two doses of P. aeruginosa. Virgin flies (5–6 days post-eclosion) were injected midway along the dorsolateral line of the thorax with 9.6 nl of inoculum using a Nanoject microprocessor-controlled microinjection pipette (Drummond Scientific) with a pulled-glass capillary tip, resulting in systemic infection with minimal wounding [27].
Flies were inoculated approximately 24 h prior to fitness and plating assays (two blocks). A subset of flies were plated immediately following inoculation and incubated for approximately 18 h at 37°C. Bacteria were absent on all plates from the sham treatment, indicating that our inoculation method was free from contamination. Average initial load for a given infection level across j replicates was calculated as E[log(colj + 1)], where colj is the number of colonies in replicate j. As initial load did not differ significantly between males and females, initial load represents the average across sexes for a given infection level. On average, 1.85 and 2.75 log10[col + 1] colonies were observed for the low (OD600 nm 0.001) and high (OD600 nm 0.002) doses, respectively, and these initial loads were used in subsequent analyses.
To assess fitness, inoculated flies were placed in mating groups with competitors of the same sex and flies of the opposite sex. The MA and control chromosomes carry the recessive marker bw. Individual focal males and females were housed for 3 days with two outbred virgin bw/bw flies of the opposite sex and one outbred virgin wild-type fly of the same sex. At the end of day three, flies were anaesthetized and discarded. During preliminary tests, we found that flies infected with P. aeruginosa rarely survived more than 4 days; thus, our fitness assay likely captured total post-infection fitness. Offspring of focal individuals versus competitors (bw/bw versus bw/+) were scored by eye colour on days 12 and 15 following vial initiation. Multiple replicates (mean 5.83) were conducted for each combination of sex, genotype and infection level. Our measure of absolute fitness is the number of offspring from focal individuals relative to the total number of offspring, across replicates (see below). Over 175 000 offspring were scored from 1749 replicate mating trials. Mating groups are shown in detail in the electronic supplementary material, figure S1.
We used published methods to assess bacterial load [27]. Briefly, inoculated flies were individually homogenized in sterile 10 mM MgSO4, serially diluted and plated onto LB medium. Plates were incubated for approximately 18 h at 37°C, and the number of colonies was quantified. In five cases, there were too many colonies to count; these replicates were assigned the maximum recorded number of colonies. Bacterial load was assessed for 801 infected flies in total (four on average for each combination of sex, genotype and infection level). Bacterial load and reproductive fitness assays were performed on separate flies for each sex and genotype.
We operationally defined resistance as the ability to limit the growth of bacteria, and tolerance as the ability to maintain fitness in the presence of bacteria, relative to fitness in the absence of bacteria. For each combination of genotype, sex and infection level, we first calculated mean bacterial load and relative mean fitness across i replicates, where fitness assays and load assays were replicated separately. Mean bacterial load was calculated as d = E[log10(coli + 1)], where coli is the number of colonies scored in replicate i. The absence of bacteria in sham-treated flies was confirmed both immediately following inoculations and in the bacterial load assays. Mean fitness was calculated as W = Σbwi/Σtotali, where bwi and totali are the number of brown-eyed and total number of offspring scored in replicate i, respectively. Relative fitness at infection level k was then calculated as wk = Wk/W0, where W0 is fitness in the absence of infection (sham treatment).
Resistance was calculated as 1/m, where m is the linear slope of d on average initial load, with a fixed intercept of (0, 0) (i.e. when initial load is zero, bacterial load is always zero). Transforming the slope as –m instead of 1/m produced the same results. Initial loads were constant across groups, represented by 0, 1.85 and 2.75; thus, flies with relatively larger bacterial loads were said to have relatively lower resistance. Tolerance was calculated as the linear slope of w on d, with a fixed intercept of (0,1) (i.e. sham-treated flies, with d = 0, have relative fitness of 1). Thus, where increasing bacterial load resulted in greater loss of fitness, relative to fitness when uninfected, those flies were said to have lower tolerance. In a few cases, we were unable to determine tolerance in males (n = 2), females (n = 4) or either sex (n = 1), because W0 was zero, i.e. flies had zero fitness when uninfected. These tolerance values were treated as missing.
Data were analysed in R (v. 3.0.0 [28]). Differences among groups were assessed using analysis of variance. Non-parametric Kruskal–Wallis rank-sum tests produced similar results. Correlations were assessed using Spearman's rank correlation (rs). We applied the Z-transform approach [29] to test the combined significance of some statistics across groups. Statistical approaches to testing for genetic variance in each trait and an ad hoc procedure for an unbiased test of a genetic correlation between traits are described in the electronic supplementary material.
3. Results
We detected significant sexual dimorphism in immunity. Males were both more tolerant (F1,89 = 5.51, p = 0.0211; figure 1a) and resistant (F1,97 = 4.09, p = 0.0457; figure 1b) than females. There was no effect of mutation (tolerance: F1,89 = 0.033, p = 0.856; resistance: F1,97 = 1.71, p = 0.194) nor the interaction between sex and mutation (tolerance: F1,88 = 0.00, p = 0.989; resistance: F1,96 = 0.210, p = 0.648; figure 1) on tolerance or resistance.
Figure 1.
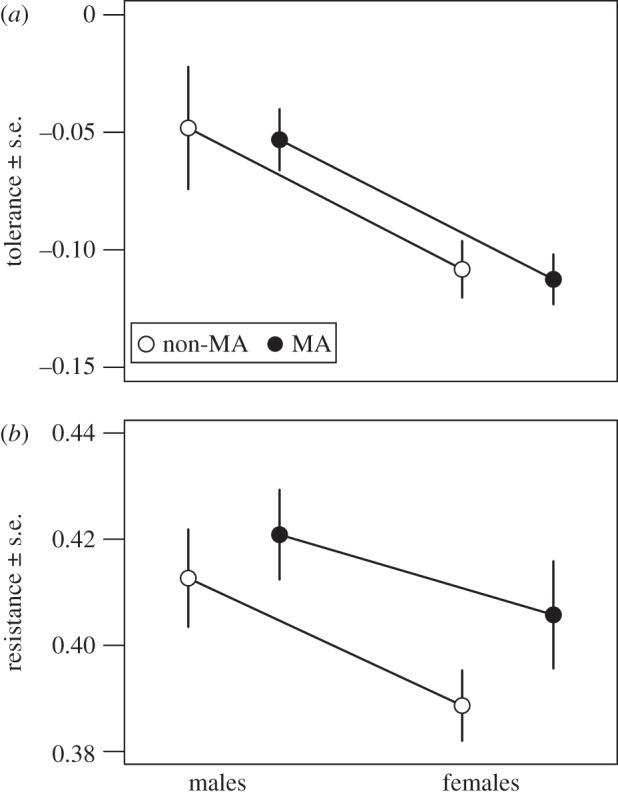
Resistance and tolerance in males and females. Markers indicate the mean ± s.e.m. among lines for either non-MA or MA lines. (a) Tolerance was calculated as the slope of the regression of relative fitness on bacterial load. Negative values of tolerance indicate decreasing fitness with increasing bacterial load; less-negative values indicate higher tolerance. (b) Resistance was calculated using the inverse slope of observed bacterial load on initial load; greater values indicate higher resistance, i.e. lower growth of bacteria following infection.
To test for sexual antagonism in immunity, we examined the intersexual genetic correlation [30] for resistance and tolerance. We found evidence for sexually antagonistic genetic effects on both resistance (rs = −0.43, p = 0.031, n = 25) and tolerance (rs = −0.34, p = 0.027, n = 43) with the exception of resistance in non-MA lines (rs = 0.102, p = 0.63, n = 25; figure 2). After removing outlier tolerance values, we still detected significant sexual dimorphism (F1,85 = 5.45, p = 0.022) and a significant negative intersexual genetic correlation for tolerance (rs = –0.33, p = 0.041, n = 39).
Figure 2.
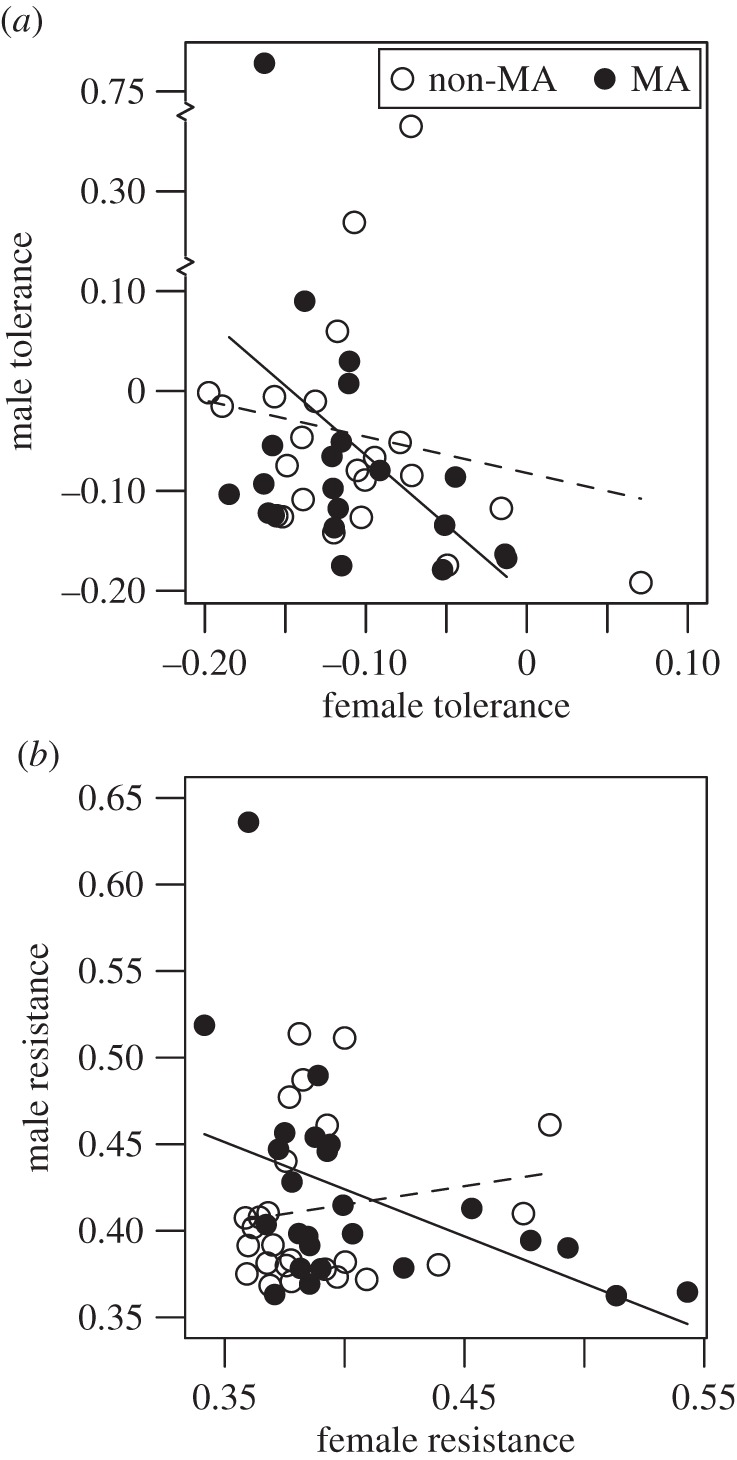
Intersexual genetic correlation for tolerance and resistance. Solid and dashed lines are linear trend lines for MA and non-MA lines, respectively. (a) Across all lines there was a significant negative correlation for tolerance. Breaks are shown in the y-axis to accommodate unusually high values of male tolerance. (b) Across all lines, there was a non-significant negative correlation for resistance. We detected a significant negative correlation for resistance in MA lines, and a non-significant positive correlation in non-MA lines.
In addition to sexual antagonism, we observed a negative correlation between resistance and tolerance (males: rs = −0.17, p = 0.25, n = 47; females: rs = −0.32, p = 0.028, n = 45; combined p-values: Z = −2.36, p = 0.018; figure 3), providing evidence for antagonistic pleiotropy between immune traits. We found significant genetic variance for both traits in both sexes, with the exception of tolerance in non-MA males (electronic supplementary material, table S1).
Figure 3.
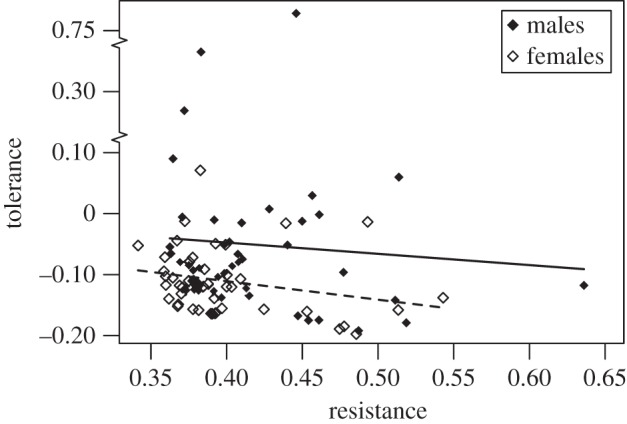
Correlation between resistance and tolerance. Solid and dashed lines are linear trend lines for males and females, respectively. Breaks are shown in the y-axis to accommodate unusually high values of male tolerance. We observed a negative genetic correlation between resistance and tolerance in both sexes. We expect a negative correlation between these traits by chance owing to their shared reliance on measures of bacterial load. An alternative unbiased analysis of the relationship between fitness and load supports the conclusion that resistance and tolerance are negatively correlated (see electronic supplementary material for details).
4. Discussion
We found evidence of sexual dimorphism and sexual antagonism for resistance and tolerance, and our results demonstrate a trade-off between the two traits. Some of our lines exhibited positive tolerance values (one female, seven male), suggesting increased fitness under infection. While this may reflect measurement error, an alternate explanation for positive tolerance is that the decreased likelihood of survival following an immune challenge led flies to increase their investment in current reproductive activity (terminal investment [31]). In our mating assays, infected males might have continued to sire offspring post-mortem provided that their sperm were stored and used by females. By contrast, fitness of infected females in our study may have been limited by post-mating longevity, which could explain the apparent sex difference in positive tolerance values we observed. Because we measured fitness as competitive reproductive success, our results may reflect variation in multiple adult life-history traits, including survivorship, fecundity, mating success and sperm competition. A disadvantage of this approach is that we cannot identify which of these specific fitness components gave rise to the patterns we observed.
Owing to the relatively greater reproductive investment by females, female fitness may be more negatively affected by stress, and as a result females could be more likely to invest in immunity, and particularly in resistance mechanisms [32]. Most eco-immunology studies assess resistance only, and, to date, no comparison of tolerance in male and female animals has been undertaken; we suggest that both resistance and tolerance should be assessed prior to concluding that females are more immunocompetent than males. Our results suggest that in D. melanogaster, males are the more immunocompetent sex. When infected with the bacterium P. aeruginosa, males outperformed females in their ability both to restrict the spread of infection (resistance), and to minimize pathology (tolerance). Furthermore, we observed an unexpected level of sexual antagonism for immunity, particularly for tolerance, resulting from both standing variation and new mutations.
Previous studies have reported male-biased resistance [33,34], but these are usually thought to be exceptions to the general rule of female-biased resistance. Intriguingly, these exceptions are often reported in studies that measure an individual's success (e.g. survival, fitness, pathogen clearance rates) following exposure to an actual pathogen [33,34], whereas female-biased immunity abounds when immunocompetence is estimated in the absence of a pathogen [35–37]. Thus, sex-biases in immunity may result from differences in realized (survival and reproductive success once infected) and constitutive (estimates of circulating immune effectors) immunity across the sexes. Because male-biased immunity is most frequently observed in studies involving actual pathogens and tolerance is necessarily measured during infection (a ‘realized’ response), it is perhaps unsurprising that we detected male-biased tolerance. Even so, the possibility of greater tolerance and resistance in males warrants further exploration.
In addition to sexual dimorphism and sexual antagonism, our findings provide evidence for antagonistic pleiotropy between immune traits (figure 3). The evolution of increased tolerance at the expense of resistance, which would slow host–parasite coevolutionary dynamics [4], may be prevented by sexual antagonism for immunity, leading to the maintenance of genetic variation in both traits. We found genetic and mutational variation in both traits, corroborating previous studies in animals that found genetic variance in resistance [3,20,24] and tolerance [20], and indicating that genetic variance in immunity is also introduced by new mutations. We found that spontaneous mutations contributed genetic variance, but did not affect the trait means. This is consistent with the idea that the ancestral genotype of the MA lines was not at the optimum for either males or females, owing to sexually antagonistic balancing selection, causing new mutations to have bidirectional effects [23].
We find that antagonistic pleiotropy, and particularly sexual antagonism, may be important factors in the maintenance of genetic variation in resistance and tolerance. The presence of two sexes may therefore have profound consequences for host–parasite coevolution.
Supplementary Material
Acknowledgements
We are grateful to D. Guttman for providing Pseudomonas stocks, and to A. F. Agrawal, R. L. Baker and A. Cutter for providing experimental materials and equipment. Thanks to Y. Apidianakis, C. Diegel, J. Kozlowska and K. Schreiber for discussion, and to L. St Amant, H. Kwok, M. Nambakkam and F. So for laboratory assistance.
Data accessibility
The data presented are available at Dryad doi:10.5061/dryad.t7t5r.
Funding statement
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) IPS and L'Oreal–UNESCO scholarships to C.M.V., an NSERC Vanier Scholarship to N.P.S. and NSERC Discovery Grants to A. F. Agrawal and R. L. Baker. D. T. Gwynne, N. Mideo, L. Rowe, J. Stinchcombe, M. L. Wayne and two anonymous reviewers provided helpful feedback on a previous version of the manuscript.
References
- 1.Kraaijeveld AR, Godfray HC. 1997. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389, 278–280. ( 10.1038/38483) [DOI] [PubMed] [Google Scholar]
- 2.Kover PX. 2006. Evolutionary genetics of host–parasite interactions. In Evolutionary genetics: concepts and case studies (eds Fox CW, Wolf JB.), pp. 447–463. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Vale PF, Little TJ. 2012. Fecundity compensation and tolerance to a sterilizing pathogen in Daphnia. J. Evol. Biol. 25, 1888–1896. ( 10.1111/j.1420-9101.2012.02579.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy BA, Kirchner JW. 2000. Evolutionary dynamics of pathogen resistance and tolerance. Evol. Int. J. Org. Evol. 54, 51–63. ( 10.1111/j.0014-3820.2000.tb00007.x) [DOI] [PubMed] [Google Scholar]
- 5.Svensson EI, Råberg L. 2010. Resistance and tolerance in animal enemy–victim coevolution. Trends Ecol. Evol. 25, 267–274. ( 10.1016/j.tree.2009.12.005) [DOI] [PubMed] [Google Scholar]
- 6.Stinchcombe JR. 2002. Can tolerance traits impose selection on herbivores? Evol. Ecol. 16, 595–602. ( 10.1023/A:1021617418037) [DOI] [Google Scholar]
- 7.Baucom RS, de Roode JC. 2011. Ecological immunology and tolerance in plants and animals. Funct. Ecol. 25, 18–28. ( 10.1111/j.1365-2435.2010.01742.x) [DOI] [Google Scholar]
- 8.Ashman T-L, Cole DH, Bradburn M. 2004. Sex-differential resistance and tolerance to herbivory in a gynodioecious wild strawberry. Ecology 85, 2550–2559. ( 10.1890/03-0495) [DOI] [Google Scholar]
- 9.Kover PX, Schaal BA. 2002. Genetic variation for disease resistance and tolerance among Arabidopsis thaliana accessions. Proc. Natl Acad. Sci. USA 99, 11 270–11 274. ( 10.1073/pnas.102288999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fineblum WL, Rausher MD. 1995. Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature 377, 517–520. ( 10.1038/377517a0) [DOI] [Google Scholar]
- 11.Strauss SY, Agrawal AA. 1999. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 14, 179–185. ( 10.1016/S0169-5347(98)01576-6) [DOI] [PubMed] [Google Scholar]
- 12.Simms EL. 2000. Defining tolerance as a norm of reaction. Evol. Ecol. 14, 563–570. ( 10.1023/A:1010956716539) [DOI] [Google Scholar]
- 13.Nunn CL, Lindenfors P, Pursall ER, Rolff J. 2009. On sexual dimorphism in immune function. Phil. Trans. R. Soc. B 364, 61–69. ( 10.1098/rstb.2008.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuk M, McKean KA. 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26, 1009–1023. ( 10.1016/S0020-7519(96)00086-0) [DOI] [PubMed] [Google Scholar]
- 15.Poulin R. 1996. Sexual inequalities in helminth infections: a cost of being a male? Am. Nat. 147, 287–295. ( 10.1086/285851) [DOI] [Google Scholar]
- 16.Braune P, Rolff J. 2001. Parasitism and survival in a damselfly: does host sex matter? Proc. R. Soc. Lond. B 268, 1133–1137. ( 10.1098/rspb.2001.1641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstad I, Karter AJ. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622. ( 10.1086/285346) [DOI] [Google Scholar]
- 18.Rolff J. 2002. Bateman's principle and immunity. Proc. R. Soc. Lond. B 269, 867–872. ( 10.1098/rspb.2002.1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKean KA, Nunney L. 2005. Bateman's principle and immunity: phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution 59, 1510 ( 10.1554/04-657) [DOI] [PubMed] [Google Scholar]
- 20.Råberg L, Sim D, Read AF. 2007. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814. ( 10.1126/science.1148526) [DOI] [PubMed] [Google Scholar]
- 21.Cox RM, Calsbeek R. 2009. Sexually antagonistic selection, sexual dimorphism, and the resolution of intralocus sexual conflict. Am. Nat. 173, 176–187. ( 10.1086/595841) [DOI] [PubMed] [Google Scholar]
- 22.Houle D. 1998. How should we explain variation in the genetic variance of traits? Genetica 102–103, 241–253. ( 10.1023/A:1017034925212) [DOI] [PubMed] [Google Scholar]
- 23.Keightley PD, Lynch M. 2003. Toward a realistic model of mutations affecting fitness. Evol. Int. J. Org. Evol. 57, 683–685; discussion 686–689 ( 10.1111/j.0014-3820.2003.tb01561.x) [DOI] [PubMed] [Google Scholar]
- 24.Corby-Harris V, Habel KE, Ali FG, Promislow DEL. 2007. Alternative measures of response to Pseudomonas aeruginosa infection in Drosophila melanogaster. J. Evol. Biol. 20, 526–533. ( 10.1111/j.1420-9101.2006.01267.x) [DOI] [PubMed] [Google Scholar]
- 25.Sharp NP, Agrawal AF. 2012. Evidence for elevated mutation rates in low-quality genotypes. Proc. Natl Acad. Sci. USA 109, 6142–6146. ( 10.1073/pnas.1118918109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp NP, Agrawal AF. 2013. Male-biased fitness effects of spontaneous mutations in Drosophila melanogaster. Evol. Int. J. Org. Evol. 67, 1189–1195. ( 10.1111/j.1558-5646.2012.01834.x) [DOI] [PubMed] [Google Scholar]
- 27.Apidianakis Y, Rahme LG. 2009. Drosophila melanogaster as a model host for studying Pseudomonas aeruginosa infection. Nat. Protoc. 4, 1285–1294. ( 10.1038/nprot.2009.124) [DOI] [PubMed] [Google Scholar]
- 28.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 29.Whitlock MC. 2005. Combining probability from independent tests: the weighted Z-method is superior to Fisher's approach. J. Evol. Biol. 18, 1368–1373. ( 10.1111/j.1420-9101.2005.00917.x) [DOI] [PubMed] [Google Scholar]
- 30.Chippindale AK, Gibson JR, Rice WR. 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA 98, 1671–1675. ( 10.1073/pnas.98.4.1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonneaud C, Mazuc J, Chastel O, Westerdahl H, Sorci G. 2004. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evol. Int. J. Org. Evol. 58, 2823–2830. ( 10.1111/j.0014-3820.2004.tb01633.x) [DOI] [PubMed] [Google Scholar]
- 32.McCall AC. 2007. Leaf damage and gender but not flower damage affect female fitness in Nemophila menziesii (Hydrophyllaceae). Am. J. Bot. 94, 445–450. ( 10.3732/ajb.94.3.445) [DOI] [PubMed] [Google Scholar]
- 33.Haine ER, Pollitt LC, Moret Y, Siva-Jothy MT, Rolff J. 2008. Temporal patterns in immune responses to a range of microbial insults (Tenebrio molitor). J. Insect Physiol. 54, 1090–1097. ( 10.1016/j.jinsphys.2008.04.013) [DOI] [PubMed] [Google Scholar]
- 34.Kelly CD, Jennions MD. 2009. Sexually dimorphic immune response in the harem polygynous Wellington tree weta Hemideina crassidens. Physiol. Entomol. 34, 174–179. ( 10.1111/j.1365-3032.2009.00671.x) [DOI] [Google Scholar]
- 35.Schwarzenbach GA, Hosken DJ, Ward PI. 2005. Sex and immunity in the yellow dung fly Scathophaga stercoraria. J. Evol. Biol. 18, 455–463. ( 10.1111/j.1420-9101.2004.00820.x) [DOI] [PubMed] [Google Scholar]
- 36.Yourth CP, Forbes MR, Baker RL. 2002. Sex differences in melanotic encapsulation responses (immunocompetence) in the damselfly Lestes forcipatus Rambur. Can. J. Zool. 80, 1578–1583. ( 10.1139/z02-159) [DOI] [Google Scholar]
- 37.Tucker TM, Stevens L. 2003. Geographical variation and sexual dimorphism of phenoloxidase levels in Japanese beetles (Popillia japonica). Proc. R. Soc. Lond. B 270, S245–S247. ( 10.1098/rsbl.2003.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented are available at Dryad doi:10.5061/dryad.t7t5r.


