Abstract
Strokes are devastating as there are no current therapies to prevent the long term neurological deficits that they cause. Soon after ischemic stroke, there is proliferation and differentiation of neural stem/progenitor cells as an important mechanism for neuronal restoration. However, endogenous neurogenesis by itself is insufficient for effective brain repair after stroke as most newborn neurons do not survive. One fascinating strategy for stroke treatment would thus be maintaining the survival and/or promoting the differentiation of endogenous neural stem/progenitor cells. Using transgenic (Tg) mice over-expressing the C. elegans fat-1 gene encoding an enzyme that converts endogenous omega-6 to omega-3 polyunsaturated fatty acids (n-3 PUFAs), we showed that fat-1 Tg mice with chronically elevated brain levels of n-3 PUFAs exhibited less brain damage and significantly improved long-term neurological performance compared to wild type littermates. Importantly, post-stroke neurogenesis occurred more robustly in fat-1 Tg mice after focal ischemia. This was manifested by enhanced neural stem cell proliferation/differentiation and increased migration of neuroblasts to the ischemic sites where neuroblasts matured into resident neurons. Moreover, these neurogenic effects were accompanied by significantly increased oligodendrogenesis. Our results suggest that n-3 PUFA supplementation is a potential neurogenic and oligodendrogenic treatment to naturally improve post-stroke brain repair and long-term functional recovery.
Keywords: omega-3 polyunsaturated fatty acids, stroke, neuroprotection, neurogenesis, oligodendrogenesis
INTRODUCTION
Stroke is a leading cause of serious long-term disability in adults. There are approximately 500,000 new cases of stroke annually and more than 6 million stroke survivors in the United States alone. No therapies are available to prevent stroke-induced neurological deficits. Recently, neurorestorative therapies that enhance neurogenesis, angiogenesis, or oligodendrogenesis have gained increasing attention in the stroke field because of their efficacy and their ability to extend the therapeutic time window to improve long-term stroke recovery (1).
In the last several decades, evidence has accumulated that continuous neurogenesis, or the production of new neurons, still occurs during adulthood and endows the brain with the capacity to replace damaged tissue after injuries such as stroke (2). Increased endogenous neurogenesis was observed within the adult brain after stroke in experimental animals and postmortem human brains (2, 3). Such increased neurogenesis initially generated optimism that this was a means of compensatory endogenous repair. Disappointingly, however, the majority of newly generated neurons die soon after stroke. Only about 20% of the new neurons in the ischemic striatum survive longer than 2 weeks (2), replacing a mere 0.2% of the lost mature neurons. Recent studies highlight the potential of exogenous neural stem cell (NSCs) transplantation therapy to enhance post-stroke neurogenesis; however, significant limitations, including unanticipated side effects and challenges with delivery, are still awaiting solutions. One alternative strategy to enhance post-stroke neurogenesis would be to maintain the survival and/or promote the differentiation of endogenous neural stem/progenitor cells (NSCs/NPCs) after stroke.
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) have long been known to be critical for nervous tissue growth and function in the developing brain (4). Docosahexaenoic acid (DHA), the most abundant n-3 PUFA in the central nervous system (CNS), promotes the differentiation of cultured rat NSCs towards a neuronal lineage (5) and improves neurite outgrowth in cultured neurons (6). Recent findings further suggest a relationship between n-3 PUFAs and neurogenesis in adulthood. Experiments in adult H. americanus demonstrate that even short-term dietary augmentation of n-3 relative to n-6 PUFAs results in a significant increase in the rate of neuronal proliferation in the olfactory system, where neurogenesis persists throughout life (7). Dietary administration of DHA also increases the number of newborn neurons in the hippocampus and improves learning and memory in both young and aged adult rats (8, 9). The effect of n-3 PUFAs on neurogenesis is further implicated in in vivo models of some chronic neurological disorders such as Alzheimer’s disease (10). The effect of n-3 PUFAs on neurogenesis and other processes of brain repair after acute brain insults such as stroke, however, remains unexplored.
Using transgenic (Tg) mice over-expressing the C. elegans fat-1 gene encoding an enzyme that converts endogenous n-6 to n-3 PUFAs, we showed that fat-1 Tg mice with elevated brain levels of n-3 PUFAs are remarkably resistant to focal cerebral ischemia compared to their wild type (Wt) littermates. Interestingly, post-stroke neurogenesis and oligodendrogenesis are robustly enhanced in fat-1 Tg mice after focal ischemia. Our results therefore suggest that n-3 PUFA supplementation is a potential treatment to naturally improve post-stroke brain repair and long-term functional recovery.
MATERIAL AND METHODS
Creation of fat-1 transgenic mice
The chimeric transgene to create the transgenic mice contains the C. elegans fat-1 gene driven by the cytomegalovirus (CMV) enhancer and a chicken β-actin promoter (Cβ-actin). The fat-1 gene encodes an n-3 PUFA desaturase that adds an extra n-3 double bond to n-6 fatty acids, hence converting n-6 PUFAs to n-3 PUFAs. To facilitate the expression of fat-1 in mammalian cells, the coding region of C. elegans fat-1 cDNA had been optimized for the construction of the chimeric transgene (11). The fat-1 heterozygote on the C57/B6 background and Wt C57/B6 mice were interbred to produce fat-1 Tg mice and Wt littermates. All lines of mice continued to be backbred to the C57/B6 background in order to minimize the potential influence of genetic heterogeneity on the susceptibility to stroke. Both fat-1 and Wt littermates were maintained on a normal lab-rodent diet (5% fat, n-6:n-3 ratio = 5:1, ProLab IsoPro RMH 3000, PMI, Brentwood, MO).
Lipid extraction and fatty acid analysis
Brain samples were collected and stored at −80 °C before fatty acid analysis. After addition of an internal standard (1,2-dinonadecanoyl-sn-glycero-3-phosphocholine), total lipid extracts were prepared by a modified Folch extraction method. Fatty acid profiles were determined by using capillary gas chromatography in the School of Public Health, University of Pittsburgh. Fatty acid concentrations were expressed as pmol/mg extracted lipid.
Murine model of transient focal ischemia
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male 10–12 week old mice (25–30g) were anesthetized with 1.5% isoflurane in a 30% O2/68.5% N2O mixture under spontaneous breathing. Focal cerebral ischemia was produced by intraluminal occlusion of the left middle cerebral artery (MCA) for 60 min as described previously (12). Rectal temperature was controlled at ~37.0°C throughout the experiment via a temperature-regulated heating pad. To confirm the induction of ischemia and successful reperfusion, changes in regional cerebral blood flow (rCBF) before, during and after MCA occlusion were evaluated in animals using laser-Doppler flowmetry. In two groups of mice (Wt or Tg-fat-1), quantitative rCBF measurement was performed at 15 min after the onset of MCA occlusion using [14C]-iodoantipyrine autoradiography as described previously (13). In all experiments, the surgeon was blinded to mouse genotype.
Measurement of infarct volume
For 2,3,5-triphenyltetrazolium chloride (TTC) staining, brains were removed and sliced into 7 coronal sections, each 1mm thick. Sections were immersed in prewarmed 2% TTC (Sigma) in saline for 10 min, and then fixed in 4% paraformaldehyde in PBS, pH 7.4. For cresyl violet staining, animals were sacrificed and perfused transcardially with 0.9% normal saline and 4% paraformaldehyde in PBS. Free floating sections from the fixed and dehydrated brains were stained with cresyl violet (Sigma, St. Louis, MO). Infarct volume was determined with NIH Image J analysis by an observer blinded to the experimental group.
Corner test
The corner test was performed as described previously (14). In this test the ischemic mouse turns preferentially toward the non-impaired (left) side. Corner test performance was expressed by the number of left turns out of 10 turn trials per day.
BrdU injections
To label newly generated cells, we injected the S-phase marker 5-bromo-2-deoxyuridine (BrdU) (50 mg/kg body weight, Sigma) intraperitoneally twice per day, 8 hrs apart at day 10–13 after MCAO. Animals were sacrificed 14 day after MCAO.
Immunohistochemistry
Animals were deeply anesthetized before intracardiac perfusion with 0.9% saline followed by 4% paraformaldehyde. Brains were cryoprotected in 30% sucrose in PBS. Immunohistochemistry staining was performed on 30μm free-floating sections. Sections for BrdU staining were pretreated with 1 N HCl for 1 hr at 37°C followed with 0.1 mol/L boric acid (pH 8.5) for 10 minutes at room temperature. After blocking with 10% goat serum albumin in PBS for 1 hr, sections were incubated with rat anti-BrdU (1:400; Abcam, Cambridge, MA) antibody at 4°C overnight. After washing, DyLight™ 488-conjugated goat anti-rat IgG (1:2000, Jackson ImmunoResearch Laboratories, West Grove, PA) was applied to the sections for 1 hr at room temperature. Immature NPCs were visualized using anti-doublecortin (DCX) (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) followed by Cy3 conjugated secondary IgG antibody (1:2000; Jackson Immunoresearch Laboratories). Mature neurons were visualized using anti-NeuN (1:1000; Millipore, Billerica, MA) immunostaining. Oligodendrocyte progenitor cells (OPCs) were visualized with anti-NG2 (1:200; Cell Signaling) immunostaining. Immunopositive cell densities were calculated as the number of cells in the designated fields divided by the area measured by the MCID image analysis system (Imaging Research, G.E. Healthcare Biosciences, Pittsburgh, PA). The fields were randomly sampled by an investigator who was blinded of the experiment groups. All positive cells in each field were counted.
Statistical analysis
All data are reported as mean ± SEM. Significant differences between means were assessed by ANOVA and post hoc Scheffe tests for multiple comparisons unless otherwise indicated. For some experiments, significance was assessed by the chi-square or the t-test. p<0.05 was considered statistically significant.
RESULTS
Expression of the fat-1 transgene elevated brain levels of n-3 PUFAs
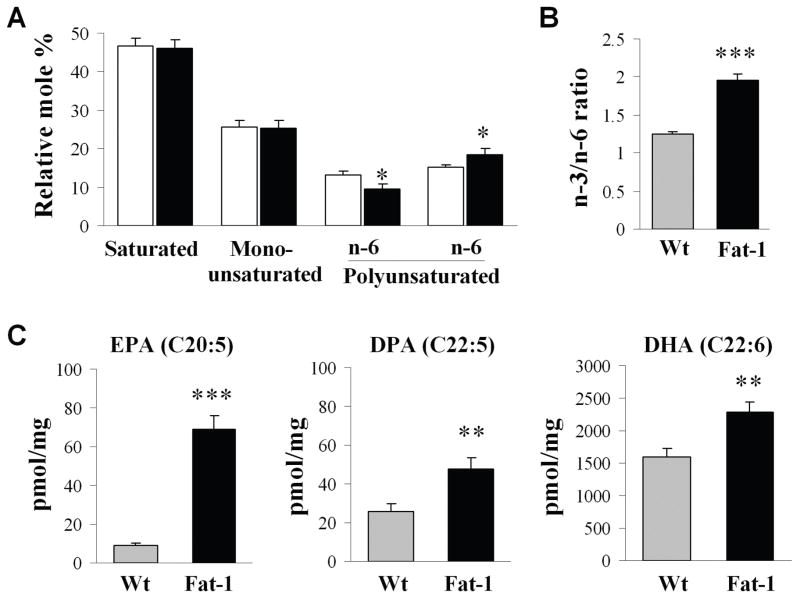
Fatty acid analysis of forebrains was performed on the brain samples from Wt and fat-1 mice using gas chromatography. As shown in Fig. 1A, no difference was detected between Wt and fat-1 mice in the proportions of total saturated fatty acids and mono-unsaturated fatty acids. In contrast, compared to Wt mice, the n-3 PUFA fraction in fat-1 mice was significantly increased, whereas the n-6 PUFA fraction was significantly decreased, leading to an increase in the overall n-3/n-6 PUFA ratio (Fig. 1B). Individually, the contents of the three major n-3 fatty acids, including EPA (eicosapentaenoic acid, C20:5), DPA (docosapentaenoic acid, C22:5) and DHA (docosahexaenoic acid, C22:6) were all significantly increased in the fat-1 mice compared to their Wt littermates (Fig. 1C).
Fig. 1. Lipid profiles of forebrains from wild-type (Wt) and fat-1 mice.
(A) Total lipid profile of mouse forebrains. Data are mean ± SEM, n=5; *p<0.05 vs. Wt by ANOVA and post hoc test. (B) n-3/n-6 ratio, n=5, ***p<0.001 vs. Wt by chi-square test. (C) Individual n-3 PUFAs. Data are mean ± SD, n=6; **p<0.01 ***p<0.001 vs. Wt by t-tests. EPA: eicosapentaenoic acid; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid.
Fat-1 Tg mice showed reduced brain damage after stroke compared to Wt littermates
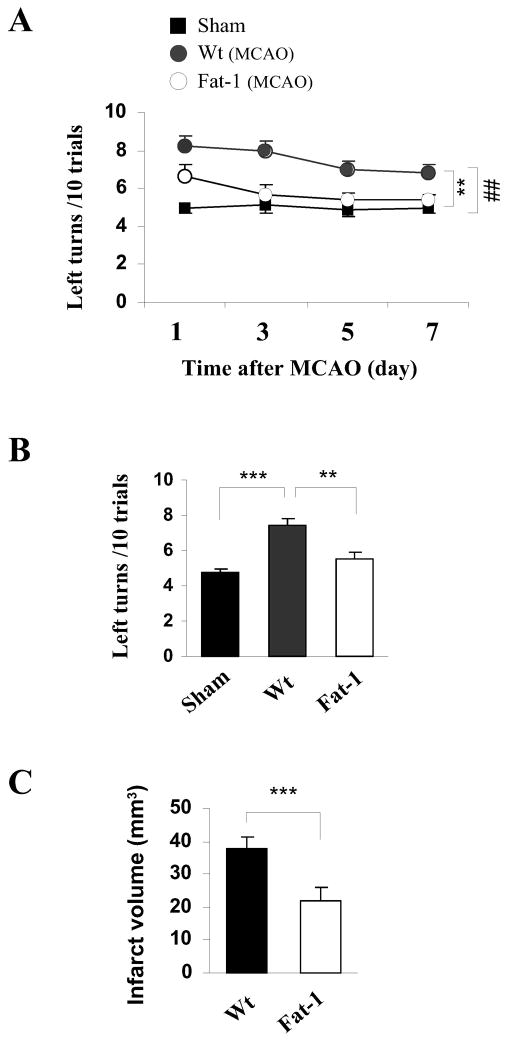
To determine whether n-3 PUFAs protect against cerebral ischemia, adult male fat-1 and Wt littermates were subjected to MCAO and sacrificed 48 hrs later. As shown in Fig. 2A–C, fat-1 mice developed a significantly smaller infarct (47.6% reduction) than their Wt littermates. To rule out the possibility that the protection is associated with a change in regional cerebral blood flow (rCBF) in fat-1 mice, we quantitatively measured rCBF at 15 min after the onset of MCAO. As shown in Fig. 2D–F, no significant difference was detected between Wt and fat-1 mice in the contralateral hemisphere, ischemic boundary and ischemic core regions, indicating that fat-1 mice had ischemic areas and intensities similar to those of Wt mice after MCAO. Similarly, as determined using laser Doppler flowmetry, no difference in cortical rCBF was found between Wt and fat-1 mice during MCAO or reperfusion (not shown). No significant alterations in arterial blood pressure and blood gases were found between Wt and fat-1 mice during MCAO either (not shown). These findings indicate that n-3 PUFAs protect against ischemia independent of changes in rCBF or other physiological variables that are known to affect stroke outcomes.
Fig. 2. n-3 PUFAs protect against ischemia induced by MCAO in mice.

(A) Infarct volume at 48 hrs after 60 min of MCAO in fat-1 and Wt mice. (B) Infarct areas of consecutive coronal sections every 1 mm throughout the MCA territory. Data are mean ± SE, n=6 per group. * p<0.05, **: p<0.01 vs. Wt. (C) TTC staining showing smaller infarct in a fat-1 mouse brain than in Wt mouse brain. (D) Representative autoradiography of C14-IAP, showing rCBF reduction in the ischemic boundary (area 2) and ischemic core (area 3) regions. (E) Wt and fat-1 mice demonstrated similar ischemic areas (CBF was less than 30% of baseline). (F) Wt and fat-1 mice demonstrated similar levels of CBF reduction in the ischemic boundary and ischemic core regions, compared to the contralateral hemisphere (area 1).
The fat-1 transgene improved functional outcomes and conferred prolonged protection after stroke
To determine the impact of n-3 PUFAs on neurofunctional outcomes after stroke, the corner test was performed during both the acute (1–7 days) and late (14 days) recovery stages following transient focal ischemia. Sham control mice showed no significant difference in neurobehavioral performance regardless of their phenotype; thus, data from both sham groups were combined. Acute sensorimotor dysfunction (1–7 days after ischemia) was significantly ameliorated in fat-1 mice compared to their Wt littermates (Fig. 3A). More importantly, the fat-1 mice also showed improved long-term sensorimotor performance (Fig. 3B), together with reduced infarct volume (Fig. 3C) at 14 days after MCAO.
Fig. 3. fat-1 mice showed improved neurological outcomes and long term protection against focal ischemia.
(A,B) Corner test at 1–7 days (A) and 14 days (B) after ischemia or sham operation following 60 min of MCAO (n=7 per group). (E) Infarct volume determined at 14 days after ischemia in cresyl violet-stained brain sections (n=7 per group). ## p<0.01 between Wt MCAO and sham control mice; *p<0.05; **p<0.01; ***p<0.001 between Wt MCAO and fat-1 MCAO mice.
Fat-1 Tg mice showed enhanced neurogenesis after stroke compared to Wt littermates
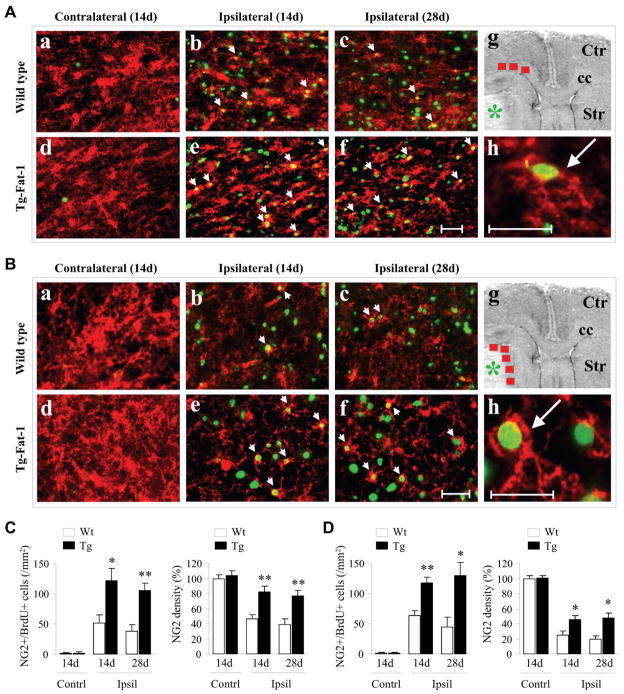
Neurogenesis induced by ischemic injury involves the proliferation of NSCs/NPCs, differentiation of NPCs, and migration of neuroblasts to the ischemic areas. Upon arrival, the neuroblasts mature into resident neurons and integrate into the parenchymal tissue (15–17). The subventricular zone (SVZ) lining the lateral ventricles is a critical source of neurogenesis in the adult brain. To determine the effect of n-3 PUFAs on post-stroke neurogenesis in the SVZ, Wt and fat-1 Tg mice were subjected to 60 min of MCAO. BrdU (50 mg/kg) was injected (i.p.) twice a day at 10–13 days after ischemia. Animals were sacrificed 14 days after ischemia. BrdU staining was performed to reveal NSCs/NPCs proliferation; and Brdu/DCX double staining was performed to reveal newly generated NPCs in the SVZ (Fig. 4A and 4B). We found that: 1) both SVZ volume and the number of BrdU+ cells increased significantly in ipsilateral SVZ of Wt mice after ischemia; and 2) the fat-1 Tg mice showed enlarged SVZ volume and amplified Brdu staining of the same region compared to Wt littermates. These results suggest increased NSCs/NPCs proliferation in the SVZ of the ischemic hemisphere in Wt mice, and that this is even further enhanced by a rise in n-3 PUFAs in transgenics.
Fig. 4. Enhanced post-stroke neurogenesis in fat-1 mice.
(A) Representative images of BrdU labeling (a–c) and Brdu/DCX double labeling (d–f) in the dorsolateral SVZ (marked with yellow arrows) of either the contralateral hemisphere of wild-type (Wt) mice or the ipsilateral hemisphere of both Wt and fat-1 mice. All images were taken from animals sacrificed 14 days after 60 min of MCAO (from 5 mice/group with similar results). (B) Quantification of SVZ volume expressed as percentage of Wt contralateral (Top), and the number of Brdu+ cells in the SVZ (bottom). n=5/group. Note that the ipsilateral SVZ is further enlarged with more Brdu+ cells in fat-1 mice compared to the Wt mice after ischemia. (C) Representative images of double-label immunofluorescence for BrdU (green) and DCX (red) in a peri-infarct region in the striatum at 14 days after 60 min of MCAO. The BrdU+/DCX+ dual labeled cells show yellow fluorescence (arrow). (a–b) Lower magnification. (c) Image analysis in 4 ipsilateral peri-infarct areas in striatum (red boxes), and the non-ischemic contralateral counterparts. (d) Higher magnification. (e) Three-dimensional confocal image of double-labeled immunofluorescence for DCX and Brdu. (D) Quantification of the number of BrdU+/DCX+ cells per mm2 area in the peri-infarct region in the striatum of Wt and fat-1 mice at 14 days after ischemia. n=5/group. (E) Representative images of double-label immunofluorescence for BrdU (red) and NeuN (green) in a peri-infarct region in the striatum at 14 days and 28 days after MCAO. The BrdU+/NeuN+ dual labeled cells show yellow fluorescence (arrow). Insets show higher magnification of BrdU+/NeuN+ double labeling. (F) Quantification of the number of BrdU+/NeuN+ cells per mm2 area in the peri-infarct region in the striatum of Wt and fat-1 mice at 14 and 28 days after ischemia. Data are mean ± SE, n=5/group; *p<0.05; **p<0.01.
Double immunofluorescence staining for BrdU and DCX was then performed to determine whether the n-3 PUFA-induced newborn cells in the SVZ successfully migrated into striatum at 14 days after MCAO (Fig. 4C and 4D). In the contralateral hemisphere of the MCAO brain, few cells were immunoreactive for DCX and Brdu. Ischemia gave rise to a marked increase of Brdu/DCX double labeling in the ipsilateral striatum in Wt mice. Fat-1 transgene expression resulted in further augmentation in the number of Brdu+/DCX+ cells in the same region after ischemia, suggesting that more newly generated NPCs migrated successfully into the injured striatum.
Finally, we performed BrdU and NeuN double staining to determine the effect of n-3 PUFAs on the maturation of newly generated NPCs after focal ischemia. As shown in Fig. 4E and 4F, the differentiation of newly proliferated NPCs into mature neural phenotypes (BrdU+NeuN+) was significantly enhanced in the ipsilateral striatum of fat-1 mice at 14 days and 28 days after MCAO. These results suggest that an elevated cerebral level of n-3 PUFA may be associated with improved neuronal replacement after ischemic brain injury. It is noted that numerous BrdU-labeled cells do not express NeuN, which may reflect active gliosis and inflammatory response in the ischemic brain.
Fat-1 Tg mice showed enhanced oligodendrogenesis after stroke compared to Wt littermates
White matter injury is an important component of post-stroke brain damage (18) and is expected to disrupt normal communication between brain regions. The presence of OPCs in the mature brain provides the opportunity for oligodendrocyte replenishment and white matter repair after injury. To characterize the effect of n-3 PUFAs on oligodendrogenesis after ischemic stroke, we examined progenitor cells committed to oligodendrocyte differentiation in the corpus callosum (CC, Fig 5A) and striatum (Fig. 5B) by Brdu and NG2 double staining. We found that NG2 was constitutively expressed in the brains of both Wt and fat-1 Tg animals. Focal ischemia resulted in dramatic decreases in the density of NG2 staining at 14 and 28 days following MCAO, suggesting a stroke-induced loss of OPCs. However, the number of newly generated OPCs, represented by BrdU-labeled NG2+ cells, increased in both the CC and striatum of ipsilateral hemispheres in Wt mice, suggesting that ischemic stress induces endogenous proliferation or repair of OPCs. The expression of fat-1 transgene led to significant increases of NG2 density and the number of NG2+Brdu+ cells at 14 and 28 days following MCAO, indicating a preserved population of OPCs and increased oligodendrogenesis (Fig 5C–5D).
Fig. 5. Enhanced oligodendrogenesis after stroke in fat-1 Tg mice.
(A and B) Immunostaining of NG2 (red) and BrdU (green) in the corpus callosum (CC, A) and striatum (B) of either the ipsilateral and/or the contralateral hemisphere of Wt and fat-1 mice at 14 and 28 days after MCAO. (a–f) Lower magnification. Scale bar: 50μm. (h) Higher magnification. Scale bar: 30μm. (g) Image analysis in 3 ipsilateral CC regions or 5 ipsilateral peri-infarct areas in striatum (red boxes), and the non-ischemic contralateral counterparts. Green * indicate infarct areas. (C) Quantification of NG2+/BrdU+ cells, expressed as the number of NG2+/BrdU+ cells/mm2 and NG2 density in the peri-infarct region and contralateral counterparts in the CC. (D) Quantification of NG2+/BrdU+ cells and NG2 density in the peri-infarct region and contralateral counterparts in the striatum. Data are mean ± SE, n=5/group; *p<0.05; **p<0.01 vs. ipsilateral hemisphere of Wt group.
DISCUSSION
Western diets poor in n-3 PUFAs are becoming increasingly implicated in poor neural development and function. Information has accumulated from epidemiological observations and intervention studies to show that lower n-3 PUFA intake increases the risk of many neurological disorders including ischemic stroke (19–21). It has been estimated that the ratio of n-6 to n-3 fatty acids in the diet used to be 1:1, but the ratio in the Western diet nowadays is about 10:1 due to increased use of vegetable oils and reduced fish consumption. A large body of evidence suggests that increasing the relative abundance of dietary n-3 PUFAs may have multiple beneficial effects.
Prophylactic or therapeutic supplementation of n-3 PUFAs has emerged as a highly promising neuroprotective strategy for stroke. Epidemiological studies reveal an inverse association between n-3 PUFA intake and the risk of ischemic stroke (19–21). Supplementation with n-3 PUFAs, especially DHA, effectively reduces the extent of brain damage and neurological deficits in the acute phase of ischemic injury in animals (17). However, the long-term effect of n-3 PUFAs on brain damage and post-stroke neurological recovery is not thoroughly researched, because short-term supplementation of n-3 PUFAs before or after ischemic insult is the most popular regime. In the human condition, however, one would expect long-term supplementation with n-3 PUFAs as a prophylactic against stroke risk. Aside from its transient nature, another problem with food supplementation of n-3 PUFA is that control and experimental animals are often maintained on different diets that may contain other bioactive components. All of these issues have introduced variability in both the treatment regimen and the results and confound the true effect of chronic n-3 PUFA supplementation. In the current study, we relied on the fat-1 Tg mouse to study the protective effect of n-3 PUFAs against ischemia. The fat-1 mice showed elevated n-3 PUFAs level, reduced n-6 PUFAs, and increased n-3/n-6 PUFA ratio due to endogenous n-6 PUFAs conversion, which could represent the adjusted PUFA composition after dietary interventions. Importantly, this model allows well-controlled animal studies to be performed without the confounding factors from long-term dietary interference of other bioactive compounds. We demonstrated the protective effect of n-3 PUFAs in not only the acute phase (48 hrs) after focal cerebral ischemia but also at the delayed phase (14 days) after ischemia, manifested by reduced infarct size and improved neurological functional performance in fat-1 Tg mice. These results support the notion that chronic supplementation with n-3 PUFAs may be a potential therapy to enhance functional recovery and long-term rehabilitation after stroke.
Another important finding in this study is that neurogenesis in the ischemic brain was enhanced in fat-1 Tg mice compared to Wt littermates, suggesting that n-3 PUFAs promote endogenous post-stroke brain repair. Several lines of evidence now underscore the importance of neurogenesis in long-term functional recovery after stroke. Ionizing radiation applied to the SGZ reduces neurogenesis and impairs functional recovery after global ischemia (22). Similarly, transgenic ablation of neurogenesis by neuronal precursor cell depletion worsens stroke outcomes in mice (22). These studies all imply a direct and causal relationship between neurogenesis and functional recovery after stroke. Accordingly, some cell-based and pharmacological strategies to induce or enhance neurogenesis in experimental stroke models have been developed (23). For example, administration of VEGF on days 1–3 of reperfusion after transient ischemia enhances the survival of newborn neurons in the dentate gyrus and SVZ, resulting in reduced infarct size and improved neurological performance (22). Over-expression of IGF-1 through adeno-associated virus (AAV)-mediated gene transfer effectively increases newly formed neurons in the SVZ and enhances motor functions assessed at 3 weeks after ischemia (23). A variety of stem cells, including NSCs cultured from fetal tissue, immortalized neural cell lines and haematopoietic/endothelial progenitors isolated from bone marrow or umbilical cord blood, also show efficacy in experimental stroke models (24–26). However, some limitations, including intolerable side effects and barriers to effective drug delivery, have restricted their clinical application, particularly the long-term application to prevent post-stroke disability. In this regard, orally delivered n-3 PUFAs are superior to many other strategies in that they are easy to administer, readily across the blood brain barrier and are relatively inexpensive. In addition, n-3 PUFAs have already been used for other clinical indications such as cardiovascular diseases, and have good safety records, permitting their long-term use. Finally, they are also superior to many other therapies in that they are originally a natural part of the normal human diet. These clear advantages support their rapid translation into the clinic to prevent or reduce post-stroke disability.
To fully restore neurological function after cerebral ischemia, rescued or regenerated neurons must also be adequately myelinated. Remyelination in the CNS is an effective regenerative process mediated by OPCs (27). Consistent with previous reports, we showed that OPCs are widely distributed throughout the adult CNS and readily proliferate after ischemic injury. Remarkably, a long term elevation in brain levels of n-3 PUFAs augmented the proliferation or repair of OPCs following ischemic stroke. As a result, the reduction in OPC density following stroke was almost fully abolished in fat-1 Tg mice. The concerted oligodendrogenesis and neurogenesis enhancement in fat-1 mice is expected to optimize their brain repair processes and improve functional recovery after ischemic stroke based on our knowledge of these two protective processes.
Previous studies have documented several mechanisms that underlie n-3 PUFA-mediated neuroprotection, such as the suppression of neuronal cell death and the inhibition of intracerebral inflammation. In the current study, our data demonstrated for the first time that chronically elevated brain levels of n-3 PUFAs confer long-term protection against ischemic brain damage and improved neurological performance and that they prime the brain for an enhancement of post-stroke neurogenesis as well as oligodendrogenesis. Given their ability to protect against stroke through multiple mechanisms, to benefit overall heart health, and to be administered safely as a potential prophylaxis, further translational and clinical studies on n-3 PUFAs are warranted to further evaluate their clinical value for long-term stroke management.
Acknowledgments
This work was supported by the VA RR&D Merit Review (to J.C.), NIH grants NS036736, NS045048, and NS056118 (to JC), and AHA grants 10POST4150028 (to X.H.) and 10SDG2560122 (to F.Z.). Y.G. was supported by Chinese Natural Science Foundation grants No 30670642, 30870794, and 81020108021. J.C. is a recipient of the VA Career Research Scientist Award and also supported by the RK Mellon Endowed Chair for Cerebrovascular Disease Research at the University of Pittsburgh.
Footnotes
The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet neurology. 2009 May;8(5):491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine. 2002 Sep;8(9):963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 3.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Annals of neurology. 2002 Dec;52(6):802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 4.Coti Bertrand P, O’Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. The Journal of nutrition. 2006 Jun;136(6):1570–5. doi: 10.1093/jn/136.6.1570. [DOI] [PubMed] [Google Scholar]
- 5.Katakura M, Hashimoto M, Shahdat HM, Gamoh S, Okui T, Matsuzaki K, et al. Docosahexaenoic acid promotes neuronal differentiation by regulating basic helix-loop-helix transcription factors and cell cycle in neural stem cells. Neuroscience. 2009 May 19;160(3):651–60. doi: 10.1016/j.neuroscience.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 6.Cao D, Xue R, Xu J, Liu Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. The Journal of nutritional biochemistry. 2005 Sep;16(9):538–46. doi: 10.1016/j.jnutbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neuroscience letters. 2007 Mar 26;415(2):154–8. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamoh S, Hashimoto M, Hossain S, Masumura S. Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clinical and experimental pharmacology & physiology. 2001 Apr;28(4):266–70. doi: 10.1046/j.1440-1681.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 9.Gamoh S, Hashimoto M, Sugioka K, Shahdat Hossain M, Hata N, Misawa Y, et al. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93(1):237–41. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 10.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959 Aug;37(8):911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 11.Wei D, Li J, Shen M, Jia W, Chen N, Chen T, et al. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic beta-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes. 2010 Feb;59(2):471–8. doi: 10.2337/db09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, et al. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000 Jan;20(1):112–8. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008 Dec 3;28(49):13038–55. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwai M, Sato K, Omori N, Nagano I, Manabe Y, Shoji M, et al. Three steps of neural stem cells development in gerbil dentate gyrus after transient ischemia. J Cereb Blood Flow Metab. 2002 Apr;22(4):411–9. doi: 10.1097/00004647-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003 Oct 31;278(44):43807–17. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 16.Bas O, Songur A, Sahin O, Mollaoglu H, Ozen OA, Yaman M, et al. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat hippocampus. Neurochem Int. 2007 Feb;50(3):548–54. doi: 10.1016/j.neuint.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2009 Sep;40(9):3121–6. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke; a journal of cerebral circulation. 1996 Sep;27(9):1641–6. doi: 10.1161/01.str.27.9.1641. discussion 7. [DOI] [PubMed] [Google Scholar]
- 19.Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. Jama. 2001 Jan 17;285(3):304–12. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 20.He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, et al. Fish consumption and risk of stroke in men. Jama. 2002 Dec 25;288(24):3130–6. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Longstreth WT, Jr, Lemaitre RN, Manolio TA, Kuller LH, Burke GL, et al. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005 Jan 24;165(2):200–6. doi: 10.1001/archinte.165.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. The Journal of clinical investigation. 2003 Jun;111(12):1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, et al. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke; a journal of cerebral circulation. 2008 Apr;39(4):1254–61. doi: 10.1161/STROKEAHA.107.500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Yasuhara T, Hara K, Matsukawa N, Maki M, Yu G, et al. Transplantation of bone marrow-derived stem cells: a promising therapy for stroke. Cell transplantation. 2007;16(2):159–69. [PubMed] [Google Scholar]
- 25.Jin K, Mao X, Xie L, Greenberg RB, Peng B, Moore A, et al. Delayed transplantation of human neural precursor cells improves outcome from focal cerebral ischemia in aged rats. Aging Cell. 2010 Dec;9(6):1076–83. doi: 10.1111/j.1474-9726.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HJ, Lim IJ, Lee MC, Kim SU. Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res. 2010 Nov 15;88(15):3282–94. doi: 10.1002/jnr.22474. [DOI] [PubMed] [Google Scholar]
- 27.Franklin RJ, Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. Journal of neurology. 2008 Mar;255( Suppl 1):19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]