Abstract
Potassium and sodium share a yin/yang relationship in the regulation of blood pressure (BP). BP is directly associated with the total body sodium and negatively correlated with the total body potassium. Epidemiologic, experimental, and clinical studies have demonstrated that potassium is a significant regulator of BP and further improves cardiovascular outcomes. Hypertensive cardiovascular damage, stroke and stroke-related death are accelerated by salt intake but could be prevented by increased dietary potassium intake. The antihypertensive effect of potassium supplementation appears to occur through several mechanisms that include regulation of vascular sensitivity to catecholamines, promotion of natriuresis, limiting plasma renin activity, and improving endothelial function. In the absence of chronic kidney disease, the combined evidence supports a diet high in potassium content serves a vasculoprotective function, especially in the setting of salt-sensitive hypertension and prehypertension.
Keywords: dietary potassium, blood pressure, natriuresis, sodium chloride, renin, endothelium
Introduction
Hypertension is a global health problem, affecting one-fourth of adults in the general population; the prevalence continues to increase in industrialized countries (1).Hypertension is also a preventable risk factor, since epidemiological evidence suggests that the prevalence of cardiovascular, cerebrovascular, and chronic kidney disease (CKD) is reduced if hypertension is well controlled (2). With a few exceptions, hypertension is a polygenic trait with phenotypic manifestations that are dependent upon interactions between genes and the environment. In one study, for example, heritability of blood pressure (systolic, diastolic and mean) responses to dietary salt intake was 0.49 to 0.51, indicating that in this Chinese population, variation in blood pressure responses to salt intake was influenced almost equally by genetic and environmental factors (3). Relevant environmental factors include not only dietary sodium but also dietary potassium intake. The aim of this review is to analyze the effect of high potassium intake on the pathogenesis, prevention, and treatment of hypertension and cardiovascular outcomes specifically in the setting of normal renal function. Data regarding the role of dietary potassium intake in blood pressure regulation in CKD are generally lacking, although serum potassium levels contribute to cardiovascular mortality in CKD associated with heart failure, with an increase in mortality related to both hypokalemia (K < 4.0 mEq/L) (4) and hyperkalemia (K > 5.5 mEq/L) (5).
Epidemiological studies and clinical trials demonstrate an association between potassium intake and blood pressure
INTERSALT demonstrated for the first time an inverse relationship between urinary potassium excretion rates and blood pressure in a large diverse population (6). In a double-blind, randomized, crossover study, Krishna et al. (7), reported that a low potassium diet in hypertensive and normotensive patients caused significant elevations in systolic BP (6 mm Hg and 7 mm Hg) and diastolic BP (4 mm Hg and 7 mm Hg), respectively. In another randomized crossover study design using 10 healthy, normotensive men, Krishna et al. (8), found a significant increase in the mean arterial blood pressure from 90.9 ± 2.2 mm Hg to 95.0 ± 2.2 mm Hg when dietary potassium was reduced from normal (90 mmol/d) to very low (10 mmol/d) levels.
A randomized controlled trial (RCT) by Siani et al. (9), investigated the intensity of antihypertensive medication use during an increase in dietary potassium intake from natural foods. By the end of the study, the initial antihypertensive therapy was successfully decreased in 81% of the patients in the high potassium group (Cl, 66% to 96%) as compared with a reduction of 29% in patients in the low potassium group (Cl, 10% to 48%) (P = 0.001). The authors concluded that increasing dietary potassium intake from natural foods was a feasible and effective measure to reduce antihypertensive drug treatment in patients with normal renal function (9).
Appel et al. (10), designed a seminal RCT (Dietary Approaches to Stop Hypertension (DASH)) to test the hypothesis that increased intake of dietary potassium may lower BP significantly. A total of 459 adults with systolic blood pressures of less than 160 mm Hg and diastolic blood pressures between 80 to 95 mm Hg were randomly assigned to receive one of three types of diet. Groups included a control diet (low in fruits, vegetables and dairy products with high-fat content), a diet rich in fruits and vegetables, and a “combination” diet rich in fruits, vegetables and low-fat dairy products with reduced saturated and total fat. Over the eight-week course of the study, the investigators observed a reduction both in systolic and diastolic blood pressure by 11.4 and 5.5 mm Hg, respectively, in the 133 hypertensive patients who received the combination diet, compared with those patients who received the control diet (p<0.001 for each). In addition, there were 3.5 mm Hg (p<0.001) and 2.1 mm Hg (P=0.003) reductions in systolic and diastolic blood pressures, respectively, among 326 normotensive subjects receiving the combination diet. While these reductions may appear to be small, they are sufficient to support the conclusion that increasing dietary intake of potassium reduces blood pressure and likely associated cardiovascular diseases such as stroke and coronary heart disease (10). One point to note in this trial is the low content of sodium (3 grams) in combination diet and diet rich in fruit and vegetables. An earlier trial suggested that supplemental potassium provided no additional hypotensive effect in hypertensive men on a salt-restricted diet (11). However, a subsequent follow-up study revealed that the DASH diet provided an additional BP benefit for participants at three different levels of salt intake (50, 100, 150 mmol of sodium daily), although participants consuming a diet lowest in salt content (50 mmol/d) demonstrated the least amount of mean reduction in systolic and diastolic pressures (12). Nevertheless, it is difficult to conclude that increased potassium intake is the sole factor to explain reduction of blood pressure by DASH type diet, because the antioxidant content of the diet is high and that may also favorably influence the blood pressure.
A meta-analysis incorporating 33 RCTs (2609 participants) in which potassium supplementation was the only difference between the intervention and the control conditions showed that potassium supplementation was associated with a significant decrease in mean systolic and diastolic blood pressures, by 3.11 mm Hg and 1.97 mm Hg, respectively (13). One interesting aspect of the results was that this beneficial effect of potassium supplementation seemed particularly enhanced in studies in which participants remained on a high salt diet. The authors concluded that dietary potassium supplementation should be considered especially for patients who are unable to effectively limit their sodium intake. On the other hand a more recent systematic review of 6 RCTs (483 participants) found a large, but not statistically significant, reduction in mean systolic and diastolic BP with dietary potassium supplementation (14). The participant number was small and follow-up periods were relatively short in the RCTs used in this latter meta-analysis.
Potential reasons for the discrepancies between the conclusions of these meta-analyses include the heterogeneous nature of the response to dietary potassium supplementation and the potential variations in experimental protocols of the different RCTs, such as the amount of salt in the diet. He et al. (15), showed that, analogous to salt-sensitivity, the systolic and diastolic blood pressure responses to dietary potassium supplementation assume a Gaussian distribution about a mean of nearly zero change, reflecting significant individual variations in the response. Interestingly, Kelly et al. (16), observed that potassium supplementation reduced blood pressure specifically among those participants who had a maternal history of hypertension. The odds ratios for potassium sensitivity were 1.36 and 1.60 for those with maternal hypertension and early onset maternal hypertension, respectively. Montasser et al. (17) conducted an observational study to determine if single nucleotide polymorphisms (SNP) of specific genes that included EDN1 (endothelin-1), NOS3 (endothelial nitric oxide synthase) and SELE (E-selectin) associated with the phenotype of blood pressure changes in response to dietary potassium intake. These authors found that genetic variations in EDN1 and SELE associated with the blood pressure response to potassium intake. Thus, a combination of hereditary and acquired traits appears to determine the vascular response to potassium intake.
Clinical trials and epidemiological studies support an effect of potassium intake on cardiovascular outcomes
Studies have demonstrated the beneficial effects of high potassium diet on clinical cardiovascular endpoints. Khaw et al. (18), prospectively examined the relationship between dietary potassium intake per day obtained at entry into the study and subsequent stroke-associated mortality. In multivariate analysis of the data, the risk of stroke-related death decreased by 40% with a 10-mmol increase in daily potassium intake. The authors concluded that potassium-rich foods such as fruits and vegetables offer protection against stroke-related deaths. A recent meta-analysis of prospective studies also suggested that an increase in the daily intake of dietary potassium of 1.64 g (42 mmol) provided a statistically significant 21% risk reduction in stroke (19). The relationship appeared to be partly mediated by the higher potassium intake effect on BP.
A prospective cohort study has shown the positive impact of fruits and vegetables on the cardiovascular disease. The INTERHEART study (20) compared fruit and vegetable intake in a group of 15,152 patients with acute myocardial infarction and a group of 14,820 control patients from 52 countries. After modifying potential confounding factors, the authors observed a 30% decrease in the risk of acute myocardial infarction in patients who consumed a diet containing fruits and vegetables. In a meta-analysis, McKee et al estimated 2.6 million deaths per year worldwide from inadequate consumption of fruit and vegetables. They also concluded that increasing fruit and vegetable intake up to 600 g per day (the baseline of choice) could reduce the risk of coronary heart disease and ischemic stroke by 31% and 19%, respectively (21). A recent analysis of the NHANES III study showed that the sodium-to-potassium ratio in the highest compared with lowest quartiles was associated with a hazard ratio of 1.46, 1.46, and 2.15 for all-cause, cardiovascular disease, and ischemic heart disease mortality, respectively (22). The authors concluded that a higher sodium-potassium ratio is associated with significantly increased risk of CVD and all-cause mortality, and higher sodium intake is associated with increased total mortality in the general US population.
Perhaps the most compelling findings to date derive from the work of Chang et al. (23). In this study, kitchens of retirement home were randomized into 2 groups, with the 1981 patients who were assigned to those kitchens given either potassium-enriched salt (experimental group) or regular salt (control group). Patients whose serum creatinine concentrations were ≥ 3.5 mg/dl were excluded from the study. In the subsequent 31 months, the incidence of CVD-related deaths was 13.1 per 1000 persons (27 deaths in 2057 person-years) in the experimental group and 20.5 per 1000 (66 deaths in 3218 person-years) in the control group (Figure 1). The experimental group demonstrated 59% reduction in CVD mortality. These findings demonstrated an important long-term beneficial effect of potassium supplementation in this elderly cohort.
Figure 1.
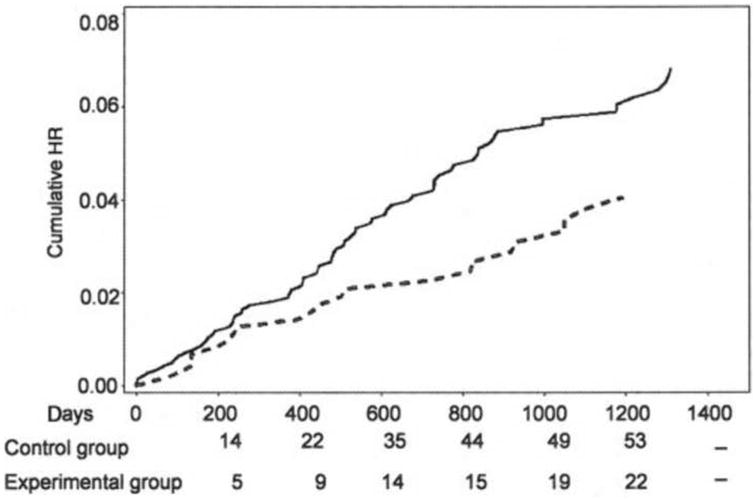
Effect of potassium supplementation on prevention of cardiovascular event rates. A long-term benefit of potassium supplementation on cardiovascular mortality was observed. Figure adapted from Chang, et al., Am. J. Clin. Nutr. 83:1289, 2006, with permission.
Evidence demonstrating a role for hypokalemia and potassium depletion in adverse clinical outcomes also comes from studies of antihypertensive treatments that include thiazide diuretics. In a sub-analysis of Systolic Hypertension in the Elderly Program (SHEP), a five year prospective study, the authors found that the participants who had hypokalemia after 1 year of treatment with a low-dose diuretic did not experience the reduction in cardiovascular events achieved among those who did not have hypokalemia (24). These findings emphasize that, regardless of the underlying pathogenesis, serial examination of the serum potassium concentration and correction of hypokalemia are essential during treatment with diuretics.
Mechanisms of potassium-induced blood pressure and end-organ effects
Reduced potassium intake leads to renal sodium retention (7, 8). Conversely, potassium seems to have natriuretic effects both in hypertensive and normotensive subjects. In 1935, Keith and Binger (25) demonstrated that ingestion of about 150 mmoles of potassium chloride a day produced a diuretic effect in 80% of patients. Brunner at al. (26), observed that in normal participants potassium supplementation sufficient to increase plasma potassium concentration also stimulated natriuresis and suppressed plasma renin activity without changing aldosterone production. Conversely, potassium deprivation stimulated plasma renin activity, but not aldosterone production, despite a tendency toward sodium retention (26). Thus, potassium supplementation might exert an antihypertensive effect by promoting natriuresis and limiting plasma renin activity, although the mechanism(s) involved remain poorly defined.
Potassium intake also affects vasomotor tone by modulating the vascular sensitivity to catecholamines (27). Skrabal et al. (28), examined the blood pressure effects of salt and potassium in a small group of prehypertensive subjects. Twenty normotensive subjects were given diets that contained varying amounts of sodium and potassium. In every subject, reduction of salt intake from 200 to 50 mmol/day over 2 weeks reduced the rise in blood pressure induced by various doses of noradrenaline (0.1 to 0.4 microgram/kg/min). A high potassium intake reduced diastolic blood pressure by at least 5 mm Hg in 10 out of 20 subjects; of the 10 patients who responded, 7 had a family history of hypertension and 9 responded to salt restriction. The authors concluded that the mechanism of benefit of dietary potassium intake on blood pressure related to improvement in adherence to a low salt diet, promotion of sodium loss, prevention of a rise in plasma catecholamines induced by a low salt intake, and increased sensitivity of the baroreceptor reflex. The short duration (2 weeks) limits this study, but the findings provide important clues to the putative mechanisms of benefit of potassium supplementation on BP regulation and further supports this approach in the management of hypertension, especially in salt-sensitive patients (28).
Pre-clinical studies have demonstrated an effect of potassium on vascular function. Sodium and potassium also have independent roles in determining endothelial function. In healthy humans (29) and rats (30, 31), high salt intake increases nitric oxide (NO) production. However, this response is impaired or absent in salt-sensitive rats (30, 31). Potassium supplementation increases endothelium-dependent NO production. Raij, et al. (32), demonstrated that a diet containing 8.0% NaCl supplemented with 3.6% potassium citrate developed hypertension but improved endothelium-dependent relaxation in the Dahl rat. Along with NO, the endothelium also elaborates Transforming Growth Factor-β (TGF-β) in response to high salt intake (33-37). Endothelium-derived TGF-β1 is a locally acting molecule with autacrine and paracrine actions on neighboring endothelium and vascular smooth muscle and is involved in blood pressure regulation. TGF-β is considered an important determinant of arterial stiffness, by promoting hypertrophy of vascular smooth muscle (38), increasing the local production of extracellular matrix proteins (39) and inhibiting metalloproteinases involved in collagen degradation and remodeling (40). Importantly, increased dietary potassium intake promotes a dose-dependent inhibition of dietary salt-induced endothelial production of TGF-β (34). Studies in vitro demonstrate that the extracellular potassium concentration alters endothelial cell function through the BKCa channel (41). Thus, dietary potassium may have a salutary effect on vascular function during high-salt intake, perhaps mediated through regulation of endothelial production of TGF-β.
Finally, potassium may be important in mitigating the end-organ effects of hypertension. In experimental studies, the incidence of stroke, stroke-related death, development of cardiac hypertrophy, mesenteric vascular and renal damage were significantly reduced in hypertensive rats on high sodium diet with increased potassium intake (42, 43). Potassium may have effects that are independent of blood pressure, because a high potassium diet protected against stroke but not hypertension in hypertensive rats (18, 45).
Conclusion
Multiple clinical studies demonstrate strong associations among dietary potassium intake, the blood pressure response to dietary salt intake, and cardiovascular outcomes. There is evidence for a genetic as well as acquired predisposition to the vascular effects of potassium intake. This review points out limitations of existing studies and the need to enhance efforts to explore these relationships in greater detail. Potassium supplementation mitigates the blood pressure response associated with a high sodium diet and further attenuates the progression of end-organ damage particularly in the setting of high salt intake. This vasoprotective effect is mediated in part by endothelial cell production of NO and TGF-β.
The combined evidence is sufficiently compelling for the Institute of Medicine to recommend that all adults, who do not have chronic kidney disease, should consume daily a diet containing at least 120 mmol (about 4.7 grams) of potassium (48). Perhaps the best strategy to achieve a high-potassium intake is to raise the consumption of fruits and vegetables. Achieving this goal of adequate dietary intake of potassium may serve a vasculo-protective role for the population. Reinforcing a diet high in potassium with periodic monitoring of serum potassium levels in patients at risk for hypo- or hyperkalemia should be considered essential particularly in the management of patients who are salt-sensitive.
Acknowledgments
Dr. Sanders' research is supported by grants (5 IO1 BX001192 and 1 IP1 BX001595) from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and National Institutes of Health grants (R01 DK046199 and P30 DK079337 (George M. O'Brien Kidney and Urological Research Centers Program)).
Footnotes
The authors declare there are no conflicts of interest.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. doi: 10.1016/S0140-6736(05)17741-1. Epub 2005/01/18. [DOI] [PubMed] [Google Scholar]
- 2.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–74. doi: 10.1016/0140-6736(90)90878-9. Epub 1990/03/31. [DOI] [PubMed] [Google Scholar]
- 3.Gu D, Rice T, Wang S, Yang W, Gu C, Chen CS, et al. Heritability of blood pressure responses to dietary sodium and potassium intake in a Chinese population. Hypertension. 2007;50(1):116–22. doi: 10.1161/HYPERTENSIONAHA.107.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowling CB, Pitt B, Ahmed MI, Aban IB, Sanders PW, Mujib M, et al. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: findings from propensity-matched studies. Circ Heart Fail. 3(2):253–60. doi: 10.1161/CIRCHEARTFAILURE.109.899526. Epub 2010/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed MI, Ekundayo OJ, Mujib M, Campbell RC, Sanders PW, Pitt B, et al. Mild hyperkalemia and outcomes in chronic heart failure: A propensity matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.04.041. Epub 2009/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. Brit Med J. 1988;297(6644):319–28. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishna GG, Kapoor SC. Potassium depletion exacerbates essential hypertension. Ann Intern Med. 1991;115(2):77–83. doi: 10.7326/0003-4819-115-2-77. Epub 1991/07/15. [DOI] [PubMed] [Google Scholar]
- 8.Krishna GG, Miller E, Kapoor S. Increased blood pressure during potassium depletion in normotensive men. N Engl J Med. 1989;320(18):1177–82. doi: 10.1056/NEJM198905043201804. Epub 1989/05/04. [DOI] [PubMed] [Google Scholar]
- 9.Siani A, Strazzullo P, Giacco A, Pacioni D, Celentano E, Mancini M. Increasing the dietary potassium intake reduces the need for antihypertensive medication. Ann Intern Med. 1991;115(10):753–9. doi: 10.7326/0003-4819-115-10-753. Epub 1991/11/15. [DOI] [PubMed] [Google Scholar]
- 10.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. doi: 10.1056/NEJM199704173361601. Epub 1997/04/17. [DOI] [PubMed] [Google Scholar]
- 11.Grimm RH, Jr, Neaton JD, Elmer PJ, Svendsen KH, Levin J, Segal M, et al. The influence of o12. ral potassium chloride on blood pressure in hypertensive men on a low-sodium diet. N Engl J Med. 1990;322(9):569–74. doi: 10.1056/NEJM199003013220901. Epub 1990/03/01. [DOI] [PubMed] [Google Scholar]
- 12.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 13.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277(20):1624–32. doi: 10.1001/jama.1997.03540440058033. Epub 1997/05/28. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson HO, Nicolson DJ, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;(3):CD004641. doi: 10.1002/14651858.CD004641.pub2. Epub 2006/07/21. [DOI] [PubMed] [Google Scholar]
- 15.He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27(1):48–54. doi: 10.1097/hjh.0b013e328316bb87. Epub 2009/01/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly TN, Gu D, Rao DC, Chen J, Chen J, Cao J, et al. Maternal history of hypertension and blood pressure response to potassium intake: the GenSalt Study. American journal of epidemiology. 2012;176(Suppl 7):S55–63. doi: 10.1093/aje/kws272. Epub 2012/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montasser ME, Shimmin LC, Gu D, Chen J, Gu C, Kelly TN, et al. Blood pressure response to potassium supplementation is associated with genetic variation in endothelin 1 and interactions with E selectin in rural Chinese. J Hypertens. 2010;28(4):748–55. doi: 10.1097/HJH.0b013e3283355672. Epub 2009/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaw KT, Barrett-Connor E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N Engl J Med. 1987;316(5):235–40. doi: 10.1056/NEJM198701293160502. Epub 1987/01/29. [DOI] [PubMed] [Google Scholar]
- 19.D'Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57(10):1210–9. doi: 10.1016/j.jacc.2010.09.070. Epub 2011/03/05. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. Epub 2004/09/15. [DOI] [PubMed] [Google Scholar]
- 21.McKee M, Lock K, Pomerleau J. Fruit and vegetable consumption and stroke. Lancet. 2006;367(9516):1056. doi: 10.1016/S0140-6736(06)68470-5. Epub 2006/04/04. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171(13):1183–91. doi: 10.1001/archinternmed.2011.257. Epub 2011/07/13. [DOI] [PubMed] [Google Scholar]
- 23.Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83(6):1289–96. doi: 10.1093/ajcn/83.6.1289. Epub 2006/06/10. [DOI] [PubMed] [Google Scholar]
- 24.Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension. 2000;35(5):1025–30. doi: 10.1161/01.hyp.35.5.1025. Epub 2000/05/20. [DOI] [PubMed] [Google Scholar]
- 25.Keith NM, Binger MW. Diuretic action of potassium salts. J Am Med Assoc. 1935;105:1584–91. [Google Scholar]
- 26.Brunner HR, Baer L, Sealey JE, Ledingham JG, Laragh JH. The influence of potassium administration and of potassium deprivation on plasma renin in normal and hypertensive subjects. J Clin Invest. 1970;49(11):2128–38. doi: 10.1172/JCI106430. Epub 1970/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barri YM, Wingo CS. The effects of potassium depletion and supplementation on blood pressure: a clinical review. Am J Med Sci. 1997;314(1):37–40. doi: 10.1097/00000441-199707000-00008. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 28.Skrabal F, Aubock J, Hortnagl H. Low sodium/high potassium diet for prevention of hypertension: probable mechanisms of action. Lancet. 1981;2(8252):895–900. doi: 10.1016/s0140-6736(81)91392-1. Epub 1981/10/24. [DOI] [PubMed] [Google Scholar]
- 29.Bech JN, Nielsen CB, Ivarsen P, Jensen KT, Pedersen EB. Dietary sodium affects systemic and renal hemodynamic response to NO inhibition in healthy humans. Am J Physiol. 1998;274(5 Pt 2):F914–F23. doi: 10.1152/ajprenal.1998.274.5.F914. [DOI] [PubMed] [Google Scholar]
- 30.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–67. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension. 1993;22:812–8. doi: 10.1161/01.hyp.22.6.812. [DOI] [PubMed] [Google Scholar]
- 32.Raij L, Luscher TF, Vanhoutte PM. High potassium diet augments endothelium-dependent relaxations in the Dahl rat. Hypertension. 1988;12(6):562–7. doi: 10.1161/01.hyp.12.6.562. Epub 1988/12/01. [DOI] [PubMed] [Google Scholar]
- 33.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-beta1. Am J Physiol Renal Physiol. 2008;295(2):F406–14. doi: 10.1152/ajprenal.90294.2008. Epub 2008/06/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying WZ, Aaron K, Wang PX, Sanders PW. Potassium inhibits dietary salt-induced transforming growth factor-beta production. Hypertension. 2009;54(5):1159–63. doi: 10.1161/HYPERTENSIONAHA.109.138255. Epub 2009/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-beta in rats. Am J Physiol. 1998;274(4 Pt 2):F635–41. doi: 10.1152/ajprenal.1998.274.4.F635. Epub 1998/05/12. [DOI] [PubMed] [Google Scholar]
- 36.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-beta1 in rat aortic endothelium. Am J Physiol. 1999;277(4 Pt 2):H1293–8. doi: 10.1152/ajpheart.1999.277.4.H1293. Epub 1999/10/12. [DOI] [PubMed] [Google Scholar]
- 37.Ying WZ, Sanders PW. The interrelationship between TGF-beta1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol. 2003;285(5):F902–8. doi: 10.1152/ajprenal.00177.2003. Epub 2003/07/17. [DOI] [PubMed] [Google Scholar]
- 38.Owens GK, Geisterfer AA, Yang YW, Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol. 1988;107(2):771–80. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987;262(14):6443–6. [PubMed] [Google Scholar]
- 40.Mozes MM, Bottinger EP, Jacot TA, Kopp JB. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. J Am Soc Nephrol. 1999;10:271–80. doi: 10.1681/ASN.V102271. [DOI] [PubMed] [Google Scholar]
- 41.Ying WZ, Aaron KJ, Sanders PW. Effect of Aging and Dietary Salt and Potassium Intake on Endothelial PTEN (Phosphatase and Tensin Homolog on Chromosome 10) Function. PLoS One. 2012;7(11):e48715. doi: 10.1371/journal.pone.0048715. Epub 2012/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobian L, MacNeill D, Johnson MA, Ganguli MC, Iwai J. Potassium protection against lesions of the renal tubules, arteries, and glomeruli and nephron loss in salt-loaded hypertensive Dahl S rats. Hypertension. 1984;6(2 Pt 2):I170–6. doi: 10.1161/01.hyp.6.2_pt_2.i170. Epub 1984/03/01. [DOI] [PubMed] [Google Scholar]
- 43.Liu DT, Wang MX, Kincaid-Smith P, Whitworth JA. The effects of dietary potassium on vascular and glomerular lesions in hypertensive rats. Clin Exp Hypertens. 1994;16(4):391–414. doi: 10.3109/10641969409067953. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 44.Zhou MS, Kosaka H, Yoneyama H. Potassium augments vascular relaxation mediated by nitric oxide in the carotid arteries of hypertensive Dahl rats. Am J Hypertens. 2000;13(6 Pt 1):666–72. doi: 10.1016/s0895-7061(99)00269-1. Epub 2000/07/27. [DOI] [PubMed] [Google Scholar]
- 45.Acheson RM, Williams DR. Does consumption of fruit and vegetables protect against stroke? Lancet. 1983;1(8335):1191–3. doi: 10.1016/s0140-6736(83)92467-4. Epub 1983/05/28. [DOI] [PubMed] [Google Scholar]
- 46.O'Shaughnessy KM, Karet FE. Salt handling and hypertension. J Clin Invest. 2004;113(8):1075–81. doi: 10.1172/JCI21560. Epub 2004/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keith NM, Binger MW. Diuretic action of potassium salts. JAMA. 1935;105:1584. [Google Scholar]
- 48.Ekundayo OJ, Dell'italia LJ, Sanders PW, Arnett D, Aban I, Love TE, et al. Association between hyperuricemia and incident heart failure among older adults: A propensity-matched study. Int J Cardiol. 2010;142:279–87. doi: 10.1016/j.ijcard.2009.01.010. Epub 2009/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]


