Abstract
POSH (Plenty of SH3 domains) is a scaffold for signaling proteins regulating cell survival. Specifically, POSH promotes assembly of a complex including Rac GTPase, mixed lineage kinase (MLK), MKK7, and Jun kinase (JNK). In Drosophila, genetic analysis implicated POSH in Tak1-dependent innate immune response, in part through regulation of JNK signaling. Homologs of the POSH signaling complex components, MLK and MKK7, are essential in Drosophila embryonic dorsal closure. Using a gain-of-function approach, we tested whether POSH plays a role in this process. Ectopic expression of POSH in the embryo causes dorsal closure defects due to apoptosis of the amnioserosa, but ectodermal JNK signaling is normal. Phenotypic consequences of POSH expression were found to be dependent on Drosophila Nc, the caspase-9 homolog, but only partially on Tak1 and not at all on Slpr and Hep. These results suggest that POSH may use different signaling complexes to promote cell death in distinct contexts.
Keywords: scaffold, JNK pathway, caspase
INTRODUCTION
Scaffold proteins act as platforms for the assembly of functional multiprotein signaling complexes. In doing so, they facilitate desired signaling outcomes while limiting inappropriate pathway crosstalk. Accordingly, the role of scaffolds in signal transduction has emerged as a critical regulatory mechanism to promote pathway activity and specificity (Morrison and Davis, 2003; Dhanasekaran et al., 2007). This has been extensively characterized in relation to mitogen-activated protein kinase (MAPK) signaling pathways, with Ste5p being a paradigmatic scaffold regulating pheromone response in Saccharomyces cerevisiae (Elion, 2001). Given the tendency for individual cells to have multiple MAPK pathways, such as extracellular signal-regulated kinase (ERK), and stress-activated protein kinases (SAPKs): Jun-NH2-terminal kinase (JNK) and p38, several distinct classes of scaffold proteins have been described that partition these highly related signaling cascades into functional groups. For example, the POSH (Plenty of SH3 domains) protein is proposed to be a scaffold to assemble and stimulate pro-apoptotic JNK/SAPK signaling (Tapon et al., 1998; Xu et al., 2003).
The POSH protein was first identified by yeast two-hybrid analysis as a binding partner of Rac GTPase (Tapon et al., 1998). This initial study further demonstrated that POSH overexpression in fibroblasts stimulated both NF-κB and JNK signaling to trigger cell death. Subsequent studies provided additional evidence supporting a role for POSH in apoptosis, particularly in neuronal cells subjected to growth factor withdrawal (Xu et al., 2003). Upon NGF withdrawal, cultured sympathetic or PC12 neurons induce cell death in a Rac/Cdc42 and JNK-dependent manner (Maroney et al., 1999; Xu et al., 2001, 2003). Knockdown of POSH is neuroprotective in this context, while POSH overexpression mimics NGF deprivation by stimulating JNK signaling. Consequently, POSH-induced cell death correlates with increased cJun phosphorylation, and can be blocked by various treatments, which disable JNK pathway signal transducers, such as RNAi knockdown, small molecule inhibition, or expression of dominant-negative proteins. With these strategies, a mechanistic model has emerged whereby POSH serves as a scaffold protein linking Rac to mixed lineage kinases (MLKs), MAP3K components of the JNK cascade (Xu et al., 2001, 2003), as well as JNK-interacting protein (JIP), another scaffold for the downstream ki-nases, MKK7 and JNKs in the pathway (Yasuda et al., 1999; Xu et al., 2005; Kukekov et al., 2006). Together, this protein assemblage has been referred to as the POSH-JIP apoptotic complex (PJAC; Kukekov et al., 2006). Consistent with a role in pro-survival signaling, Akt/PKB is reported to bind and negatively regulate POSH interactions with JNK pathway components, thus counteracting formation of the PJAC (Figueroa et al., 2003; Lyons et al., 2007). Ultimately though, in neurons and fibroblasts, execution of cell death downstream of JNK signaling involves the intrinsic (mitochondrial) pathway, relying primarily on the initiator caspase-9 (Tournier et al., 2000; Xu et al., 2001).
POSH proteins (SH3RF1 in human) are composed of multiple SH3 domains, a Rac-binding domain, and an NH2-terminal RING finger domain that confers E3 ubiquitin ligase activity (Tapon et al., 1998; Xu et al., 2003; Tsuda et al., 2005; Kim et al., 2006). The SH3 domains are docking sites for binding partners such as AKT/PKB. The RING domain is important for regulating levels of POSH protein, and possibly its binding partners, by means of ubiquitination and subsequent proteasomal degradation. Moreover, POSH E3 ligase activity appears to regulate levels and localization of additional substrates independent of JNK signaling (Kim et al., 2006; Tuvia et al., 2007). Loss of POSH RING domain function by deletion or mutation stabilizes the POSH protein (Xu et al., 2003; Tsuda et al., 2005), even though the RING domain itself is not required for proapoptotic activity or MLK binding (Xu et al., 2003). Under conditions that induce apoptosis however, stabilization of POSH and MLKs occurs posttranscriptionally and facilitates a positive feedback mechanism by means of increased JNK activation (Tsuda et al., 2005; Xu et al., 2005). This feed-forward mechanism likely contributes to cells reaching a point-of-no-return where they are irreversibly committed to die.
In Drosophila POSH was identified in several independent genetic screens. One allele was found to modify a Ras1/MAPK-dependent patterning phenotype (Schnorr et al., 2001). A different insertion allele was found to confer longevity to adult flies upon overexpression (Seong et al., 2001a,b). Despite the proapoptotic function of POSH and the PJAC in mammalian neurons, postmitotic, pan-neural expression of Drosophila POSH results in viable, morphologically normal, long-lived flies (Seong et al., 2001a). In contrast, strong overexpression of POSH in select non-neuronal tissues during development induces visible morphological phenotypes, in some instances, suggesting inappropriate cell death with concomitant up-regulation of a JNK pathway target gene reporter (Seong et al., 2001a). Drosophila POSH null mutants are viable but display innate immune defects in response to Gram-negative bacterial infection due to delayed and abnormally sustained JNK and relish/NF-κB signaling (Tsuda et al., 2005). What accounts for the abnormal signaling dynamics in POSH mutants is thought to be altered protein turnover of the MAP3K, Tak1, a substrate for POSH RING domain directed ubiquitination and an upstream transducer in immune signaling (Silverman et al., 2003; Tsuda et al., 2005; Delaney et al., 2006). Altogether, the observations from gain- and loss-of-function studies in Drosophila implicate POSH primarily in regulation of organismal homeostasis and lifespan, with the JNK signaling pathway being a possible target of POSH-dependent regulation. Nevertheless, it is still unclear to what extent POSH is involved in the numerous other MAPK- or JNK-dependent processes elucidated to date.
The JNK pathway in Drosophila has been implicated in various developmental and homeostatic processes, such as tissue morphogenesis and polarity, wound healing, innate immunity, cell death, and oxidative stress, among others (Sykiotis and Bohmann, 2008). The core JNK pathway consists of basket (bsk), encoding JNK, which is the substrate for a JNK kinase, the MKK7 homolog hemipterous (hep). Hep is similarly phosphorylated and selectively activated by upstream kinases of the MAP3K family that consists of several members (Stronach, 2005). These include slipper (slpr/MLK), wallenda (wnd/DLK), Protein kinase at 92B (Pk92B/Ask1), Mekk1, TGF-β activated kinase 1 (Tak1), Tak1-like 1 and 2 (Takl1,2). Slpr, the MLK homolog, is important for several major morphogenetic processes in development including embryonic dorsal closure and pupal thorax closure (Stronach and Perrimon, 2002; Polaski et al., 2006), while Tak1 and Mekk1 are primarily involved in homeostatic processes, such as immune and stress responses, respectively (Inoue et al., 2001; Vidal et al., 2001; Brun et al., 2006; Delaney et al., 2006). Notably, Tak1 is also considered to be a transducer of proapoptotic JNK signaling activated by the Drosophila tumor necrosis factor (TNF) homolog, eiger (Igaki et al., 2002; Polaski et al., 2006). Pk92B/Ask1 mutants have not yet been characterized, but this protein has also been linked to cell death by its association with TNF-receptor-associated-factor 4 (Traf4) and by genetic suppression of proapoptotic signaling with a dominant-negative Ask1 construct (Kuranaga et al., 2002). Wnd is required in the nervous system for JNK-dependent regulation of synaptic growth (Collins et al., 2006) and the roles of the Tak-like kinases are currently unknown.
Given that mammalian POSH is part of a signaling complex with Rac, MLK, MKK7, and JNK, we considered whether Drosophila POSH might modulate signaling through a Rac1, Slpr, Hep, and Bsk pathway. A well-known role for these JNK pathway components in Drosophila is to promote embryonic dorsal closure morphogenesis through activation of gene expression in the dorsal ectoderm. Loss of JNK signaling due to mutations in these components results in embryonic lethality because tissue closure fails and therefore the embryos are “open” dorsally (Glise et al., 1995; Riesgo-Escovar et al., 1996; Sluss et al., 1996; Stronach and Perrimon, 2002; Woolner et al., 2005). Even though POSH mutants are viable, it is formally possible that POSH is functionally redundant with other scaffold proteins, masking its role in JNK-dependent processes like dorsal closure. Thus, a gain-of-function approach may reveal whether POSH is involved in JNK signaling in the embryo.
Here, we describe the consequences of POSH overexpression in the embryo and larval eye disc tissues during development and elaborate on the molecular mechanism of POSH-induced cell death. We find that, in the embryo, the extraembryonic amnioserosa tissue is differentially sensitive to increased POSH levels. In particular, POSH overexpression can induce dorsal closure defects through premature loss of amnioserosa integrity, rather than through interference of normal ectodermal JNK signaling. Moreover, cell death stimulated by POSH is dependent on the caspase-9 homolog, Drosophila Nc (Dronc) suggesting conservation of the execution of apoptosis in response to an increase in cellular POSH levels.
RESULTS
POSH Overexpression Leads to Dorsal Closure Defects
Given that Rac1, MLK, and JNK participate in regulating the morphogenetic movements of embryonic dorsal closure in Drosophila (Sluss et al., 1996; Riesgo-Escovar and Hafen, 1997; Harden et al., 1999; Stronach and Perrimon, 2002; Woolner et al., 2005), we sought to determine whether POSH may also play a role in this process. One prediction is that POSH expression would facilitate JNK signaling due to its scaffold function or through protein stabilization as described above. Hyperactive JNK signaling in the embryo leads to up-regulation of JNK target genes and excessive tissue closure giving a puckered phenotype (Polaski et al., 2006), reminiscent of mutations in the JNK phosphatase, puckered (Martin-Blanco et al., 1998). Alternatively, POSH may cause turnover of its E3 ligase substrates or engage in unproductive protein complexes, which down-regulate JNK signaling causing a dorsal open phenotype.
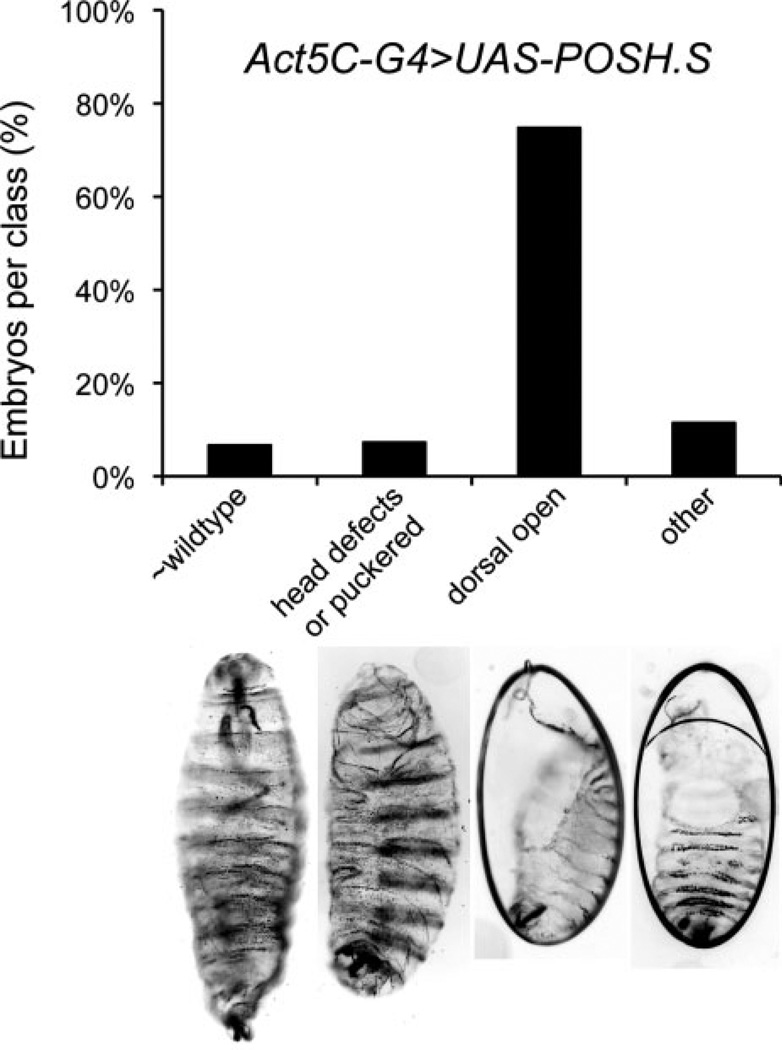
To determine the consequences of Drosophila POSH overexpression in the embryo, the Gal4/UAS system of targeted gene expression was used (Brand and Perrimon, 1993). We found that expression of UAS-POSH under the control of numerous Gal4 drivers with broad embryonic expression patterns (e.g., tubulinP-Gal4, Actin5C-Gal4, armadillo-Gal4) resulted in variable embryonic lethality but a considerable percentage of dorsal closure defects as indicated by dorsally oriented holes in the larval cuticle. Figure 1 shows representative data with Act5C-Gal4. arm- and tubP-Gal4 crosses produced qualitatively similar results. The phenotypes were also observed upon expression of two independent UAS-POSH trangenes (POSH.S and POSH.W) as well as a UAS-containing P-element (Gene Search-GS) inserted upstream of the endogenous locus (POSHGS2207; data not shown; Seong et al., 2001a,b). Despite ubiquitous overexpression of POSH in the embryo, the dorsal open cuticle phenotype was the predominant defect occurring among the dead embryos. A few additional defects were observed in the experimental cuticle populations, such as segmental misalignments of the ventral denticle belts, ventral holes, or head defects; however, these additional defects occurred in a low percentage of the population (Fig. 1).
Fig. 1.
Ubiquitous expression of POSH (Plenty of SH3 domains) in embryos results in a majority with dorsal open cuticle defects. Larval cuticles are shown which exemplify the classes of defects observed when POSH expression is directed by the Actin5C-Gal4 driver. Mean percent embryonic lethality from six trials (Ntot > 800 embryos) is 17%; the range is 7–28%. Over 75% percent of the dead embryos recovered (Ntot = 166) show substantial dorsal closure defects revealed by the dorsal open phenotype.
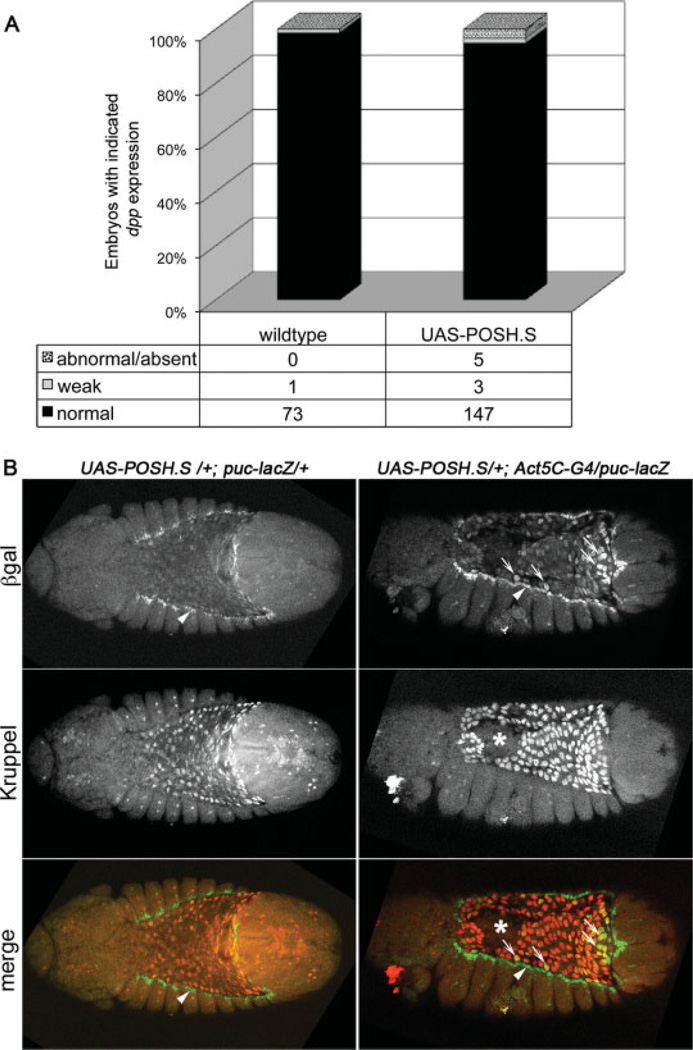
The observation that embryonic POSH overexpression resulted in dorsal open cuticles led us to test whether JNK signaling in the dorsal ectoderm was also perturbed, which might provide a molecular explanation for the dorsal closure defects. To this end, expression of known transcriptional targets of JNK signaling, dpp and puclacZ, were assessed by in situ hybridization and β-gal immunofluorescence respectively, in embryos ectopically expressing POSH. We found that both markers were essentially normal, neither up- nor down-regulated, in the dorsal ectoderm of experimental embryos (Fig. 2A dpp, 2B, S1A,B puc-lacZ arrowheads), leading us to conclude that forced expression of POSH results in a failure of dorsal closure by a mechanism distinct from inactivation of the JNK pathway in the ectodermal leading edge.
Fig. 2.
Ubiquitous expression of POSH (Plenty of SH3 domains) in embryos does not perturb Jun kinase (JNK) pathway target gene expression in ectodermal leading edge cells. A: Quantitative results of dpp RNA expression by in situ hybridization in the leading edge cells of stage 11–13 embryos. Genotypes are w1118 for wild-type vs. UAS-POSH.S/+;Actin5C-Gal4 (or TM3)/+. Fifty percent of these embryos should express POSH, yet only 5% have altered dpp expression. B: Dual-label immunofluorescence staining of late stage 12 embryos with the indicated genotypes. These are dorsal or dorsolateral views of embryos oriented with anterior to the left. βgal expression (green in merged image) reveals the puc-lacZ reporter indicative of JNK pathway activity and amnioserosa is visualized by nuclear Kruppel expression (red in merged image). Note that puc-lacZ expression is present at the ectodermal leading edge in both embryos (arrowheads). Moreover, JNK pathway activity is modestly up-regulated in amnioserosa cells expressing POSH (small arrows), though the reporter would normally be down-regulated in the amnioserosa by the start of dorsal closure in wild-type embryos. Finally, loss of amnioserosa integrity in the POSH expressing embryo is marked with an asterisk.
Given that transcriptional targets of JNK signaling in the leading edge appear relatively normal in POSH-expressing embryos, we considered whether the defects in dorsal closure required POSH expression in the ectoderm at all. To localize the effect to a particular tissue, additional Gal4 drivers with progressively restricted spatial expression were used to direct POSH expression. The c381-Gal4 driver, with expression predominantly in the amnioserosa tissue from stage 10 (Manseau et al., 1997; Schock and Perrimon, 2003; Scuderi and Letsou, 2005), produced moderate embryonic lethality (36% mean lethality), but among the dead embryos, there were a high percentage of cuticles with dorsal holes (72%, Ntot = 232). These results suggest that the effect of POSH overexpression on dorsal closure may involve the amnioserosa tissue.
POSH Expression Leads to Disruption of Amnioserosa Integrity and Premature Loss of Tissue
To understand why POSH expression in amnioserosa tissue results in defective dorsal closure, we used molecular markers to assess amnioserosa cell differentiation and maintenance, as well as cell shape. Expression of the Dorsocross (Doc) genes in the amnioserosa, under the control of early dorsal patterning genes, correlates with amnioserosa differentiation (Reim et al., 2003). Doc proteins are also required for maintenance of amnioserosa tissue in part due to their regulation of downstream genes such as kruppel and pebbled/hindsight (Reim et al., 2003). Consequently, Doc mutant embryos show defective germ-band retraction, where the caudal end of the embryo remains folded anteriorly, resulting in a “u-shaped” cuticle. The “u-shaped” phenotype is often indicative of abnormal amnioserosa form or function (Frank and Rushlow, 1996; Schock and Perrimon, 2003).
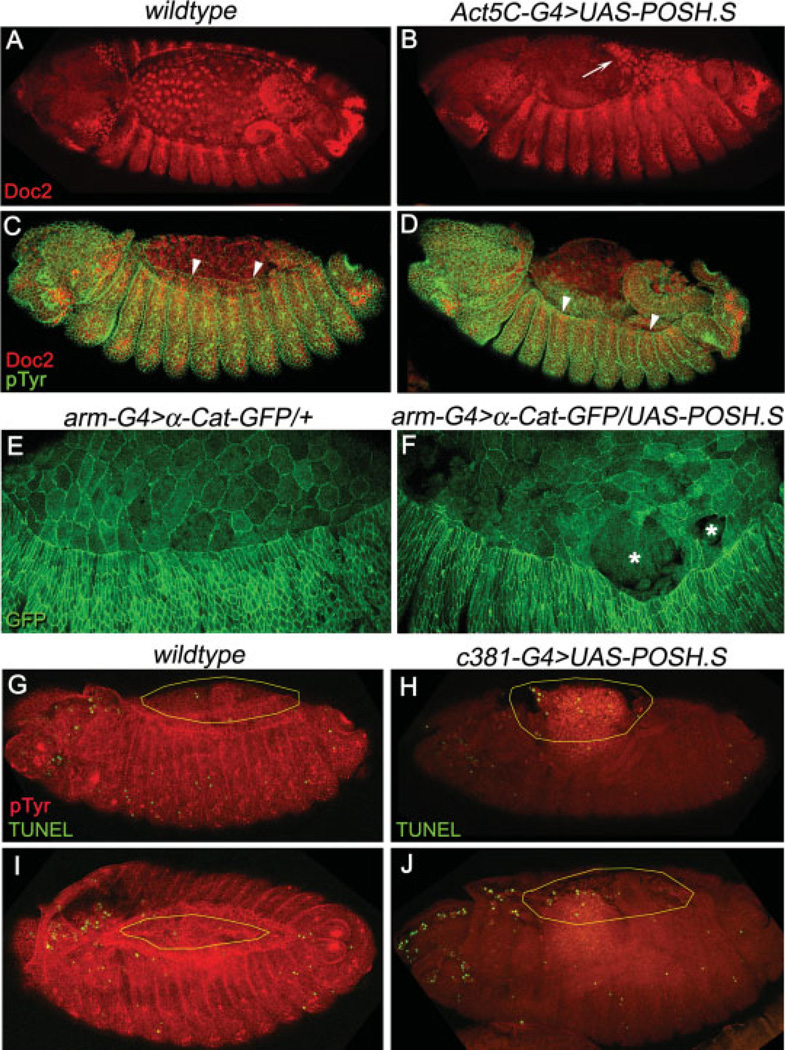
In embryos that express POSH ubiquitously, Doc2 protein expression commences properly and persists in amnioserosa cells, as it does in wild-type; however, as dorsal closure proceeds, Doc2 immunostaining is reduced dorsally in POSH overexpression embryos. Rather than down-regulation of Doc2 protein per se, the reduced staining is likely due to premature loss of amnioserosa tissue, as evidenced by embryos where the amnioserosa appears to be detaching from the ectoderm or ripping apart, creating holes (Fig. 3A,B,E,F; see also Fig. 2B). We assume that amnioserosa holes and detachment from the leading edge could compromise further closure of the dorsal epidermis. Indeed, various physical and genetic ablation studies targeting the amnioserosa have previously demonstrated that its integrity contributes to proper dorsal closure (Kiehart et al., 2000; Reed et al., 2001; Scuderi and Letsou, 2005). Consistent with previous studies, we find that in many of our preparations, numerous experimental embryos appear to have no amnioserosa covering the internal organs at all, and the dorsal aspect of the epidermis remains laterally positioned (Fig. 3C,D, arrowheads).
Fig. 3.
Loss of amnioserosa integrity and dorsal closure defects in embryos that overexpress POSH (Plenty of SH3 domains). A,B: Expression pattern of Dorsocross (Doc2) protein in stage 13+ wild-type (A) or POSH expressing (B) embryos. C,D: Dual-labeling for phosphotyrosine (green) and Doc2 (red) in dorsal closure stage embryos. E,F: Visualization of amnioserosa integrity in living embryos with D-α-catenin-GFP. G–J: TUNEL labeling (green) in stage 13 and 14 wild-type (G,I) or amnioserosa specific UAS-POSH (H,J) embryos. Note amnioserosa cell morphology and adhesion are aberrant, exemplified by a free tissue edge (arrow in B) that has pulled away from the ectoderm or rips in the tissue (asterisks in F). Dorsal advancement of the leading edge cells during dorsal closure (arrowheads in C,D) is normal in wild-type embryos (C), but fails to be maintained in embryos expressing POSH (D). Also, exposure of the internal organs is pronounced (D,H,J) due to loss of amnioserosa integrity and apoptosis. Yellow ovals in G–J outline the extent of amnioserosa tissue. Embryos are viewed dorsolaterally with anterior to the left.
To visualize cell death directly, we performed a terminal deoxynucleotidyl transferase–mediated deoxyuridinetriphosphate nick end-labeling (TUNEL) assay to detect DNA fragmentation on wild-type embryos in comparison to those with expression of POSH in the amnioserosa tissue using the c381-G4 driver. Wild-type embryos undergo substantial programmed cell death according to a dynamic spatiotemporal pattern (Abrams et al., 1993). In fact, it is normal for a small fraction of apoptotic amnioserosa cells to be extruded basally during dorsal closure followed by large-scale disintegration of the tissue after internalization (Toyama et al., 2008; Gorfinkiel et al., 2009; Mohseni et al., 2009). Indeed, we observed the occasional TUNEL-positive amnioserosa cells in dorsal closure stage wild-type embryos (Fig. 3G,I). Upon expression of POSH in amnioserosa tissue, we observed an increase in TUNEL-positive cells dorsally, especially in those embryos that were clearly failing in dorsal closure during stages 13 and 14 (Fig. 3H,J), before the bulk degeneration of the internalized amnioserosa of wild-type embryos (Mohseni et al., 2009).
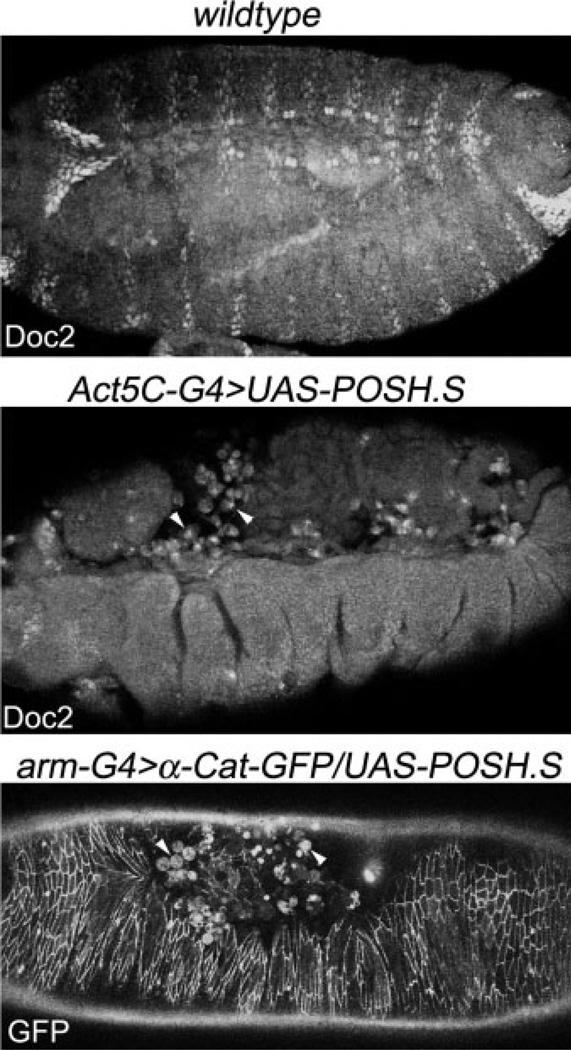
Consistent with these observations, in late stage experimental embryos expressing POSH ubiquitously and where dorsal closure is compromised, the internal tissues are exposed and become infiltrated with large round vacuolated cells characteristic of phagocytic hemocytes (Bruckner et al., 2004; Fig. 4, arrowheads). These infiltrating cells cluster dorsally where the amnioserosa has been compromised and many stain positively for Doc2 (Fig. 4, middle panel), in line with the notion that the observed reduction in Doc2 staining at the dorsal surface results from death and subsequent engulfment of the Doc2-positive amnioserosa corpses. Together, these observations suggest that POSH triggers a disruption of the amnioserosa, correlating with death of those cells and disruption of the progress of dorsal closure.
Fig. 4.
Late stage embryos that express POSH (Plenty of SH3 domains) and have prematurely lost amnioserosa tissue display robust phagocytic infiltration, dorsally. Doc2 staining labels amnioserosa tissue and segmentally repeated cells in the ectoderm of wild-type and POSH-expressing embryos as indicated. In the middle panel, Doc-positive fluorescent signal can be seen inside macrophages of this dorsal open embryo. Similar phagocytic activity is observed in live embryos of the genotype arm-G4, α-catenin-GFP/UAS-POSH.S. All panels are dorsolateral views of embryos oriented anterior to the left.
Prior studies have also reported that abnormal persistence of JNK signaling in amnioserosa tissue markedly increases the likelihood that dorsal closure will fail (Reed et al., 2001). In our studies, puc-lacZ reporter expression is only moderately up-regulated in amnioserosa cells of embryos with ectopic POSH expression (Fig. 2B, arrows and S1); however, if additional POSH transcripts are driven from two UAS sources, the intensity of puc-lacZ expression in the amnioserosa and the severity of phenotypic consequences increases accordingly (Supp. Fig. S1C,D, which is available online). Although puclacZ induction is compatible with the idea that JNK activity is stimulated or prolonged by POSH expression in the amnioserosa, the failure to induce ectopic puc-lacZ when expressed outside the amnioserosa argues against a wholesale activation of the JNK pathway by POSH. Moreover, whether POSH expression results in up-regulation of JNK pathway activity directly or indirectly, for instance by means of loss of tissue integrity, failure to down-regulate amnioserosa JNK signaling ultimately promotes premature apoptosis and closure defects (Reed et al., 2001, 2004). Thus, although the terminal arrest of dorsal closure can be incurred by an increase in amnioserosa JNK signaling or a loss of ectodermal JNK signaling, the cellular phenotypes are distinctive. Here, we observe that POSH induces a loss of amnioserosa integrity through dissolution of cell contacts internal to the tissue (Fig. 2B) and at the interface with leading edge cells (Fig. 3B,F). In JNK pathway mutants, amnioserosa tissue constricts but eventually loses adhesion with the leading edge of the ectoderm as a primary defect (Homsy et al., 2006; Fernandez et al., 2007).
Requirements for POSH-Dependent Cell Death
Mammalian POSH stimulates cell death through its membership in a multiprotein complex recently termed the POSH-JIP apoptotic complex or PJAC (Kukekov et al., 2006). The PJAC includes the scaffolds, POSH and JIP, and their binding partners, Rac-GTP, MAP3K mixed lineage kinase (MLK) family members, MAP2K member MKK7, and JNKs (Whitmarsh et al., 1998; Xu et al., 2001; Kukekov et al., 2006; Xu and Greene, 2006). Selective association of these JNK pathway components under certain conditions promotes transcription-dependent and -independent proapoptotic signals (Wang et al., 2007). To probe further the response of cells to overexpression of Drosophila POSH, we turned to the developing larval imaginal discs as tissues to more easily observe cell death and genetic interactions with putative pathway components.
POSH Expression in the Eye Stimulates Caspase-9–Dependent Cell Death
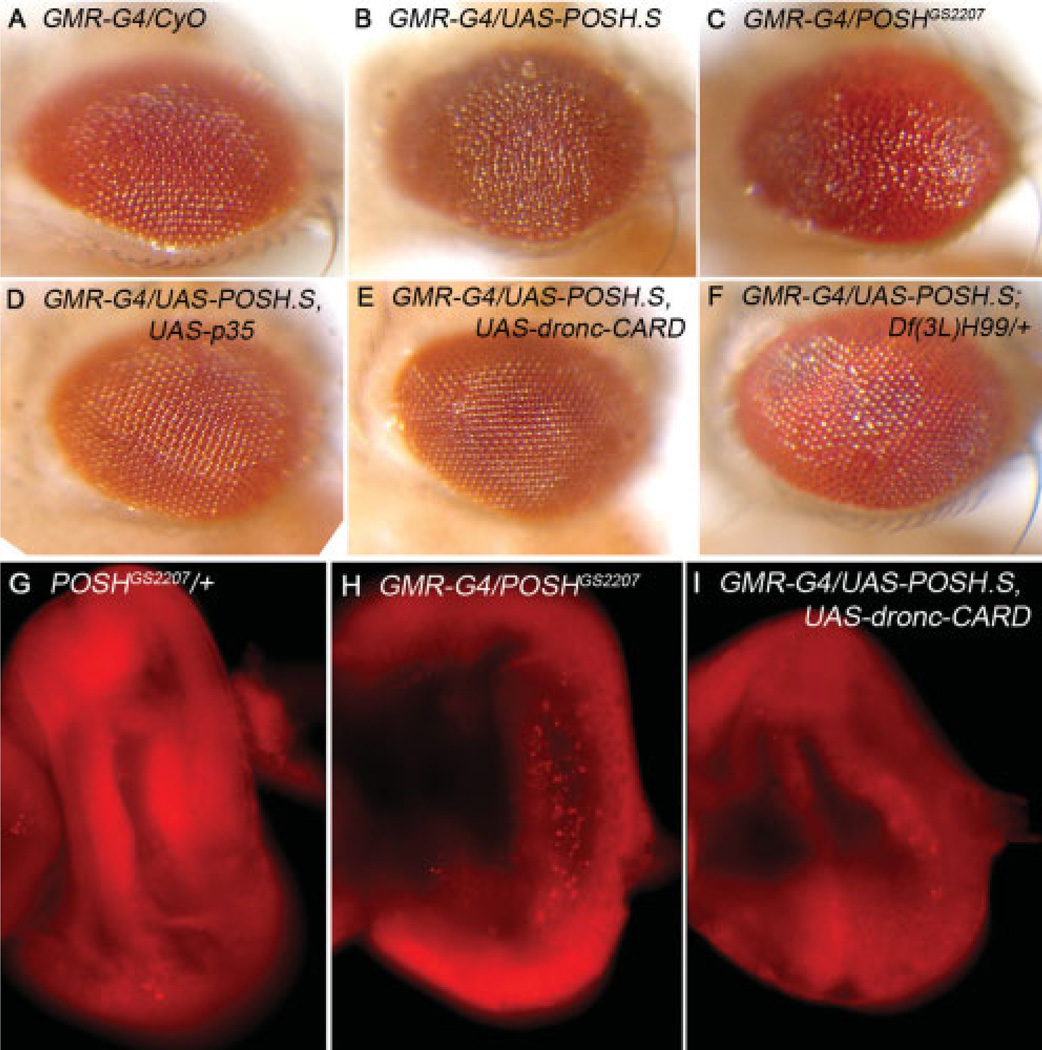
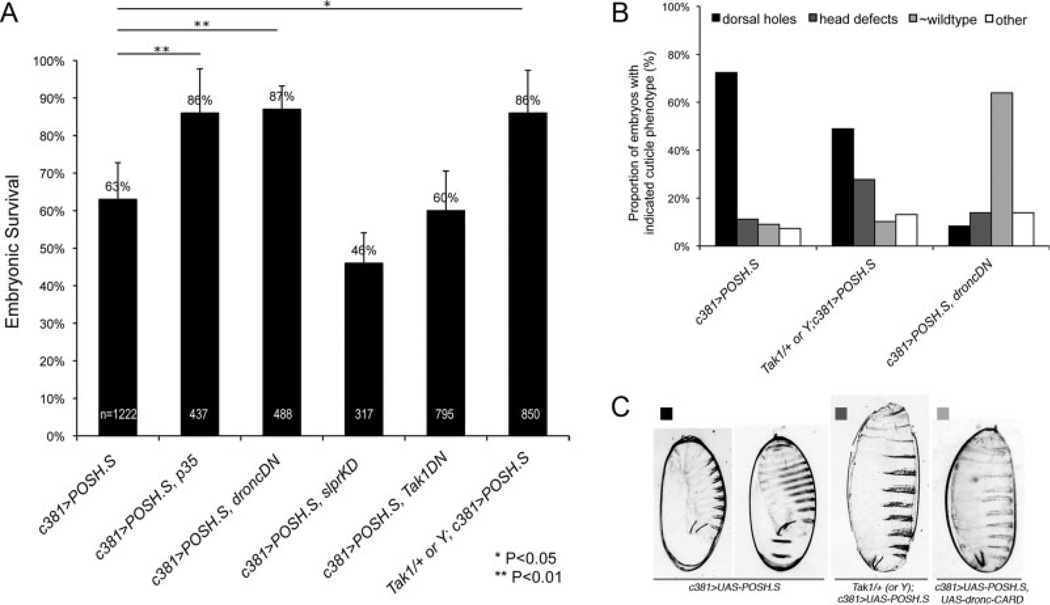
Overexpression of POSH in the larval imaginal eye disc with GMR-Gal4, results in a roughening of the adult compound eye (Fig. 5A–C, and Seong et al., 2001a). To determine whether the adult rough eye results from excessive cell death during an earlier developmental period, larval eye discs were dissected and bathed in acridine orange (AO), a vital dye that reproducibly stains apoptotic cells in live Drosophila tissue (Abrams et al., 1993). A small number of AO-positive cells are present in eye discs from a UAS-POSH heterozygote, lacking Gal4 (Fig. 5G), which may reveal the elimination of cells as part of normal eye development (Hay et al., 1994). Brightly stained AO-positive cells are much more prevalent in GMR-G4>POSH eye discs (Fig. 5H). Coexpression of a dominant-negative form of Dronc (UAS-dronc-CARD; Meier et al., 2000), the Drosophila caspase-9 homolog, suppresses the increase in AO-positive cells providing further evidence that the AO staining reflects POSH-expressing apoptotic cells (Fig. 5I). Corroborating these data, we also observed an increase in active effector caspase staining, dependent on over-expression of POSH (Supp. Fig. S3). Expression of the CARD prodomain of Dronc also suppresses the adult rough eye phenotype of POSH overexpression (Fig. 5E), confirming that the phenotype is related to excessive cell death. In addition, we find that coexpression of baculovirus caspase inhibitor p35 also suppresses the POSH-induced rough eye (Fig. 5D). p35 inhibits Dronc-dependent cell death but not Dronc itself (Meier et al., 2000), implicating an effector caspase in addition to Dronc. Finally, a deletion (H99) removing a genomic region that contains rpr, hid, and grim (RHG genes; White et al., 1994; Grether et al., 1995; Chen et al., 1996) dominantly suppresses the POSH-induced eye phenotype (Fig. 5F). The proteins encoded by these genes promote death by displacing inhibitory DIAP1 interactions with caspases, freeing them for activation (Wang et al., 1999; Goyal et al., 2000). Thus, reduction in RHG gene dosage is sufficient to substantially mitigate the consequences of POSH overexpression in the eye. Testing these requirements in the embryonic amnioserosa produced similar results, i.e., a dependence of POSH-induced amnioserosa loss and embryonic lethality on Dronc and a p35-sensitive caspase (Fig. 7A). Consistent with a significant increase in survival of embryos upon genetic inhibition of apoptosis, we also observed a concomitant reduction in the proportion of embryos displaying dorsal holes (Fig. 7B,C). Together these data implicate a proapoptotic axis of activity (RHG proteins, Dronc, effector caspase) required to mediate cell death by POSH in several Drosophila tissues.
Fig. 5.
POSH (Plenty of SH3 domains) expression in the developing eye results in caspase-dependent cell death. A–F: Adult eyes with the indicated genotypes. G–I: Larval eye imaginal discs stained with acridine orange. POSH expression with the GMR-G4 driver results in disrupted ommatidial organization (B,C), while the GMR-G4 driver alone is nearly wild-type (A). Coexpression of POSH with viral p35 (D) or dominant-negative Dronc (E) substantially rescues the POSH-induced eye phenotype. F: Reduced dosage of RHG genes with Df(3L)H99 also suppresses the effect of POSH overexpression. The eye phenotype is likely due to cell death because there is an increase in acridine orange-positive cells in larval eye imaginal discs that overexpress POSH (compare H with G). Coexpression of the Dronc Card domain largely prevents the increase in cell death (I).
Fig. 7.
Genetic suppression of embryonic lethality and dorsal closure defects associated with targeted expression of POSH (Plenty of SH3 domains) in the amnioserosa. A: Embryonic survival is plotted for embryos expressing POSH alone under the c381-Gal4 driver or in combination with other dominant-negative constructs or loss-of-function alleles as indicated (n = total embryos counted). All of the crosses were from homozygous parents except for Tak1DN; thus the value for this genotype has been scaled to account for a CyO balancer present in the maternal genotype. Results show that coexpression of p35 or dominant-negative dronc (droncDN) significantly rescues POSH-induced lethality (P < 0.01). Expression of slprKD or Tak1DN alone is not sufficient however, to block the effect of POSH. Reduced maternal Tak1 has a moderate effect (P < 0.05). Error bars represent a 95% confidence interval based on the standard error of the mean. P values reflecting significant differences among the data were determined by independent two-tailed t-test. B,C: Proportion of embryos (B; percent of total y-axis) with the indicated genotypes (x-axis) displaying the cuticle phenotypes as shown in C. n = 232, 137, and 36, respectively, for the indicated genotypes.
Involvement of JNK Pathway Components in POSH-Induced Cell Death?
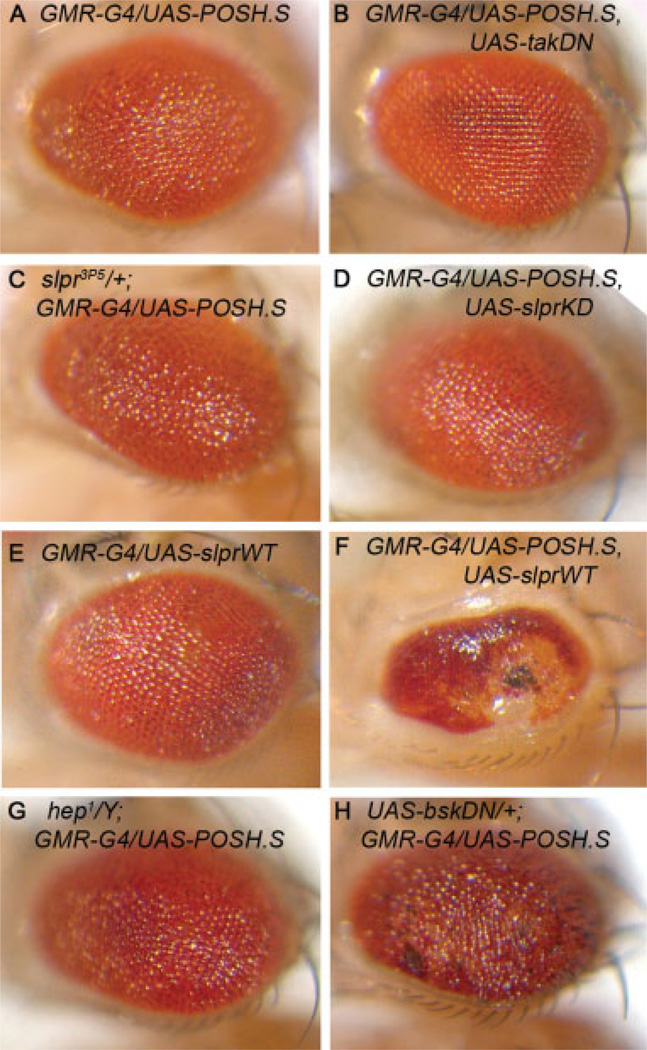
Initial studies in Drosophila showed that ectopic POSH expression resulted in up-regulation of the JNK pathway reporter, puc-lacZ, suggesting possible conservation of function between flies and vertebrates (Seong et al., 2001a). Cumulative evidence in vertebrate systems demonstrates that mammalian POSH interacts directly with the MLK family of MAP3K proteins (MLK1-3 and DLK) to regulate JNK-dependent apoptosis (Tapon et al., 1998; Figueroa et al., 2003; Xu et al., 2003); yet in Drosophila, POSH regulates innate immune signaling by means of a distinct MAP3K, Tak1 (Tsuda et al., 2005). That POSH is a proposed scaffold for the JNK signaling pathway, but has been shown to interact with different MAP3K family members, albeit in different organisms and distinct contexts, raises the question of which transducers are mobilized by POSH. To address this question, we altered the dosage of slpr and Tak1 along with POSH overexpression in the eye. Results show that reduced Slpr signaling, either by expression of a kinase-dead transgene or by introducing a mutant allele (Polaski et al., 2006), fails to visibly modify the GMR-G4>POSH rough eye phenotype (Fig. 6A,C,D). Yet coexpression of POSH with wild-type Slpr results in a severe reduction in eye tissue (Fig. 6A,E,F). This synergistic interaction indicates that, although reduced slpr function cannot dominantly modify the effects of POSH overexpression in the eye, POSH and Slpr can nevertheless cooperate in driving cell death.
Fig. 6.
Dominant-negative Tak1, but not other JNK pathway signal transducers, rescues the POSH-induced eye phenotype. A–D: Coexpression of a kinase-dead form of Tak1 substantially suppresses the GMR>POSH phenotype (compare B with A), yet reducing slpr activity with a nonsense allele (C) or coexpression of a kinase-dead (D) form has no effect on POSH-induced disorganization of the eye. E,F: Expression of slprWT alone has mild effect on eye development (E), but strongly synergizes with POSH when they are coexpressed with GMR-G4 (F). G,H: The GMR>POSH eye phenotype is not suppressed in hep1 hemizygous males (G) and appears moderately enhanced by coexpression of a dominant-negative bsk transgene (H).
In contrast, coexpression of a dominant-negative Tak1 transgene suppresses the GMR-G4>POSH phenotype substantially (Fig. 6A,B). Though these data suggest that Tak1 may selectively participate with POSH to induce cell death, Tak1 mutant animals (either heterozygous females or hemizygous males) do not show appreciable rescue of the eye phenotype (data not shown). Moreover, neither hep mutants nor coexpression of a dominant-negative bsk transgene rescue the GMR-G4>POSH phenotype; rather bskDN alters and enhances the eye phenotype (Fig. 6A,G,H). We confirmed the activity of the bskDN transgene in a separate cell death assay (Supp. Fig. S2). Thus, these results raise the prospect that POSH works through alternative JNK-independent pathways that may or may not rely exclusively on Tak1.
In the embryonic amnioserosa, neither dominant-negative slpr nor Tak1 were able to suppress the lethality of c381-G4>POSH embryos to the extent seen with coexpression of dominant-negative Dronc, for instance (Fig. 7A). However, absence of maternal Tak1 from homozygous mutant females and reduced zygotic Tak1 in the progeny expressing POSH did provide moderate suppression of lethality and cuticle phenotype (Fig. 7B,C). Taken together, results from assays in the eye and in the embryo are suggestive that Tak1 might mediate POSH-induced cell death; however, the differences in potency between the Tak1 transgene vs. mutant in the two tissues also leaves open the possibility that additional factors are involved, that there are several genetically separable consequences of POSH overexpression, or that there are tissue specific complexities to POSH function.
DISCUSSION
Previous studies have suggested that Drosophila POSH overexpression induces JNK signaling and apoptosis. For instance, Seong et al. reported several phenotypes associated with POSH misexpression during development including genital rotation defects, wing blistering and notching, and rough eyes (Seong et al., 2001a), defects often associated with cell death. In addition, they observed puclacZ up-regulation as a result of POSH overexpression in larval imaginal discs and implicated bsk (JNK) and hep (JNKK) as relevant transducers (Seong et al., 2001a). These data, coupled with differences in the mechanistic details of apoptosis between invertebrates and vertebrates, led us to examine additional players contributing to POSH misexpression phenotypes in Drosophila.
In the present study, we explore the consequences and requirements of POSH overexpression in Drosophila. We demonstrate that POSH stimulates Dronc (caspase-9)-dependent apoptosis selectively in several Drosophila tissues including the developing eye and extraembryonic amnioserosa (Fig. 3, Fig. 5). POSH-MLK directed apoptosis in mammalian neurons has been linked to mitochondrial cytochrome c release and caspase activation by direct visualization (Xu et al., 2001) and inhibitor studies, respectively (Xu et al., 2003). Drosophila encodes two initiator caspases, Dronc (caspase-9) and Dredd (caspase-8; Salvesen and Abrams, 2004). Dronc appears to be the primary caspase required for developmental and stress-induced cell death in the fly (Quinn et al., 2000; Chew et al., 2004) while Dredd functions primarily in innate immunity (Leulier et al., 2000). Dronc associates with an adaptor protein, Drosophila Apaf-1 related killer (Ark), which forms a mulitmeric complex referred to as the apoptosome (Yu et al., 2006). Although the Drosophila apoptosome is distinct from the mammalian version, with respect to three-dimensional structure and lack of cytochrome c binding, it is thought to be functionally equivalent as a platform for caspase processing and activation (Yu et al., 2006). Despite these differences, we infer the requirement for Dronc, and presumably the apoptosome, with POSH-induced cell death because a dominant-negative form of Dronc (the CARD domain) is inhibitory in both the embryonic amnioserosa tissue and in larval eye discs of Drosophila. These results suggest a conserved function of POSH involving a caspase-9–dependent pathway.
Whether or not JNK signaling is required for Drosophila POSH-dependent cell death upstream of caspase activation remains unresolved. In favor of the JNK pathway playing a role, we see that (1) the JNK reporter puc-lacZ is induced by POSH in the amnioserosa and the eye disc, albeit modestly (Fig. 2B, Supp. Fig. S1, Fig. S3); and (2) Tak1DN suppresses the POSH eye phenotype (Fig. 6). Evidence that JNK signaling does not play a role is that the POSH-induced eye and amnioserosa phenotypes are not suppressed by reduction of JNK pathway signaling (Fig. 6, Fig. 7). How are these results reconciled with previously reported genetic interactions implicating hep and bsk? Dominant genetic suppression of adult POSH overexpression phenotypes by hep and bsk were demonstrated in a puc heterozygous background (Seong et al., 2001a). With reduced Puc phosphatase function, JNK activity would be elevated, serving to sensitize the genotype to further changes in JNK signaling. Thus, it has not been formally demonstrated whether hep and bsk on their own can modify POSH-dependent phenotypes in a dosage-sensitive manner. We were surprised to find that they did not noticeably alleviate the GMR-G4>POSH eye phenotype as would be expected if they transduced POSH-dependent signals. Although the extent of functional reduction of slpr, hep, and bsk, might have been insufficient for visible suppression in the eye, slpr and hep are on the X chromosome and were assayed in viable male hemizygotes. Moreover, coexpression of POSH with a bskDN transgene under GMR-G4, worsens the eye phenotype instead of alleviating it, as predicted. Importantly, GMR-G4>bskDN expression alone shows essentially no phenotype in the adult eye, but coexpression of bskDN with GMR-G4>egr, which nearly ablates the developing eye due to JNK-dependent apoptosis (Igaki et al., 2002; Polaski et al., 2006), strongly suppresses the egr cell death phenotype confirming the potency of the bskDN transgene (Supp. Fig. S2). Thus, our results raise the possibility that POSH misexpression phenotypes are mediated by redundant components, alternative signaling pathways, tissue specific modulators, or effectors downstream of bsk.
Given that MLK is a direct binding partner of POSH and Rac in the mammalian PJAC (Xu et al., 2001; Kukekov et al., 2006), we were surprised to find that reduction of Slpr function alone did not mitigate POSH overexpression phenotypes, although we cannot rule out that in these contexts, Slpr is redundant with another transducer. At the level of the MAP3Ks that are postulated to function in the stress signaling pathways, there are at least seven genes in Drosophila (Stronach, 2005), so functional redundancy could mask individual requirements as shown in cell culture experiments (Chen et al., 2002; Silverman et al., 2003; Zhuang et al., 2006). Tak1 is a logical candidate for a redundant transducer with Slpr. First, POSH and Tak1 physically interact in vitro; and second, in a separate assay for innate immune response, Tak1-dependent signaling requires POSH function (Tsuda et al., 2005). Third, Drosophila POSH apparently lacks the Rac binding domain described in mammalian POSH (Seong et al., 2001a), which is consistent with a role for POSH in scaffolding Tak1 complexes rather than Slpr/MLK, whose activity is dependent on Rac in several assays (Xu et al., 2001; Stronach and Perrimon, 2002). Finally, other MAP3K members may be involved individually or combinatorially. For instance, Pk92B/Ask1, might be required for POSH-induced apoptosis because it has been implicated in cell death in other contexts (Kuranaga et al., 2002). Thus, POSH misexpression phenotypes are either mediated by a combination of MAP3K transducers simultaneously or by noncanonical components.
Taken together, we conclude that the downstream effect of POSH expression in the GMR-G4 expression domain, although sensitive to some apoptotic effectors like Dronc, is different than egr signaling in the requirements for JNK pathway activity. It is also possible that POSH and Egr trigger JNK-dependent apoptosis at different thresholds. Within the framework of a threshold model, it is intriguing that when POSH and wild-type Slpr are coexpressed simultaneously with GMR-G4, the eye phenotype is strongly enhanced. A synergistic effect upon co-overexpression often suggests cooperation between proteins, and this result is consistent with the notion that Slpr may be limiting for POSH-induced cell death; nevertheless, this explanation is hard to reconcile with the observation that blocking slpr function has no effect. Alternatively, overexpression of POSH and Slpr each stimulate pathways converging on cell death, though independently.
Why are there tissue specific effects of POSH expression? In this work, multiple Gal4 lines were used to target POSH expression in different spatial patterns revealing that tissues are differentially sensitive to POSH overexpression. Among embryonic tissues, the amnioserosa is particularly sensitive to POSH expression. What accounts for the difference in potency of POSH or sensitivity of the amnioserosa tissue to POSH? Amnioserosa is not thought to contribute to larval tissues; instead, it is internalized during the process of dorsal closure and degenerates (Reed et al., 2004; Mohseni et al., 2009). In fact, a fraction of amnioserosa cells undergo apoptosis normally during dorsal closure (Kiehart et al., 2000; Reed et al., 2004). This is thought to provide a force that can be harnessed to contract the amnioserosa tissue as a whole and promote the progression of closure (Toyama et al., 2008). Consistent with this, recent evidence demonstrated that prevention of cell death with p35 delays dorsal closure (Toyama et al., 2008; Gorfinkiel et al., 2009). In our experiments, p35 expression in the amnioserosa rescues the lethality associated with POSH overexpression supporting the argument that POSH stimulates cell death, and that amnioserosa cells may be sensitized to death inducing signals or pathways more so than the ectoderm. This is also borne out by numerous treatments that are associated with amnioserosa death and dorsal closure defects, such as genetic loss of hindsight or myospheroid function (Reed et al., 2004), overexpression of ricin toxin (Scuderi and Letsou, 2005), dominant-negative forms of Rho GTPases (Harden et al., 2002; Schock and Perrimon, 2003), wild-type Crumbs (Harden et al., 2002), or Unpaired (Brown et al., 2003), and more recently the Atg1 autophagy kinase (Mohseni et al., 2009). Together, it appears that there are multiple ways to perturb essential functions necessary for amnioserosa cell adhesion, survival, signaling, and morphogenesis. Presumably POSH overexpression influences one or more of these essential functions.
One of the earliest events we have observed upon POSH expression is the appearance of holes in the amnioserosa (Figs. 2, Fig. 3). Notably, βPS integrin (myospheroid) mutants display similar amnioserosa “rips” (Narasimha and Brown, 2004). Integrin-mediated adhesion is observed in several distinct complexes between amnioserosa cells and with surrounding tissues (Narasimha and Brown, 2004). Consequently, premature loss of integrin function disrupts morphogenesis (Schock and Perrimon, 2003; Narasimha and Brown, 2004) and stimulates anoikis, a form of cell death related to loss of matrix attachment (Reed et al., 2004). It has also been suggested that cell death in hindsight mutants is likely to be secondary to loss of tissue integrity (Reed et al., 2004), but the causal relationship between events is vague. Hindsight antagonizes JNK signaling to maintain amnioserosa differentiation and adhesive complexes at the interface between amnioserosa and dorsal ectoderm, which exhibits strong JNK signaling activity (Reed et al., 2001). Indeed, in hindsight mutants, JNK signaling becomes derepressed in the amnioserosa, creating parity in JNK activity across the interface, rather than a sharp boundary defining on/off states of signaling (Reed et al., 2001). Normalization in levels of JNK activity (whether high–high or low–low) across this interface correlates with loss of adhesion and eventual cell death of the amnioserosa. Thus, cumulative evidence reveals that adhesion, JNK signaling, and cell death are all correlated. Here, our results demonstrate that loss of amnioserosa integrity, JNK reporter gene expression, and amnioserosa apoptosis are also cooccurring with POSH overexpression, but they do not indicate the sequence or proximity of the events. The question remains, does POSH overexpression directly cause cell death or is it a secondary consequence of an activated signaling pathway such as JNK?
If POSH overexpression promotes apoptosis, then maintenance of POSH levels below a critical threshold would be important for cell survival (Xu et al., 2005); and the RING-domain directed autoubiquitination and degradation of POSH would be necessary in normal cells to keep them alive. In fact, forms of POSH with a mutant RING domain are more stable and potent apoptosis inducers than wild-type (Xu et al., 2003). During the course of this work, a description of a mutant allele and phenotype for Drosophila POSH were published (Tsuda et al., 2005). POSH mutants are viable, precluding an essential role for POSH in dorsal closure, and development in general, but lack of a visible phenotype may reflect a redundant mechanism or might underscore subtler roles in regulating homeostatic processes. If POSH protein destabilization is a constitutive activity in cells under normal conditions, then further reduction in POSH levels owing to a mutation, may not lead to apparent phenotypes. In contrast, forced expression overcomes the protective cellular degradation mechanism, revealing visible morphological effects. Targeted overexpression also uncouples POSH mediated signaling from its relevant stimulus, which is ill defined in this system.
Curiously, several other proapoptotic genes, including egr (TNF), wgn (TNFR), and Tak1 (MAP3K), are also viable as mutants, yet show strong killing activity when overexpressed (Igaki et al., 2002; Kanda et al., 2002; Moreno et al., 2002; Polaski et al., 2006). We may therefore consider that POSH homozygous viability is compatible with POSH functioning as a homeostatic apoptotic inducer, and although our evidence challenges the involvement of various JNK signaling components, subsequent apoptosis is dependent on caspase-9/Dronc. Future studies will address whether subtle defects in developmental or inducible cell death processes are affected in POSH mutants.
EXPERIMENTAL PROCEDURES
Drosophila Strains
The following fly stocks were used (chromosome location and Bloomington Stock numbers are indicated where appropriate): UAS-POSH.S (II), POSHGS2207 (II) (Seong et al., 2001a,b); POSHEP1206 (II) BL#16997; UAS-dronc-CARD (II) (Meier et al., 2000); UAS-Tak1DN (II) (Mihaly et al., 2001); UAS-slprKD (II&III), UAS-slprWT (II), slpr3P5/FM7, Tak12527 (Polaski et al., 2006); hep1/FM6 (Glise et al., 1995); UAS-bskDN (X) BL#6409 (Adachi-Yamada et al., 1999); UAS-p35 (II) BL#5072 (Zhou et al., 1997); Actin5C-Gal4/TM3Sb,ftz-lacZ derived from BL#3954; arm-Gal4 (II) BL#1560; c381-Gal4 (IV) BL#3734 (Manseau et al., 1997); pnrMD237 /TM3Ser1 {UAS-y+} (pnr-Gal4) BL#3039 (Calleja et al., 1996); arm-Gal4>UAS-D-α-catenin-green fluorescent protein (GFP) (II) (Oda and Tsukita, 1999); GMR-Gal4 (II) BL#1104; Df(3L) H99/TM6B Hu Tb1 derived from BL#1576; pucE69 (puc-lacZ) (Martin-Blanco et al., 1998); egrGS9830 (II) (Igaki et al., 2002).
Second chromosome recombinants were generated for co-expression of many of the above listed transgenes with POSH.S. All crosses and collections were performed at 25°C on standard fly media.
Tissue Preparation and Imaging
For in situ hybridization, a dpp cDNA was transcribed in vitro to produce an antisense, digoxigenin-labeled riboprobe according to the manufacturer (Roche). Hybridization to embryos was carried out essentially as described (Wolff, 2000). For immunofluorescence, embryos were collected overnight, fixed with 4% formaldehyde in PEM buffer (Pipes, ethylene-diaminetetraacetic acid, magnesium sulfate) and processed for indirect immunofluorescence (Patel, 1994). Larval eye discs were dissected in Ringer’s solution and fixed in 4% formaldehyde in phosphate buffered saline (PBS) with 0.1% Triton, 20 min. Primary antibodies were guinea pig α-Kruppel (573) at 1:300 (Kosman et al., 1998), rabbit α-DOC at 1:2,000 (Reim et al., 2003), rabbit α-(β-galactosidase at 1:1,000 (Cappel), mouse α-phosphotyrosine, clone 4G10, at 1:1,000 (Upstate Biotech), mouse α-GFP at 1:1,000 (Molecular Probes) and rabbit α-active caspase3 at 1:100 (Cell Signaling). All fluorophore-conjugated secondary antibodies were used at 1:200 (Jackson Immunoresearch). Images were collected by confocal laser scanning microscopy using Bio-Rad Radiance 2000 equipment. For fixed embryos, multiple optical sections were projected and merged for the final image. Confocal microscopy was also used to visualize GFP in dechorionated live embryos mounted in Halocarbon oil. Single optical sections of GFP-expressing embryos are shown.
For larval cuticle preparations, overnight egg collections were aged for an additional 24 hr at which point the unhatched, brown embryos were counted and recovered. Dead embryos were dechorionated in diluted bleach for 5 min, and rinsed extensively in PBS plus 0.1% Triton detergent. After removing the wash buffer, larvae were fixed in acetic acid:glycerin (4:1) for 30 min at 60°C and then left at room temperature for at least 24 hr. Larvae were then mounted in Polysciences CMCP-10 mounting media:lactic acid (3:1) on a dust free slide, topped with a coverslip, and placed on a 50°C slide warmer overnight. Cuticle phenotypes were scored and binned as indicated in Figure 1.
For acridine orange staining, larvae of the appropriate genotype were selected by the absence of GFP on balancer chromosomes. Third-instar larval eye discs were dissected in PBS and then bathed in 0.6 µg/ml solution of acridine orange in PBS for 5 min. Discs were washed briefly in PBS and mounted in PBS on a slide treated with a dilute BSA solution to minimize tissue sticking. Spacers were also used under the coverslip. Discs were visualized and photographed immediately with a SPOT™ camera attached to a Nikon E800 compound fluorescence microscope. Images of adult eyes were taken on a Leica MZ16 dissecting stereomicroscope while the flies were anesthetized with CO2.
TUNEL staining using the Roche In Situ Cell Death Detection Kit was carried out on fixed embryos washed extensively in ethanol, rehydrated, and permeabilized with phosphate buffered saline supplemented with 0.3% Triton X-100. TUNEL reactions were performed with 100 µl of staining solution at room temperature for 1 hr.
Supplementary Material
ACKNOWLEDGMENTS
We thank C. Pugar, S. Polaski, J. Matous, and D. Tso for their contributions to quantifying embryonic lethality in several experiments and to G. Campbell for use of the SPOT camera. Also, we thank M. Miura and T. Aigaki for POSH and Eiger stocks, P. Meier for Dronc-CARD, L. Kockel for Tak1DN flies, and M. Frasch for DOC antisera. Many thanks to Vern Twombly for comments on the manuscript.
Grant sponsor: Samuel and Emma Winters Foundation; Grant sponsor: University of Pittsburgh Central Research Development Fund; Grant sponsor: NIH; Grant number: HD045836.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Abrams JM, White K, Fessler LI, Steller H. Programmed cell death during Drosophila embryogenesis. Development. 1993;117:29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brown S, Hu N, Hombria JC. Novel level of signalling control in the JAK/STAT pathway revealed by in situ visualisation of protein-protein interaction during Drosophila development. Development. 2003;130:3077–3084. doi: 10.1242/dev.00535. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Brun S, Vidal S, Spellman P, Takahashi K, Tricoire H, Lemaitre B. The MAPKKK Mekk1 regulates the expression of Turandot stress genes in response to septic injury in Drosophila. Genes Cells. 2006;11:397–407. doi: 10.1111/j.1365-2443.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Chen P, Nordstrom W, Gish B, Abrams JM. grim, a novel cell death gene in Drosophila. Genes Dev. 1996;10:1773–1782. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- Chen W, White MA, Cobb MH. Stimulus-specific requirements for MAP3 kinases in activating the JNK pathway. J Biol Chem. 2002;277:49105–49110. doi: 10.1074/jbc.M204934200. [DOI] [PubMed] [Google Scholar]
- Chew SK, Akdemir F, Chen P, Lu WJ, Mills K, Daish T, Kumar S, Rodriguez A, Abrams JM. The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev Cell. 2004;7:897–907. doi: 10.1016/j.devcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Collins CA, Wairkar YP, Johnson SL, DiA-ntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Delaney JR, Stoven S, Uvell H, Anderson KV, Engstrom Y, Mlodzik M. Cooperative control of Drosophila immune responses by the JNK and NF-kappaB signaling pathways. EMBO J. 2006;25:3068–3077. doi: 10.1038/sj.emboj.7601182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- Elion EA. The Ste5p scaffold. J Cell Sci. 2001;114:3967–3978. doi: 10.1242/jcs.114.22.3967. [DOI] [PubMed] [Google Scholar]
- Fernandez BG, Arias AM, Jacinto A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev. 2007;124:884–897. doi: 10.1016/j.mod.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Figueroa C, Tarras S, Taylor J, Vojtek AB. Akt2 negatively regulates assembly of the POSH-MLK-JNK signaling complex. J Biol Chem. 2003;278:47922–47927. doi: 10.1074/jbc.M307357200. [DOI] [PubMed] [Google Scholar]
- Frank LH, Rushlow C. A group of genes required for maintenance of the amnioserosa tissue in Drosophila. Development. 1996;122:1343–1352. doi: 10.1242/dev.122.5.1343. [DOI] [PubMed] [Google Scholar]
- Glise B, Bourbon H, Noselli S. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Blanchard GB, Adams RJ, Martinez Arias A. Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development. 2009;136:1889–1898. doi: 10.1242/dev.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal L, McCall K, Agapite J, Hartwieg E, Steller H. Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J. 2000;19:589–597. doi: 10.1093/emboj/19.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- Harden N, Ricos M, Ong YM, Chia W, Lim L. Participation of small GTPases in dorsal closure of the Drosophila embryo: distinct roles for Rho subfamily proteins in epithelial morphogenesis. J Cell Sci. 1999;112:273–284. doi: 10.1242/jcs.112.3.273. [DOI] [PubMed] [Google Scholar]
- Harden N, Ricos M, Yee K, Sanny J, Langmann C, Yu H, Chia W, Lim L. Drac1 and Crumbs participate in amnioserosa morphogenesis during dorsal closure in Drosophila. J Cell Sci. 2002;115:2119–2129. doi: 10.1242/jcs.115.10.2119. [DOI] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Homsy JG, Jasper H, Peralta XG, Wu H, Kiehart DP, Bohmann D. JNK signaling coordinates integrin and actin functions during Drosophila embryogenesis. Dev Dyn. 2006;235:427–434. doi: 10.1002/dvdy.20649. [DOI] [PubMed] [Google Scholar]
- Igaki T, Kanda H, Yamamoto-Goto Y, Kanuka H, Kuranaga E, Aigaki T, Miura M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 2002;21:3009–3018. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Tateno M, Fujimura-Kamada K, Takaesu G, Adachi-Yamada T, Ninomiya-Tsuji J, Irie K, Nishida Y, Matsumoto K. A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. EMBO J. 2001;20:5421–5430. doi: 10.1093/emboj/20.19.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Igaki T, Kanuka H, Yagi T, Miura M. Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J Biol Chem. 2002;277:28372–28375. doi: 10.1074/jbc.C200324200. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GH, Park E, Kong YY, Han JK. Novel function of POSH, a JNK scaffold, as an E3 ubiquitin ligase for the Hrs stability on early endosomes. Cell Signal. 2006;18:553–563. doi: 10.1016/j.cellsig.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kosman D, Small S, Reinitz J. Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev Genes Evol. 1998;208:290–294. doi: 10.1007/s004270050184. [DOI] [PubMed] [Google Scholar]
- Kukekov NV, Xu Z, Greene LA. Direct interaction of the molecular scaffolds POSH and JIP is required for apoptotic activation of JNKs. J Biol Chem. 2006;281:15517–15524. doi: 10.1074/jbc.M601056200. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Kanuka H, Igaki T, Sawamoto K, Ichijo H, Okano H, Miura M. Reaper-mediated inhibition of DIAP1-induced DTRAF1 degradation results in activation of JNK in Drosophila. Nat Cell Biol. 2002;4:705–710. doi: 10.1038/ncb842. [DOI] [PubMed] [Google Scholar]
- Leulier F, Rodriguez A, Khush RS, Abrams JM, Lemaitre B. The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep. 2000;1:353–358. doi: 10.1093/embo-reports/kvd073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons TR, Thorburn J, Ryan PW, Thorburn A, Anderson SM, Kassenbrock CK. Regulation of the pro-apoptotic scaffolding protein POSH by Akt. J Biol Chem. 2007;282:21987–21997. doi: 10.1074/jbc.M704321200. [DOI] [PubMed] [Google Scholar]
- Manseau L, Baradaran A, Brower D, Budhu A, Elefant F, Phan H, Philp AV, Yang M, Glover D, Kaiser K, Palter K, Selleck S. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev Dyn. 1997;209:310–322. doi: 10.1002/(SICI)1097-0177(199707)209:3<310::AID-AJA6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Maroney AC, Finn JP, Bozyczko-Coyne D, O’Kane TM, Neff NT, Tolkovsky AM, Park DS, Yan CY, Troy CM, Greene LA. CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally differentiated PC12 cells from death evoked by three distinct insults. J Neurochem. 1999;73:1901–1912. [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P, Silke J, Leevers SJ, Evan GI. The Drosophila caspase DRONC is regulated by DIAP1. EMBO J. 2000;19:598–611. doi: 10.1093/emboj/19.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly J, Kockel L, Gaengel K, Weber U, Bohmann D, Mlodzik M. The role of the Drosophila TAK homologue dTAK during development. Mech Dev. 2001;102:67–79. doi: 10.1016/s0925-4773(01)00285-4. [DOI] [PubMed] [Google Scholar]
- Mohseni N, McMillan SC, Chaudhary R, Mok J, Reed BH. Autophagy promotes caspase-dependent cell death during Drosophila development. Autophagy. 2009;5:329–338. doi: 10.4161/auto.5.3.7444. [DOI] [PubMed] [Google Scholar]
- Moreno E, Yan M, Basler K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr Biol. 2002;12:1263–1268. doi: 10.1016/s0960-9822(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Narasimha M, Brown NH. Novel functions for integrins in epithelial morphogenesis. Curr Biol. 2004;14:381–385. doi: 10.1016/j.cub.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Dynamic features of adherens junctions during Drosophila embryonic epithelial morphogenesis revealed by a Dalpha-catenin-GFP fusion protein. Dev Genes Evol. 1999;209:218–225. doi: 10.1007/s004270050246. [DOI] [PubMed] [Google Scholar]
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. In: Goldstein LSB, Fyrberg EA, editors. Drosophila melanogaster: practical uses in cell and molecular biology. San Diego: Academic Press; 1994. pp. p446–p487. [DOI] [PubMed] [Google Scholar]
- Polaski S, Whitney L, Barker BW, Stronach B. Genetic analysis of slipper/mixed lineage kinase reveals requirements in multiple Jun-N-terminal kinase-dependent morphogenetic events during Drosophila development. Genetics. 2006;174:719–733. doi: 10.1534/genetics.106.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LM, Dorstyn L, Mills K, Colussi PA, Chen P, Coombe M, Abrams J, Kumar S, Richardson H. An essential role for the caspase dronc in developmentally programmed cell death in Drosophila. J Biol Chem. 2000;275:40416–40424. doi: 10.1074/jbc.M002935200. [DOI] [PubMed] [Google Scholar]
- Reed BH, Wilk R, Lipshitz HD. Downregulation of Jun kinase signaling in the amnioserosa is essential for dorsal closure of the Drosophila embryo. Curr Biol. 2001;11:1098–1108. doi: 10.1016/s0960-9822(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Reed BH, Wilk R, Schock F, Lipshitz HD. Integrin-dependent apposition of Drosophila extraembryonic membranes promotes morphogenesis and prevents anoikis. Curr Biol. 2004;14:372–380. doi: 10.1016/j.cub.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Reim I, Lee HH, Frasch M. The T-box-encoding Dorsocross genes function in amnioserosa development and the patterning of the dorsolateral germ band downstream of Dpp. Development. 2003;130:3187–3204. doi: 10.1242/dev.00548. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar JR, Hafen E. Drosophila Jun kinase regulates expression of decapentaplegic via the ETS-domain protein Aop and the AP-1 transcription factor DJun during dorsal closure. Genes Dev. 1997;11:1717–1727. doi: 10.1101/gad.11.13.1717. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar JR, Jenni M, Fritz A, Hafen E. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Abrams JM. Caspase activation - stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene. 2004;23:2774–2784. doi: 10.1038/sj.onc.1207522. [DOI] [PubMed] [Google Scholar]
- Schnorr JD, Holdcraft R, Chevalier B, Berg CA. Ras1 interacts with multiple new signaling and cytoskeletal loci in Drosophila eggshell patterning and morphogenesis. Genetics. 2001;159:609–622. doi: 10.1093/genetics/159.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock F, Perrimon N. Retraction of the Drosophila germ band requires cell-matrix interaction. Genes Dev. 2003;17:597–602. doi: 10.1101/gad.1068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi A, Letsou A. Amnioserosa is required for dorsal closure in Drosophila. Dev Dyn. 2005;232:791–800. doi: 10.1002/dvdy.20306. [DOI] [PubMed] [Google Scholar]
- Seong KH, Matsuo T, Fuyama Y, Aigaki T. Neural-specific overexpression of drosophila plenty of SH3s (DPOSH) extends the longevity of adult flies. Biogerontology. 2001a;2:271–281. doi: 10.1023/a:1013249326285. [DOI] [PubMed] [Google Scholar]
- Seong KH, Ogashiwa T, Matsuo T, Fuyama Y, Aigaki T. Application of the gene search system to screen for longevity genes in Drosophila. Biogerontology. 2001b;2:209–217. doi: 10.1023/a:1011517325711. [DOI] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, Maniatis T. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278:48928–48934. doi: 10.1074/jbc.M304802200. [DOI] [PubMed] [Google Scholar]
- Sluss HK, Han Z, Barrett T, Goberdhan DC, Wilson C, Davis RJ, Ip YT. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- Stronach B. Dissecting JNK signaling, one KKKinase at a time. Dev Dyn. 2005;232:575–584. doi: 10.1002/dvdy.20283. [DOI] [PubMed] [Google Scholar]
- Stronach B, Perrimon N. Activation of the JNK pathway during dorsal closure in Drosophila requires the mixed lineage kinase, slipper. Genes Dev. 2002;16:377–387. doi: 10.1101/gad.953002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Stress-activated protein kinase signaling in Drosophila. In: Posas F, Nebreda AR, editors. Stress activated protein kinases. Berlin: Springer; 2008. pp. 225–241. [Google Scholar]
- Tapon N, Nagata K, Lamarche N, Hall A. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kappaB signalling pathways. EMBO J. 1998;17:1395–1404. doi: 10.1093/emboj/17.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Langmann C, Harden N, Aigaki T. The RING-finger scaffold protein plenty of SH3s targets TAK1 to control immunity signalling in Drosophila. EMBO Rep. 2005;6:1082–1087. doi: 10.1038/sj.embor.7400537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvia S, Taglicht D, Erez O, Alroy I, Alchanati I, Bicoviski V, Dori-Bachash M, Ben-Avraham D, Reiss Y. The ubiquitin E3 ligase POSH regulates calcium homeostasis through spatial control of Herp. J Cell Biol. 2007;177:51–61. doi: 10.1083/jcb.200611036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S, Khush RS, Leulier F, Tzou P, Nakamura M, Lemaitre B. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev. 2001;15:1900–1912. doi: 10.1101/gad.203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98:453–463. doi: 10.1016/s0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Destrument A, Tournier C. Physiological roles of MKK4 and MKK7: insights from animal models. Biochim Biophys Acta. 2007;1773:1349–1357. doi: 10.1016/j.bbamcr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- Wolff T. Histological Techniques for the Drosophila Eye. Part I: larva and pupa. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila protocols. Cold Spring Harbor, NY: CSHL Press; 2000. p. 728. [Google Scholar]
- Woolner S, Jacinto A, Martin P. The small GTPase Rac plays multiple roles in epithelial sheet fusion-dynamic studies of Drosophila dorsal closure. Dev Biol. 2005;282:163–173. doi: 10.1016/j.ydbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Xu Z, Greene LA. Activation of the apoptotic JNK pathway through the Rac1-binding scaffold protein POSH. Methods Enzymol. 2006;406:479–489. doi: 10.1016/S0076-6879(06)06036-8. [DOI] [PubMed] [Google Scholar]
- Xu Z, Kukekov NV, Greene LA. POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J. 2003;22:252–261. doi: 10.1093/emboj/cdg021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Kukekov NV, Greene LA. Regulation of apoptotic c-Jun N-terminal kinase signaling by a stabilization-based feed-forward loop. Mol Cell Biol. 2005;25:9949–9959. doi: 10.1128/MCB.25.22.9949-9959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol. 2001;21:4713–4724. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol. 1999;19:7245–7254. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang L, Acehan D, Wang X, Akey CW. Three-dimensional structure of a double apoptosome formed by the Drosophila Apaf-1 related killer. J Mol Biol. 2006;355:577–589. doi: 10.1016/j.jmb.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc Natl Acad Sci U S A. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZH, Zhou Y, Yu MC, Silverman N, Ge BX. Regulation of Drosophila p38 activation by specific MAP2 kinase and MAP3 kinase in response to different stimuli. Cell Signal. 2006;18:441–448. doi: 10.1016/j.cellsig.2005.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.