Abstract
Current dogma favors elimination of therapy-resistant cancer stem cells (bCSC) for chemoprevention of breast cancer. We showed recently that mammary cancer development in a transgenic mouse model (mouse mammary tumor virus-neu; MMTV-neu) was inhibited significantly upon treatment with withaferin A (WA), a steroidal lactone derived from a medicinal plant. Herein, we demonstrate that the mammary cancer prevention by WA is accompanied by in vivo suppression of bCSC. In vitro mammosphere formation was dose-dependently inhibited by WA treatment in MCF-7 and SUM159 human breast cancer cells. Other markers of bCSC including aldehyde dehydrogenase 1 (ALDH1) activity and CD44high/CD24low/epithelial specific antigen-positive (ESA+) fraction were also decreased significantly in the presence of plasma achievable doses of WA. On the other hand, WA exposure resulted in cell line-specific changes in Oct4, SOX-2, and Nanog mRNA expression. WA administration to MMTV-neu mice (0.1 mg/mouse, three times/week for 28 weeks) resulted in inhibition of mammosphere number and ALDH1 activity in vivo. Mechanistic studies revealed that while urokinase-type plasminogen activator receptor overexpression conferred partial protection against bCSC inhibition by WA, Notch4 was largely dispensable for this response. WA treatment also resulted in sustained (MCF-7) or transient (SUM159) downregulation of Bmi-1 (B cell-specific Moloney murine leukemia virus insertion region-1) protein. Ectopic expression of Bmi-1 conferred partial but significant protection against ALDH1 activity inhibition by WA. Interestingly, WA treatment caused induction of Kruppel-like factor 4 (KLF4) and its knockdown augmented bCSC inhibition by WA. In conclusion, the present study shows in vivo effectiveness of WA against bCSC.
Keywords: Withaferin A, Breast Cancer, Cancer Stem Cells, Chemoprevention
Introduction
Breast cancer affects thousands of families each year worldwide. Nearly 40,000 women succumb to this disease every year in the United States alone (1). Substantial reduction in mortality and morbidity from breast cancer and improvement in quality of life for women diagnosed with this disease is possible with non-toxic preventive interventions. Currently available preventive interventions, including selective estrogen receptor (ER) modulators (e.g., tamoxifen and raloxifene) and aromatase inhibitors (e.g., exemestane), have undoubtedly demonstrated clinical benefit against ER-positive breast cancers (2–4). These preventive interventions, however, are not perfect for several reasons, including: (a) a subset of ER-positive breast cancer is not responsive to some of these interventions (2, 3); (b) these agents are ineffective against ER-negative or triple-negative breast cancers (2–4), and (c) selective ER modulators as well as aromatase inhibitors have some side effects (2–6).
Phytochemicals derived from edible and medicinal plants are attractive for chemoprevention of breast and other cancers because of their efficacy in preclinical models and favorable safety profile (7, 8). Protective effect of some of these plants or their constituents against cancer (e.g., isothiocyanates from cruciferous vegetables) is substantiated by population-based epidemiological studies as well as preclinical data in experimental animals (7–9). It is interesting to note that a majority of naturally-occurring phytochemicals exhibit selectivity towards cancer cells, which likely contributes to their favorable safety profile (7, 8).
Withania somnifera plant is a key ingredient of the Ayurvedic remedies used in Indian sub-continent for alleviation of different chronic health problems (10, 11). Root extract of W. somnifera was shown to be effective for prevention of chemically-induced cancer in experimental animals (12, 13). Alleviation of cancer chemotherapy-induced toxicity and fatigue and improvement in quality of life in cancer patients by administration of W. somnifera were also shown (14, 15). Health promoting effects of W. somnifera are attributed to steroidal lactones collectively referred to as withanolides (16). Withaferin A (WA) is one of the withanolides that has been studied extensively for its anticancer properties using cultured cancer cells and xenograft models (8). We showed recently that WA administration resulted in significant inhibition of mammary tumor burden as well as pulmonary metastasis incidence in mouse mammary tumor virus-neu (MMTV-neu) transgenic mice without any apparent side effects (17). The chemopreventive effect of WA in MMTV-neu mice was associated with tumor cell apoptosis induction and inhibition of glycolysis (reversal of Warburg effect) (17). A similar dosing regimen was also effective in retarding growth of MDA-MB-231 human breast cancer xenografts in athymic mice (18). Previous studies have also identified novel targets of WA in breast cancer cells, including FOXO3a (18), complex III of the electron transport chain (17, 19), estrogen receptor-α (20), signal transducer and activator of transcription 3 (21), and Notch family of transcription factors (22).
Recent studies suggest that a small subset of tumor initiating cells or breast cancer stem cells (bCSC), which were first identified by Al-Hajj et al. (23), may be responsible not only for tumor initiation and progression but also for treatment failure (24, 25). Consistent with this notion, removal of both therapy-sensitive tumor cells constituting bulk of the tumor mass and bCSC may be necessary to achieve chemopreventive response (24, 25). In the present study, we have determined the effect of WA on bCSC using cellular and in vivo models of breast cancer.
Materials and Methods
Ethical considerations for animal studies and in vivo effect of WA on bCSC fraction
Freshly dissected breast tumor samples from our previous study on mammary cancer chemoprevention by WA in MMTV-neu mice (17) were used for in vivo analysis of bCSC. Care of animals was consistent with the Institutional Animal Care and Use Committee guidelines. Briefly, mammary cancer incidence and burden were determined in female MMTV-neu mice after 28 weeks of intraperitoneal treatment with 0.1 mg WA/mouse (three times per week) or vehicle (control). The overall tumor incidence was not different between the control and the WA treatment groups (17). On the other hand, the palpable tumor size was decreased by 50% upon WA administration in comparison with control (P = 0.03 by two-sided Student’s t-test) (17). The mean area of microscopic invasive carcinoma was lower by >95% in the WA treatment group compared with control (17). Mechanistic studies revealed increased apoptosis, inhibition of complex III activity of the electron transport chain, and reduced levels of glycolysis and tricarboxylic acid cycle intermediates in the tumors of WA-treated mice when compared with those of control mice (17).
Reagents and cell lines
WA (purity 99%) was purchased from Enzo Life Sciences and dissolved in dimethyl sulfoxide (DMSO). Final concentration of DMSO for the in vitro experiments did not exceed 0.1%. Cell culture medium, fetal bovine serum, and antibiotics were purchased from Invitrogen-Life Technologies. Antibodies against B cell-specific Moloney murine leukemia virus insertion region-1 (Bmi-1) and Kruppel-like factor 4 (KLF4) were from Cell Signaling Technology, whereas anti-actin and anti-cleaved Notch4 antibodies were purchased from Sigma-Aldrich. Small interfering RNA (siRNA) targeted against Notch4 was purchased from Santa Cruz Biotechnology; KLF4-targeted siRNA was from Abnova, and a control (nonspecific) siRNA was from Qiagen. MCF-7 cell line was purchased from the American Type Culture Collection and last authenticated in February 2012. Frozen stocks of the authenticated MCF-7 cells were used in the present study. Monolayer cultures of MCF-7 cells were maintained in MEM supplemented with 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, 1.5 g/L sodium bicarbonate, 10% fetal bovine serum, and antibiotics. SUM159 cell line was purchased from Asterand (Detroit, MI) and authenticated by the provider. The SUM159 cultures were maintained in Ham’s F-12 media supplemented with 5% fetal bovine serum, 1 μg/mL hydrocortisone, 5 μg/mL insulin, and 10 mM HEPES. MCF-7 cells stably transfected with a plasmid encoding for urokinase-type plasminogen activator receptor (uPAR) were generously provided by Dr. Steven L Gonias (University of California, San Diego, CA), and maintained as recommended by the provider (26). MCF-7 cells were stably transfected with empty pcDNA3.1 vector or the same vector encoding for Bmi-1 using FuGENE6. The pcDNA3.1-Bmi-1 plasmid was a generous gift from Dr. M. H. Yang (National Yang-Ming University, Taipei, Taiwan). Clones with stable overexpression of Bmi-1 were selected in the presence of 800 μg/mL of G418 over a period of 8 weeks. Each cell line was maintained at 37°C.
Mammosphere formation assay
Mammosphere assay was performed as described by us previously (27). The first generation mammospheres of >50 μm in size were scored under an inverted microscope after 5 days of cell seeding. The first generation mammospheres were disaggregated and single cell suspensions were re-plated without further treatment with DMSO or WA for the second generation mammosphere formation. The second generation mammospheres were scored after 7 days of cell plating.
Flow cytometric analysis of aldehyde dehydrogenase (ALDH1) activity and CD44high/CD24low/epithelial specific antigen-positive (ESA+) population
The ALDH1 activity was quantified by using the ALDEFLUOR™ assay kit from Stem Cell Technologies and by following the supplier’s instructions. Diethylaminobenzaldehyde (DEAB), a specific ALDH1 inhibitor, was used as a control. The CD44high/CD24low/ESA+ population was analyzed as previously described (27).
Real-time quantitative polymerase chain reaction (qPCR)
Total RNA from cells was isolated using RNeasy kit (Qiagen). First-strand cDNA was synthesized using superscript reverse-transcriptase (Invitrogen-Life Technologies) with oligo (dT)20 primer. Primers for stemness-related genes including Oct4, Nanog, and SOX-2 are described in Kim et al. (27). Relative gene expression was calculated by the method described by Livak and Schmittgen (28).
RT2 profiler PCR array
MCF-7 cells were treated with 0.5 μmol/L WA or DMSO for 24 h or 48 h. Total RNA was extracted using RNeasy kit (Qiagen) followed by reverse transcription with 1 μg of total RNA and RT2 First-Strand kit (Qiagen) as suggested by the supplier. To evaluate the effect of WA treatment on expression of a panel of genes involved in cancer stemness, Human Cancer Stem Cell RT2 Profiler™ PCR Array (Qiagen) was used. Briefly, 25 μL reaction mixture containing cDNA and RT2 SYBR® GREEN ROX™ qPCR master mix was prepared immediately before the real-time PCR and loaded into each well of the PCR array plate (96-well). Real-time PCR was performed using an ABI StepOne PLUS™ instrument with 10 min at 95°C followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. Change in gene expression was quantitated using web-based software provided by the manufacturer. Gene expression with Ct value above 35 was considered undetectable. The cut-off was at least 1.5-fold change (up- or down-regulation) and P ≤ 0.05 (by Student’s t-test).
In vivo analysis of bCSC from MMTV-neu tumors
Primary tumors from control and WA-treated MMTV-neu mice after their sacrifice (17) were washed with phosphate-buffered saline and digested in DMEM supplemented with 300 units/mL collagenase and 100 units/mL hyaluronidase for 3–4 h at 37°C. The resultant cells were suspended in Hank’s balanced salt solution supplemented with 2% fetal bovine serum and ammonium chloride. The cell suspension was re-suspended in 0.25% Trypsin-EDTA, 5 mg/mL dispase, 0.1 mg/mL DNase1 in Hank’s balanced salt solution and passed through a 40 μm strainer. The cells were used for mammosphere formation assay and determination of ALDH1 activity.
Western blot analysis
Details of cell lysate preparation and western blotting have been described by us previously (29, 30).
Ectopic expression of Bmi-1 by transient transfection
SUM159 cells were transiently transfected at ~50 % confluence with pcDNA3.1 empty vector or the same vector encoding for Bmi-1 using FuGENE6. Twenty-four hours after transfection, the cells were treated with DMSO (control) or WA for 24 h and then processed for flow cytometric analysis of ALDH1 activity.
RNA Interference
Cells were transiently transfected with Notch4-targeted siRNA, KLF4-targeted siRNA, or a control (nonspecific) siRNA using Oligofectamine. After 24 h, cells were treated with DMSO (control) or WA for 48 h (for Notch4) or 72 h (for KLF4). Subsequently, the cells were collected and processed for flow cytometric analysis of ALDH1 activity or mammosphere formation.
Statistical analyses
One-way analysis of variance (ANOVA) with Dunnett’s adjustment or multiple comparisons tests were used to determine statistical significance of difference between groups. Student’s t-test was employed for binary comparisons. Difference was considered significant at P < 0.05.
Results
WA treatment inhibited self-renewal of bCSC in vitro
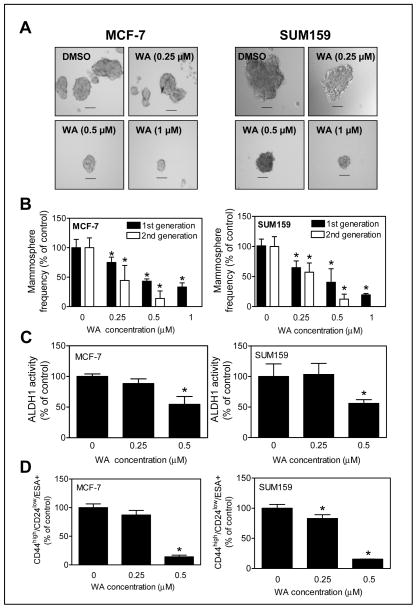
MCF-7 (ER-positive) and SUM159 (triple-negative) human breast cancer cell lines were used to determine the in vitro effect of WA on bCSC population. Fig. 1A shows representative first generation mammospheres resulting from MCF-7 and SUM159 cells after 5 days of cell seeding and treatment with DMSO or WA. Of note, the WA concentrations used herein were well within the plasma achievable level of 1.8 μM based on a pharmacokinetic study in mice (31). WA treatment resulted in a dose-dependent and statistically significant decrease in first and second generation mammosphere number in both cell lines (Fig. 1B). These results indicated inhibition of bCSC self-renewal by WA treatment.
Figure 1.
WA treatment inhibited bCSC in vitro in MCF-7 and SUM159 human breast cancer cells. A, representative images of first generation mammospheres after treatment with DMSO or WA (50× magnification; scale bar- 100 μm). B, percentage of first generation and second generation mammospheres relative to DMSO-treated control. C, percentage of ALDH1 activity relative to DMSO-treated control (72 h treatment with DMSO or WA) in MCF-7 and SUM159 cells. D, quantitation of CD44high/CD24low/ESA+ population relative to DMSO-treated control (72 h treatment with DMSO or WA) in MCF-7 and SUM159 cells. The results shown (mean ± SD, n = 3) are representative of at least two independent experiments. *Significantly different (P < 0.05) compared with DMSO-treated control by one-way ANOVA with Dunnett’s adjustment.
Inhibitory effect of WA treatment on bCSC fraction was confirmed by flow cytometric analysis of ALDH1 activity and CD44high/CD24low/ESA+ fraction. The ALDH1 activity was decreased significantly in the presence of 0.5 μmol/L of WA in both cell lines when compared with control (Fig. 1C). In comparison with DMSO-treated control, the CD44high/CD24low/ESA+ fraction was significantly lower in the WA-treated MCF-7 (0.5 μmol/L WA) and SUM159 (0.25 and 0.5 μmol/L WA) cultures (Fig. 1D).
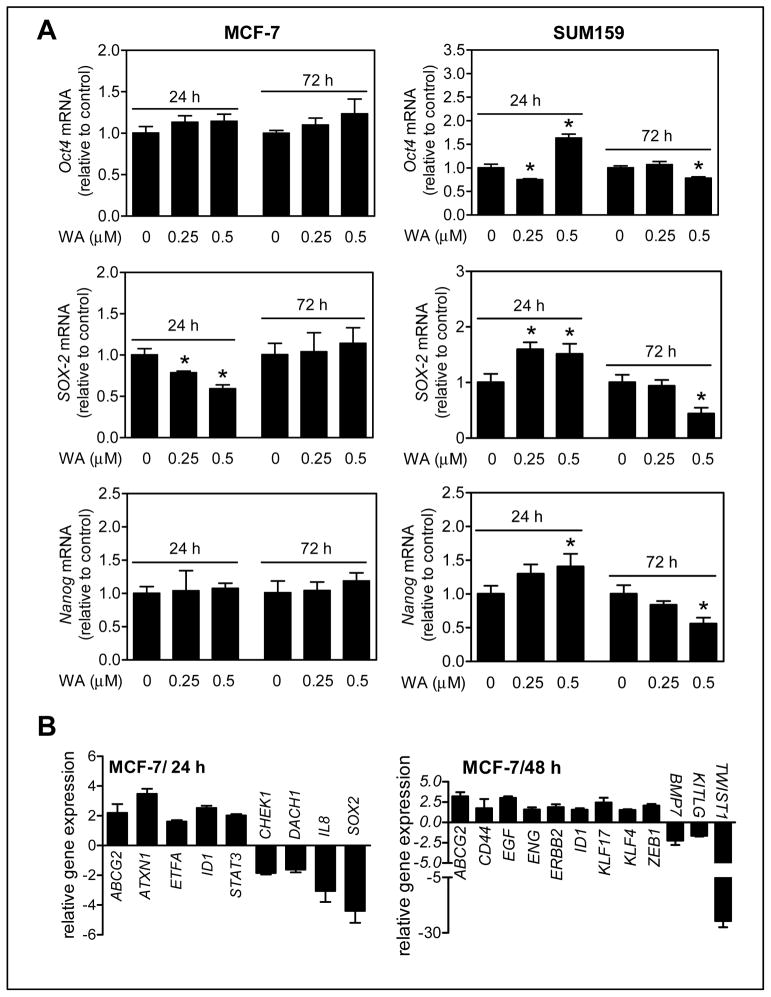
Expression of stemness-related genes, including Oct4, SOX-2, and Nanog, was determined after 24 h or 72 h treatment of MCF-7 and SUM159 cells with DMSO or WA (0.25 or 0.5 μmol/L). The expression of only SOX-2 mRNA was reduced dose-dependently after 24 h treatment with WA in MCF-7 cells; but this effect was abolished at the 72 h time point (Fig. 2A). In SUM159 cells, reduced expression of Oct4, SOX-2, and Nanog was clearly evident at the 72 h time point with 0.5 μmol/L of WA (Fig. 2A).
Figure 2.
WA treatment decreased mRNA expression of stemness-related genes in MCF-7 and SUM159 cells. A, quantitation of Oct4, SOX-2 and Nanog mRNA expression by qPCR in MCF-7 and SUM159 cells relative to DMSO control after treatment with DMSO or WA. The results shown are mean ± SD (n = 3). *Significantly different (P < 0.05) compared with control by one-way ANOVA with Dunnett’s adjustment. Representative data from replicate experiments are shown. B, changes in expression of genes related to cancer stemness in MCF-7 cells after treatment with 0.5 μmol/L WA when compared with DMSO-treated control (cut-off 1.5-fold change and P ≤ 0.05). The results shown are mean ± SD (n = 3).
Because of cell line-specific differences, targeted PCR array was performed for a panel of cancer stemness related genes using MCF-7 cells (Fig. 2B). Expression of several genes was affected significantly (P ≤ 0.05 by two-sided Student’s t-test) by 1.5-fold upon 24 h or 48 h treatment with WA (Fig. 2B). In agreement with data shown in Fig. 2A, SOX-2 expression was significantly decreased after 24 h treatment of MCF-7 cells with WA when compared with control. Collectively, these results provided evidence for in vitro inhibition of bCSC by WA treatment in MCF-7 and SUM159 cells.
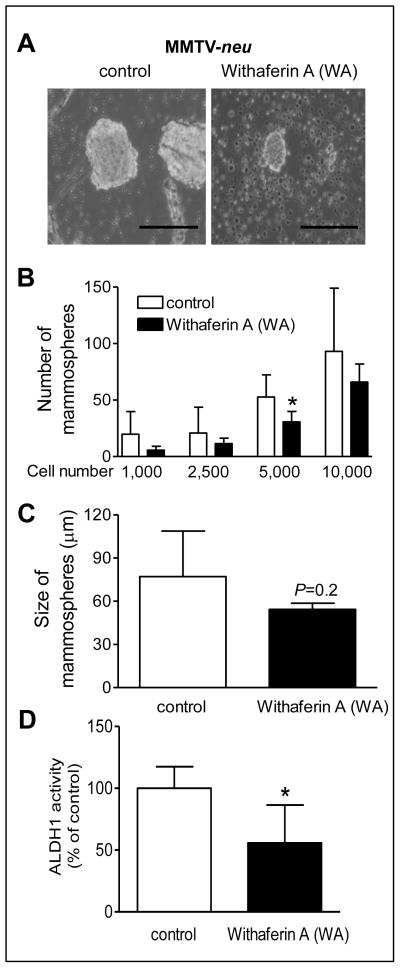
WA administration suppressed bCSC in tumors of MMTV-neu mice
We have shown previously that WA administration significantly reduces mammary tumor burden in MMTV-neu mice (17). Freshly harvested tumors from 5 mice of each group (at the time of sacrifice) from this study were used to determine the in vivo effect of WA on bCSC. Fig. 3A depicts primary mammospheres resulting from representative tumor of a control mouse and that from a WA-treated MMTV-neu mouse after 7 days of cell plating. The Number of mammospheres from tumors of WA-treated mice was lower compared with control at each cell density, but the difference was significant only at 5,000 cell density (Fig. 3B). The mean mammosphere size from tumors of WA-treated mice was also smaller by about 30% compared with control; although the difference was not significant due to large data scatter especially in the control group (Fig. 3C). Five tumors from each group were also used for measurement of ALDH1 activity. The ALDH1 activity was unusually very high in one tumor sample from the control group, and this sample was not included in the statistical analysis. The ALDH1 activity was lower by about 44% (P <0.05 by two-sided Student’s t-test) in the tumors from WA treatment group in comparison with the control (Fig. 3D). Together, these results provided evidence for in vivo inhibition of bCSC by WA.
Figure 3.
Mammary cancer chemoprevention by WA administration in MMTV-neu mice was accompanied by in vivo inhibition of bCSC. A, representative photomicrographs showing mammospheres formed by isolated primary tumor cells from MMTV-neu transgenic mice (100× magnification, scale bar- 200 μm). B, mammosphere number from tumor cells of control and WA-treated MMTV-neu mice. Results shown are mean ± SD (n = 5, except for 1,000 cells where mammospheres were observed only from 2–3 samples). C, The bar graph shows quantitation of mammosphere size from the 5,000 cell density group. Mammosphere size was determined from at least five-non overlapping regions of each sample. D, flow cytometric quantitation of ALDH1 activity in tumor cells (relative to control) from control and WA-treated (n = 4–5) MMTV-neu mice. The results shown are mean ± SD. *Significantly different (P < 0.05) compared with control by two-sided Student’s t-test.
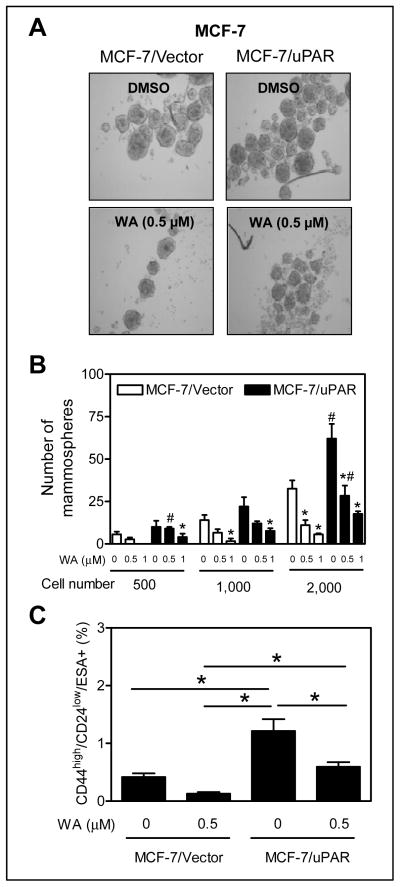
Effect of uPAR overexpression on WA-mediated inhibition of bCSC
Overexpression of uPAR alone is sufficient to drive stemness in MCF-7 cells (26). Overexpression of uPAR in MCF-7 cells was confirmed by western blotting (results not shown). Consistent with published findings (26), mammosphere number was markedly higher from uPAR overexpressing MCF-7 cells compared with vector control (Fig. 4A,B). WA treatment inhibited mammosphere number in both empty vector transfected control cells and uPAR overexpressing MCF-7 cells (Fig. 4B). However, the difference in mammosphere number in the presence of WA between empty vector transfected cells and uPAR overexpressing MCF-7 cells was not significant except at 500 and 2,000 cell density with 0.5 μmol/L WA. WA treatment caused about 69% decrease in CD44high/CD24low/ESA+ fraction compared with DMSO control in empty vector transfected MCF-7 cells (Fig. 4C). Percent decrease in CD44high/CD24low/ESA+ fraction after WA treatment in uPAR overexpressing MCF-7 cells was about 51% and the difference was significant from empty vector transfected MCF-7 cells (Fig. 4C). Based on these results, we conclude that uPAR overexpression confers partial protection against bCSC inhibition by WA.
Figure 4.
uPAR overexpression conferred partial protection against bCSC inhibition by WA. A, representative photomicrographs depicting first generation mammospheres from MCF-7 cells stably transfected with empty vector (MCF-7/Vector) or uPAR plasmid (MCF-7/uPAR) after 5 days of cell seeding and treatment with DMSO or WA (50× magnification; scale bar- 100 μm). B, mammosphere number in MCF-7/Vector or MCF-7/uPAR cells. Significantly different (P < 0.05) compared with *respective DMSO control and #between MCF-7/Vector and MCF-7/uPAR cells by one-way ANOVA followed by Bonferroni’s test. C, percentage of CD44high/CD24low/ESA+ fraction in MCF-7/Vector and MCF-7/uPAR cells after 72 h treatment with DMSO or 0.5 μmol/L WA. The results shown are mean ± SD (n = 3). *Significantly different (P < 0.05) between the identified groups by one-way ANOVA followed by Bonferroni’s test. The experiments were repeated twice with comparable results.
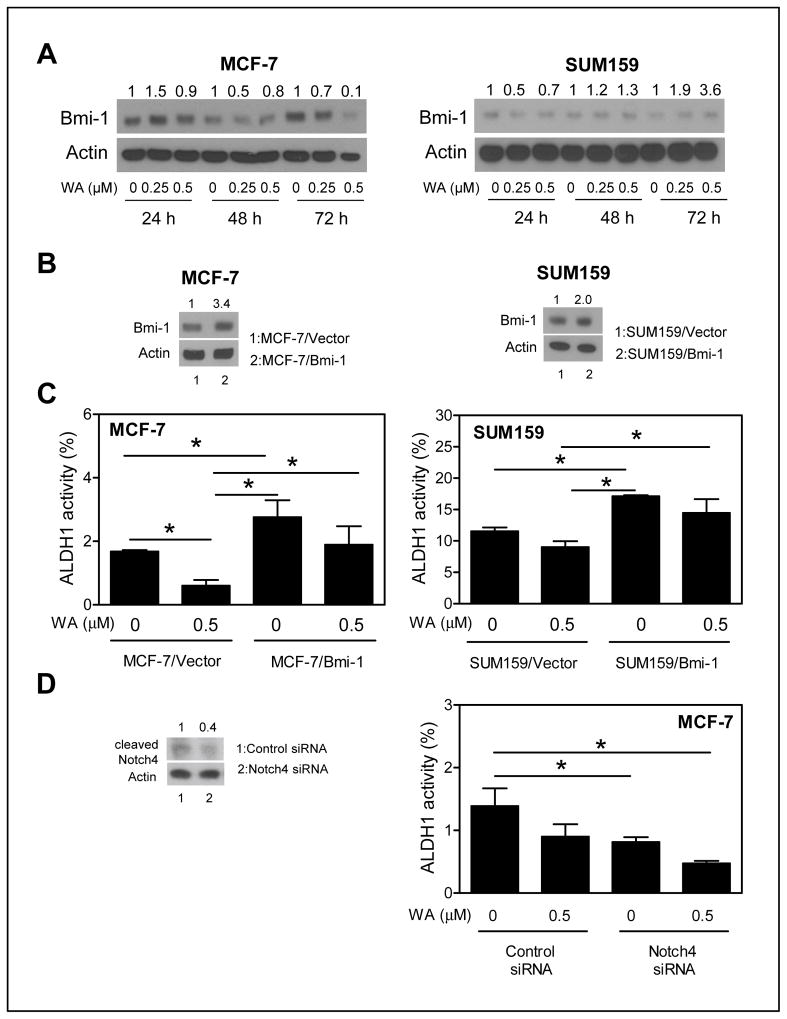
The role of Bmi-1 in WA-mediated inhibition of bCSC
The polycomb group protein Bmi-1 has been implicated in self-renewal of bCSC (32). The level of Bmi-1 protein was decreased markedly after treatment of MCF-7 cells with WA (Fig. 5A). Stable overexpression of Bmi-1 protein in MCF-7 cells was confirmed by western blotting (Fig. 5B). Treatment with 0.5 μmol/L WA for 72 h resulted in a significant decrease (64% decrease compared with DMSO-treated control; P < 0.05) in ALDH1 activity in empty vector transfected MCF-7 cells (Fig. 5C). The ALDH1 activity was about 1.6-fold higher in Bmi-1 overexpressing MCF-7 cells compared with the empty vector transfected cells in the absence of WA treatment (Fig. 5C). The ALDH1 activity was decreased by only about 31% compared with DMSO control after 72 h treatment of Bmi-1 overexpressing MCF-7 cells with 0.5 μmol/L WA (Fig. 5C). The difference in ALDH1 activity in the presence of WA was statistically significant between empty vector transfected cells and Bmi-1 overexpressing MCF-7 cells (Fig. 5C).
Figure 5.
Ectopic expression of Bmi-1 conferred partial protection against ALDH1 activity inhibition by WA. A, western blotting for Bmi-1 protein using cell lysates from MCF-7 and SUM159 cells after treatment with DMSO or WA. Densitometric quantitation relative to respective DMSO control and after normalization for protein loading (actin) is shown above the band. B, western blotting for protein levels of Bmi-1 in MCF-7 (stable transfection) or SUM159 cells (transient transfection) transfected with empty vector (lane 1) or Bmi-1 plasmid (lane 2). C, percentage of ALDH1 activity in empty vector transfected or Bmi-1 overexpressing MCF-7 and SUM159 cells after 72 h (MCF-7) or 24 h (SUM159) treatment with DMSO or 0.5 μmol/L WA. The results shown are mean ± SD (n = 3). *Significantly different (P < 0.05) between the indicated groups by one-way ANOVA with Tukey’s post-hoc analysis. D, ALDH1 activity in MCF-7 cells transfected with a control siRNA or a Notch4 targeted siRNA after 48 h treatment with DMSO or 0.5 μmol/L WA. The western blot shows knockdown of cleaved Notch4. The results shown are mean ± SD (n = 3). *Significantly different (P < 0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparison test. Comparable results were observed in two independent experiments
WA-mediated decrease in Bmi-1 protein level was transient in SUM159 cells (24 h only; Fig. 5A). However, a 2-fold overexpression of Bmi-1 protein in SUM159 cells after transient transfection (Fig. 5B) resulted in significant abrogation of ALDH1 activity inhibition by WA (Fig. 5C). Collectively, these results indicated that Bmi-1 suppression was partly responsible for bCSC inhibition resulting from WA exposure.
Notch4 activation by WA was dispensable for its inhibitory effect on bCSC
Signaling through the Notch4 receptor has been implicated in regulation of bCSC activity (33). Previous work from our own laboratory has indicated cleavage (activation) of Notch4 upon treatment of breast cancer cells with WA (22). Thus, it was of interest to determine if Notch4 activation by WA affected its activity against bCSC. Knockdown of Notch4 itself inhibited ALDH1 activity by about 41% in MCF-7 cells (Figure 5D). However, knockdown of Notch4 did not significantly augment WA-mediated inhibition of ALDH1 activity.
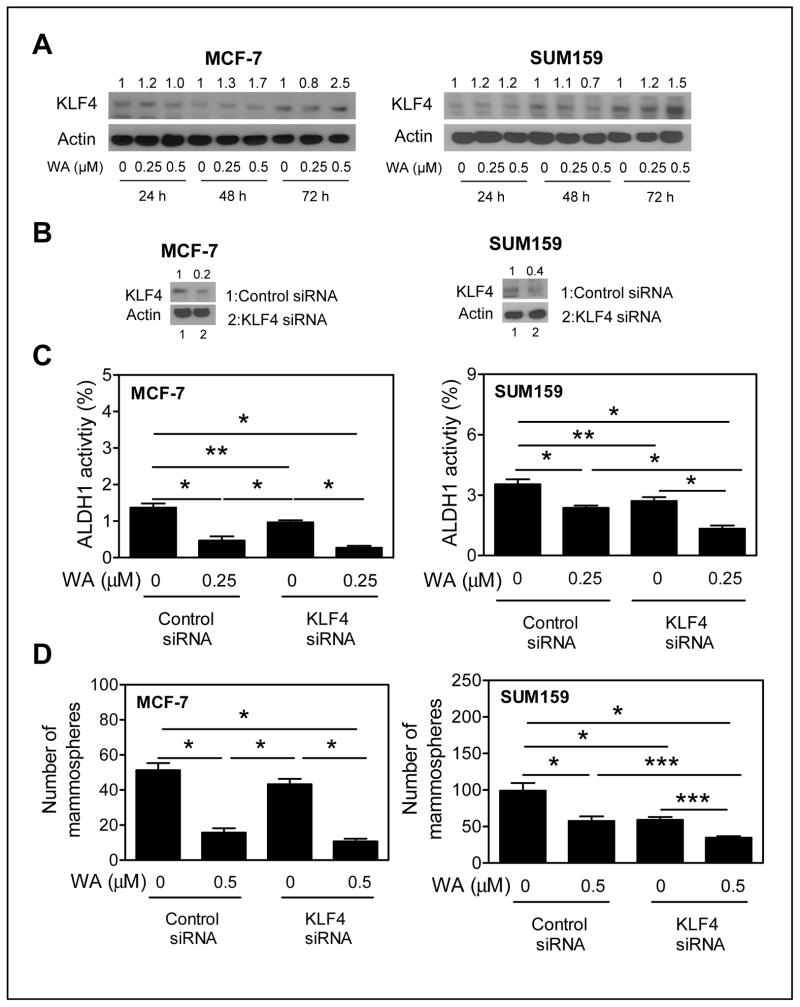
KLF4 knockdown augmented WA-mediated inhibition of bCSC
KLF4 is required for maintenance of bCSC and mammary cancer cell migration and invasion (34). Because expression of KLF4 was modestly but significantly increased after 48 h treatment of MCF-7 cells with WA (Fig. 2B), we first determined the effect of WA on KLF4 protein level using MCF-7 and SUM159 cells (Fig. 6A). WA treatment resulted in induction of KLF4 protein in MCF-7 and SUM159 cells especially at the 72 h time point with 0.5 μmol/L dose (Fig. 6A). Expression of KLF4 was decreased by 60–80 % in MCF-7 and SUM159 cells transfected with the KLF4-targeted siRNA compared with corresponding control siRNA transfected cells (Fig. 6B). Knockdown of KLF4 itself significantly inhibited ALDH1 activity in both MCF-7 and SUM159 cells (Fig. 6C). In addition, KLF4 knockdown significantly augmented WA-mediated inhibition of ALDH1 activity at least in the SUM159 cells; a similar trend was discernible in the MCF-7 cells but the difference was not significant (Fig. 6C). Mammosphere inhibition by WA treatment was also significantly augmented by RNA interference of KLF4 in the SUM159 cell line (Fig. 6D). Together, these results indicated that KLF4 induction by WA treatment modestly impeded its inhibitory effect on bCSC.
Figure 6.
KLF4 status affected WA-mediated inhibition of bCSC in SUM159 cells. A, western blotting for KLF4 protein using cell lysates from MCF-7 and SUM159 cells after treatment with DMSO or WA. Densitometric quantitation relative to respective DMSO control and after normalization for protein loading (actin) is shown above the band. B, western blotting for KLF4 protein expression in MCF-7 and SUM159 cells transfected with a control siRNA (lane 1) or KLF4 siRNA (lane 2). C, percentage of ALDH1 activity in MCF-7 and SUM159 cells transiently transfected with a control siRNA or a KLF4-targeted siRNA after 72 h treatment with DMSO or WA. D, number of primary mammospheres (after 5 days of cell seeding and treatment with DMSO or WA) from MCF-7 and SUM159 cells transfected with a control siRNA or KLF4 siRNA. The results shown are mean ± SD (n = 3). Significantly different (*P < 0.001; ** P<0.01; ***P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each experiment was repeated twice with comparable results.
Discussion
An emerging hypothesis argues for removal of tumor cells constituting bulk of the tumor mass as well as bCSC for effective prevention and treatment of breast cancers (24, 25). The role of bCSC in mammary carcinogenesis has been reviewed extensively (24, 25), but a few examples to support its role in cancer metastasis follow: (a) presence of CD44-positive/CD24−/low fraction in primary human breast tumors may favor distant metastasis (35), and (b) the ALDH1 expression has been shown to correlate with metastasis and poor clinical outcome in inflammatory breast cancers (36). The present study demonstrates, for the first time, that WA administration inhibits self-renewal capacity of bCSC in vivo in HER-2-driven mammary cancer. Mechanistic studies demonstrate a role for uPAR and the polycomb group protein Bmi-1 in bCSC inhibition by WA.
We show that WA can overcome Notch4 activation for inhibition of bCSC. These observations have clinical ramifications as Notch4 is implicated in regulation of bCSC (33). Notch4 activity is significantly higher in bCSC in comparison with differentiated cancer cells (33). Pharmacologic as well as genetic inhibition of Notch4 decreases stem cell activity in vitro and reduces tumor formation in vivo (33). In addition, Notch4 overexpression has been observed in triple-negative human breast cancer specimens (37). The present study reveals decrease in ALDH1 activity after Notch4 knockdown in MCF-7 cells. However, knockdown of Notch4 protein does not synergize with WA for inhibition of ALDH1 activity.
KLF4, a zinc finger transcription factor, plays an important role in regulating various cellular processes including apoptosis, migration, and caner stem cells (34, 38). Earlier studies have shown that KLF4 can either function as a tumor suppressor or can be oncogenic depending on type of cancer (38). However, many studies point to an oncogenic role for KLF4 in breast cancer. For example, >70% of breast cancers exhibit elevated KLF4 expression and increased nuclear staining for KLF4 is associated with an aggressive phenotype in early stage breast cancer (39, 40). Second, KLF4 knockdown inhibits mammary tumor development in vitro and in vivo as well as suppresses migration and bCSC fraction (34). In agreement with these published findings, results shown herein demonstrate an oncogenic role for KLF4 in breast cancer cells. Knockdown of KLF4 itself inhibits stemness in MCF-7 and SUM159 cells, but only SUM159 cells exhibit augmentation with WA for inhibition of ALDH1 activity and formation of mammospheres. Even though the molecular basis for differential sensitivity of MCF-7 versus SUM159 cells to WA-mediated augmentation of bCSC inhibition by KLF4 knockdown is still unclear, these observations have clinical implications. SUM159 is a triple-negative cell line and triple-negative breast cancers have worse prognosis (41). We are tempted to speculate that WA likely exhibits greater efficacy against triple-negative breast cancers. While experimental validation of this contention requires further experimentation, our more recent studies have revealed that the SUM159 cell line (IC50 after 48 h treatment- about 1 μmol/L) is relatively more sensitive to cell proliferation inhibition by WA in comparison with MCF-7 cells (IC50 after 48 h treatment- >2 μmol/L) (42).
A clinically viable preventive intervention targeting both ER-positive and ER-negative breast cancers is still not available. The present study together with our previous observations (17) suggest that WA may be useful for prevention of hormone-dependent as well as hormone-independent breast cancers because: (a) WA administration significantly inhibits ER-negative mammary cancer development in MMTV-neu mice (17); (b) incidence and multiplicity of methylnitrosourea-induced rat mammary cancer, which is ER-positive, is reduced by gavage with W. somnifera root extract (13); (c) WA functions as an anti-estrogen and pro-apoptotic effect of WA is partially but significantly attenuated by overexpression of ER-α in MDA-MB-231 cells (20); and (d) WA treatment inhibits self-renewal of bCSC in both ER-positive (MCF-7) and triple-negative (SUM159) breast cancer cells (present study).
The present study reveals cell line-specific differences in the mechanisms by which WA may inhibit bCSC. A molecular target approach (43) may be necessary to gain insights into the mechanisms underlying cell line-specific differences. As suggested by Lee et al. (43), the overall approach for identification of molecular target(s) of phytochemicals may involve RNA interference screen followed by validation using in vitro, ex vivo and/or in vivo models. In silico virtual screening based on natural product libraries and diverse ligand databases including the ZINC database (which contains ‘ready-to-dock’ searchable libraries of 4.6 million three-dimensional compounds) and the Asinex database is also useful for identification of potential chemopreventive phytochemicals.
In conclusion, the results of the present study indicate that: (a) WA treatment inhibits bCSC in vitro in MCF-7 and SUM159 cells; (b) mammary cancer prevention by WA administration in MMTV-neu mice is associated with inhibition of self-renewal of bCSC in vivo; and (c) Bmi-1 plays a role in WA-mediated inhibition of bCSC in MCF-7 cells, whereas KLF4 status affects bCSC inhibition by WA in SUM159 cell line. The results shown herein together with our previously published in vivo efficacy data (17, 18) warrants clinical investigation of WA for prevention and/or treatment of human breast cancer.
Acknowledgments
Grant Support
This work was supported by the grant RO1 CA142604-04 awarded by the National Cancer Institute. This research used the Animal Facility and the Flow Cytometry Facility supported in part by a grant from the National Cancer Institute at the National Institutes of Health (P30 CA047904).
Footnotes
Conflict of Interest: None
Disclosure of Potential Conflicts of Interest:
No potential conflicts of interests were disclosed.
Authors’ Contributions
Conception and design: S.H. Kim, S.V. Singh
Development of methodology: S.H. Kim
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): S.H. Kim
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): S.H. Kim, S.V. Singh
Writing, review and/or revision of the manuscript: S.H. Kim, S.V. Singh
Study supervision: S.V. Singh
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125–34. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 5.Obiorah I, Jordan VC. Progress in endocrine approaches to the treatment and prevention of breast cancer. Maturitas. 2011;70:315–21. doi: 10.1016/j.maturitas.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boccardo F, Rubagotti A, Guglielmini P, Fini A, Paladini G, Mesiti M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol. 2006;17(Suppl 7):vii10–4. doi: 10.1093/annonc/mdl941. [DOI] [PubMed] [Google Scholar]
- 7.Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33:1833–42. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J. 2014;16:1–10. doi: 10.1208/s12248-013-9531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–8. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 10.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–46. [PubMed] [Google Scholar]
- 11.Winters M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern Med Rev. 2006;11:269–77. [PubMed] [Google Scholar]
- 12.Padmavathi B, Rath PC, Rao AR, Singh RP. Roots of Withania somnifera inhibit forestomach and skin carcinogenesis in mice. Evid Based Complement Alternat Med. 2005;2:99–105. doi: 10.1093/ecam/neh064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khazal KF, Samuel T, Hill DL, Grubbs CJ. Effect of an extract of Withania somnifera root on estrogen receptor-positive mammary carcinomas. Anticancer Res. 2013;33:1519–23. [PMC free article] [PubMed] [Google Scholar]
- 14.Hamza A, Amin A, Daoud S. The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol Toxicol. 2008;24:63–73. doi: 10.1007/s10565-007-9016-z. [DOI] [PubMed] [Google Scholar]
- 15.Biswal BM, Sulaiman SA, Ismail HC, Zakaria H, Musa KI. Effect of Withania somnifera (Ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr Cancer Ther. 2013;12:312–22. doi: 10.1177/1534735412464551. [DOI] [PubMed] [Google Scholar]
- 16.Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–93. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahm ER, Lee J, Kim SH, Sehrawat A, Arlotti JA, Shiva SS, et al. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst. 2013;105:1111–22. doi: 10.1093/jnci/djt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stan SD, Hahm E-R, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–9. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6:e23354. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahm ER, Lee J, Huang Y, Singh SV. Withaferin A suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog. 2011;50:614–24. doi: 10.1002/mc.20760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;31:1991–8. doi: 10.1093/carcin/bgq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Sehrawat A, Singh SV. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res Treat. 2012;136:45–56. doi: 10.1007/s10549-012-2239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velasco-Velázquez MA, Homsi N, De La Fuente M, Pestell RG. Breast cancer stem cells. Int J Biochem Cell Biol. 2012;44:573–7. doi: 10.1016/j.biocel.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien CS, Farnie G, Howell SJ, Clarke RB. Breast cancer stem cells and their role in resistance to endocrine therapy. Horm Cancer. 2011;2:91–103. doi: 10.1007/s12672-011-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo M, Eastman BM, Webb DL, Stoletov K, Klemke R, Gonias SL. Cell signaling by urokinase-type plasminogen activator receptor induces stem cell-like properties in breast cancer cells. Cancer Res. 2010;70:8948–58. doi: 10.1158/0008-5472.CAN-10-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Sehrawat A, Singh SV. Dietary Chemopreventative benzyl isothiocyanate inhibits breast cancer stem cells in vitro and in vivo. Cancer Prev Res. 2013;6:782–90. doi: 10.1158/1940-6207.CAPR-13-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 30.Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, et al. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst. 2011;103:571–84. doi: 10.1093/jnci/djr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129:2744–55. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–18. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu F, Li J, Chen H, Fu J, Ray S, Huang S, et al. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30:2161–72. doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–9. [PubMed] [Google Scholar]
- 36.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speiser J, Foreman K, Drinka E, Godellas C, Perez C, Salhadar A, et al. Notch-1 and Notch-4 biomarker expression in triple-negative breast cancer. Int J Surg Pathol. 2012;20:139–45. doi: 10.1177/1066896911427035. [DOI] [PubMed] [Google Scholar]
- 38.Rowland BD, Peeper DS. KLF4, p21, and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 39.Foster KW, Frost AR, McKie-Bell P, Lin CY, Engler JA, Grizzle WE, et al. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488–95. [PubMed] [Google Scholar]
- 40.Pandya AY, Talley LI, Frost AR, Fitzgerald TJ, Trivedi V, Chakravarthy M, et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10:2709–2719. doi: 10.1158/1078-0432.ccr-03-0484. [DOI] [PubMed] [Google Scholar]
- 41.Bayraktar S, Glück S. Molecularly targeted therapies for metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2013;138:21–35. doi: 10.1007/s10549-013-2421-5. [DOI] [PubMed] [Google Scholar]
- 42.Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, Kumari V, et al. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with downregulation and covalent binding at cysteine-303 of β-tubulin. J Biol Chem. 2014;289:1852–65. doi: 10.1074/jbc.M113.496844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee KW, Bode AM, Dong Z. Molecular targets of phytochemicals for cancer prevention. Nat Rev Cancer. 2011;11:211–8. doi: 10.1038/nrc3017. [DOI] [PubMed] [Google Scholar]