Abstract
Mast cells play a significant role in both the innate and adaptive immune response; however, the tissue-bound nature of mast cells presents an experimental roadblock to performing physiologically relevant mast cell experiments. In this work, a heterogeneous cell culture containing primary culture murine peritoneal mast cells (MPMCs) was studied to characterize the time-dependence of mast cell response to allergen stimulation, and the time- and concentration-dependence of the ability of the heterogeneous MPMC culture to uptake and degranulate exogenous serotonin using high performance liquid chromatography (HPLC) coupled to an electrochemical detector. Additionally, because mast cells play a central role in asthma, MPMCs were exposed to CXCL10 and CCL5, two important asthma-related inflammatory cytokines that have recently been shown to induce mast cell degranulation. MPMC response to both allergen exposure and cytokine exposure was evaluated for 5-HT secretion and bioactive lipid formation using ultraperformance liquid chromatography coupled to an electrospray ionization triple quadrupole mass spectrometer (UPLC-MS/MS). In this work, MPMC response was shown to be highly regulated and responsive to subtle alterations in a complex environment through time and concentration dependent degranulation and bioactive lipid formation. These results highlight the importance of selecting an appropriate mast cell model when studying mast cell involvement in allergic response and inflammation.
Introduction
Mast cells are tissue-bound cells of hematopoetic origin widely known for their roles in inflammation and allergic response. They also have roles in innate immunity, host defense against parasitic and bacterial infection, wound healing, tissue homeostasis, and disease states such as vasculitis and fibrosis.1–3 Mast cell function is often thought to be dominated by their cytosolic granules that contain inflammatory mediators, including enzymes such as tryptase and chymase, highly charged biopolymers such as heparin or chondroitin sulfate, and small molecule messengers such as serotonin (5-hydroxytryptamine, 5-HT) and histamine. In addition to granule-stored mediators, mast cells produce and secrete bioactive lipid compounds via enzymatic transformations of their phospholipid membranes. In vivo mast cell secretion of both granule-stored and de novo manufactured inflammatory mediators influence surrounding cell types, leading to symptoms commonly associated with allergic response, including mucus hypersecretion, bronchoconstriction, and vasodilation.4, 5 Based on their significant involvement in both innate and adaptive immune response, there are many research groups that aim to study mast cell behavior; however, there are several experimental roadblocks to performing physiologically relevant mast cell experiments.
Mature mast cells are tissue bound, and as such, it is difficult to isolate large numbers of pure populations for study; due to this challenge, several different in vitro strategies for mast cell culture are commonly used in experimental work. Isolation of pure populations of mast cells typically requires tissue homogenization followed by several immunomagnetic separation steps, which can affect the activation state of the mast cells and gives low cell yields. To circumvent this challenge, studies of mast cells are often performed using immortal tumor-derived cell lines such as rat basophilic leukemia 2H3 (RBL) cells or the human mast cell lines HMC-1 or LAD2. Benefits of immortal cell lines include homogeneity and ease of culture; however, studies of adherence, receptor expression, and enzyme content have shown each of these mast cell-like cells lines to be only marginally representative of mature, tissue bound, non-transformed mast cells.6–8 Another strategy for mast cell studies involve culturing mast cell-like cells from bone marrow or blood-derived immature precursors for 4–6 weeks with chemokines to drive mast cell maturation. Such cell cultures produce generally homogeneous mast cell-like cell populations, but they are not ideal due to length of culture time, expense of culture media, scarcity of precursor cells, and disparity between in vivo and in vitro culture conditions. Studies of primary culture mast cells employ the isolation of a cell suspension often from a mouse or rat peritoneal cavity. Cell suspensions generally contain a mixture of cells including ~3% mast cells, ~ 30% macrophages ~55% B cells and ~7% T cells and are co-cultured with fibroblasts to maintain mast cell viability.9,10These heterogeneous cell cultures more closely model the in vivo environment of mast cells, which are in contact with macrophages and connective tissue cells but present challenges because mast cells make up less than 3% of the total cell population.It is already well known that IgE-sentisized mast cells release serotonin in several different cell models. However, how mast cell models change their response to their environment and the effects that the environment plays in IgE-allergen release over a period of time, in which mediators can stimulate other cell types or be cleared from the extracellular milieu, has not been evaluated. In this work, a heterogeneous cell culture containing primary culture murine peritoneal mast cells (MPMCs) is studied to characterize the time-dependence of mast cell response to allergen stimulation. The serotonin secretion behavior of MPMCs in a heterogeneous cell culture was compared to that from immortal RBL cells. When an unexpected 5-HT secretion trend was observed in the primary culture MPMCs, the time- and concentration-dependence of serotonin uptake in the heterogeneous MPMC culture was examined. Additionally, because mast cells play a central role in inflammatory diseases such as asthma, primary culture mast cells were exposed to CXCL10 and CCL5, two important asthma-related inflammatory cytokines that have recently been shown to induce mast cell degranulation at the single-cell level with different degranulation kinetics than IgE-mediated response to allergen.11 To further understand these differences in time-dependent mast cell communication mechanisms, MPMC secretion of 5-HT and bioactive lipids was measured at various time points from 10 – 90 min following exogenous 5-HT exposure. These experiments aim to reveal differences in mast cell biochemical pathways leading to exocytosis.
MATERIALS AND METHODS
Reagents
All materials used for cell culture were obtained from HyClone. Recombinant murine CXCL10 and CCL5 were purchased from Shenandoah Biotechnology Inc. and stored as suggested. LTC4, LTD4, LTE4, PGD2, and PAF and internal standards LTC4-d5, LTD4-d5, LTE4-d5, PGD2-d9, and PAF-d4 were purchased from Cayman Chemical and used as received. LC/MS-grade acetonitrile (ACN) and water were purchased from JT Baker. LC/MS grade isopropyl alcohol (IPA) and a penicillin/streptomycin mixture were purchased from Fisher Scientific. Anti-trinitrophenol (TNP) IgE was purchased from BD Biosciences, while TNP-ovalabumin (TNP-ova) was purchased from Fisher Scientific.
Mast Cell Isolation and Exposure to Chemokines
MPMCs were harvested from 10 week old C57BL/6J mice purchased from The Jackson Laboratory. Briefly, 8–9 mL of DMEM supplemented with 10% bovine calf serum and 1% penicillin and streptomycin (henceforth referred to as media) were injected into the peritoneal cavity of each mouse immediately following euthanasia (by CO2 asphyxiation according to IACUC approved protocol #0807A40164). Following a 30 second abdominal massage, approximately 6–7 mL of peritoneal lavage fluid per mouse were recovered. The collected cell suspension was pelleted by centrifugation at 450×g for 10 minutes, resuspended at 2 × 106 cells mL−1 in fresh media, and plated over Swiss albino 3t3 fibroblasts (purchased from ATCC). Cells were then cultured overnight with additional 500 µL of fresh media containing anti-TNP IgE such that the concentration of IgE in each well was 0.5 Ig mL−1.
After overnight incubation, MPMCs were washed three times with tris buffer (12.5 mM Trizma-HCl, 150 mM NaCl, 4.2 mM KCl, 5.6 mM glucose, 1.5 mMCaCl2, 1.4mM MgCl2) at 37 °C to remove serum-containing media. To investigate 5-HT processing by the cell culture, MPMCs were incubated with 0.1–0.55 µM 5-HT or tris buffer as a control, the supernatant was collected at specific time points and the cells were lysed with 0.5 µM HClO4 for 30 min. The supernatant and lysate 5-HT content was assessed by HPLC at various timepoints. For experiments evaluating the effect of stimulation with TNP-ova, CXCL10, or CCL5 on MPMCs, cells were incubated for either 2 hours or particular time points of 10, 30, 60, and 90 minutes with tris buffer or with tris buffer containing 0.2–200 ng mL−1 CXCL10, 0.2–200 ng mL−1 CCL5, or 100 ng mL−1 TNP-ova. Following incubation, degranulation was terminated by incubating the cells on ice for 10 minutes, and supernatants were collected for analysis by UPLC-MS/MS or HPLC with electrochemical detection.
HPLC Analysis of Serotonin
MPMC-secreted serotonin was detected and quantified by HPLC coupled to an electrochemical detector using a modified version of a previously published protocol.12 Briefly, following MPMC incubation, 250 µL of supernatant was collected and filtered using a 0.45µm filter plate from Millipore (Billerica, MA) at 3000×g for 5 min. 180 µL of the filtered supernatant was added to 20 µL of 5 µM dopamine (used as an internal standard) in 0.5M HClO4 for final concentrations of 0.5 µM dopamine and 500 mM HClO4. The samples were vortexed and injected by an autosampler into an Agilent 1200 HPLC. Separation was achieved using a 5µm, 4.6 × 150 mm Eclipse XDB C18 column attached to a Waters 2465 electrochemical detector with a glassy carbon-based electrode. The working potential was set at 0.7 V vs. an in situ Ag/AgCl reference electrode with a current range of 50 nA. The HPLC flow rate was 2 mL/min using a mobile phase mixture consisting of 11.6 mg L−1 of the surfactant sodium octyl sulfate, 170µL L−1 dibutylamine, 55.8 mg L−1 Na2EDTA, 10% methanol, 203 mg L−1 sodium acetate anhydrous, 0.1M citric acid, and 120 mg L−1 sodium chloride.
A 5 point calibration curve was constructed by plotting the ratio of serotonin to dopamine (internal standard) peak areas in solutions containing 500 nM dopamine and 28–1000 nM serotonin in 0.5 M HClO4. The line of best fit for an average calibration curve for several representative experiments was y = 2.0578+0.0088 with R2=0.9992, where y = peak area of 5-HT/peak area of internal standard and × = 5-HT concentration. Cell-secreted serotonin was quantified using the experimentally generated calibration curve.
UPLC-MS/MS Determination of Secreted Lipids
UPLC-MS/MS analysis was performed using a previously published method.13 Briefly, supernatants from MPMCs were collected, spiked with 25 ppb of each of the internal standards LTC4-d5, LTD4-d5, LTE4-d5, PGD2-d9, and PAF-d4 and concentrated in a centrifugal evaporator until the remaining volume was approximately 50 µL. After concentrating, salts were precipitated by the addition of 100 µL ice-cold ethanol containing 0.1% formic acid (FA) and 200 µL mobile phase B (90/10 ACN/IPA containing 0.1% FA). Precipitated salts were removed by centrifuging for 10 minutes at 12,000 RCF, and supernatants were again concentrated to approximately 20 µL. Samples were then resuspended in 100 µL 70/30 mobile phase A/mobile phase B (with A: water containing 0.1% formic acid and B: 90/10 acetonitrile/ isopropyl alcohol containing 0.1% formic acid). For UPLC-MS/MS analysis, a Waters Acquity UPLC coupled to a Waters triple quadrupole mass spectrometer (Acquity TQD) were used. Separation was performed on a Waters BEH C-18 2.1 mm × 50 mm column at 45 °C employing the following 5 min gradient separation at a flow rate of 0.6 mL per minute: 30% B, 0 min to 0.5 min; 30% B to 50% B, 0.5 min to 1.0 min; 50% B 1.0 min to 2.0 min; 50% B to 90% B, 2.0 min to 2.2 min; 90% B, 2.2 min to 3.2 min; 90% B to 30% B, 3.2 min to 3.5 min; and 30% B, 3.5 min to 5.0 min.
Assay of Secreted β-Hexosaminidase
To determine if incubation with 5-HT induced MPMC degranulation, supernatant content of the granule-stored enzyme β-Hexosaminidase (β-Hex) was assessed as described previously.13, 14 Briefly, 50 µL supernatant samples were collected in a 96 well plate and stored overnight at −80°C. When thawed, supernatants were incubated for 60 min at 37°C with 100 µL 1 mM 4-nitrophenyl N-acetyl-β-D-glucosaminide in 0.1 M citrate buffer at pH 4.5. After 60 min, 150 µL ice cold 0.1 M carbonate buffer at pH 9 was added, and absorbance of each well was recorded at 405 nm with a background subtraction absorbance reading at 630 nm.
RESULTS AND DISCUSSION
Time-dependent MPMC and RBL cell response to IgE-mediated stimulation
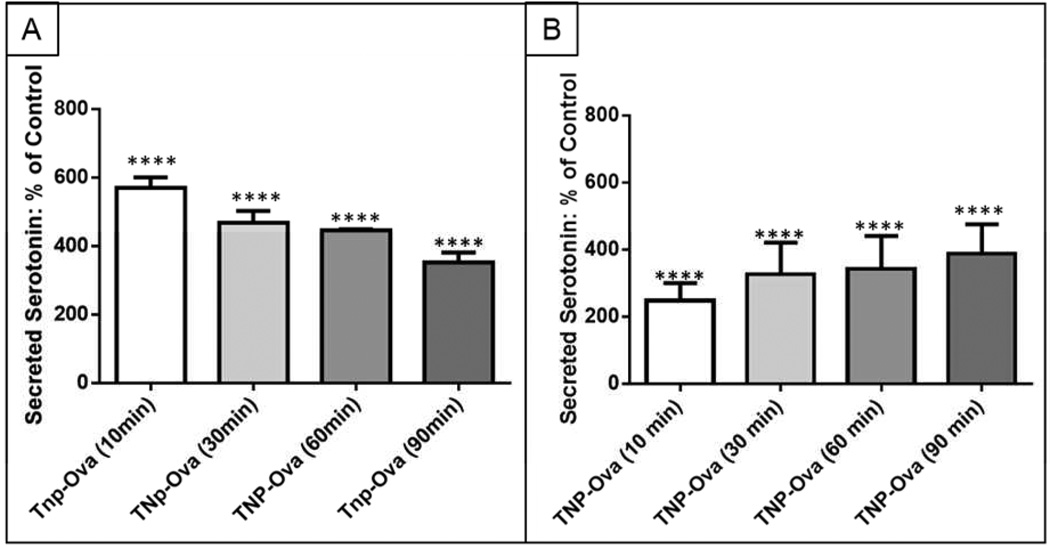
While MPMCs have been used for many studies of single mast cell secretion kinetics (usually tracking events on the ms to s timescale), the longer term (min-hrs) serotonin secretion of MPMCs in a heterogeneous cell culture has not been explored. To examine the time-dependence of mast cell response to IgE-mediated allergen stimulation, MPMCs were incubated overnight with anti-TNP IgE, washed three times, then exposed to 100 ng mL−1 TNP-ova. After exposure to TNP-ova, supernatants from the adherent cell culture were collected and analyzed for secreted mediators at discrete time points to probe the kinetics of long-term release of preformed granules. As previous research has indicated that exocytotic cells only partially secrete their granule contents upon direct stimulation,15 it was hypothesized that supernatant 5-HT concentration would steadily increase over the course of 90 min exposure to allergen stimulation. However, Fig. 1A shows that over the course of 90 min, exposure to TNP-ova resulted in an increase followed by a decrease in secreted serotonin. Unsurprisingly, after 10 min of incubation with TNP-ova, secreted serotonin increased to 571 ± 30% of control serotonin. After 10 min, however, serotonin concentration in MPMC supernatants ([5-HT]supernatant) steadily decreased to 351 ± 30% of 5-HT control cell concentration at 90 min (Fig. 1A). MPMCs are cultured in a heterogeneous cellular environment, and although this type of cell culture more closely models the in vivo cellular environment than homogeneous immortal cell lines, heterogeneity can obscure the fate of the secreted serotonin. To probe the effect of the heterogeneous cell culture on secreted serotonin, MPMC 5-HT secretion was compared to that of the homogenous RBL cell line. RBL cells, while widely accepted as different from mast cells, share the common mast cell traits of adherence, FcεRI receptor expression, and degranulation in response to IgE-mediated stimulation.8 Figure 1 shows that the RBL cells continually release more serotonin over the course of 90 min, quite distinct from the serotonin secretion trend exhibited by MPMCs. When compared to the steadily increasing RBL cell [5-HT]supernatant concentration (Fig. 1B), the decreasing trend in MPMC [5-HT]supernatant (Fig. 1A) indicates a process of metabolism or reuptake by the cells in culture. This difference demonstrates the importance of the choice of cell culture when examining cell-to-cell communication behavior and interactions in response to changes in the environment.
Figure 1.
Time-dependent effects of serotonin secretion from A. MPMCs and B. RBL cells. ****p < 0.0001 vs. supernatant 5-HT concentration of control (unactivated) MPMCs or RBL cells. Control refers to the concentration of 5-HT in the supernatant of MPMCs for A RBL cells for B exposed to tris buffer, normalized to 100%.
Exogenous 5-HT effects on MPMC culture
Mast cell secretion of 5-HT (along with other granule stored and de novo manufactured mediators critical to cell-cell communication and inflammation) has several known downstream effects and potentially many other effects on mast cells themselves and surrounding cell types. Expression of the 5-HT specific reuptake transporter (SERT) has been detected on mast cells, macrophages, and B cells.16–18 To explore the role that the cell culture microenvironment plays in MPMC 5-HT secretion, MPMCs were incubated with tris buffer containing concentrations of 5-HT ranging from 0.1 µM to 0.55 µM. These incubations were intended to model the environment that un-activated (but IgE-sensitized) cells would experience when in contact with activated MPMCs. Data represented in Figures 1 and 4 presented as percent of control showthat TNP-ova stimulated MPMCs generate supernatant 5-HT concentrations of 0.4 – 5 µM, and MPMCs exposed to inflammation- relevant cytokines generate 0.1 – 0.2 µM 5-HT in the cell culture supernatant. To create a similar environment, the number of mice and conditions were accounted for to achieve similar ratios and amount of cells. At 10, 30, and 60 min of serotonin incubation, the cell culture supernatants were collected and analyzed for 5-HT, and the adherent cells were lysed and analyzed for 5-HT content. In Fig. 2, the total system 5-HT is presented as a percentage of the sum of the control supernatant, control cell lysate, and incubation 5-HT concentrations. For this work, the total system 5-HT refers to the 5-HT in the cell supernatants and cell lysates. To account for the exogenous concentration of 5-HT in the cell cultures, Equation 1 was used to calculate the total system 5-HT.
| Equation 1 |
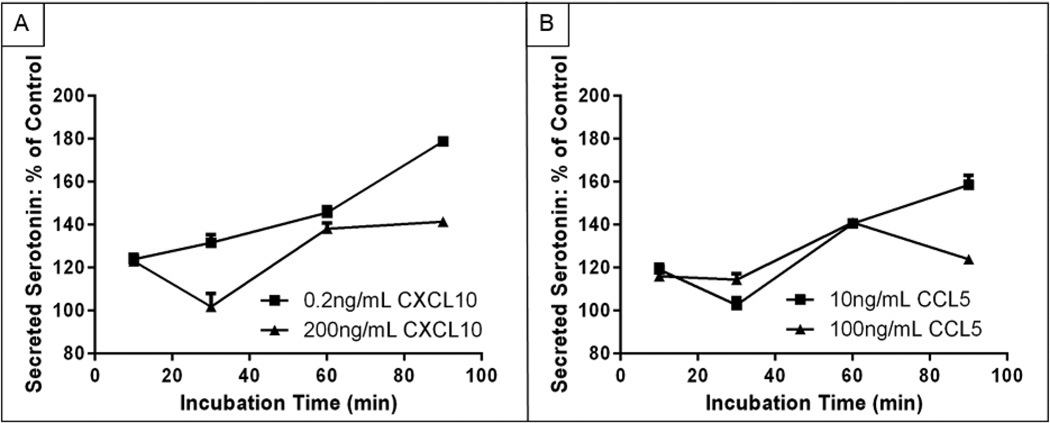
Figure 4.
Supernatant 5-HT content as a percent of control MPMC supernatant 5-HT content after MPMC exposure to the inflammatory cytokines A. CXCL10 and B. CCL5.
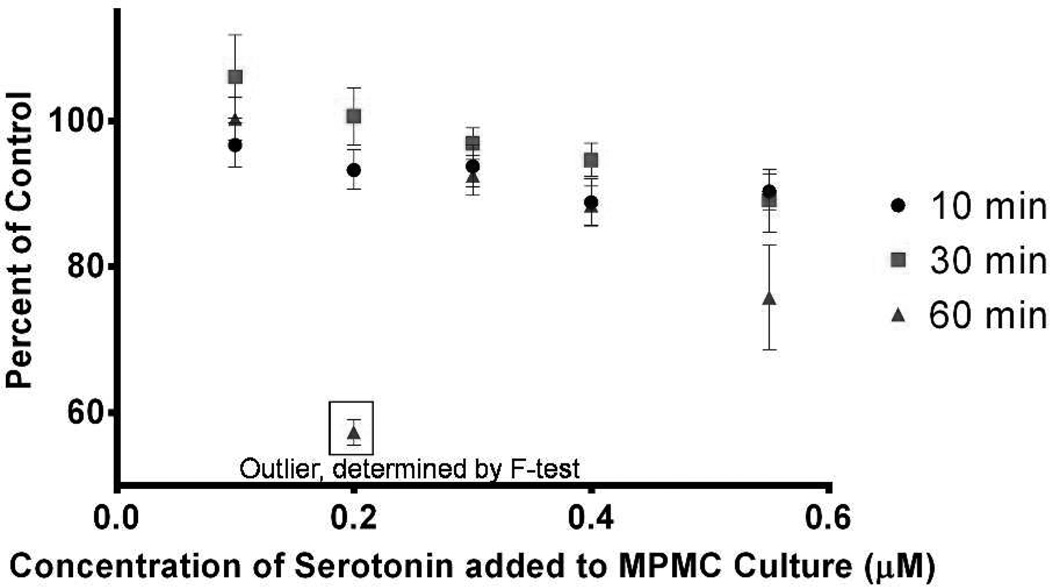
Figure 2.
Time-dependent effects of incubating MPMCs with 0.1–0.55 µM 5-HT. Percents of control were calculated using Equation 1: each data point represents the total system serotonin (the sum of 5-HT concentration in supernatant as well as in lysed cells) as a percentage of the total system serotonin in control cells. The 0.2 µM incubation condition at 60 min was determined to be an outlier using the extra-sum-of-squares F tests (p ≤ 0.05).
Surprisingly, the 5-HT content of the cell cultures exhibited a decreasing trend with increasing 5-HT incubation concentrations. The correlation between 5-HT as a percent of control and 5-HT incubation concentration was significant at 30 min (p = 0.0009) and 60 min (p = 0.02) but not at the earliest time point of 10 min (p = 0.07). This indicates that, over the course of 30–60 min, the heterogeneous cell culture, including the macrophages, B cells, and mast cells which have been shown to contain SERT, processes 5-HT.17–19 Exposing the cell culture to higher concentrations of exogenous 5-HT leads to a proportionally greater degree of cellular processing of 5-HT. To determine if supernatant-contained species were causing degranulation and/or caused the release of serotonin to be higher than that of our controls, a β-Hex assay was used to indicate extents of cell degranulation. When the β-Hex data were analyzed using a one-way ANOVA, the supernatants of MPMCs incubated with 0.4 µM and 0.55 µM 5-HT for 60 min released significantly more β-Hex than control MPMCs at the same timepoints (p = 0.03 and p = 0.02 for 0.4 µM 5-HT and 0.55 µM 5-HT incubation, respectively). No significant difference was detected between control β-Hex values and β-Hex values for the 0.1 – 0.55 µM 5-HT incubation at 10 min or 30 min or between controls and 0.1 – 0.3 µM 5-HT incubation at 60 min (p > 0.05). So, only the highest exogenous 5-HT concentrations and the longest incubation times induced significant increases in mast cell degranulation, meaning that degranulation alone does not account for the cells’ 5-HT processing behavior (all β-Hex data can be found in Supplementary Information). Previous studies on the effects of exogenous 5-HT on mast cells using bone marrow-derived or blood progenitor-derived mast cell models determined that 5-HT affects mast cell adhesion but does not induce mast cell degranulation.17 It is likely that mast cells in culture with a heterogeneous mix of other cells (as would be present in vivo) experience a different chemical microenvironment than that of mast cells derived from blood or bone marrow progenitor cells, and this may result in different autocrine and/or paracrine effects of exposure to cell-cell communication species, including 5-HT. Changes in chemical microenvironment as a result of incubation with 5-HT after 60 minutes may induce release in mediators by surrounding cells, which stimulates mast cell degranulation of β-Hex and 5-HT.
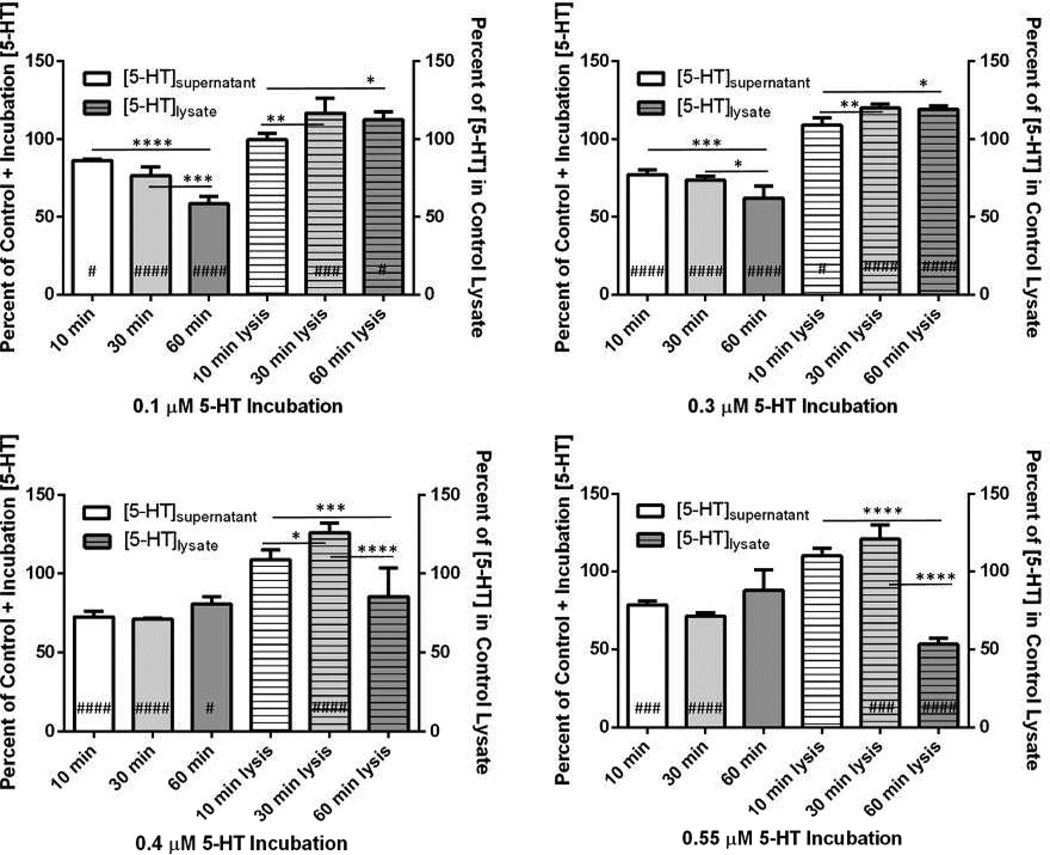
To evaluate whether the trend in cellular processing of 5-HT shown in Fig. 2 was a result of supernatant 5-HT metabolism, intracellular 5-HT up-take and metabolism (indicating intracellular MPMC 5-HT metabolism) or a combination of both, Fig. 3 shows the MPMC supernatant and lysate 5-HT trends for each 5-HT incubation condition separately. To compare the supernatant 5-HT concentrations of MPMCs incubated with exogenous 5-HT to control MPMC supernatants, the 5-HT values in the solid bars of Fig. 3 are represented as a percentage of the sum of the control (MPMC supernatant 5-HT + 5-HT concentration of the MPMC incubation). Trends in MPMC supernatant 5-HT content are shown in Table 1: after 10 min, the supernatant concentrations of 5-HT were significantly decreased in comparison to the 5-HT content of the supernatant of MPMCs exposed to no exogenous 5-HT (p ≤ 0.05 using one-way ANOVA) at all exogenous 5-HT concentrations examined, when controlling for the concentration of 5-HT added to the cells. After 30 min of incubation with 5-HT, the supernatant 5-HT content did not change significantly from the 10 min timepoint supernatant 5-HT content at any of the exogenous 5-HT conditions (p > 0.05). After 60 min of incubation, however, the 5-HT content of MPMC supernatants exhibited a dependence on exogenous [5-HT]. The [5-HT] of supernatants of MPMCs incubated with lower concentrations of 5-HT, 0.1 and 0.3 µM, decreased significantly at 60 min vs. 10 min (p < 0.0001 for both). However, as the exogenous 5-HT concentration increased, the reverse effect was observed: at 0.4 µM and 0.55 µM exogenous 5-HT, supernatant 5-HT content did not change significantly at 60 min incubation vs. at 10 min of incubation (p > 0.05). In summary, assessing supernatant β-Hex content indicated that at the longest incubation times and highest incubation concentrations, MPMC degranulation was induced, which would increase the 5-HT content of the supernatant. This may explain the trend of decreased supernatant 5-HT in MPMCs incubated with low concentrations of exogenous 5-HT and the opposite trend observed at higher concentrations of exogenous 5-HT. Cells exposed to the 0.4 –0.55 µM exogenous 5-HT for 60 min likely experience a chemical microenvironment similar to what they would experience when MPMCs are responding to allergen (as many mast cells degranulate). MPMC secretion of 5-HT must induce surrounding cells to secrete chemical species that in turn can induce further activation of mast cells. The difference between trends in supernatant 5-HT decrease at low (0.1 and 0.3 µM) and high (0.4 and 0.55 µM) 5-HT incubations indicate that 5-HT metabolism by the cell culture has both time and concentration dependence. Several studies have reported on the mechanisms of clearance of 5-HT in the brain19–21 and determined that, while SERT has a high binding affinity for 5-HT (Kd = 6.7 nM22), there are also other mechanisms by which 5-HT is cleared, as SERT-knockout mice demonstrate the ability to clear 5-HT.21 Although they have lower binding affinity for 5-HT than SERT, the extraneuronal monoamine transporter, plasmalemmal monoamine transporter, and the organic cation transporters 1–3 are several receptors with the ability to clear extracellular 5-HT efficiently.21 While it is known that mast cells and macrophages express SERT, little is known about the non-SERT clearance of 5-HT by macrophages and mast cells. Additionally, as 5-HT clearance varies by local area within the brain,23 it is highly likely that 5-HT clearance in non-neuronal tissue by SERT and non-SERT mechanisms will differ from brain synaptosomes. Clearly, further study on the clearance of extracellular 5-HT by peripheral tissues is warranted.
Figure 3.
Supernatant and lysate 5-HT content of MPMCs incubated with 0.1 – 0.55 µM 5-HT for 10–60 min. #p ≤ 0.05 vs. control; ###p < 0.001 vs. control; ####p < 0.0001 vs. control; *p ≤ 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Table 1.
Trends in MPMC supernatant 5-HT content over the course of 60 min at varying exogenous concentrations of 5-HT.
| Exogenous [5-HT] (µM) |
10 min vs. Control MPMCs |
10 min vs. 30 min |
30 min vs. 60 min |
10 min vs. 60 min |
|---|---|---|---|---|
| 0.1 | −13.7% | ns | −23.5% | −32.0 |
| 0.2 | −16.9% | ns | ||
| 0.3 | −22.9% | ns | −15.4% | −19.2% |
| 0.4 | −27.7% | ns | ns | ns |
| 0.55 | −21.5% | ns | +23.2% | ns |
Where significant (p ≤ 0.05 using one-way ANOVA), the percent increase or decrease is indicated, and ns = not significant, p > 0.05 using one-way ANOVA
Analysis of the cell lysate 5-HT content revealed interesting trends in the uptake of 5-HT by the cell culture, which are detailed in Table 2. Of the 5-HT incubation concentrations examined, only 0.3 µM 5-HT produced a significant increase in cell-stored 5-HT concentration (compared to controls) after 10 min of incubation (p = 0.05). The trends in cell-stored 5-HT over the course of 10 to 60 min indicate significant increases at 5-HT incubation concentrations of 0.1 and 0.3 µM and significant decreases for 5-HT incubation concentrations of 0.4 and 0.55 µM. The overall 60 min decrease at the higher exogenous concentrations of 5-HT is likely due in part to the induction of MPMC degranulation by the incubation itself.
Table 2.
Trends in MPMC lysate 5-HT content over the course of 60 min at varying exogenous concentrations of 5-HT.
| Exogenous [5-HT] (µM) |
10 min vs. Control MPMCs |
10 min to 30 min |
30 min to 60 min |
10 min to 60 min |
|---|---|---|---|---|
| 0.1 | ns | +17.1% | ns | +13.0% |
| 0.2 | ns | +20.0% | ||
| 0.3 | +9.01% | +9.97% | ns | +9.31% |
| 0.4 | ns | +15.8% | −21.4% | −21.4% |
| 0.55 | ns | ns | −55.8% | −51.5% |
Where significant, (p ≤ 0.05 using one-way ANOVA), the percent increase or decrease is indicated, and ns = not significant, p > 0.05 using one-way ANOVA
Stimulation Effects: Inflammatory Cytokines CXCL10 and CCL5
The concentration and time-dependent trends in supernatant and lysate 5-HT concentration of MPMCs exposed to exogenous 5-HT have potential implications for in vivo effects of intercellular 5-HT. Mast cells are a critical cell type in the pathogenesis of inflammatory diseases such as asthma.3 Their involvement in inflammatory diseases is influenced by cell-to-cell communication with surrounding cell types, which release chemokines recently shown to directly induce mast cell degranulation. Mast cells undergoing degranulation in response to IgE-mediated stimulation secrete more 5-HT than mast cells responding to inflammatory chemokines such as CXCL10 or CCL5.11 The trends in Tables 1 and 2 indicate that the cells that surround MPMCs (and potentially mast cells localized to other tissues) will have different rates of uptake or metabolism of 5-HT, and this will likely affect the pathophysiology of surrounding cell response to MPMC secretory behavior following exposure to chemokines vs. allergen.
To further explore MPMC response to stimulation over the course of min-hrs and environmental effects, the long term time-dependent response of MPMCs to inflammatory chemokines was compared to TNP-ova stimulation. Mast cells are most commonly known for their central roles in allergic response, and the TNP-ova models that response. In this case, the MPMCs were incubated overnight with anti-TNP IgE, and then exposing them to TNP-ova induces IgE-mediated degranulation. Although response to allergen stimulation is the most widely known function of mast cells, these cells have diverse and complex roles in a number of inflammatory diseases. By comparing the effects of inflammatory disease-relevant chemokines on MPMCs to the widely studied allergen response of MPMCs, this work attempts to probe the complex response of mast cells to subtle changes in chemical microenvironments. The inflammatory chemokines CXCL10 and CCL5 were selected because of their roles in mediating the cell-to-cell communication processes that give rise to asthma. CXCL10 in particular has a central role in the pathogenesis ofasthma. IgE-sensitized MPMCs were exposed to 0.2–200 ng mL−1 CXCL10 or CCL5, and the 5-HT content of the cell supernatants were measured after 2 hrs of incubation. Direct stimulation of MPMCs was observed at a wide range of concentrations of both CXCL10 and CCL5 (results can be found in Supplementary Information). Based on the results after 2 hrs of exposure to a wide range of concentrations, two concentrations each of CXCL10 and CCL5 were selected for further time-dependent study based on the robustness of the MPMC response following exposure to them.
MPMCs were exposed to CXCL10 at 0.2 and 200 ng mL−1 or CCL5 at 10 and 100 ng mL−1, and supernatants were sampled at 10, 30, 60, and 90 min for 5-HT content measurement. Trends in 5-HT secretion in response to the cytokines are shown in Fig. 4. In contrast to the robust degranulation response that decreased over the course of 90 min following MPMC exposure to TNP-ova, exposing MPMCs to CXCL10 or CCL5 generated a smaller degranulation response with an overall increasing trend in secreted 5-HT over the course of 90 min. Stimulation with CXCL10 induced mast cell degranulation to a lesser extent than TNP-ova stimulation from the same mast cell population, which on average induced a 4.6 times greater secretion of 5-HT than CXCL10. The two stimulant concentrations of CXCL10 also induced different time-dependent trends in 5-HT secretion. Upon stimulation with 0.2 ng mL−1 CXCL10, supernatant 5-HT increased continuously over 90 min. MPMC exposure to 200 ng mL−1 CXCL10 resulted in an initial increase in supernatant 5-HT content at 10 min, followed by a return to control 5-HT levels at 30 min and another increase in 5-HT levels from 30–90 min. Interestingly, MPMC stimulation with CCL5 resulted in similar overall trends to stimulation with CXCL10, but the chemokine concentration dependence was reversed: 5-HT secretion following stimulation with 100 ng mL−1 CCL5 followed the same general trend as 5-HT secretion in response to 0.2 ng mL−1 CXCL10, and stimulation with 10 ng mL−1 CCL5 resulted in similar 5-HT concentration fluctuations as MPMC exposure to 200 ng mL−1 CXCL10. Both CXCL10 and CCL5 are mast cell chemoattractants that induce MPMC degranulation over a wide range of chemokine concentrations. The contrast in temporal response of MPMCs to low and high concentrations of these chemokines highlights the complex role of mast cells in inflammatory diseases. While in vivo mast cells are likely exposed simultaneously to multiple chemokines as they move toward the site of inflammation, examining the response of mast cells to individual mediators of inflammatory diseases can reveal information about MPMC response to different aspects of inflammation.
The response of surrounding cells to different concentrations of exogenous 5-HT is also informative of the diverse role of mast cells in inflammation. TNP-ova stimulation of MPMCs generates supernatant 5-HT concentrations of 0.4–0.55 µM, and stimulation with CXCL10 or CCL5 generates supernatant 5-HT concentrations of 0.1–0.2 µM. As the trends in Table 1 highlight, exposing MPMCs to 0.4–0.55 µM 5-HT results in an initial decrease in supernatant 5-HT concentration, likely due to MPMCs and macrophages taking up and/or processing the 5-HT, followed by an increase or no change in the supernatant 5-HT concentration. When the heterogeneous cultures were exposed to lower concentrations of 5-HT, the decrease in supernatant 5-HT concentration was more pronounced and continued for 60 min. When MPMCs are exposed to TNP-ova, the concentrations of secreted 5-HT are high enough that the surrounding cell culture does not process the secreted 5-HT as efficiently as the lower secreted 5-HT concentrations induced by the inflammatory chemokines CXCL10 and CCL5.
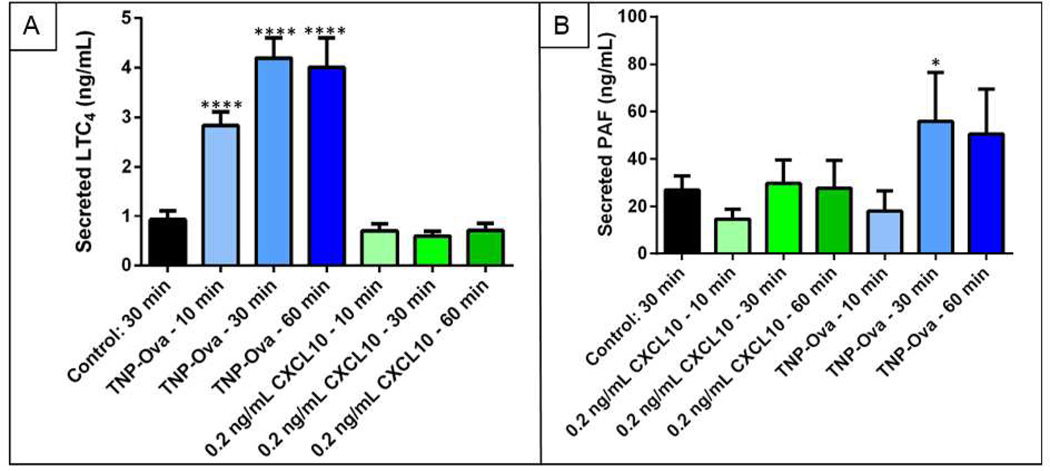
The secretion of 5-HT by mast cells is an important component of understanding mast cell function in both allergic response and inflammatory disease; however, mast cells secrete many other chemical species that contribute to their roles in physiological processes. In asthma, the effect of CXCL10 on mast cells is particularly important,24, 25 and previous studies have shown that CXCL10 potentially activates MPMCs through a different pathway than IgE-mediated allergen stimulation.11 To further examine the effects of stimulation by response to allergen vs. inflammatory chemokines on MPMC function, the secretion of bioactive lipids from MPMCs exposed to 0.2 ng mL−1 CXCL10 or 100 ng mL−1 TNP-ova was also examined. MPMC supernatant concentrations of LTC4 and PAF were determined using UPLC-MS/MS, and results are shown in Fig. 5. As expected, TNP-ova activation of MPMCs induced an increase (vs. unactivated MPMCs) of LTC4 secretion and PAF secretion. CXCL10 exposure, however, did not significantly increase the secretion of LTC4 or PAF (p > 0.05 vs. control MPMCs). In addition to the differences between CXCL10 and TNP-ova-induced degranulation of MPMCs, the mechanism by which CXCL10 activates mast cells likely does not induce bioactive lipid secretion. Further studies on the effects of cell-secreted cytokines are clearly needed to better understand the complex role that mast cell activation plays in inflammatory diseases.
Figure 5.
MPMC secretion of the bioactive lipids A. LTC4 and B. PAF in response to TNP-ova or 0.2 ng mL−1 CXCL10. *p = 0.02; ****p < 0.0001.
Conclusions
In this work, MPMC response is shown to be highly regulated and responsive to subtle alterations in a complex environment through time and concentration dependent degranulation and bioactive lipid formation. These results highlight the importance of selecting an appropriate mast cell model when studying mast cell involvement in allergic response and inflammation. This work probed both the effects of 5-HT concentration and time on surrounding cells in culture with MPMCs as well as mast cell response to different stimuli. The MPMC co-culture system actively responds to the presence of 5-HT, which in vivo may correspond to the ability of cells localized near mast cells to limit the concentration of inflammatory mediators in the extracellular microenvironment. The correlation between the processing of exogenous serotonin and time supports that heterogeneous cellular systems are able to efficiently respond to inflammation. When attempting to apply in vitro mast cell results to in vivo systems, considering the environment that the mast cell is experiencing is important for understanding mast cell contribution to inflammation.
MPMCs exhibited a controlled release dependence when exposed to different agents of mast cell activation. The relatively small degree of degranulation of MPMCs in response to the cytokines CXCL10 and CCL5 in contrast to the robust response of MPMCs to TNP-ova (allergen) are potentially due to the chemoattractant roles of the cytokines. Mast cell contact with chemoattractants in vivo generally occurs when mast cells are localized away from the site of inflammation. Mast cell secretion of small amounts of serotonin and other granule-associated mediators increases vascular dilation, which allows chemoattractants including CXCL10 and CCL5 to enable mast cells to move more efficiently to the site of inflammation.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to acknowledge funding from the National Institutes of Health (DP2 OD004258-01) as well as B. Meyer and B. Petkov for their work on flow cytometry of the peritoneal cell culture.
ABBREVIATIONS
- MPMC
murine peritoneal mast cell
- TNP-ova
trinitrophenol
- 5-HT, serotonin
5-hydroxytryptamine
- LT
leukotriene
- PAF
platelet-activating factor
- PG
prostaglandin
Footnotes
ASSOCIATED CONTENT
Supporting Information
Supporting Information contains a sample HPLC chromatogram, CXCL10 and CCL5 preliminary data, and β-Hex data for MPMCs incubated with exogenous 5-HT. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interests.
REFERENCES
- 1.Page S, Ammit AJ, Black JL, Armour CL. Human mast cell and airway smooth muscle cell interactions: implications for asthma. Am. J. Physiol Lung Cell Mol Physiol. 2001;281:L1313–L1323. doi: 10.1152/ajplung.2001.281.6.L1313. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol. Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 3.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang BD, Asadi S, Vasiadi M, Weng ZY, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim. Biophys. Acta, Mol. Basis Dis. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Neuroendocrine and Immune Crosstalk. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 6.Guhl S, Babina M, Neou A, Zuberbier T, Artuc M. Mast cell lines HMC-1 and LAD2 in comparison with mature human skin mast cells - drastically reduced levels of tryptase and chymase in mast cell lines. Exp. Dermatol. 2010;19:845–847. doi: 10.1111/j.1600-0625.2010.01103.x. [DOI] [PubMed] [Google Scholar]
- 7.Passante E, Ehrhardt C, Sheridan H, Frankish N. RBL-2H3 cells are an imprecise model for mast cell mediator release. Inflammation Res. 2009;58:611–618. doi: 10.1007/s00011-009-0028-4. [DOI] [PubMed] [Google Scholar]
- 8.Passante E, Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflammation Res. 2009;58:737–745. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- 9.Marquis BJ, Haynes CL. The effects of co-culture of fibroblasts on mast cell exocytotic release characteristics as evaluated by carbon-fiber microelectrode amperometry. Biophys. Chem. 2008;137:63–69. doi: 10.1016/j.bpc.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J. Visualized Exp. 2010;35 doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning BM, Meyer AF, Gruba SM, Haynes CL. Single cell analysis of mast cell exocytosis reveals direct induction of mast cell degranulation by the airway smooth muscle-associated chemokines CXCL10 and RANTES. Revision. 2013 [Google Scholar]
- 12.Koseoglu S, Gruba SM, Maurer-Jones MA, Haynes CL. Comparison of platelet delta-granule secretion from different species using single cell measurements. Revision. 2013 [Google Scholar]
- 13.Meyer AF, Thompson JT, Wang Y, Koseoglu S, Haynes CL, Dalluge JJ. Isotope-dilution UPLC-MS/MS determination of mast cell-secreted bioactive lipids. Analyst. 2013;138:5697–5705. doi: 10.1039/c3an00875d. [DOI] [PubMed] [Google Scholar]
- 14.Blank U, Rivera J. Current Protocols in Cell Biology. John Wiley & Sons, Inc.; 2006. Assays for regulated exocytosis of mast cells. [DOI] [PubMed] [Google Scholar]
- 15.Omiatek DM, Dong Y, Heien ML, Ewing AG. Only a fraction of quantal content is released during exocytosis as revealed by electrochemical cytometry of secretory vesicles. ACS Chem. Neurosci. 2010;1:234–245. doi: 10.1021/cn900040e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connell PJ, Wang XB, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudd ML, Nicolas AN, Brown BL, Fischer-Stenger K, Stewart JK. Peritoneal macrophages express the serotonin transporter. J. Neuroimmunol. 2005;159:113–118. doi: 10.1016/j.jneuroim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Ahern GP. 5-HT and the immune system. Curr. Opin. Pharmacol. 2011;11:29–33. doi: 10.1016/j.coph.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn KJ, French PC, Merrills RJ. 5-hydroxytryptamine uptake by rat brain in vitro. Life Sci. 1967;6:1653–1663. doi: 10.1016/0024-3205(67)90176-2. [DOI] [PubMed] [Google Scholar]
- 20.Blakely RD, Berson HE, Fremeau RT, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat-brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- 21.Hagan CE, Schenk JO, Neumaier JF. The Contribution of low-affinity transport mechanisms to serotonin clearance in synaptosomes. Synapse. 2011;65:1015–1023. doi: 10.1002/syn.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neubauer HA, Hansen CG, Wiborg O. Dissection of an allosteric mechanism on the serotonin transporter: A cross-species study. Mol. Pharmacol. 2006;69:1242–1250. doi: 10.1124/mol.105.018507. [DOI] [PubMed] [Google Scholar]
- 23.Daws LC, Toney GM. High-speed chronoamperometry to study kinetics and mechanisms for serotonin clearance in vivo. Electrochem. Methods for Neurosci. 2007;1:63–81. [PubMed] [Google Scholar]
- 24.Bradding P. Asthma: eosinophil disease, mast cell disease, or both? Allergy, Asthma, and Clin. Immunol. 2008;4:84–90. doi: 10.1186/1710-1492-4-2-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughs JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am. J. Respir. and Crit. Care Med. 2005;171:1103–1108. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.