Abstract
Dynorphin (dyn) is suggested to excite the auditory nerve (AN) when released by the lateral olivocochlear (LOC) efferents. However, previous studies evaluated either intravenously delivered dyn-like agents, raising the potential for systemic (central) effects, or agent concentrations unlikely to be achieved via endogenous cochlear release. This study tested the hypothesis that biologically relevant increases in dyn levels in the cochlea achieved via diffusion of the drug of (−)pentazocine across the round window membrane enhances AN firing. In general, amplitude of the cochlear whole-nerve action potential (CAP) was depressed following drug application. These results suggest that dyn released by the LOC neurons would likely act as an inhibitory transmitter substance in the LOC system; neurotransmission is one of the LOC system's vast unknowns.
Keywords: lateral olivocochlear efferent, auditory nerve, cochlear whole nerve action potential, dynorphin, kappa opioid receptor
INTRODUCTION
Dynorphin (dyn) was suggested as a cochlear transmitter after dyn-B detection in homogenized cochlear tissue [15]. Dyn-like immunoreactivity is localized to the lateral superior olive (LSO), origin of lateral olivocochlear (LOC) efferents innervating the cochlea [1, 3], and LOC terminations on auditory nerve (AN) dendrites [2]. Some LSO cell bodies co-localize enkephalin (enk) and dyn [1, 3] or dyn and choline acetyltransferase [ChAT: enzyme responsible for synthesizing acetylcholine (ACh)] [1]. Dyn-B and α-neoendorphin immunostaining are found under the cochlear inner hair cells (IHCs), and in tunnel spiral bundle [TSB, containing LOC neurons traveling from LSO to cochlea] and inner spiral bundle [ISB, composed of both LOC neurons at bases of IHCs and medial (MOC) olivocochlear neurons under outer hair cells (OHCs)] [2, 42–44]. Dyn opioid peptides primarily act as agonists at kappa-opioid receptors (KOR) but also have some affinity for μ-opioid receptors (MOR), which they antagonize. KOR and MOR immunoreactivity have been confirmed in rat [17] and guinea pig [16] cochleae. Unlike the MOC system, very little is known about the LOC system, including LOC neurotransmission.
Dyn-like substances delivered intravenously have robust effects on sound-evoked neural potentials. Lower (better) sound-evoked compound-action-potential (CAP) thresholds and enhanced (larger) CAP amplitude were observed for stimuli at 0–20 dB sensation level (SL) after 8–16 mg/kg (−)pentazocine or 20 mg/kg U50488 delivered intravenously (but not 6 mg/kg U50488) [34, 37]. Excitation was attributed to KOR binding in the cochlea as selective KOR antagonists naloxone [applied intravenously, see 35] and norbinaltorphimine (norBNI) [applied to the round window membrane (RWM), see 38] blocked these effects.
When 50-mM U50488 was applied to the RWM to eliminate potential systemic effects, CAP amplitude increased at 0–10 dB SL but decreased at 20–40 dB SL [39]. “Biphasic” effects were replicated using 50-mM (−)pentazocine and 100-mM U50488 [32]; however, high drug concentrations may induce non-selective effects. Endogenous cochlear opioid release is suggested to achieve nanomolar, or perhaps micromolar, concentrations [28]. Sharply contrasting with this, 50-mM (−) U50488 applied to chinchilla RWM might produce 1.1 millimolar cochlear drug concentrations [Washington University Cochlear Fluids Simulator (version 1.6i, December 2005; http://oto.wustl.edu/cochlea/)]. The current study assessed whether micromolar cochlear dosing of (−)pentazocine mediates AN activity.
METHODS
Subjects
Male and female pigmented guinea pigs (Elm Hill Breeding Labs, Chelmsford, MA) were maintained with free access to food (Guinea Pig Chow, PMI Nutrition International Inc., Brentwood, MO) and water (n=11). Animal weights were ~250–300 grams on arrival and ~350–500 grams at study entry. The animal care program was AALAC accredited. Husbandry met or exceeded all applicable standards; the University (of Michigan) Committee on Use and Care of Animals approved all protocols.
Surgical Procedure
Guinea pigs were anesthetized (108 mg/kg ketamine, 14 mg/kg xylazine) and the cochlea exposed via post-auricular incision. A platinum-iridium-wire ballelectrode (diameter=0.2–0.25 mm) was placed through the wall of the cochlea into scala tympani. A silastic bead 0.5-mm distal to the end of the electrode prevented over-insertion and cochlear fluid loss [as in 23]. The electrode was secured temporarily using cyanoacrylate (VetBond) at the bulla, then cemented securely to the bulla (Durelon). CAP was assessed immediately after securing the electrode (“Baseline”), and 30-min after application of artificial perilymph (AP) (145-mM NaCl, 2.7-mM KCl, 2.0-mM MgSO4, 1.2-mM CaCl2, 5.0-mM HEPES; pH=7.40, osmolality=280–285 mOsm) or increasing concentrations (1.0, 5.0 mM) of (−)pentazocine (Sigma #P-134, CAS 66429-56-9) to the RWM. (−)Pentazocine was initially dissolved in a small volume of dilute (0.1M) hydrochloric acid then brought to the appropriate concentration using AP (final pH=6.73–7.08). RWM applications were ~6-µl, and the middle ear was carefully dried prior to CAP tests using fine tip cotton points.
CAP
Acoustic stimuli were tone-pips from 0 to 100-dB SPL (5-dB increments, 5-msec duration, 0.5-msec rise-fall; 10/sec). Control subjects receiving AP were tested from 2–18 kHz in 2-kHz increments (nominally 9-minutes); (−)pentazocine subjects were tested at 2, 4, 8, and 16 kHz (nominally 4-minutes). Signals were generated using Tucker-Davis Technology (TDT; Alachua, FL) hardware and SigGen32 software. Signals were converted to analog (DA1), filtered (FT6-2, Fc=40 kHz), attenuated (PA5), and presented using a 200-Ohm transducer (Beyer Dynamic, Farmingdale, NY) coupled to the ear canal via vinyl tubing. Cochlear potentials were filtered (300–3000 Hz) and amplified (1000×) using a Grass P55 amplifier. BioSig32 was used to average 25 evoked responses per frequency/level combination. CAP threshold was defined as the level producing a 10-µV response using linear interpolation. All subjects were required to be within 2-SD of the mean for the population of animals tested in this laboratory previously, for both threshold and amplitude, at baseline.
Statistical analysis
All values are mean ±S.D.; statistical reliability of group differences was analyzed via ANOVA using SPSS 13.0. Statistical reliability of drug effects was evaluated at each frequency using mixed-effects ANOVA for the between-subjects condition (baseline, 1-mM, 5-mM) and within-subjects effect of stimulus level (0, 5, 10, …, and 100-dB SPL). Greenhouse-Geisser corrections adjusted for sphericity violations; Bonferroni corrections were applied for multiple comparisons.
RESULTS
CAP Threshold
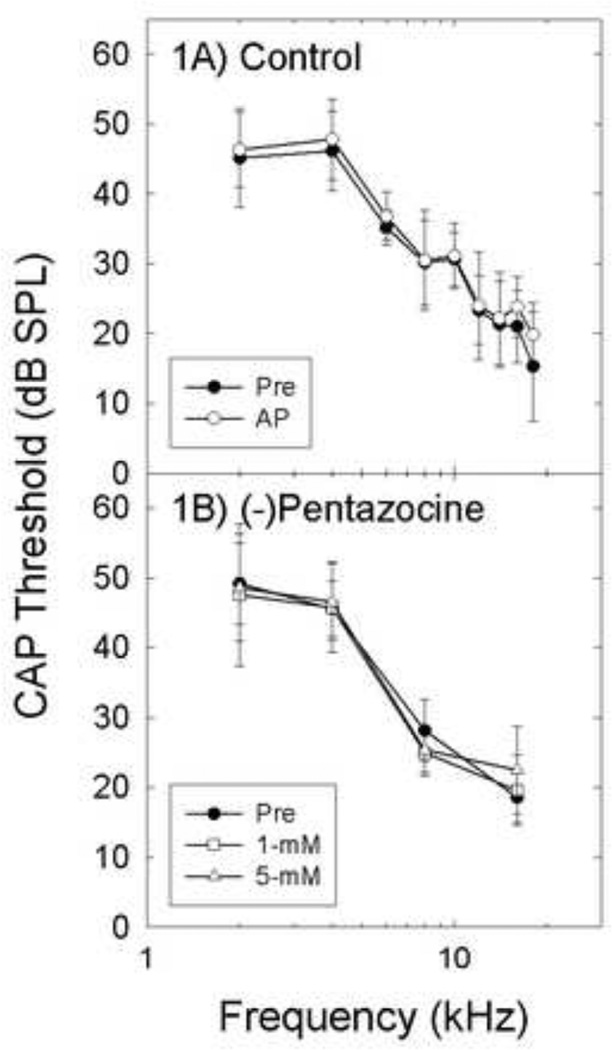
Threshold sensitivity was not reliably affected by AP (Figure 1A) or 1- or 5-mM (−)pentazocine (Figure 1B). There was no statistically reliable difference between Baseline versus AP (F=1.008, df=1, 7, p=0.349) or Baseline vs 1- or 5-mM (−)pentazocine (F=1.813, df=1.775, 7.098, p=0.230).
Figure 1.
There were no statistically reliable changes in CAP threshold (mean± S.D.) after artificial perilymph (AP) control (1A, n=6), or (−)pentazocine (1B, n=5).
CAP Amplitude (dB SPL)
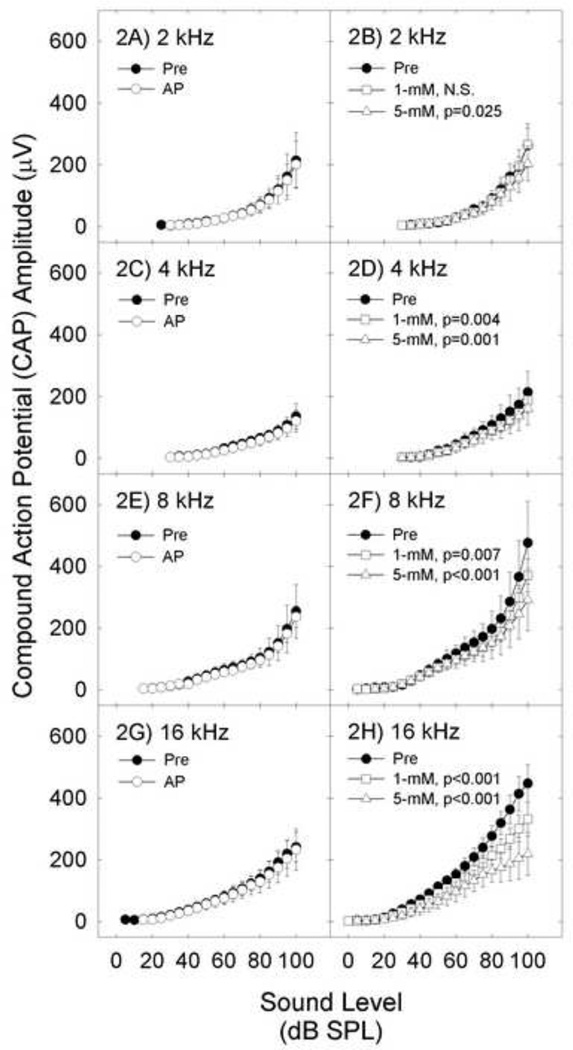
Both 1- and 5-mM (−)pentazocine depressed CAP amplitude (Figure 2). All pair-wise comparisons between drugs and baseline were statistically significant after Bonferroni corrections, except Baseline vs 1-mM (−)pentazocine at 2-kHz. Drug effects were most robust at higher frequencies [expected with RWM delivery, see 6] and higher sound levels [Treatment×Level interaction significant for pre vs 5-mM at 8- and 16-kHz (all p’s<0.05)]. In contrast to (−)pentazocine, AP did not affect CAP amplitude (all p’s >0.05). Although thresholds were equivalent for the groups treated with AP and (−)pentazocine, average CAP amplitude growth was smaller in the AP group. When animals matched for baseline amplitude were individually examined from each group, there was no effect of AP on CAP amplitude, and there was a dose-dependent effect of (−)pentazocine on CAP amplitude, consistent with group results.
Figure 2.
Sound-evoked cochlear action potential (CAP) amplitude (mean+/−S.D.) is depicted immediately after cementing recording electrode in place (Pre), and 30-min after applying test agents. CAP amplitude was not affected by artificial perilymph (AP) (2A, 2C, 2E, and 2G). Responses were reliably depressed by 1- and 5-mM (−)pentazocine (2B, 2D, 2F, and 2H). Effects were dose-dependent and frequency-specific.
CAP Amplitude (dB SL)
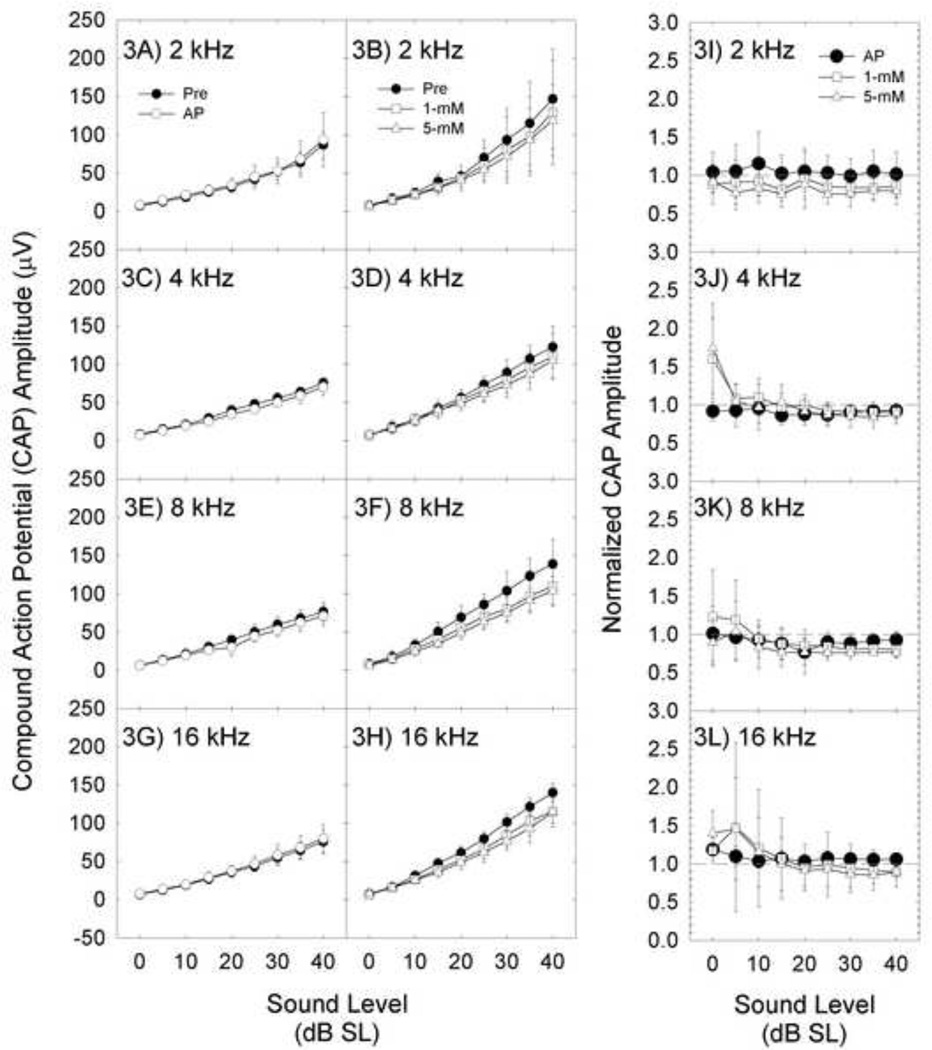
CAP amplitude measured within tests was normalized to threshold; 0-dB SL was defined as the sound level yielding a 10-µV CAP amplitude during that level series. There was no effect of AP at any frequency. CAP amplitude appeared depressed after (−)pentazocine; however, the difference between pre- and post-drug CAP amplitude was not statistically significant (all p’s>0.10) (Figures 3A–3H). The negative outcome should be interpreted cautiously; achieved power was less than 0.2 at 2-, 4-, and 16-kHz. The highest power was at 8-kHz (power=0.425, p-value=0.11).
Figure 3.
CAP amplitude was expressed as dB sensation level (dB SL) from 0–40 dB SL. CAP amplitude (mean+/−S.D.) is depicted immediately after cementing the recording electrode in place (Pre), and 30-min after applying artificial perilymph (AP) or increasing concentrations of (−)pentazocine (1-, 5-mM) to the round window membrane. Response amplitude was not affected by AP (3A, 3C, 3E, and 3G). Although response amplitude seemingly decreased after (−)pentazocine (3B, 3D, 3F, and 3H), the effect was not statistically reliable. To be consistent with other reports, we normalized AP and (−)pentazocine CAP functions to baseline amplitude (3I, 3J, 3K, 3L). Normalized amplitude of 1.0 (dashed-line) represents no change in CAP amplitude post-treatment; normalized amplitude of 0.5 represents CAP amplitude that is 50% of baseline. There were no statistically reliable changes in amplitude as a function of treatment.
In earlier studies reporting excitatory [34, 37] or biphasic [32, 39] changes in CAP amplitude, post-drug CAP amplitudes were normalized using a single pre-drug threshold as 0-dB SL. In a final analysis, we normalized post-drug CAP amplitude data using pre-drug baseline as in earlier reports (Figure 3I–3L). There was a trend towards increased CAP amplitude at/near threshold, particularly at 4-kHz, but the effect varied across subjects and was not statistically reliable. There was little or no decrease in CAP amplitude as SL increased up to 40-dB SL. Thus, the current data did not show reliable excitation, or biphasic effects, for 1- and 5-mM concentrations of (−)pentazocine on the RWM.
DISCUSSION
Effects of dyn agonists on auditory nerve activity
The LOC transmitter dyn has the potential to depress AN activity during endogenous transmitter release. We report that RWM application of ligands at concentrations that might be achieved via endogenous dyn release depressed sound-driven AN activity at the highest test frequencies. The lack of effect at 2-kHz likely reflects minimal drug distribution to this region [based on 41]. Effects were robust when CAP amplitude was evaluated strictly as a function of sound level (dB-SPL). When neural response amplitude was normalized (dB-SL), statistical reliability was reduced, although there was still a downwards trend with CAP amplitude appearing reduced after (−)pentazocine. The observed effects are not unique to (−)pentazocine. Dose-dependent CAP amplitude depression was described when U-62066 (Spiradoline; 1-, 2.5-, 5-, or 10-mM) and U50488 (1-, 2.5-, and 5-mM) were applied to the RWM in smaller groups of animals [22]. U62066 and U50488 are highly selective KOR agonists without MOR antagonist effects.
Biological relevance
Lioudyno et al. [28] have shown that micromolar concentrations of endomorphin-1 and dynorphin-B inhibit ACh-evoked cell responses in oocytes and cochlear cell cultures. The actual concentrations achieved in each tonotopic location of the cochlea at the specific time each frequency was tested cannot be determined based on unknown RWM permeability, scala tympani drug elimination constants, and drug entry through both the RWM and the stapes [40, 41]. Based on the predicted concentrations calculated using a variety of biologically plausible assumptions in the models submitted to the Washington University Cochlear Fluids Simulator, the 5-mM (−)pentazocine solutions on the RWM of the guinea pig cochlea concentrations might be on the order of 150-µM at/near 16-kHz and 30-µM at/near 8- kHz, with minimal (<5-µM) diffusion reaching regions at/near 4-kHz, and closer to the apex. These concentrations better approximate the micromolar levels suggested by Lioudyno et al. [28], although the effects of (−)pentazocine may not be identical to those of endogenous dyn peptides.
Biphasic Dose Effects?
Threshold improved and evoked potential amplitude increased for 0–20 dB SL clicks after intravenous 8–20 mg/kg (−)pentazocine or U50488 [34, 37], with no changes in amplitude at 40-dB SL [36]. With 100-mM U50488 applied to the RWM, effects were biphasic: amplitude increased 200% for 0–10 dB SL clicks, with 50% decreases in amplitude for 20–40 dB SL clicks [see 32, 39]. With application of significantly lower concentrations of (−)pentazocine to the RWM here, CAP amplitude appeared to be enhanced up to 50% for 0–5 dB SL signals (at 16-kHz), with responses reduced some 10–20% for 10–40 dB SL signals (Figure 3I–3L), but those results were not statistically different from AP control. To draw conclusions at near-threshold stimulus levels, a greater number of averages may be required. In future investigations, it would be worthwhile to include higher doses as tested by others, as well as lower more biologically relevant concentrations as used here. Taken together, the main effect of the current manipulation was reduction of CAP amplitude as sound level increased (Figure 2A–2D). Higher intra-cochlear concentrations of (−)pentazocine and other more selective KOR-agonists may indeed produce excitatory and/or biphasic effects as described previously. However, the current data reflect down-regulation of AN responses to higher-level sound stimulation to be the only reliable effect of (−)pentazocine on sound-evoked AN responses.
Implications for LOC modulation of auditory nerve activity
The current data show inhibitory effects of (−)pentazocine on sound-evoked AN activity. While this report focused selectively on effects of this dyn-like ligand, immunocytochemical labeling studies clearly reveal LOC neurons are chemically rich, with co-localized transmitters [10]. They contain ACh, dopamine (DA), enk, γ-aminobutyric acid (GABA), and calcitonin-gene-related-peptide (CGRP) [for reviews, see 20, 21, 31]. DA agonist infusion decreases AN single-unit driven rates [29, 30], AN whole-nerve response [7, 8, 30], and spontaneous AN firing (according to [30], but not [29]). These effects are increasingly understood to vary with action at specific receptor subtypes, with D1/5 agonists suppressing CAP amplitude, but no effect of D2 and D3 agonists on CAP amplitude [12]. Because a D2 antagonist suppressed CAP amplitude, tonic endogenous DA release has been suggested [12]. Limited results are also available for enk and GABA agonists. Microiontophoresis of enk strongly depressed AN firing induced by α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), kainate (KA), and, to a lesser extent, N-methyl-Daspartate [NMDA, see 5]. Microiontophoresis of GABA had little effect on spontaneous AN firing, but inhibited glutamate-induced activity [11]. Finally, the GABAA agonist muscimol, but not the GABAB agonist baclofen, reduced NMDA- and AMPA-induced neural activity [4]. Taken together, DA [7, 8, 12, 30], enk [5], GABA [4, 11], and now dyn [in the present data set] have all inhibited sound-evoked AN activity.
These inhibitory effects of LOC transmitters are notably consistent with data from the mouse, where LSO lesions enhanced sound-evoked AN activity; an effect that is consistent with disruption to inhibitory neurotransmitter systems [9]. These sharply contrast with the observed effects of LSO lesions and LOC damage in cats, chinchillas, and guinea pigs, however. Cutting the combined LOC and MOC pathways, which travel together in the olivocochlear bundle (OCB), decreased spontaneous AN activity in cats and chinchillas [25, 45]. Similarly, selective LOC disruption decreased CAP amplitude in guinea pigs [23, 24]. Species differences may explain some of the observed differences [19], and the relative placement of lesions may also influence the outcome, based on data from electrical stimulation studies which showed either excitation or inhibition depending on location [13]. In contrast to the MOC system, there are clearly major gaps remaining in our understanding of the LOC system. Level-dependent effects of the LOC system also remain poorly understood. The current data show seemingly more robust effects of dyn agonists on AN responses at higher stimulus levels, although previous data suggest effects of LOC damage are not level-dependent [23]. Effects at higher levels are relatively well-explained anatomically. Most LOC fibers synapse on afferent fibers on the modiolar side of IHCs, and fewer synapse on afferents on the pillar side [27]. High-threshold, low-SR afferent fibers connect to the modiolar side of the IHCs, while low-threshold, high-SR afferent fibers connect to the pillar side [26]. A greater number of LOC synapses on highthreshold, low-SR fibers may provide a mechanistic explanation for larger effect at higher SPLs. It is of interest that Guinan and Stankovic [14] previously reported greater MOC effects on high threshold, low-SR fibers, an effect for which there is no accepted mechanistic explanation as these data do not fit with the known effects of MOC inhibition of basilar-membrane motion.
Summary and Conclusions
The current data emphasize the importance of using biologically plausible concentrations of active experimental agents when assessing the role of a chemical in normal biological function. Although data from studies using high drug concentrations suggest dyn is an LOC excitatory substance [32, 34, 35, 37–39], dyn was inhibitory when delivered at biologically plausible concentrations. Based on reports that dyn has excitatory effects in the inner ear, loss of endogenous dyn was suggested to contribute to the net depression of spontaneous and sound-driven AN activity after LOC disruption [23–25, 45]. This interpretation is not supported by the current data. Based on excitatory action, dyn has also been proposed as underlying human tinnitus; i.e., the perception of “phantom sound” [29, 33, 36, 39]. The current data instead suggest endogenous dyn would in fact down-regulate afferent response. Interestingly, this does not negate the possibility that endogenous dyn might influence tinnitus, as decreased peripheral input has been suggested to underlie tinnitus [18]. Although the current studies do not confirm the conditions under which dyn is in fact released endogenously (i.e., tonically, or in response to sound), the effects obtained using biologically relevant micromolar drug levels are clearly inhibitory in nature.
Highlights.
The kappa opioid receptor agonist (−) pentazocine was applied to the cochlea.
Sound evoked neural potentials were reduced in amplitude after drug treatment.
Endogenous dynorphin at biologically plausible levels has an inhibitory effect.
Higher (nonbiological) concentrations of drugs excite the auditory nerve.
Transmitter effects should be assessed at biologically plausible concentrations.
ACKNOWLEDGEMENTS
C. G. L. contributed to study design, collected data, and had primary responsibility for manuscript preparation; L. F. H. completed and interpreted all statistical analyses and contributed to manuscript; S. C. B. contributed to study design, interpretation of data, and contributed to manuscript. Financial support was provided by NIH-NIDCD grants R03-DC007342 (CGL), P01-DC00078 (SCB), and P30-DC05188. We thank K. Halsey, R. Chavez and J. McLaren for technical help, and D. Dolan for comments on an earlier manuscript. We are grateful to A. Salt for modeling drug diffusion in the cochlea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Abou-Madi L, Pontarotti P, Tramu G, Cupo A, Eybalin M. Coexistence of putative neuroactive substances in lateral olivocochlear neurons of rat and guinea pig. Hear. Res. 1987;30:135–146. doi: 10.1016/0378-5955(87)90131-6. [DOI] [PubMed] [Google Scholar]
- 2.Altschuler RA, Hoffman DW, Reeks KA, Fex J. Localization of dynorphin B-like and alpha-neoendorphin-like immunoreactivities in the guinea pig organ of Corti. Hear. Res. 1985;17:249–258. doi: 10.1016/0378-5955(85)90069-3. [DOI] [PubMed] [Google Scholar]
- 3.Altschuler RA, Reeks KA, Fex J, Hoffman DW. Lateral olivocochlear neurons contain both enkephalin and dynorphin immunoreactivities: immunocytochemical co-localization studies. J. Histochem. Cytochem. 1988;36:797–801. doi: 10.1177/36.7.2898496. [DOI] [PubMed] [Google Scholar]
- 4.Arnold T, Oestreicher E, Ehrenberger K, Felix D. GABA(A) receptor modulates the activity of inner hair cell afferents in guinea pig cochlea. Hear. Res. 1998;125:147–153. doi: 10.1016/s0378-5955(98)00144-0. [DOI] [PubMed] [Google Scholar]
- 5.Burki C, Felix D, Ehrenberger K. Enkephalin suppresses afferent cochlear neurotransmission. ORL. J. Otorhinolaryngol. Relat. Spec. 1993;55:3–6. doi: 10.1159/000276344. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Kujawa SG, McKenna MJ, Fiering JO, Mescher MJ, Borenstein JT, Swan EE, Sewell WF. Inner ear drug delivery via a reciprocating perfusion system in the guinea pig. Journal of controlled release : official journal of the Controlled Release Society. 2005;110:1–19. doi: 10.1016/j.jconrel.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.d'Aldin C, Eybalin M, Puel JL, Charachon G, Ladrech S, Renard N, Pujol R. Synaptic connections and putative functions of the dopaminergic innervation of the guinea pig cochlea. Eur. Arch. Otorhinolaryngol. 1995;252:270–274. doi: 10.1007/BF00185388. [DOI] [PubMed] [Google Scholar]
- 8.d'Aldin C, Puel JL, Leducq R, Crambes O, Eybalin M, Pujol R. Effects of a dopaminergic agonist in the guinea pig cochlea. Hear. Res. 1995;90:202–211. doi: 10.1016/0378-5955(95)00167-5. [DOI] [PubMed] [Google Scholar]
- 9.Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J. Neurophysiol. 2007;97:1775–1785. doi: 10.1152/jn.00955.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darrow KN, Simons EJ, Dodds LW, Liberman MC. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J. Comp. Neurol. 2006;498:403–414. doi: 10.1002/cne.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felix D, Ehrenberger K. The efferent modulation of mammalian inner hair cell afferents. Hear. Res. 1992;64:1–5. doi: 10.1016/0378-5955(92)90163-h. [DOI] [PubMed] [Google Scholar]
- 12.Garrett AR, Robertson D, Sellick PM, Mulders WH. The actions of dopamine receptors in the guinea pig cochlea. Audiol. Neurootol. 2011;16:145–157. doi: 10.1159/000316674. [DOI] [PubMed] [Google Scholar]
- 13.Groff JA, Liberman MC. Modulation of cochlear afferent response by the lateral olivocochlear system: activation via electrical stimulation of the inferior colliculus. J. Neurophysiol. 2003;90:3178–3200. doi: 10.1152/jn.00537.2003. [DOI] [PubMed] [Google Scholar]
- 14.Guinan JJ, Jr, Stankovic KM. Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J. Acoust. Soc. Am. 1996;100:1680–1690. doi: 10.1121/1.416066. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman DW, Zamir N, Rubio JA, Altschuler RA, Fex J. Proenkephalin and prodynorphin related neuropeptides in the cochlea. Hear. Res. 1985;17:47–50. doi: 10.1016/0378-5955(85)90129-7. [DOI] [PubMed] [Google Scholar]
- 16.Jongkamonwiwat N, Phansuwan-Pujito P, Casalotti SO, Forge A, Dodson H, Govitrapong P. The existence of opioid receptors in the cochlea of guinea pigs. Eur. J. Neurosci. 2006;23:2701–2711. doi: 10.1111/j.1460-9568.2006.04810.x. [DOI] [PubMed] [Google Scholar]
- 17.Jongkamonwiwat N, Phansuwan-Pujito P, Sarapoke P, Chetsawang B, Casalotti SO, Forge A, Dodson H, Govitrapong P. The presence of opioid receptors in rat inner ear. Hear. Res. 2003;181:85–93. doi: 10.1016/s0378-5955(03)00175-8. [DOI] [PubMed] [Google Scholar]
- 18.Kaltenbach JA. Tinnitus: Models and mechanisms. Hear. Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Prell CG. A role for the lateral olivocochlear neurons in auditory function. Focus on “Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury”. J. Neurophysiol. 2007;97:963–965. doi: 10.1152/jn.01223.2006. [DOI] [PubMed] [Google Scholar]
- 20.Le Prell CG, Bledsoe SC, Jr, Bobbin RP, Puel JL. In: Neurotransmission in the inner ear: Functional and molecular analyses. Jahn AF, Santos-Sacchi J, editors. New York: Physiology of the Ear, Singular Publishing; 2001. pp. 575–611. [Google Scholar]
- 21.Le Prell CG, Dolan DF, Schacht J, Miller JM, Lomax MI, Altschuler RA. Pathways for protection from noise-induced hearing loss. Noise Health. 2003;5:1–17. [PubMed] [Google Scholar]
- 22.Le Prell CG, Halsey K, Dolan DF, Bledsoe SCJ. Kappa opioid-receptor agonists depress compound action potential amplitude. Abs. Assoc. Res. Otolaryngol. 2004;27:329–330. [Google Scholar]
- 23.Le Prell CG, Halsey K, Hughes LF, Dolan DF, Bledsoe SC., Jr Disruption of lateral olivocochlear neurons via a dopaminergic neurotoxin depresses sound-evoked auditory nerve activity. J. Assoc. Res. Otolaryngol. 2005;6:48–62. doi: 10.1007/s10162-004-5009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Prell CG, Shore SE, Hughes LF, Bledsoe SC., Jr Disruption of lateral efferent pathways: Functional changes in auditory evoked responses. J. Assoc. Res. Otolaryngol. 2003;4:276–290. doi: 10.1007/s10162-002-3018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberman MC. Effects of chronic cochlear de-efferentation on auditory-nerve response. Hear. Res. 1990;49:209–223. doi: 10.1016/0378-5955(90)90105-x. [DOI] [PubMed] [Google Scholar]
- 26.Liberman MC. Single-neuron labeling in the cat auditory nerve. Science. 1982;216:1239–1241. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- 27.Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J. Comp. Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- 28.Lioudyno MI, Verbitsky M, Glowatzki E, Holt JC, Boulter J, Zadina JE, Elgoyhen AB, Guth PS. The alpha9/alpha10-containing nicotinic ACh receptor is directly modulated by opioid peptides, endomorphin-1,a nd dynorphin B, proposed efferent cotransmitters in the inner ear. Mol. Cell. Neurosci. 2002;20:695–711. doi: 10.1006/mcne.2002.1150. [DOI] [PubMed] [Google Scholar]
- 29.Oestreicher E, Arnold W, Ehrenberger K, Felix D. Dopamine regulates the glutamatergic inner hair cell activity in guinea pigs. Hear. Res. 1997;107:46–52. doi: 10.1016/s0378-5955(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 30.Ruel J, Nouvian R, Gervais d'Aldin C, Pujol R, Eybalin M, Puel JL. Dopamine inhibition of auditory nerve activity in the adult mammalian cochlea. Eur. J. Neurosci. 2001;14:977–986. doi: 10.1046/j.0953-816x.2001.01721.x. [DOI] [PubMed] [Google Scholar]
- 31.Ruel J, Wang J, Rebillard G, Eybalin M, Lloyd R, Pujol R, Puel JL. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear. Res. 2007;227:19–27. doi: 10.1016/j.heares.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Sahley TL, Anderson DJ, Chernicky CL. Bi-phasic intensity-dependent opioid-mediated neural amplitude changes in the chinchilla cochlea: partial blockade by an N-Methyl-D-Aspartate (NMDA)-receptor antagonist. Eur. J. Pharmacol. 2008;580:100–115. doi: 10.1016/j.ejphar.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahley TL, Hammonds MD, Musiek FE. Endogenous dynorphins, glutamate and N-methyl-d-aspartate (NMDA) receptors may participate in a stress-mediated Type-I auditory neural exacerbation of tinnitus. Brain Res. 2013;1499:80–108. doi: 10.1016/j.brainres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Sahley TL, Kalish RB, Musiek FE, Hoffman DW. Effects of opioid be drugs on auditory evoked potentials suggest a role of lateral olivocochlear dynorphins in auditory function. Hear. Res. 1991;55:133–142. doi: 10.1016/0378-5955(91)90099-u. [DOI] [PubMed] [Google Scholar]
- 35.Sahley TL, Musiek FE, Nodar RH. Naloxone blockade of (−)pentazocine-induced changes in auditory function. Ear Hear. 1996;17:341–353. doi: 10.1097/00003446-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Sahley TL, Nodar RH. A biochemical model of peripheral tinnitus. Hear. Res. 2001;152:43–54. doi: 10.1016/s0378-5955(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 37.Sahley TL, Nodar RH. Improvement in auditory function following pentazocine suggests a role for dynorphins in auditory sensitivity. Ear Hear. 1994;15:422–431. doi: 10.1097/00003446-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Sahley TL, Nodar RH, Musiek FE. Blockade of opioid-induced changes in auditory function at the level of the cochlea. Ear Hear. 1996;17:552–558. doi: 10.1097/00003446-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Sahley TL, Nodar RH, Musiek FE. Endogenous dynorphins: Possible role in peripheral tinnitus. Int. Tinnitus J. 1999;5:76–91. [PubMed] [Google Scholar]
- 40.Salt AN, King EB, Hartsock JJ, Gill RM, O'Leary SJ. Marker entry into vestibular perilymph via the stapes following applications to the round window niche of guinea pigs. Hear. Res. 2012;283:14–23. doi: 10.1016/j.heares.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear. Res. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 42.Satake M, Liberman MC. Morphological subclasses of lateral olivocochlear terminals? Ultrastructural analysis of inner spiral bundle in cat and guinea pig. J. Comp. Neurol. 1996;371:621–632. doi: 10.1002/(SICI)1096-9861(19960805)371:4<621::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 43.Warr WB. Organization of olivocochlear efferent systems in mammals. In: Webster DB, Popper AN, Fay RR, editors. Mammalian Auditory Pathway. Boston: Neuroanatomy, Little, Brown, and Co.; 1992. pp. 410–448. [Google Scholar]
- 44.Warr WB, Guinan JJ, White JS. Organization of efferent fibers: The lateral and medial olivocochlear systems. In: Altschuler RA, Hoffman DW, Bobbin RP, editors. Neurobiology of Hearing. New York: The Cochlea, Raven Press; 1986. pp. 333–348. [Google Scholar]
- 45.Zheng XY, Henderson D, McFadden SL, Ding DL, Salvi RJ. Auditory nerve fiber responses following chronic cochlear de-efferentation. J. Comp. Neurol. 1999;406:72–86. doi: 10.1002/(sici)1096-9861(19990329)406:1<72::aid-cne5>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]