Abstract
Excessive activation of glutamate receptors in spinal dorsal horn neurons is a key mechanism leading to abnormal neuronal activation in pathological pain conditions. Previous studies have shown that activation of glutamate receptors in the spinal dorsal horn is enhanced by impaired glial glutamate transporter functions and pro-inflammatory cytokines including interleukin-1 beta (IL-1β). In this study, we for the first time revealed that spinal glial glutamate transporter activities in the neuropathic animals are attenuated by endogenous IL-1β. Specifically, we demonstrated that nerve injury results in an increased expression of IL-1β and activation of PKC in the spinal dorsal horn as well as suppression of glial glutamate uptake activities. We provided evidence that the nerve-injury induced suppression of glial glutamate uptake is at least in part ascribed to endogenous IL-1β and activation of PKC in the spinal dorsal horn. IL-1β reduces glial glutamate transporter activities through enhancing the endocytosis of both GLT-1 and GLAST glial glutamate transporters. The IL-1β induced trafficking of glial glutamate transporters is through the calcium/PKC signaling pathway, and the dynamin-dependent endocytosis, which is dependent on the integrity of actin filaments. The signaling pathway regulating glial glutamate transporters revealed in this study provides novel targets to attenuate aberrant activation of glutamate receptors in the spinal dorsal horn, which could ultimately help the development of analgesics.
Introduction
Interactions between neurons and glial cells are crucial mechanisms underlying synaptic plasticity in the spinal dorsal horn in pathological pain conditions (Ren and Dubner, 2010, Chen et al., 2012, Kanda et al., 2013, Tsuda et al., 2013, Grace et al., 2014). Excessive activation of glutamate receptors in spinal dorsal horn neurons is a key mechanism leading to abnormal neuronal activation in the pain signaling system (Moore et al., 2000, Salter, 2004, Nie and Weng, 2010). Previous studies have shown that glial cells can enhance activation of glutamate receptors in the spinal dorsal horn by reducing glial glutamate transporter functions (Sung et al., 2003, Weng et al., 2006, Nie and Weng, 2010, Weng et al., 2014) and releasing pro-inflammatory cytokines including interleukin-1beta (IL-1β) (Sweitzer et al., 1999, Yan and Weng, 2013). Currently, mechanisms leading to reduced glial glutamate transporter functions and molecular mechanism by which IL-1β alters synaptic transmission in the spinal dorsal horn remain obscure. Two types of glial glutamate transporters (GLAST and GLT-1 located in astrocytes) and one neuronal glutamate transporters (EAAC1) exist in the spinal dorsal horn (Furuta et al., 1997, Mao et al., 2002, Weng et al., 2005). Downregulation of glial glutamate transporter protein expression in the spinal dorsal horn is associated with hyperalgesia induced by chronic nerve injury (Sung et al., 2003, Nie and Weng, 2010, Weng et al., 2014), chemotherapy (Weng et al., 2005, Doyle et al., 2012, Gao et al., 2013), and opioids (Mao et al., 2002). We and others have shown that pharmacological inhibition of glial glutamate transporters in the spinal dorsal horn makes animals hypersensitive to peripheral stimulation (Liaw et al., 2005, Weng et al., 2006). Deficient glial glutamate uptake enhances activation of AMPA and NMDA glutamate receptors, and causes glutamate to spill to the extrasynaptic space and activation of extrasynaptic NMDA receptors in spinal sensory neurons (Weng et al., 2007, Nie and Weng, 2009; 2010). Further, impairment of glial glutamate transporters reduces GABAergic synaptic activities in the spinal dorsal horn due to insufficient GABA synthesis through the glutamate-glutamine cycle between astrocytes and neurons (Jiang et al., 2012). Selectively enhanced protein expression of glial glutamate transporters by ceftriaxone treatment (Hu et al., 2010) or gene transfer (Maeda et al., 2008) can effectively prevent the development of pathological pain induced by nerve injury. Despite the critical role of glial glutamate transporters in spinal nociceptive sensory processing, the molecular mechanisms regulating activities of glial glutamate transporters remains poorly understood.
Activation of glial cells and the subsequent release of pro-inflammatory cytokines including IL-1β in the spinal dorsal horn are critically implicated in the development and maintenance of many types of pathological conditions (Coyle, 1998, Ren and Dubner, 2010, Grace et al., 2014). For example, following peripheral nerve injury, activation of glial cells (microglia and astrocytes) in the spinal dorsal horn results in the increased production and subsequent release of proinflammatory cytokines from glial cells (Sweitzer et al., 2001, Raghavendra et al., 2003, Tsuda et al., 2004, Yan and Weng, 2013). Endogenous IL-1β in neuropathic rats enhances non-NMDA glutamate receptor activities in postsynaptic neurons and glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic NMDA receptors (Yan and Weng, 2013). Treatment with IL-1β antagonists (Sommer et al., 1999, Milligan and Watkins, 2009) or knocking out IL-1β receptors (Wolf et al., 2006, Kleibeuker et al., 2008) reduces behavioral hypersensitivity induced by nerve injury. Currently, it is unknown whether activities of glial glutamate transporters in the spinal dorsal horn are regulated by IL-1β.
Protein kinase C (PKC) is one of most important kinases involved the genesis of pathological pain. Activation of PKC in the spinal dorsal horn is observed in animals with neuropathic pain induced by peripheral nerve injury (Mao et al., 1992), persistent inflammation pain induced by complete Freund's adjuvant (Park et al., 2009), formalin-induced pain (Yashpal et al., 2001), pain induced by capsaicin (Lin et al., 1996), or long-term potentiation (LTP) in the spinal dorsal horn (Yang et al., 2004). Studies of the impacts produced by PKC activation on the pain signaling system are mainly focused on receptors and signaling molecules on neurons. Whether glial glutamate transporter activities in the spinal dorsal horn of neuropathic animals are regulated by PKC remains unknown.
In this study, we for the first time demonstrated that IL-1β increases the internalization of glial glutamate transporters, which is mediated by the calcium/PKC/dynamin signaling pathway and depends on the integrity of actin filaments.
Methods
Animals
Young adult male Sprague-Dawley rats or GFAP-GFP transgenic mice (6–8 weeks old) were used. GFAP-GFP transgenic mice were obtained from the Jackson Laboratory. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Georgia and were fully compliant with the National Institutes of Health Guidelines for the Use and Care of Laboratory Animals.
Ligation of the L5 spinal nerve and behavioral tests
Animals were randomly divided into partial sciatic nerve ligation (pSNL) or sham-operated groups. Briefly, under isoflurane-induced (2–3%) anesthesia, the left sciatic nerve at the upper thigh was exposed and ligated approximately two-thirds the thickness of the sciatic nerve with a 5-0 silk suture as previously described (Seltzer et al., 1990, Nie and Weng, 2010). In sham-operated animals, the left sciatic nerve was exposed but not ligated. Following surgery, the wound was closed with skin stables. Behavioral tests were performed to determine the hind paw mechanical sensitivity 1 day before operation, and on day 10 post-surgery, prior to electrophysiological and molecular experiments. Briefly, the animals were placed on wire mesh, loosely restrained under a Plexiglas cage, and allowed to accommodate for at least 30 min. Von Frey monofilaments with bending forces ranging from 0.1 to 12.4 g were applied from below through the mesh onto the mid-plantar side of each hind paw to evoke paw withdrawal responses. Each hind paw was stimulated 10 times with each Von Frey monofilament, and the frequency (percentage) of paw withdrawal responses to 10 stimulations was recorded. The least bending force that evoked withdrawal responses in more than half the trials was assigned as the 50% withdrawal threshold (Weng et al., 2006, Yan et al., 2013).
Spinal slice preparations, recording and analysis of glial glutamate transporter currents (GTCs) from astrocytes in the spinal dorsal horn
Adult male GFAP-GFP transgenic mice were used. These transgenic mice overexpressed Green Fluorescent Protein (GFP) under the control of the astrocyte-specific glial fibrillary acidic protein (GFAP) promoter. Transverse mouse spinal cord slices of the L4–5 segment were prepared as described previously (Weng et al., 2007). Transverse spinal cord slices (400 µm thick) were cut in the ice-cold sucrose aCSF and then pre-incubated in Krebs solution oxygenated with 95% O2 and 5% CO2 at 35°C. The Krebs solution contained (mM): 117.0 NaCl, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 25.0 NaHCO3 at 35°C. To record GTCs, the spinal slice was placed in a recording chamber perfused with Krebs solution. Visualized whole-Cell patch clamp recordings were obtained from the spinal dorsal horn laminae I and II astrocytes identified by GFP under the fluorescent microscope. Borosilicate glass recording electrodes (resistance, 4–6 ML) were filled with (mM)145 Kgluconate, 5 NaCl, 1 MgCl2, 0.2 EGTA, 10 HEPES, 2 Mg-ATP and 0.1 Na-GTP (pH 7.3, 290–300 mOsm) (Zhang et al., 2009). GTCs were recorded at a holding potential of −80 mV in voltage clamp mode in the presence of blockers of GABAA receptor (10 µM bicuculline), glycine receptor (5 µM strychnine), AMPA/kainate receptors (10 µM DNQX), NMDA receptor (25 µM D-AP5), and tetrodotoxin (1 µM) at 35°C (Bergles and Jahr, 1997, Zhang et al., 2009). GTCs were evoked by puffing 50 µM L-glutamate onto the recorded astrocyte through a glass pipette connected to a Picospritzer controlled by a computer. Access resistance within the range of 10–20 MΩ was monitored continuously throughout the experiments. Recordings were abandoned when the access resistance changed more than 20%. In order to make comparison of GTCs between the controls and neuropathic mice, care was taken to ensure that the distance between the recorded astrocyte and the puffing pipette tip (about 15 µm), the pipette opening tip (3–4 µm), the puffing pressure (3 p.s.i), the puffing duration (25 ms, repeated every 30 s) (Nie and Weng, 2009), and the depth of the cell in the slice (about 50 µm below the surface) were kept constant across all experiments. In addition, the experimenters who collected the data were blinded to treatments given to the mice. All the drugs were applied through bath-perfusion unless otherwise indicated. Data were recorded using Axopatch 700B amplifiers, digitized at 10 kHz, and analyzed off-line. Four sweeps of GTCs were averaged and the mean amplitude and charge transfer of GTCs (Devaraju et al., 2013) were measured. To measure time constants for the rise phase and the decay phase of GTCs, the GTC rise or decay phase was fitted with monoexponential function, and time constants were measured (Nie and Weng, 2009). The commercial computer software Clampfit (Molecular Device, CA) was used for data analysis.
Calcium imaging
Calcium imaging procedures established by other labs (Ge et al., 2006, Gordon et al., 2009, Barabas et al., 2012) were followed. Spinal slices from GFAP-GFP mice were prepared in the same manner as those used for electrophysiological experiments stated above. A spinal slice was placed in the recording chamber perfused with Krebs solution bubbled with 95% O2 and 5% CO2 at 35°C. In order to selectively study calcium activities in astrocytes, an astrocyte identified by the green fluorescent protein in the superficial dorsal horn was patched under the microscope with a pipette filled with the intracellular solution containing the red-fluorescent calcium dye Rhod-2 (the cell impermeant tripotassium salt, Invitrogen) (200 µM) (Ge et al., 2006, Gordon et al., 2009). Twenty minutes were allowed to let Rhod-2 microdialyze into the recorded astrocyte and diffuse into the adjacent astrocytes through gap-junctions (Andersson and Hanse, 2010). The fluorescence of Rhod-2 was excited at 514 nm using a helium-neon laser line (Ge et al., 2006, Sztretye et al., 2011). Calcium imaging was performed on an Olympus fluorescent microscope under a 60X objective (numerical aperture: 1.2). Images of fluorescence signals of 548–665 nm were acquired with a Hamamatsu CDD camera (Ge et al., 2006, Sztretye et al., 2011). Metafluor imaging software was utilized in order to detect and analyze intracellular calcium changes throughout the experiment (Molecular Devices, Sunnyvale, CA) (Barabas et al., 2012).
Western blot experiments
For measuring IL-1β and phosphorylated PKC levels in neuropathic and sham-operated rats, animals were deeply anesthetized with urethane (1.3–1.5g/kg, i.p.) 10 days after the surgery. The L4 and L5 spinal segments were exposed by surgery and removed from the rats. The dorsal quadrant of the spinal cord of each segment ipsilateral to the operated side was isolated. The dorsal quadrants of the spinal cord were quickly frozen in liquid nitrogen and stored at −80 °C for later use. The frozen tissues were homogenized with a hand-held pellet pestle in lysis buffer for 0.5 hr at 37°C (Weng et al., 2014). For measuring GLT-1 and GLAST expressions in the plasma membrane and cytosol in spinal slices after IL-1β treatments, rat spinal slices of the spinal L4–5 segment were obtained in the same way as those for mouse spinal slices described above. Spinal slices were incubated with the plain aCSF or aCSF plus IL-1β (10 ng/ml) bubbled with 95% O2 and 5% CO2 at 35°C for 15 min. The dorsal halves of the spinal cord were then isolated and quickly frozen in liquid nitrogen and stored at −80 °C. The tissue was fractionated into cytosolic and membrane fractions with the cytoplasmic, nuclear, and membrane compartmental protein extraction kit (Biochain Institute, Inc.). Homogenates were then centrifuged at 14000 × g for 20 min at 4 °C and the supernatant were collected. Protein concentrations were determined using bicinchoninic acid (kit from Pierce). Protein samples (40 µg) were electrophoresed in 10 % SDS polyacrylamide gels and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membranes were blocked with 5% milk and incubated overnight at 4°C with primary antibodies against IL-1β (1:500, Millipore, Bedford, MA), phospho PKC (1:1000, Sigma-Aldrich, St. Louis, USA), GLT-1 (1:1000, Millipore, Bedford, MA), GLAST (1: 2000, Millipore, Bedford, MA ) or a monoclonal mouse anti-β-actin (1:2000, Sigma-Aldrich, St. Louis, USA) primary antibody as a loading control. The cytosolic and membrane fractions were checked for specificity by Western blotting with anti-tubulin (1:200), anti-EGFR (1:200; Santa Cruz Biotechnology). Then the blots were incubated for 1 hr at room temperature with a corresponding HRP-conjugated secondary antibody (1:5000; Santa Cruz Biotechnology, CA, USA), visualized in ECL solution (SuperSignal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA).) for 1 min, and exposed onto FluorChem HD2 System. The intensity of immunoreactive bands was quantified using ImageJ 1.46 software (NIH). Results were expressed as the ratio to control protein (Weng et al., 2014).
In vitro measurement of glutamate uptake activity
To investigate effects of IL-1β and PKC on glutamate transporter activities, the L4–L5 spinal cord was exposed by laminectomy and the spinal dura was excised in rats anesthetized with urethane (1.3–1.5 g/kg, i.p). The rate of heart beat and breathing, and the core temperature of the animals were constantly monitored and maintained in normal limits (Weng et al., 2003). IL-1β or the PKC activator (phorbol 12-myristate 13-acetate, PMA) was applied onto the L4-L5 spinal segment through a piece of cotton soaked with IL-1β (concentration: 20 ng/ml) or PMA (4 µM) in artificial CSF (aCSF) at 35°C for 30 min. Rats in the control group receiving aCSF in the same fashion. Immediately after the treatment, the dorsal half of the L4-L5 spinal segment was isolated. To determine glutamate uptake activities in the spinal dorsal horn in neuropathic rats and sham-operated rats, the dorsal quadrant of the L4-L5 spinal segment ipsilateral to the surgery side was isolated 10 days after the surgery as described above for the Western blot experiments. Synaptosome preparations were prepared immediately after the spinal tissue was isolated according to the protocol published (Rothstein et al., 1992). The spinal tissue was homogenized in an ice-cold buffer solution containing: 0.5 mM EDTA, 0.5 mM EGTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.32 M sucrose, 5 µg/ml pepstatin, 5 µg/ml aprotinin, 20 µg/ml trypsin inhibitor, 4 µg/ml leupeptin, and 0.01 M phosphate-buffered saline. The homogenates were centrifuged at 15,000 rpm for 10 min at 4°C, and the supernatant collected. The remaining pellets were resuspended in the same buffer solution and re-centrifuged at 15,000 rpm for 10 min at 4°C. The two supernatants were combined and centrifuged again at 13,000 rpm for 10 min at 4°C to obtain the synaptosomal pellets, which contained both neuronal and glial glutamate transporters (Azbill et al., 2000). The synaptosomal pellets were suspended in Locke’s buffer. The glutamate uptake activity was determined by incubating the synaptosome preparation with a solution containing [3H] L-glutamic acid (0.4 µCi/mmol) at 37°C for 5 min. The reaction was terminated by filtering the synaptosomes through a Whatman GF/B filter pre-soaked with the same buffer solution. The filter was then transferred to a vial containing scintillation cocktail and the radioactivity, which reflects glutamate uptake activities carried by both glial and neuronal glutamate transporters (Azbill et al., 2000), in the final sample was measured by a liquid scintillation counter (Beckman, LS6500).
Materials
Bicuculline, strychnine, phorbol 12-myristate 13-acetate (PMA), tetrodotoxin (TTX), L-glutamic acid, cytochalasin D, and 1,2-Bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) were obtained from Sigma (St. Louis, MO, USA). D-aminophosphonovaleric acid (D-AP5), DNQX, GF 109203X, phalloidin and dynamin inhibitory peptide were obtained from Tocris Bioscience (Minneapolis, MN, USA). Recombinant human IL-1β and IL-1ra proteins were purchased from R&D Systems (Minneapolis, MN). Rhod-2 was purchased from Invivogen (San Diego, CA). [3H] L-glutamic acid was obtained from Perkin Elmer.
Data Analysis
All data are presented as the mean ± SE. Student’s t-test was used to determine the statistical difference between data obtained from the same group (paired t-test) or between groups (non-paired t-tests). A p value less than 0.05 was considered statistically significant.
Results
All rats and mice receiving pSNL had mechanical allodynia prior to undergoing the biochemical or electrophysiological experiments that took place 10 days after the surgery. This was evident by the fact that mechanical thresholds of hind paw withdrawal responses ipsilateral to pSNL decreased significantly from 8.07 ± 1.14 g at baseline to 1.98 ± 0.50 g (p < 0.001, n=9) in neuropathic rats. The mechanical threshold in sham-operated rats was not significantly altered (from 7.86 ± 0.39 g to 8.24 ± 0.40 g, n=38). Mechanical thresholds of hind paw withdrawal responses ipsilateral to pSNL decreased significantly from 1.24 ± 0.11 g at baseline to 0.19 ± 0.04 g (p < 0.001, n=9) in neuropathic mice while the mechanical threshold in sham-operated mice was not significantly altered (from 1.17 ± 0.05 g to 1.20 ± 0.04 g, n=35).
Nerve injury results in an increased IL-1β protein expression and PKC activation in the dorsal horn, which are temporally associated with a reduction of glutamate uptake
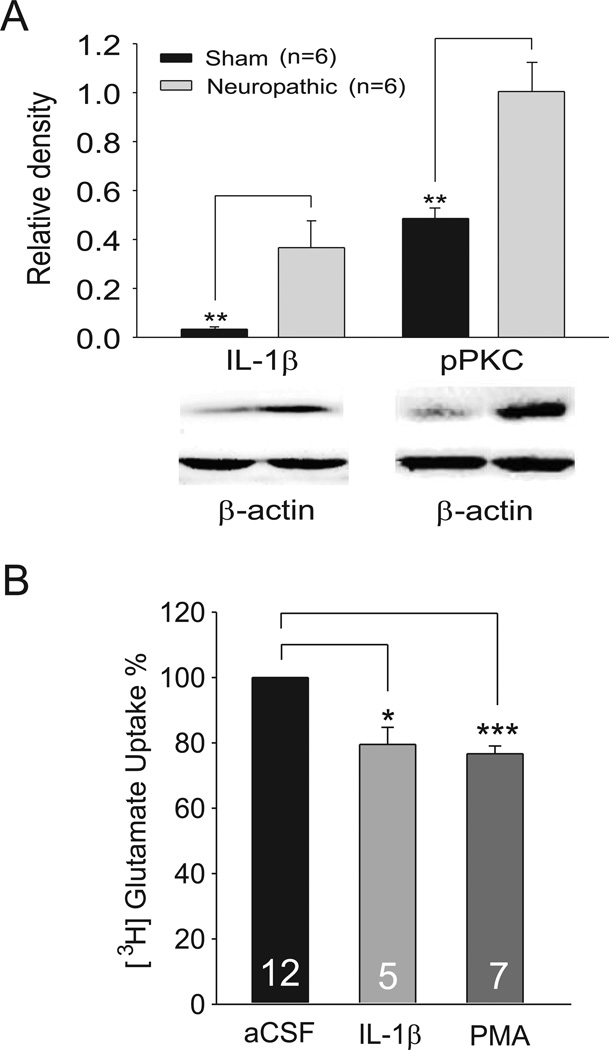
To study the mechanisms by which IL-1β and PKC regulate glutamatergic synaptic plasticity in the spinal dorsal horn induced by nerve injury, we first determined protein expression of IL-1β and the active form of PKC (phosphorylated PKC, pPKC) in the spinal dorsal horn in rats with pSNL or sham operation. As shown in Figure 1A, protein expression of IL-1β in the spinal dorsal horn of the L4-L5 spinal segment ipsilateral to the injury site was significantly (p<0.01) increased by over 9 fold (n=6) in neuropathic rats in comparison with sham-operated rats. Meanwhile, expression of pPKC in the spinal dorsal horn in rats was significantly increased by over one fold (p<0.01) (Fig. 1A). These data suggest that IL-1β and PKC are implicated in the genesis of neuropathic pain, consistent with previous studies (Mao et al., 1992, Yan and Weng, 2013).
Figure 1. Protein expressions of IL-1β and phosphorylated PKC (pPKC) in the spinal dorsal horn ipsilateral to the injury site in neuropathic rats are increased; IL-1β or activation of PKC reduces glutamate uptake activities in the rat spinal dorsal horn.
(A). Samples of IL-1β and pPKC expressions in the spinal dorsal horn at the L4 to L5 segment in neuropathic and sham-operated rats are shown. Bath graphs show the mean (+S.E.) relative density to β-actin. (B). Bar graphs show the mean (+S.E.) glutamate uptake activities obtained from the spinal cord treated with IL-1β (20 ng/ml) or PMA (4 µM) for 30 min. The glutamate uptake activities in the synaptosome preparation treated with IL-1β or PMA are normalized with those treated with aCSF measured at the same time. Number of animals included in each group for the analysis is shown in each bar. * p<0.05; ** p< 0.01;*** p<0.001.
To determine if elevated levels of IL-1β and activation of PKC in the spinal dorsal horn of neuropathic rats are associated with dysfunction of glutamate transporter functions, glutamate uptake activities of the spinal dorsal horn of the L4-L5 segment were measured in rats with pSNL or with sham-operation 10 days after surgery. Synaptosome preparations were prepared from the spinal dorsal horn ipsilateral to the operation site. Glutamate uptake activities were determined by measuring the radioactivity in the synaptosome preparation treated with a solution containing [3H] L-glutamate (0.4 µCi/mmol). We found that the overall glutamate uptake activities were reduced by 34.09 ± 5.44% in neuropathic rats (n=3, p<0.001) compared to sham-operated rats (n=3), which is consistent with previous findings (Sung et al., 2003).
Glutamate uptake activities in the rat spinal dorsal horn are reduced by IL-1β or activation of PKC
Next, the role of IL-1β in the regulation of glutamate uptake activities in the spinal dorsal horn was studied. A piece of cotton soaked with IL-1β (concentration: 20 ng/ml) in aCSF at 35°C was placed onto the dorsal surface of the L4–5 spinal segment for 30 min. Rats in the control group received aCSF treatment which was applied to the rats in the same fashion. Glutamate uptake activities in the spinal dorsal horn of the L4–5 segment were determined using synaptosome preparations. We found that in comparison with the aCSF treated rats, glutamate uptake activities in rats receiving IL-1β treatment were significantly (p<0.05) reduced by 20.51 ± 5.30% (n=5) (Fig. 1B). In another set of experiments, we analyzed the effects of PKC activation on glutamate uptake activities in the spinal dorsal horn. The PKC activator PMA (concentration: 4 µM) or aCSF was applied onto the spinal cord in the same fashion as above. We found that glutamate uptake activities in the synaptosome preparations of rats treated with PMA were significantly (p<0.001) reduced by 23.29 ± 2.54% (n=7) compared to rats treated with aCSF (Fig. 1B). These data indicate that IL-1β and PKC are key molecules regulating the overall glutamate uptake activities in the spinal dorsal horn.
Endogenous IL-1β in the spinal dorsal horn of neuropathic mice reduces glial glutamate transporter activities
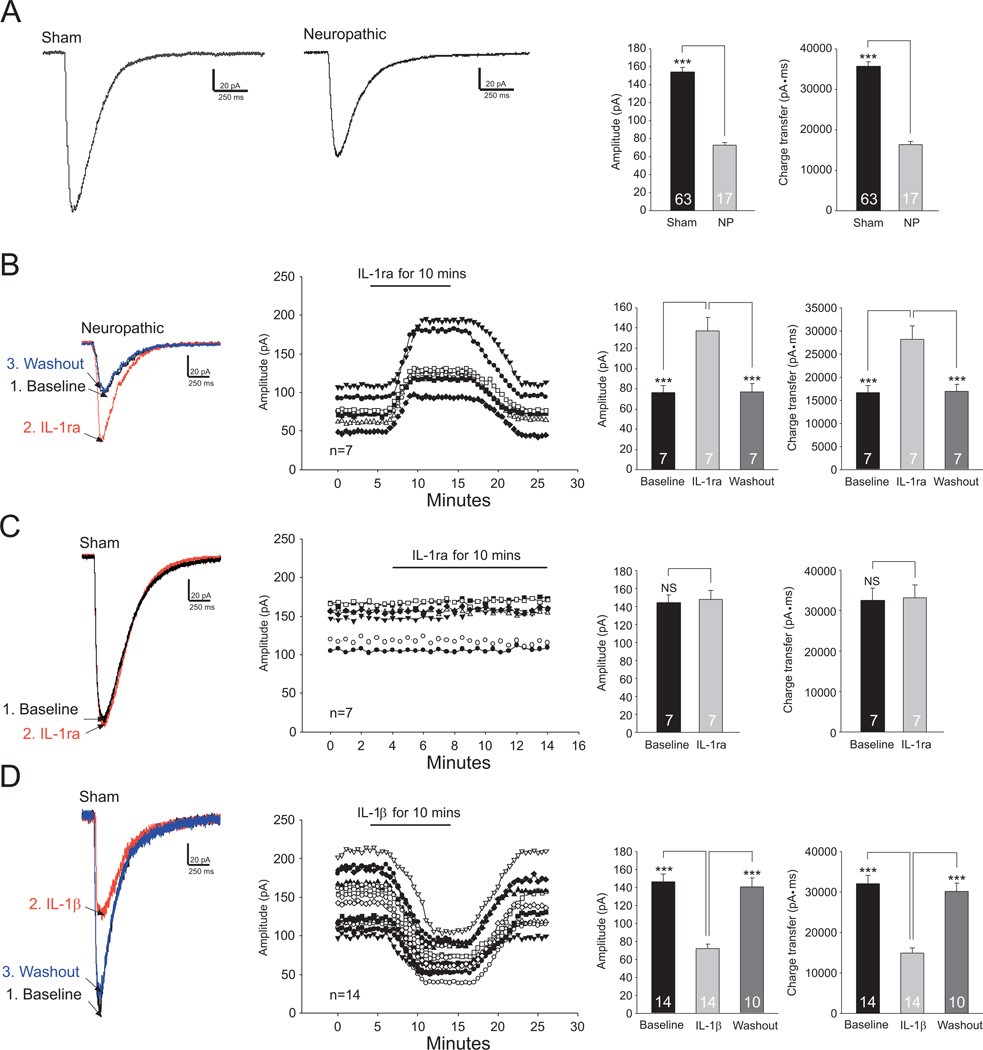
To further investigate molecular mechanisms regulating the function of glial glutamate transporters in neuropathic conditions, we directly measured and monitored activities of glial glutamate transporters in real time by recording GTCs from astrocytes. The uptake of glutamate by glial glutamate transporters is accompanied by the co-transport of two or three Na+ with one H+ and the countertransport of one K+ (Wadiche et al., 1995, Levy et al., 1998). Because of the translocation of a net positive charge during each transport cycle, glutamate uptake generates a current called GTC (glutamate transporter current). The size of GTCs reflects the amount of transported glutamate, which has been widely used as an effective tool to study the function of glial glutamate transporters (Bergles and Jahr, 1997, Adolph et al., 2007). We recorded GTCs from GFAP-GFP transgenic mice, in which astrocytes were easily identified by the expression of Green Fluorescent Protein (GFP) under a fluorescent microscope (Fig. 2A). Astrocytes displayed a linear IV relationship (a passive membrane property) (Fig. 2B) and a low input resistance (10–20 MΩ). GTCs were evoked by L-glutamate (50 µM) injected onto the astrocyte through a puff-electrode. Such currents were almost abolished in the presence of the specific glutamate transporter blocker DL-TBOA (50 µM) (Jabaudon et al., 1999) (Fig. 2C), confirming that these currents were generated from glutamate transporter activities. We then recorded GTCs from mice with pSNL and sham-operation 10 days after the operation. We first compared GTCs between mice with pSNL and sham-operation. Amplitudes and charge transfers of glutamate transporter currents (i.e., glial GT activities) (n=17) recorded from neuropathic mice were significantly (p<0.001) smaller than those (n=63) in sham-operated mice (Fig. 3A). The GTC rise time constants (22.76 ± 0.34 ms, n=17) and decay time constants (265.36 ± 6.67 ms) in neuropathic mice were not significantly different from those in sham-operated mice (rise time constants: 22.90 ± 0.17 ms, n=63, p=0.71; decay time constants: 267.04 ± 3.34 ms, n=63, p =0.82). These data indicate that glial glutamate transporter activities in the spinal dorsal horn of neuropathic mice are reduced.
Figure 2. Glutamate transporter currents in astrocytes in the spinal dorsal horn are evoked by glutamate injected onto the astrocyte.
(A) Astrocytes (indicated by arrows) in spinal slices of GFAP-GFP mice were identified by the green fluorescent protein (GFP). (B) Inward and outward currents (top) in a spinal astrocyte were evoked by voltage steps (10 mV/step) from −130 mV to +70 mV (bottom), indicating a passive membrane property of astrocytes. (C) shows GTCs in an astrocyte as baseline, during and after washout of perfusion of DL-TBOA (50 µM). Bar graphs show the mean (+S.E.) amplitude of GTCs at baseline and after blocking glutamate transporters with DL-TBOA. *** p<0.001.
Figure 3. Endogenous IL-1β in the spinal dorsal horn of neuropathic mice reduces glial glutamate transporter activities.
(A) shows samples of GTCs recorded from sham-operated and neuropathic mice and the mean (+S.E.) amplitude and charge transfer of GTCs in both sham and neuropathic (NP) mice. (B) Samples of GTCs recorded from neuropathic mice before (baseline), during and after washout of the IL-1β inhibitor (IL-1ra, 100 ng/ml) are shown. (C) Samples of GTCs recorded from sham-operated mice before and during perfusion of IL-1ra (100 ng/ml) are shown. (D) Samples of GTCs recorded from sham-operated mice before, during and after washout of IL-1β (10 ng/ml) are shown. The measurements of GTC amplitudes for each cell over time are plotted. Bar graphs show the mean (+S.E.) amplitude of GTCs before (baseline), during and after washout of each tested agent. *** p<0.001; NS: no statistical significance.
We next determined whether endogenous IL-1β in the spinal dorsal horn of neuropathic mice contributes to the reduced glial glutamate transporter activities. Blockade of IL-1β receptors with the IL-1β antagonist (IL-1ra, 100 ng/ml) significantly (p<0.001) increased GTC amplitudes by 80.90 ± 6.35% (n=7) and charge transfers by 68.27 ± 5.63% (n=7) in neuropathic mice (Fig. 3B). In contrast, GTCs (n=7) recorded from sham-operated mice were not altered by bath-perfusion of IL-1ra (100 ng/ml) (Fig. 3C). These data indicate that glial glutamate transporter activities are suppressed by endogenous IL-1β in neuropathic mice. This notion was further supported by another set of experiments where perfusion of IL-1β (10 ng/ml) into the recording bath significantly (p<0.001) reduced the amplitude by 49.12 ± 1.72% (n=14) and charge transfer of GTCs by 46.33 ± 1.89% (n=14) recorded from spinal slices of sham-operated mice (Fig. 3D).
Glial glutamate transporter activities in the spinal dorsal horn of neuropathic mice are increased by blocking PKC activities
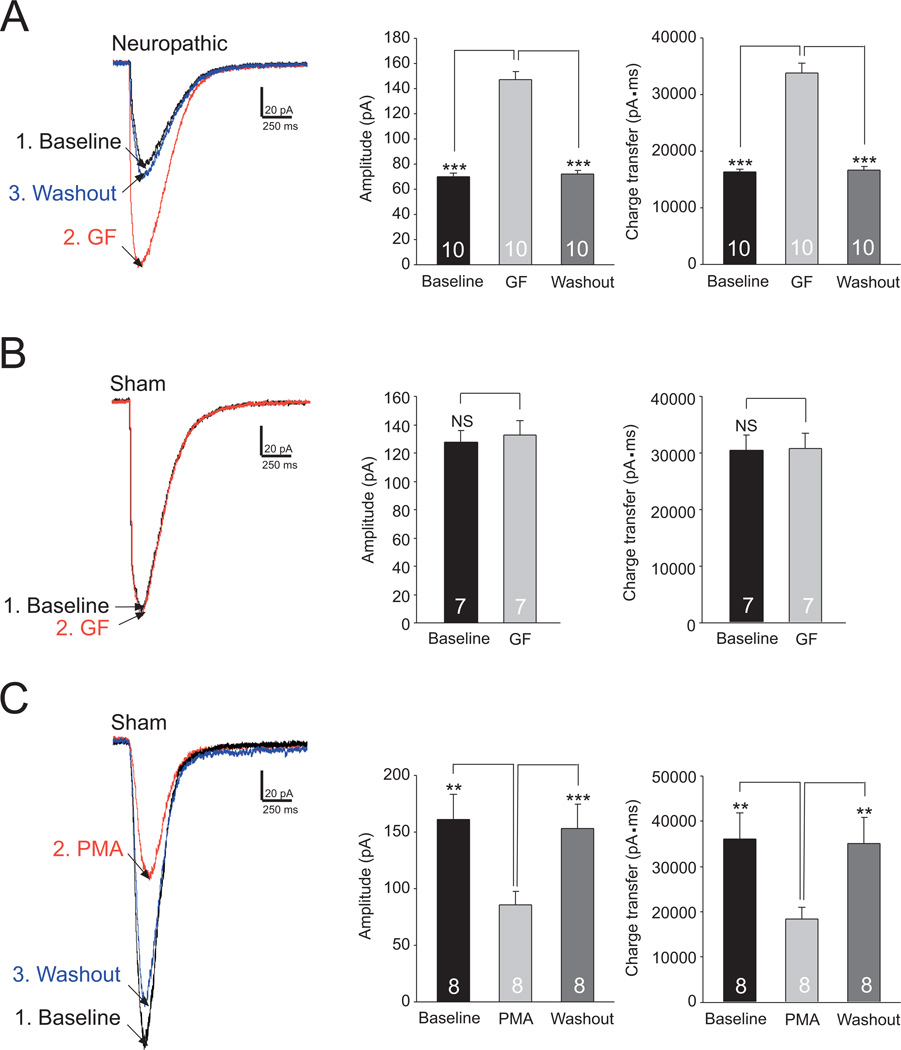
We next determined whether the activation of PKC in the spinal dorsal horn contributes to the reduced glial glutamate transporter activities in neuropathic mice. GTCs were recorded from spinal slices of neuropathic mice before and after bath-perfusion of the PKC inhibitor GF109203X (4 µM). As shown in Figure 4A, blocking PKC significantly increased the GTC amplitude by 109.73± 6.04% (n=10, p<0.001) and charge transfer by 107.35 ± 8.26% (n=10, p<0.001). In contrast, perfusion of GF109203X (4 µM) had no clear effects on the GTC amplitude and charge transfer recorded from slices of sham-operated mice (Fig. 4B). In addition, when we recorded GTCs from spinal slices of sham-operated mice and activated PKC with bath-perfusion of the PKC activator (PMA, 4 µM), we found that the amplitude and charge transfer of GTCs were significantly (n=8, p<0.01) reduced (Fig. 4C). These data indicate that glial glutamate transporter activities are suppressed by PKC activities in neuropathic mice.
Figure 4. Glial glutamate transporter activities in the spinal dorsal horn of neuropathic mice are increased by blocking PKC activities.
Samples of GTCs recorded from neuropathic mice (A) and sham-operated mice (B) before (baseline), during and after washout of the PKC inhibitor, GF109203X (GF, 4 µM) are shown. (C) shows samples of GTCs recorded from sham-operated mice before, during and after washout of the PKC activator (PMA, 4 µM). Bar graphs show the mean (+S.E.) amplitude and charge transfer of GTCs before (baseline), during and after washout of each tested agent. ** p<0.01; *** p<0.001; NS: no statistical significance.
IL-1β reduces glial glutamate transporter activities in the spinal dorsal horn through elevating calcium concentrations in astrocytes
Intracellular calcium is an important signaling molecule that is responsible for regulating activities of many second messenger pathways. IL-1β is known to increase calcium activities in C6 rat glioma cells (Pita et al., 1999). We then determined whether Ca2+ signal mediates the effects of IL-1β on GTCs. In the first set of experiments, we monitored calcium activities in astrocytes in spinal slices taken from GFAP-GFP mice before and after bath-perfusion of IL-1β. The location of astrocytes was first identified under the fluorescent microscope by the green fluorescent protein that was expressed only in astrocytes of GFAP-GFP mice (Fig. 5A). In order to selectively study calcium activities in astrocytes, a pipette filled with the intracellular solution containing the red-fluorescent calcium dye Rhod-2 (200 µM) (Ge et al., 2006, Gordon et al., 2009) was used to patch an astrocyte (Fig. 5A). To allow diffusion of the calcium sensitive dye into the patched astrocyte and the adjacent gap junction-coupled astrocytes 20 min was lapsed (Serrano et al., 2006) before calcium images were taken. The Ca2+ indicator Rhod-2 has a molecular weight of 869.06, which is well below the 1 kDa size limit for gap junction pores (Giaume et al., 2010). As shown in Figure 5, bath-perfusion of IL-1β (10 ng/ml) significantly and reversibly increased calcium activities in the astrocytes. Further, in the next set of experiments (13 astrocytes), calcium in the recorded astrocytes was chelated by a fast calcium chelator BAPTA concentration: 40 mM), which was included in the pipette intracellular solution (Gordon et al., 2005, Andersson and Hanse, 2010). Under such condition, perfusion of IL-1β (10 ng/ml) into the recording bath no longer altered GTCs recorded from spinal slices obtained from sham-operated mice (data not shown). This is in contrast with the results obtained under the control condition when BAPTA was not loaded in the electrode intracellular solution (Fig. 3D). These data indicate that IL-1β reduces glial glutamate transporter activities through elevating the intracellular Ca2+ concentration in astrocytes.
Figure 5. IL-1β increases calcium concentrations in astrocytes in the spinal dorsal horn.
(A) shows astrocytes marked by the green fluorescent protein viewed under the fluorescent microscope with a FITC cube and a patch pipette marked by dotted lines. Astrocytes used for the calcium imaging analysis were labeled by numbers. (B) Calcium imaging (red-fluorescent) pictures taken in a sequential time order before, during and after washout of IL-1β (10 ng/ml) are shown. IL-1β was applied to the bath at 100 s and washout at 220 s. (C) Line-plots show samples of quantitative analysis of astrocytic Ca2+ levels in 4 astrocytes marked by numbers in the pictures before, during and after washout of IL-1β expressed as fluorescent intensity in arbitrary units. ΔF/F = (F – Fbase)/Fbase, where F is the measured fluorescence intensity of the Ca2+ indicator, Fbase is the fluorescence intensity of the Ca2+ indicator in the cell at baseline (Takahashi et al., 1999). (D) Bar graphs show the mean (+S.E.) ΔF/F before, during and after washout of IL-1β. *** p<0.001.
IL-1β reduces glial glutamate transporter activities in the spinal dorsal horn through activating PKC
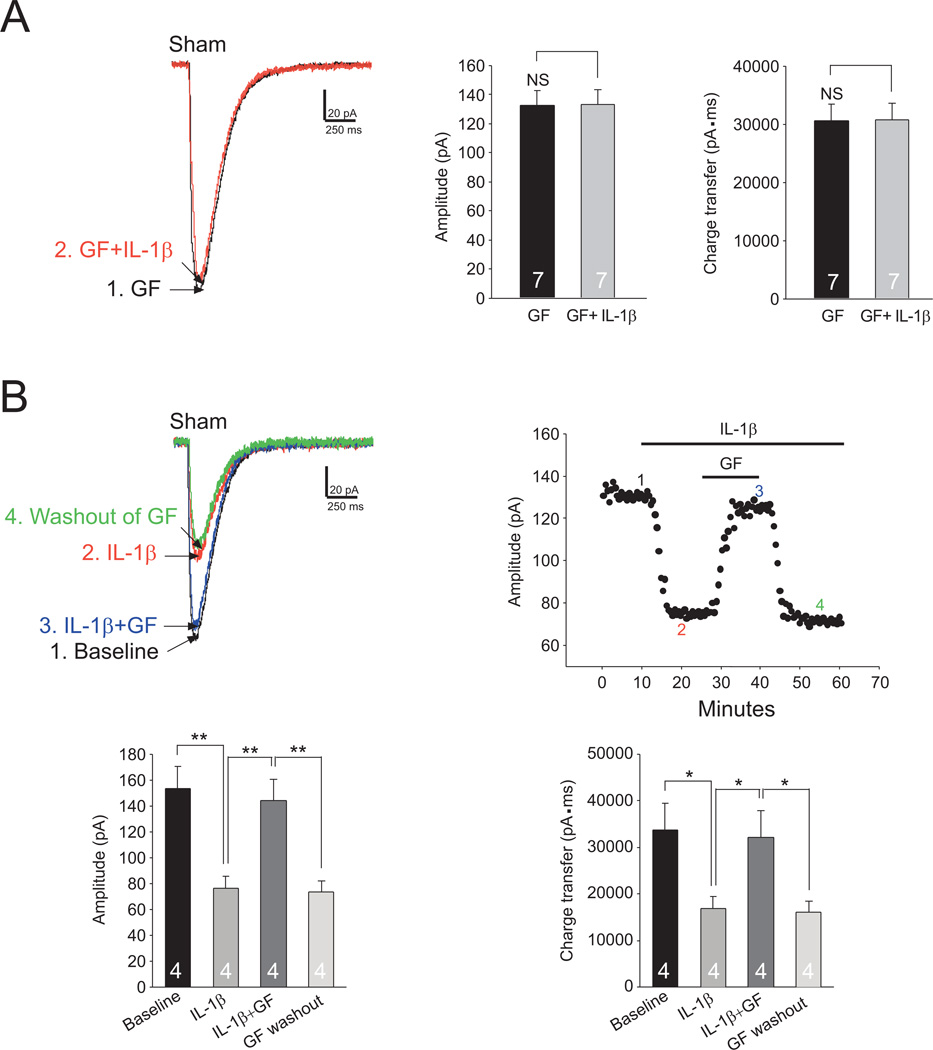
To determine if PKC is a downstream molecule used by IL-1β to suppress glial glutamate transporter activities, we bath-applied IL-1β (10 ng/ml) in the presence of the PKC inhibitor (GF109203X, 4 µM). As shown in Figure 6A, in the presence of GF109203X, perfusion of IL-1β did not significantly alter both the amplitude and charge transfer of GTCs recorded from spinal slices of sham-operated mice. In another set of experiments, after the effects of IL-1β (10 ng/ml) on GTCs were documented, we further added GF109203X (4 µM) into the recording bath in the presence of IL-1β. GF109203X reversed the attenuation of GTCs induced by IL-1β (Fig. 6B). Further, in the presence of the PKC activator (PMA, 4 µM), the GTC amplitude (75.28 ± 5.17 pA, n=6) and charge transfer (16925.41± 790.33 pA·ms, n=6) were not significantly altered when IL-1β (10 ng/ml) was further added into the recording bath (amplitudes: 75.10 ± 4.66 pA, n=6, p=0.90; charge transfers: 17085.73 ± 754.51 pA·ms, n=6, p=0.29). These data indicate that IL-1β reduces glial glutamate transporter activities through activating PKC.
Figure 6. IL-1β reduces glial glutamate transporter activities in the spinal dorsal horn through activating PKC.
(A) Raw data show samples of GTCs recorded in the presence of the PKC inhibitor, GF109203X (GF, 4 µM) from sham-operated mice before (GF) and after bath-application of IL-1β (10 ng/ml) (GF+IL-1β). Bar graphs show the mean (+S.E.) amplitude and charge transfer of GTCs before (GF) and after bath-application of IL-1β (GF+IL-1β). (B) The scatter plot to the right shows measurements of GTC amplitudes from an astrocyte from sham-operated mice at baseline, during perfusion of IL-1β (10 ng/ml), further addition of GF109203X (GF) and then washout of GF109203X with IL-1β (10 ng/ml) remaining. Samples of original recordings labeled by numbers are shown to the left. Bar graphs show the mean (+S.E.) amplitude and charge transfer of GTCs at baseline, during perfusion of IL-1β, perfusion of IL-1β+GF, and after washout of GF109203X with IL-1β (10 ng/ml) remaining (GF washout). * p<0.05; ** p<0.01; NS: no statistical significance.
IL-1β reduces glial glutamate transporter activities in the spinal dorsal horn through the dynamin-dependent endocytosis mechanism
Previous studies suggested that trafficking of glial glutamate transporters between the cytosol and plasma membrane is a major post-translational mechanism regulating the glial glutamate transporter function (Tai et al., 2007, Garcia-Tardon et al., 2012). The clathrin-dynamin dependent endocytotic process is a major mechanism regulating trafficking of neuronal transporters (Vaughan, 2004) between the plasma membrane and cytosol. To understand the possible role of the clathrin-dynamin dependent endocytotic process in regulating the glial glutamate transporter function, we microdialyzed the recorded astrocyte with the dynamin inhibitory peptide (100 µM) included in the intracellular solution in the recording pipette. As shown in Figure 7, the GTC amplitude and charge transfer became larger after rupturing of the astrocyte, indicating the function of glial glutamate transporters is regulated by the clathrin-dynamin dependent endocytotic process under normal conditions. The amplitude and charge transfer of GTCs became stable 10 to 13 min after the rupturing. Under such condition, perfusion of IL-1β (10 ng/ml) (Fig. 7A) or the PKC activator (PMA, 4 µM) (Fig. 7B) did not significantly alter GTCs. These are in contrast with the results obtained under the condition when the dynamin inhibitory peptide was not included in the electrode intracellular solution (Fig. 3D and Fig. 4C). These results indicate that IL-1β (10 ng/ml) and activation of PKC reduce the function of glial glutamate transporters in spinal dorsal horn astrocytes through enhancing the clathrin-dynamin dependent endocytosis.
Figure 7. IL-1β reduces glial glutamate transporter activities in the spinal dorsal horn through the dynamin-dependent endocytosis mechanism.
The open circles in the scatter plot (right) show measurements of GTC amplitudes before, during and after washout of IL-1β (10 ng/ml) (A) or PMA (PMA, 4 µM) (B) from an astrocyte of sham-operated mice recorded with a recording pipette filled with the intracellular solution containing the dynamin inhibitory peptide (DIP, 100 µM). Samples of original recordings labeled by numbers are shown (left). Note GTC amplitudes gradually became larger within 13 min after rupturing of the astrocyte. The filled circles in the scatter plot show that GTC amplitudes in an astrocyte recorded with the normal intracellular solution were stable during the same period. Bar graphs show the mean (+S.E.) amplitude and charge transfer of GTCs immediately after rupturing (baseline), 20 minutes after rupturing (DIP) and during perfusion of IL-1β (DIP+IL-1β) (A) or during perfusion of PMA (DIP+PMA) (B). ** p<0.01; *** p<0.001; NS: no statistical significance.
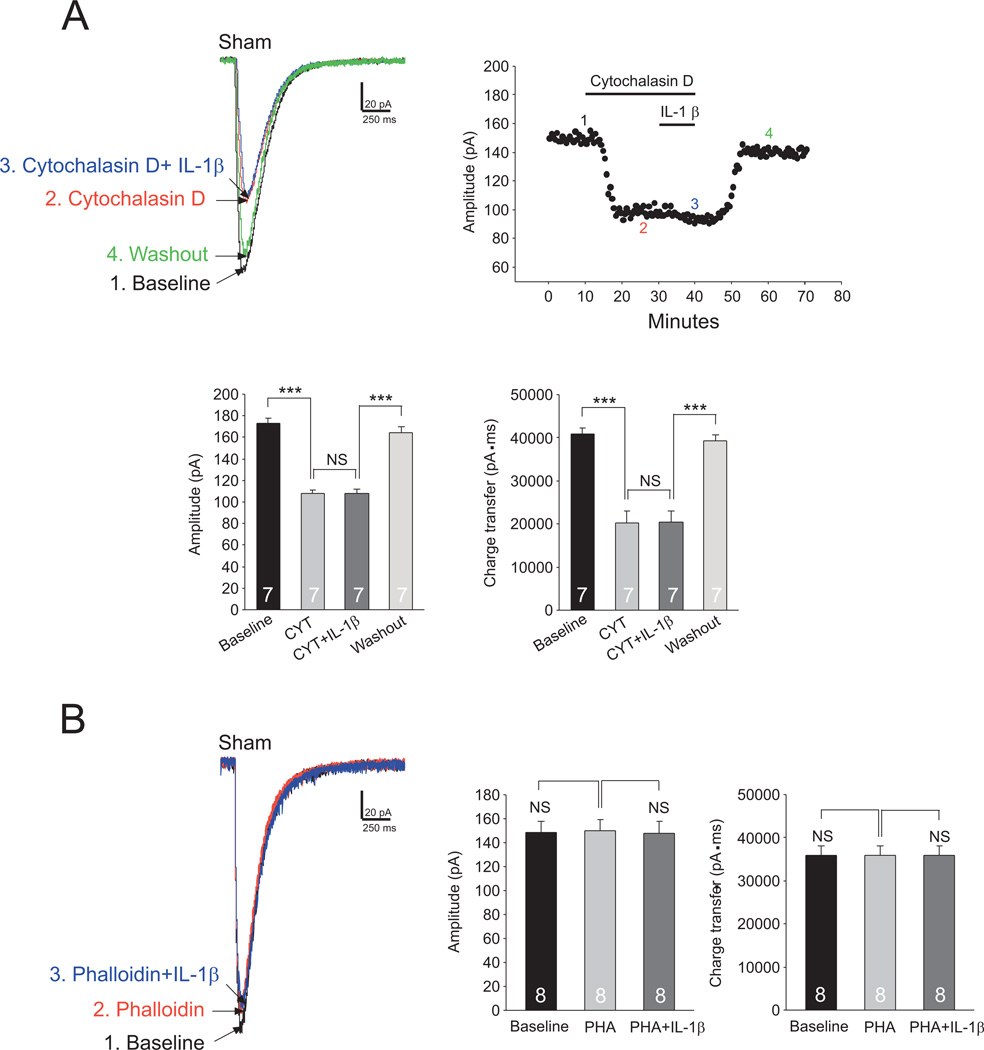
The endocytosis of glial glutamate transporters induced by IL-1β depends on the integrity of filamentous actin
Cytoskeleton proteins, like filamentous actin (F-actin), are often critically involved in the endocytosis of membrane receptors (Rogers and Gelfand, 2000) and transporters (Sakai et al., 2000). To determine the role of F-actin in the IL-1β mediated endocytosis of glial glutamate transporters, we examined the effects of IL-1β on GTCs in the presence of agents that interfere with actin filaments. Bath-application of the actin-depolymerizing agent cytochalasin D (5 µM, 20 min) (Rosenmund and Westbrook, 1993, Gu et al., 2005) significantly reduced the GTC amplitude by 37.40 ± 1.72% (n=7, p<0.001) and charge transfer by 50.73 ± 5.59% (n=7, p<0.001) (Fig. 8A). In the presence of cytochalasin D, further bath-application of IL-1β (10 ng/ml) did not alter GTCs (Fig. 8A). In another set of experiments, we microdialyzed the recorded cell with phalloidin (2 µM) included in the intracellular solution in the recording pipette (Gu et al., 2005). Phalloidin is a non-membrane permeable F-actin stabilizer (Cooper, 1987). Microdialysis of phalloidin into the cell did not significantly alter the GTC amplitude and charge transfer. Under such condition, perfusion of IL-1β (10 ng/ml) did not significantly alter GTCs (Fig.8B). Together, these results indicate that the integrity of F-actin is required for IL-1β to produce endocytosis of glial glutamate transporters in spinal dorsal horn astrocytes.
Figure 8. The endocytosis of glial glutamate transporters induced by IL-1β depends on the integrity of filamentous actin.
(A) The scatter plot shows measurements of GTC amplitudes from an astrocyte from sham-operated mice at baseline, during perfusion of cytochalasin D alone (5 µM, 20), cytochalasin D (5 µM) plus IL-1β (10 ng/ml), and then washout of both cytochalasin D and IL-1β. Samples of original recordings labeled by numbers are shown to the left. Bar graphs show the mean (+S.E.) amplitude and charge transfer of GTCs at baseline, during perfusion of cytochalasin D (CYT), perfusion of cytochalasin D+IL-1β (CYT+ IL-1β), then washout of both cytochalasin D and IL-1β (Washout). (B) The data were collected under the condition when the recorded astrocyte was microdialyzed with phalloidin (2 µM) included in the intracellular solution in the recording pipette. Raw data show GTCs recorded from sham-operated mice immediately after rupturing (baseline), 15 minutes after rupturing (Phalloidin) and during perfusion of IL-1β (10 ng/ml) (Phalloidin+IL-1β). Bar graphs show the mean (+S.E.) amplitude and charge transfer of GTCs immediately after rupturing (baseline), 15 min after rupturing (PHA) and during perfusion of IL-1β (PHA+IL-1β). *** p<0.001; NS: no statistical significance.
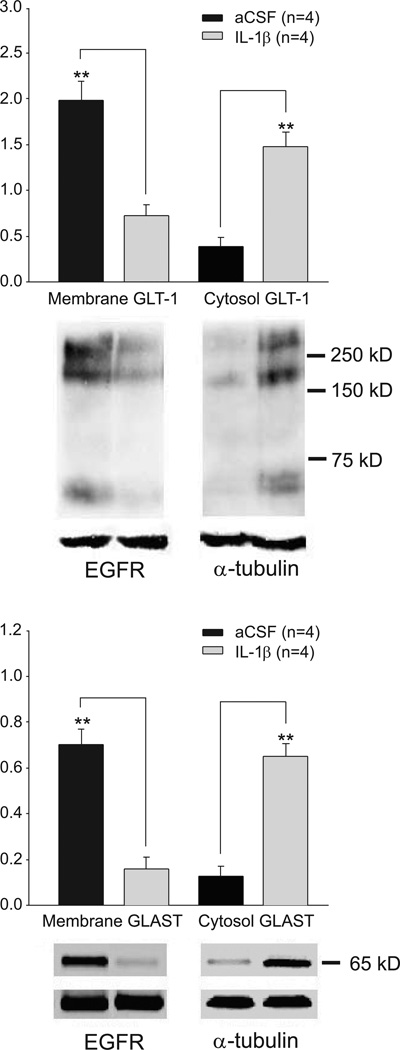
IL-1β reduces GLT-1 and GLAST protein expressions in the plasma membrane
Finally, protein expressions of GLT-1 and GLAST in the cytosol and plasma membrane in the spinal dorsal horn treated with IL−1β were measured by Western blotting. Spinal dorsal horn tissues of the L4-L5 spinal segment were obtained from rat spinal slices (450 µm thick) incubated with IL−1β (10 ng/ml) or aCSF (for controls) for 15 min. In comparison with slices treated with aCSF, spinal slices treated with IL-1β had a significant decrease (p<0.01, n=4) of GLT-1 and GLAST expressions on the cell surface (plasma membrane) and an increase of GLT-1 and GLAST expressions in the cytosol in the spinal dorsal horn (p<0.01, n=4) (Fig. 9). At the same time, the sum protein of GLT-1 (cytosol + plasma membrane) in the slices treated with aCSF (2.38 ± 0.36, n=4) was not significantly different (p=0.67) from that in slices treated with IL-1β (2.20 ± 0.20, n=4). Similarly, the total proteins of GLAST in the slices treated with aCSF (0.83 ± 0.03, n=4) and in the slices treated with IL-1β (0.81 ± 0.03, n=4) were similar (p=0.66). These data consolidate the conclusion that IL-1β reduces glial glutamate transporter activities by promoting GLT-1 and GLAST endocytosis. The lack of alteration in the total protein expressions of GLT-1 and GLAST in the IL- IL-1β treated group also rules out the possibility that changes in the protein synthesis or degradation contribute the altered glial glutamate transporter activities induced by IL-1β in these experimental conditions.
Figure 9. IL-1β reduces GLT-1 and GLAST protein expressions in the cell surface.
Samples of GLT-1 (A) and GLAST (B) protein expressions in the cell surface (plasma membrane) and cytosol in spinal dorsal horn slices treated with IL-1β (n=4 rats) and plain aCSF (n=4 rats) for 15 minutes are shown. Bath graphs show the mean (+S.E.) relative density of GLT-1 and GLAST in the plasma membrane to EGFR, and the mean (+S.E.) relative density of GLT-1 and GLAST in the cytosol to tubulin in each group. *** p< 0.001.
Discussion
Given that aberrant activation of spinal glutamate receptors and over-production of pro-inflammatory cytokines (including IL-1β) are crucial mechanisms in the genesis of pathological pain, understanding molecular signaling pathways through which pro-inflammatory cytokines alter the activation of glutamate receptors would provide therapeutic targets for the development of analgesics. In this study, we for the first time demonstrated that IL-1β reduces glial glutamate transporter activities in the spinal dorsal horn through enhancing the endocytosis of GLT-1 and GLAST glutamate transporters. The IL-1β induced trafficking of glial glutamate transporters is through the calcium/PKC signaling pathway, and the dynamin-dependent endocytosis, which is also dependent on the integrity of actin filaments. As activation of glutamate receptors in the spinal dorsal horn is restrictively controlled by the function of glial glutamate transporters, our findings reveal a novel signaling pathway by which glial cells regulate glutamatergic synaptic activation in the spinal dorsal horn in the neuropathic pain condition.
Role of glial glutamate transporters in the genesis of pathological pain
Excessive activation of glutamate receptors is a culprit in aberrant neuronal activation in pathological pain conditions including neuropathic pain (Ren and Dubner, 2010, Salter and Pitcher, 2012). Activation of glutamate receptors is governed by three key factors: the amount of glutamate release from presynaptic terminals, the function of postsynaptic glutamate receptors, and the function of glutamate transporters (Danbolt, 2001). Glutamate released from presynaptic terminals is not metabolized extracellularly. Instead, clearance of the released glutamate and homeostasis of extracellular glutamate are dependent on glutamate transporters that transport extracellular glutamate into the cell (Danbolt, 2001). It is generally believed that glial glutamate transporters up-take more than 90% of glutamate uptake in the CNS (Tanaka et al., 1997). Studies by us and others in recent years have shown that dysfunction of glial glutamate transporters contributes importantly to the development and maintenance of many types of pathological pain conditions (Mao et al., 2002, Sung et al., 2003, Weng et al., 2005, Nie and Weng, 2010). Previous studies of glutamate uptake activities are based on the synaptosome preparation which reflects the overall glutamate uptake activities by both glial and neuronal glutamate transporters (Sung et al., 2003). Our current study is in agreement with previous studies and extends to demonstrate the specific deficiency in the glial glutamate uptake in neuropathic animals. The impact of impaired glial glutamate transporters on synaptic activities was demonstrated previously by us. Activation of AMPA and NMDA glutamate receptors in spinal sensory neurons is enhanced upon impaired glial glutamate transporter activities (Weng et al., 2007, Nie and Weng, 2009; 2010). More importantly, we provided evidence that deficiency of glutamate transporter activities in animals with neuropathic pain induced by the same type of nerve injury used in this study significantly increases the decay time constants of NMDA receptor currents (Nie and Weng, 2010). Targeting of glutamate transporters to speed up the clearance of glutamate for avoiding excessive activation of glutamate receptors in the spinal dorsal horn appears to be an effective approach for the prevention of neuropathic pain. For example, the genesis of neuropathic pain can be prevented when protein expression of glial glutamate transporters is enhanced by ceftriaxone treatment (Hu et al., 2010) or gene transfer (Maeda et al., 2008). Thus, identifying endogenous molecules regulating glial glutamate transporter functions is a key step towards the development of therapeutics targeting glial glutamate transporters for the treatment of neuropathic pain.
Molecular mechanisms regulating functions of glial glutamate transporters
Glial glutamate transporter functions can be regulated by transcriptional and post-translational mechanisms. Mechanisms about the transcriptional regulation of glial glutamate transporters have been mainly derived from studies on cell cultures from forebrain areas or cellular lines. For example, increased activities of AKT enhance protein expression of GLT-1 through transcriptional regulation (Li et al., 2006). Astrocytes treated with lipopolysaccharide, and chemokines (like macrophage inflammatory protein-2γ, MIP-2γ) exhibit a decreased mRNA and protein expression of GLT-1 (Fang et al., 2012). The MIP-2γ mediated downregulation of GLT-1 expression is eliminated or partially rescued when NF-κB, PI-3 K, PKA, and MEK/ERK are pharmacologically blocked (Fang et al., 2012). In the spinal dorsal horn, modulation of glial glutamate transporter protein expression has been previously reported. Inhibition of astrocytic activation with propentofylline improve protein expression of GLT-1 and GLAST and ameliorates neuropathic pain (Tawfik et al., 2006, Tawfik et al., 2008). Downregulation of GLT-1 and GLAST protein expression in the spinal dorsal horn of rats with morphine tolerance is attenuated by amitriptyline (Tai et al., 2006). Previously we showed that suppression of glial activation with minocycline prevents the downregulation of GLT-1 protein expression in the spinal dorsal horn in rats following partial sciatic nerve ligation (Nie et al., 2010). More recently, we also reported that suppression of glucogen synthesis kinase 3β in the spinal dorsal horn ameliorates protein expression of GLT-1 in the spinal dorsal horn and allodynia in neuropathic rats (Weng et al., 2014). Changes of protein levels may results from transcriptional regulation and/or post-translational regulation like protein degradation. As these studies did not examine mRNA levels of glial glutamate transporters, mechanisms underlying transcriptional regulation of glial glutamate transporters in pathological pain conditions remain to be determined.
Mechanisms of post-translational regulation of glial glutamate transporters at least include nitration, protein degradation, and trafficking of glial of glial glutamate transporters. For example, formation of peroxynitrite in the spinal dorsal horn induced by paclitaxel treatment reduces glial glutamate transporter activities by post-translational nitration of glial glutamate transporters (Doyle et al., 2012). Down-regulation of GLT-1 and GLAST in the spinal dorsal horn of the complete Freund's adjuvant (CFA)-injected rats are ascribed to proteasome-mediated degradation of GLAST and GLT-1 (Kim et al., 2012). Our Western blot data and patch clamp recording results reveal that endocytosis is a critical mechanism by which IL-1β and activation of PKC reduce glial glutamate transporter activities at post-translational levels. Indeed, internalization of GLT-1 has been considered a mechanism by which PKC reduces GLT-1 activities in cell cultures (Garcia-Tardon et al., 2012).
To understand how the IL-1β-induced activation of PKC leads to the internalization of glial glutamate transporters, we examined if the clathrin-dynamin mediated endocytosis is involved in the reduction of GTCs induced IL-1β and the PKC activator. The clathrin-dynamin mediated endocytosis is a crucial mechanism regulating functions of GABA transporter 1 (GAT-1) in astrocytes (Vaz et al., 2011), and dopamine transporters in HEK293 cells (Sorkina et al., 2013). In this study, we found that inhibition of dynamin abolishes the inhibitory effects induced by IL-1β and the PKC activator. It is suggested that the IL-1β induced suppression of glial glutamate transporter activities is mediated through the clathrin-dynamin dependent endocytosis. This is reminiscent of a study by others demonstrating that clathrin-mediated endocytosis is the major pathway of PKC-dependent internalization of dopamine transporter (Sorkina et al., 2013). We further examined the role of cytoskeleton F-actin in the IL-1β-induced reduction of GTCs. F-actin is a major component of the cytoskeleton in the cell (Matus et al., 1982, Allison et al., 1998). The actin cytoskeleton regulates serotonin transporter (Sakai et al., 2000), and glial glutamate transporter (Adolph et al., 2007) in cell cultures. In this study, we found that the IL-1β-induced decrease of GTCs was prevented by agents interfering with actin filaments. These data suggest that IL-1β regulates glial glutamate transporter activities via a mechanism depending on the integrity of actin cytoskeleton.
Conclusions
Our study suggests that dysfunction of glial glutamate transporters contributes to mechanisms by which IL-1β and PKC activation alter glutamatergic synaptic activation in pathological pain conditions. Targeting the signaling pathway used by IL-1β to reduce glial glutamate transporter activities would be a novel approach for the development of analgesics.
Main points.
Impaired glutamate uptake enhances glutamate receptor activation in pathological pain. We found that IL-1β reduces glial glutamate transporter activities through the calcium/PKC signaling, and the dynamin- and actin filament- dependent endocytosis.
Acknowledgements
This project was supported by the National Institute of Neurological Disorders and Stroke RO1NS064289 to H.R.Weng and by the National Natural Science Foundation of China (NO. 81300662) to Xisheng Yan.
Footnotes
The authors declare that there are no conflicts of interest.
Reference List
- Adolph O, Koster S, Rath M, Georgieff M, Weigt HU, Engele J, Senftleben U, Fohr KJ. Rapid increase of glial glutamate uptake via blockade of the protein kinase A pathway. Glia. 2007;55:1699–1707. doi: 10.1002/glia.20583. [DOI] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Hanse E. Astrocytes impose postburst depression of release probability at hippocampal glutamate synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:5776–5780. doi: 10.1523/JNEUROSCI.3957-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000;871:175–180. doi: 10.1016/s0006-8993(00)02430-6. [DOI] [PubMed] [Google Scholar]
- Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One. 2012;7:e47988. doi: 10.1371/journal.pone.0047988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60:1660–1670. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA. Effects of Cytochalasin and Phalloidin on Actin. J cell Biology. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Devaraju P, Sun MY, Myers TL, Lauderdale K, Fiacco TA. Astrocytic group I mGluR-dependent potentiation of astrocytic glutamate and potassium uptake. Journal of neurophysiology. 2013;109:2404–2414. doi: 10.1152/jn.00517.2012. [DOI] [PubMed] [Google Scholar]
- Doyle T, Chen Z, Muscoli C, Bryant L, Esposito E, Cuzzocrea S, Dagostino C, Ryerse J, Rausaria S, Kamadulski A, Neumann WL, Salvemini D. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6149–6160. doi: 10.1523/JNEUROSCI.6343-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Han D, Hong J, Tan Q, Tian Y. The chemokine, macrophage inflammatory protein-2gamma, reduces the expression of glutamate transporter-1 on astrocytes and increases neuronal sensitivity to glutamate excitotoxicity. J Neuroinflammation. 2012;9:267. doi: 10.1186/1742-2094-9-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. Journal of Neuroscience. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Yan X, Weng HR. Inhibition of glycogen synthase kinase 3beta activity with lithium prevents and attenuates paclitaxel-induced neuropathic pain. Neuroscience. 2013;254:301–311. doi: 10.1016/j.neuroscience.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tardon N, Gonzalez-Gonzalez IM, Martinez-Villarreal J, Fernandez-Sanchez E, Gimenez C, Zafra F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. J Biol Chem. 2012;287:19177–19187. doi: 10.1074/jbc.M112.355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. NatNeurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014 doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li W, Lu L, Cai J, Xian X, Zhang M, Li Q, Li L. An anti-nociceptive role for ceftriaxone in chronic neuropathic pain in rats. Pain. 2010;148:284–301. doi: 10.1016/j.pain.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci U S A. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang E, Yan X, Weng HR. Glial glutamate transporter and glutamine synthetase regulate GABAergic synaptic strength in the spinal dorsal horn. J Neurochem. 2012;121:526–536. doi: 10.1111/j.1471-4159.2012.07694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Kobayashi K, Yamanaka H, Noguchi K. COX-1-dependent prostaglandin D2 in microglia contributes to neuropathic pain via DP2 receptor in spinal neurons. Glia. 2013;61:943–956. doi: 10.1002/glia.22487. [DOI] [PubMed] [Google Scholar]
- Kim HN, Kim YR, Jang JY, Shin HK, Choi BT. Electroacupuncture Confers Antinociceptive Effects via Inhibition of Glutamate Transporter Downregulation in Complete Freund's Adjuvant-Injected Rats. Evid Based Complement Alternat Med. 2012;2012:643973. doi: 10.1155/2012/643973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleibeuker W, Gabay E, Kavelaars A, Zijlstra J, Wolf G, Ziv N, Yirmiya R, Shavit Y, Tal M, Heijnen CJ. IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav Immun. 2008;22:200–208. doi: 10.1016/j.bbi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. Journal of Neuroscience. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- Liaw WJ, Stephens RL, Jr., Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Willis WD. Possible role of protein kinase c in the sensitization of primate spinothalamic tract neurons. Journal of Neuroscience. 1996;16:3026–3034. doi: 10.1523/JNEUROSCI.16-09-03026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kawamoto A, Yatani Y, Shirakawa H, Nakagawa T, Kaneko S. Gene transfer of GLT-1, a glial glutamate transporter, into the spinal cord by recombinant adenovirus attenuates inflammatory and neuropathic pain in rats. MolPain. 2008;4:65. doi: 10.1186/1744-8069-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ, Hayes RL. Pain-related increases in spinal cord membrane-bound protein kinase C following peripheral nerve injury. Brain research. 1992;588:144–149. doi: 10.1016/0006-8993(92)91354-h. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci U S A. 1982;79:7590–7594. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. NatRevNeurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Baba H, Woolf CJ. Synaptic transmission and plasticity in the superficial dorsal horn. Prog Brain Res. 2000;129:63–80. doi: 10.1016/S0079-6123(00)29006-7. [DOI] [PubMed] [Google Scholar]
- Nie H, Weng HR. Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn. Journal of neurophysiology. 2009;101:2041–2051. doi: 10.1152/jn.91138.2008. [DOI] [PubMed] [Google Scholar]
- Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. Journal of neurophysiology. 2010;103:2570–2580. doi: 10.1152/jn.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zhang H, Weng HR. Minocycline prevents impaired glial glutamate uptake in the spinal sensory synapses of neuropathic rats. Neuroscience. 2010;170:901–912. doi: 10.1016/j.neuroscience.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita I, Jelaso AM, Ide CF. IL-1beta increases intracellular calcium through an IL-1 type 1 receptor mediated mechanism in C6 astrocytic cells. Int J Dev Neurosci. 1999;17:813–820. doi: 10.1016/s0736-5748(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. JPharmacolExpTher. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Gelfand VI. Membrane trafficking, organelle transport, and the cytoskeleton. Curr Opin Cell Biol. 2000;12:57–62. doi: 10.1016/s0955-0674(99)00057-5. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-Induced Actin Depolymerization Reduces Nmda Channel Activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. The New England Journal of Medicine. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Sakai N, Kodama N, Ohmori S, Sasaki K, Saito N. Involvement of the actin cytoskeleton in the regulation of serotonin transporter (SET) activity: possible mechanism underlying SET regulation by protein kinase C. Neurochem Int. 2000;36:567–579. doi: 10.1016/s0197-0186(99)00160-6. [DOI] [PubMed] [Google Scholar]
- Salter MW. Cellular neuroplasticity mechanisms mediating pain persistence. JOrofacPain. 2004;18:318–324. [PubMed] [Google Scholar]
- Salter MW, Pitcher GM. Dysregulated Src upregulation of NMDA receptor activity: a common link in chronic pain and schizophrenia. Febs J. 2012;279:2–11. doi: 10.1111/j.1742-4658.2011.08390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Petrausch S, Lindenlaub T, Toyka KV. Neutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathy. Neuroscience Letters. 1999;270:25–28. doi: 10.1016/s0304-3940(99)00450-4. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Caltagarone J, Sorkin A. Flotillins regulate membrane mobility of the dopamine transporter but are not required for its protein kinase C dependent endocytosis. Traffic. 2013;14:709–724. doi: 10.1111/tra.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. Journal of Neuroscience. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1á expression that correlates with pain behavior in the rat. Brain Research. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. JPharmacolExpTher. 2001;297:1210–1217. [PubMed] [Google Scholar]
- Sztretye M, Yi J, Figueroa L, Zhou J, Royer L, Allen P, Brum G, Rios E. Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca(2+) release in skeletal muscle. J Gen Physiol. 2011;138:231–247. doi: 10.1085/jgp.201010592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YH, Wang YH, Tsai RY, Wang JJ, Tao PL, Liu TM, Wang YC, Wong CS. Amitriptyline preserves morphine's antinociceptive effect by regulating the glutamate transporter GLAST and GLT-1 trafficking and excitatory amino acids concentration in morphine-tolerant rats. Pain. 2007;129:343–354. doi: 10.1016/j.pain.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006 doi: 10.1016/j.pain.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev. 1999;79:1089–1125. doi: 10.1152/physrev.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Lacroix-Fralish ML, Bercury KK, Nutile-McMenemy N, Harris BT, DeLeo JA. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: heterogeneity of the quiescent phenotype. Glia. 2006;54:193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Regan MR, Haenggeli C, Lacroix-Fralish ML, Nutile-McMenemy N, Perez N, Rothstein JD, DeLeo JA. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience. 2008;152:1086–1092. doi: 10.1016/j.neuroscience.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Beggs S, Salter MW, Inoue K. Microglia and intractable chronic pain. Glia. 2013;61:55–61. doi: 10.1002/glia.22379. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Vaughan RA. Phosphorylation and regulation of psychostimulant-sensitive neurotransmitter transporters. J Pharmacol Exp Ther. 2004;310:1–7. doi: 10.1124/jpet.103.052423. [DOI] [PubMed] [Google Scholar]
- Vaz SH, Jorgensen TN, Cristovao-Ferreira S, Duflot S, Ribeiro JA, Gether U, Sebastiao AM. Brain-derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes. J Biol Chem. 2011;286:40464–40476. doi: 10.1074/jbc.M111.232009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Weng HR, Aravindan N, Cata JP, Chen JH, Shaw AD, Dougherty PM. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci Lett. 2005;386:18–22. doi: 10.1016/j.neulet.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Cata JP. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience. 2006;138:1351–1360. doi: 10.1016/j.neuroscience.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Pan ZZ, Nie H. Glial glutamate transporter 1 regulates the spatial and temporal coding of glutamatergic synaptic transmission in spinal lamina II neurons. Neuroscience. 2007;149:898–907. doi: 10.1016/j.neuroscience.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003;103:131–138. doi: 10.1016/s0304-3959(02)00445-1. [DOI] [PubMed] [Google Scholar]
- Weng HR, Gao M, Maixner DW. Glycogen synthase kinase 3 beta regulates glial glutamate transporter protein expression in the spinal dorsal horn in rats with neuropathic pain. Experimental neurology. 2014;252:18–27. doi: 10.1016/j.expneurol.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Gabay E, Tal M, Yirmiya R, Shavit Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain. 2006;120:315–324. doi: 10.1016/j.pain.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Yan X, Jiang E, Gao M, Weng HR. Endogenous activation of presynaptic NMDA receptors enhances glutamate release from the primary afferents in the spinal dorsal horn in a rat model of neuropathic pain. The Journal of physiology. 2013;591:2001–2019. doi: 10.1113/jphysiol.2012.250522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Weng HR. Endogenous interleukin-1b in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic NMDA receptors. J Biol Chem. 2013;288:30544–30557. doi: 10.1074/jbc.M113.495465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HW, Hu XD, Zhang HM, Xin WJ, Li MT, Zhang T, Zhou LJ, Liu XG. Roles of CaMKII, PKA, and PKC in the induction and maintenance of LTP of C-fiber-evoked field potentials in rat spinal dorsal horn. Journal of Neurophysiology. 2004;91:1122–1133. doi: 10.1152/jn.00735.2003. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Fisher K, Chabot JG, Coderre TJ. Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats. Pain. 2001;94:17–29. doi: 10.1016/S0304-3959(01)00337-2. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Xin WJ, Dougherty PM. Synaptically evoked glutamate transporter currents in Spinal Dorsal Horn Astrocytes. Molecular Pain. 2009;5 doi: 10.1186/1744-8069-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]