Abstract
β-thalassemia and Polycythemia vera are genetic disorders which affect the synthesis of red blood cells, also referred to as erythropoiesis. Although essentially different in clinical presentation – patients with β-thalassemia have an impairment in β-globin synthesis leading to defective erythrocytes and anemia, while patients with Polycythemia vera present with high hemoglobin levels because of excessive red blood cell synthesis – both pathologies may characterized by lasting high erythropoietic activity, i.e. chronic stress erythropoiesis. In both diseases, therapeutic strategies targeting chronic stress erythropoiesis may improve the address phenotype and prevent secondary pathology, such as iron overload. The current review will address the basic concepts of these strategies to reduce chronic stress erythropoiesis, which may have significant clinical implications in the near future.
Keywords: β-thalassemia, Polycythemia vera, chronic stress erythropoiesis, iron metabolism, macrophages
1. Introduction
β-thalassemia (BT) represents one of the most common causes of congenital anemia. While BT has an estimated world-wide incidence of 1:100.000, the genetic defects that cause it are, similar to many other hemoglobinopathies, mostly found in newborns originating from (sub)tropical regions, such as the Mediterranean, northern Africa and southeast Asia (Ginzburg and Rivella, 2011). Due to the serious anemia associated with β-thalassemia major, patients are usually diagnosed within the first two years of life – displaying general symptoms of anemia, such as paleness, irritability and failure to thrive – and require chronic treatment, including transfusions with iron-chelation therapy (Higgs et al., 2012). The anemia in β-thalassemia intermedia, which diagnosis is based on the later onset of presentation (between the age of 2 and 6 years), is generally less prominent and does not always require therapeutic intervention. However, due to the anemia-induced elevated erythropoietic activity, in some cases in combination with regular blood transfusions, these patients often develop iron overload and are consequently at risk of secondary morbidity including cardiac disease. Finally, β-thalassemia minor is only characterized by an, often asymptomatic, microcytic anemia.
Polycythemia vera (PV) is associated with an uncontrolled proliferation of cells of the myeloid linage, leading to expanded numbers of erythrocytes, megakaryocytes and granulocytes. Affecting primarily a population of over 60 years of age, PV is currently defined by the WHO by a hemoglobin (Hb) concentration higher than 18.5 g/dL (men) or 16.5 g/dL (women), or ‘other evidence of increased red cell volume’; as well as the presence of a Janus kinase 2 (Jak2) mutation (major criteria). Since thrombotic complications form the main cause of concern in patients with PV, therapeutic management is aimed at reducing the risk of major thrombosis and other cardiovascular morbidity by reducing the red blood cell volume and/or production to achieve a hematocrit below 0.45 through phlebotomy or the administration of hydroxyurea, respectively (Marchioli et al., 2013).
2. Pathogenesis
2.1. Steady-state erythropoiesis
Erythropoiesis involves the process of proliferation and differentiation of new red blood cells, which at steady-state conditions primarily occurs in the bone marrow.
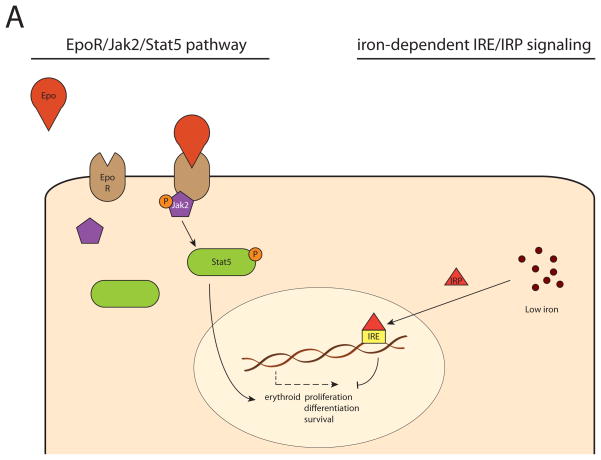
One key player is erythropoietin (Epo), in adults primarily produced in the kidney, which regulates the erythropoietic activity in response to cellular hypoxia. In turn, Epo expression is mediated by hypoxia inducible factor (HIF) (Haase, 2013).
Upon the binding of Epo to its receptor (EpoR), the tyrosine kinase Jak2 is phosphorylated, which subsequently activates Stat5. Finally, Stat5 initiates the expression of genes responsible for erythroid proliferation, differentiation and survival (Socolovsky, 2001).
Another key player is the iron pool, balanced by hepcidin, a peptide that controls iron metabolism by limiting iron absorption and cellular iron egress through degradation of ferroportin. The cellular iron balance is influenced by the iron-responsive element (IRE)-iron regulatory protein (IRP) system (Hentze et al., 2010). IRPs modulate under low-iron conditions the intracellular iron metabolism by binding IREs; depending on the exact position of the complementary IRE, they stimulate expression of genes associated with cellular iron uptake and limit expression of those associated with iron egress and utilization (Anderson et al., 2013). Low iron levels therefore induce a reduction of erythropoietic activity, and conversely, IRE/IRP-mediated iron regulation in erythroid cells depends significantly on Stat5 activity, illustrating the strong embrace between erythropoiesis and iron metabolism (Kerenyi et al., 2008).
2.2. Stress erythropoiesis
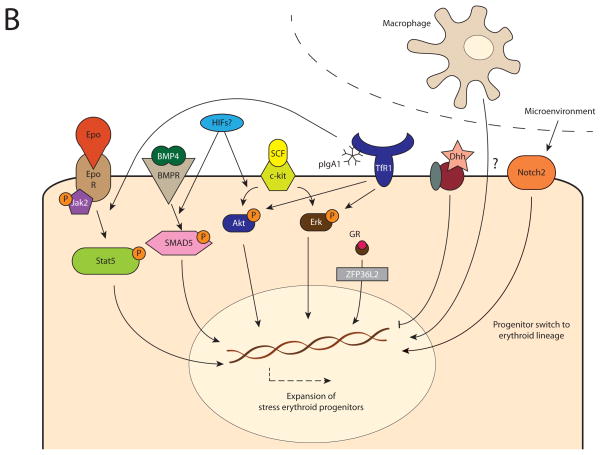
When steady-state erythropoiesis is insufficient to provide adequate levels of oxygenation, the rate of erythropoiesis may be expanded significantly, also known as stress erythropoiesis. Although there remains a clear and prominent role for the EpoR/Stat5 pathway (Goodell et al., 2012, Peslak et al., 2012), there are a number of additional mechanisms that direct stress erythropoiesis. Bone morphogenic protein 4 (BMP4) induces, through SMAD5 signaling, the proliferation of stress erythroid progenitors, which are phenotypically different from steady-state progenitors (Lenox et al., 2005). In parallel, stem cell factor (SCF) and its receptor, c-kit, have a driving role in the expansion of stress progenitors via Erk and Akt, which is potentiated by concomitant activation of the glucocorticoid receptor (GR) (Varricchio et al., 2012). Specifically, the GR stimulates, through expression of the downstream RNA-binding protein ZFP36L2, the self-renewal of erythroid progenitors by preventing their differentiation, thereby ensuring erythroid expansion (Zhang et al., 2013). Interestingly, but not surprisingly, the efficiency of BMP4 and SCF/c-kit signaling is strongly dependent on the cellular oxygenation level. In hypoxic conditions, by a mechanism that likely involves HIFs, stress progenitors are more sensitive to the proliferative induction of BMP4, SCF and GR-signaling, while the expression of BMP4 itself is modulated by the oxygen-dependent HIF-2α (Flygare et al., 2011, Perry et al., 2007).
Furthermore, several studies demonstrated a controlling role for the embryogenesis-associated hedgehog signaling pathway in stress erythropoiesis, although its exact role remains to be completely elucidated. While Desert hedgehog (Dhh) has been reported to be a negative, i.e. inhibiting, regulator of the proliferation and differentiation of steady-state and stress progenitors, it has been demonstrated that some hedgehog signaling is required for ensuring sensitivity of stress progenitors to the BMP4-induced stress response (Lau et al., 2012, Perry et al., 2009).
Another fundamental signaling pathway, the Notch pathway, has been implicated with erythropoiesis as well (Oh et al., 2013). In contrast to Notch receptor 1, which is associated with the commitment of early progenitors to the lymphoid lineage, Notch receptor 2 drives differentiation towards the erythroid phenotype. While at steady state Notch 2 receptor deficiency in mice is associated with reduced numbers of erythroid progenitors, without overt anemia, under erythropoietic stress this is associated with an adequate erythroid response and severe deficit in all stages of erythroid differentiation, indicating that Notch 2 signaling is a key factor in directing the fate of early progenitors towards an erythroid destiny.
Finally, anti-inflammatory polymeric immunoglobulin A1 (pIgA1) activates stress erythropoiesis through an alternative, transferrin receptor 1 (TfR1)-mediated, pathway (Coulon et al., 2011). Produced by B cells in response to tissue hypoxia (e.g. in anemia), pIgA1s can bind TfR1 and activate downstream Erk and Akt signaling, which is independent of, but additive to the binding of transferrin to TfR1. In addition to its induction of Erk/Akt signaling, the binding of pIgA1 to TfR1 potentiates the activity of Epo on EpoR-Stat5 signaling, but the mechanism by which this occurs remains unclear.
2.3. Macrophages and (stress) erythropoiesis
In addition to regulation on a molecular level, erythropoietic activity is also controlled by the erythroid microenvironment. The formation of new red blood cells generally takes place under the supervision of macrophages; which can be observed macroscopically as a central macrophage surrounded by differentiating erythroblasts, the erythroid island. Upon depletion of macrophages the number of erythroblasts is significantly reduced, both in steady-state and stress erythropoiesis, suggesting that these central macrophages have a supporting role in erythropoiesis, which takes place through direct cellular interactions (Chow et al., 2013, Ramos et al., 2013).
There are several candidates that may be associated with the macrophage-erythroblast crosstalk. Some adhesion molecules are under investigation, including integrin β3 (Wang et al., 2013), and integrin α4β1 which is highly expressed on developing erythroblasts and may interact with VCAM-1 present on macrophages (Ulyanova et al., 2014). In addition, focal adhesion kinase (FAK), located downstream of the integrin signaling pathway, has been associated with the stress erythroid response, possibly through mediation of the Epo/STAT5 pathway (Vemula et al., 2010).
2.4. Pathogenesis of β-thalassemia and Polycythemia vera: chronic stress erythropoiesis
In BT, homozygous or compound heterozygous mutations in the β-globin gene or promoter impair the production of β-globins. This results in the relative overproduction of α-globins and formation of insoluble hemichromes, which is associated with increased oxidative stress and erythroid apoptosis. The short life span and inefficient oxygen-carrying ability of these imperfect erythrocytes causes chronic anemia in patients with β-thalassemia, which, in turn, stimulates erythropoietic activity, resulting in a permanent state of stress erythropoiesis, i.e. chronic stress erythropoiesis. This enduring focus on red blood cell production has a counterproductive effect, and in fact promotes the α/β-globin imbalance is even further.
In PV, 98% of patients present with a mutation in JAK2, in most cases related to the V617F allele (Tefferi et al., 2013). By inducing in a gain of function of JAK2, these mutations are associated with constitutively activated downstream signaling pathways, including the JAK2/STAT5 pathway, causing chronic stress erythropoiesis and an excessive red blood cell volume.
3. Therapy
In both BT and PV, although essentially different pathologies with contrasting clinical features, there is a crucial role for chronic stress erythropoiesis (CSE) in the disease phenotype. Whereas in Polycythemia vera CSE leads to a overproduction of erythrocytes, in BT it stimulates the production of imperfect erythrocytes, also known as ineffective erythropoiesis, further aggravating the anemia. In animal models it has been demonstrated that there is a beneficial effect of reducing CSE in both PV and BT, resulting in lower and higher levels of Hb, respectively.
3.1. Targeting Epo/Jak2/Stat5 pathway
The Jak2 defects that underlie the redundant Jak2 activation in PV, suggest that Jak2 inhibition may be an excellent therapeutic strategy. Indeed, a number of Jak2 inhibitors have been evaluated in human trials, but unfortunately, high toxicity associated to a small therapeutic index forms a serious impediment for their clinical application.
Jak2 inhibition has also demonstrated successful reduction of ineffective erythropoiesis and splenomegaly in thalassemic mice (Libani et al., 2008). Although a (partial) correction of anemia by Jak2 inhibition alone could not be achieved, this therapy may have clinical significance in thalassemia patients where ineffective erythropoiesis, causing iron overload, and splenomegaly form serious problems.
3.2. Targeting iron metabolism
Reduction of erythroid iron availability limits CSE, as hemoglobin synthesis requires iron, making it a viable strategy in BT and PV. In BT, the combination of high erythroid iron demand, resulting in high systemic iron absorption, regular blood transfusions, and the lack of a physiological way to excrete iron from the body, makes iron overload a significant problem in CSE. In PV, therapeutic phlebotomies limit the total body iron accumulation. Therefore, targeting CSE through iron metabolism may limit erythroid expansion in both PV and BT, reducing iron overload in BT and normalizing iron content in PV.
In BT, this principle has been utilized by a variety of approaches. Inhibition of TMPRSS6, a serine protease involved in reducing hepcidin expression, successfully increased hepcidin expression and reduced the iron load in mice with BT, while limiting CSE and improving anemia (Guo et al., 2013, Schmidt et al., 2012). Similarly, a small hepcidin-derived peptide, minihepcidin, demonstrated iron-restricting activity in animal models of iron-overload, and may therefore be of high clinical relevance for the management of BT (Ramos et al., 2012). The administration of transferrin, the circulating protein responsible for iron transport, forms another approach to limit erythroid iron availability by reducing the amount of iron ions delivered by each transferrin molecule, which ameliorated CSE and improved anemia. (Li et al., 2010).
3.3. Targeting macrophage interaction
While having demonstrated to limit CSE in animal models of BT and PV (Ramos et. al, 2013), the depletion of macrophages severely compromises the innate immune system, increasing risk of opportunistic infections, and will therefore as such not have a clinical future. However, specifically interfering with the macrophage-erythroblast interaction, without affecting their immunological function, may be a very viable strategy to limit CSE. Studies are currently underway to identify the players involved in the cellular crosstalk, allowing the development of new therapies directed at the associated pathways, thereby limiting macrophage-induced erythropoietic activity and attenuating CSE in disorders such as β-thalassemia and Polycythemia vera.
Figure 1.
Cellular mechanisms regulating steady-state and (chronic) stress erythropoiesis. In steady-state erythropoiesis, erythroid proliferation, differentiation and survival is primarily dependent on EpoR/Jak2/Stat5 signaling and iron-dependent IRE/IRP modulation (A). In (chronic) stress erythropoiesis, the expansion of stress erythroid progenitors is regulated by several other mechanisms in addition to the EpoR/Jak2/Stat5 pathway. This includes BMP4 signaling through SMAD4 and SCF/c-kit signaling through Akt/Erk, which are both dependent on oxygenation, possibly via HIFs; pIgA1-mediated transferrin receptor 1 (TfR1) activation which stimulates Akt/Erk and potentiates EpoR/Stat5 signaling; glucocorticoid receptor (GR) signaling via ZFP36L2; and the desert Hedgehog (Dhh) pathway, which inhibits the response. Moreover, there is a regulating role of the microenvironment through Notch2 signaling, which stimulates switching of early progenitors towards the erythroid lineage, as well as macrophage-associated regulation, which stimulate erythropoiesis through a currently unknown mechanism (B).
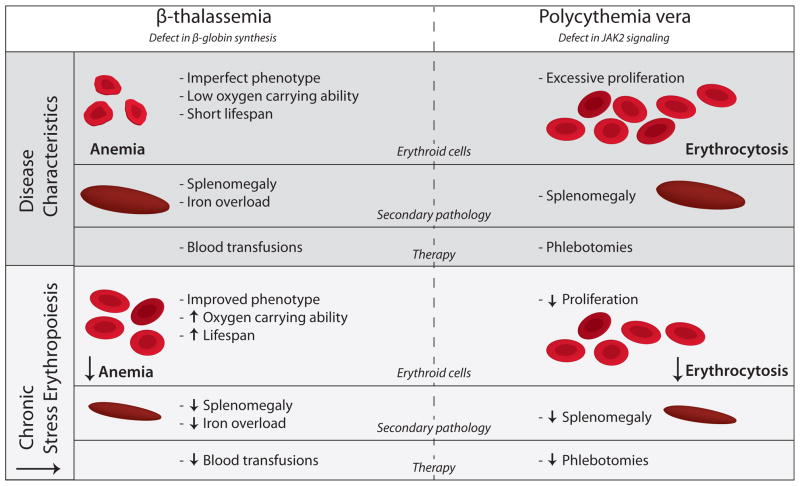
Figure 2.
Disease characteristics of β-thalassemia and Polycythemia and the effect of reducing chronic stress erythropoiesis.
β-thalassemia is characterized by a defect in β-globin synthesis, leading to the formation in imperfect erythrocytes with reduced oxygen carrying ability and short lifespan. As a result, patients have anemia, splenomegaly, and iron overload due to increased iron absorption. Therapy consists of blood transfusions, which may aggravate iron overload even further. Polycythemia vera is caused by a defect in Jak2 signaling, resulting in excessive erythrocyte production, causing erythrocytosis and splenomegaly. Therapy includes phlebotomies to reduce hematocrit and prevent secondary complications.
In both disorders reduction of chronic stress erythropoiesis improves the pathological phenotype, leading to reduction of anemia, splenomegaly and iron overload in case of β-thalassemia, and reduction of hematocrit and splenomegaly in case Polycythemia vera. As a consequence, the frequency of therapeutic interventions may be reduced.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson Sheila A, Nizzi Christopher P, Chang Y-I, Deck Kathryn M, Schmidt Paul J, Galy B, et al. The IRP1-HIF-2α Axis Coordinates Iron and Oxygen Sensing with Erythropoiesis and Iron Absorption. Cell Metabolism. 2013;17:282–90. doi: 10.1016/j.cmet.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature Medicine. 2013;19:429–36. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon S, Dussiot M, Grapton D, Maciel TT, Wang PHM, Callens C, et al. Polymeric IgA1 controls erythroblast proliferation and accelerates erythropoiesis recovery in anemia. Nature Medicine. 2011;17:1456–65. doi: 10.1038/nm.2462. [DOI] [PubMed] [Google Scholar]
- Flygare J, Estrada VR, Shin C, Gupta S, Lodish HF. HIF1 synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood. 2011;117:3435–44. doi: 10.1182/blood-2010-07-295550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg Y, Rivella S. β-thalassemia: a model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood. 2011;118:4321–30. doi: 10.1182/blood-2011-03-283614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Porpiglia E, Hidalgo D, Koulnis M, Tzafriri AR, Socolovsky M. Stat5 Signaling Specifies Basal versus Stress Erythropoietic Responses through Distinct Binary and Graded Dynamic Modalities. PLoS Biology. 2012;10:e1001383. doi: 10.1371/journal.pbio.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Casu C, Gardenghi S, Booten S, Aghajan M, Peralta R, et al. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. Journal of Clinical Investigation. 2013;123:1531–41. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Reviews. 2013;27:41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379:373–83. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- Kerenyi MA, Grebien F, Gehart H, Schifrer M, Artaker M, Kovacic B, et al. Stat5 regulates cellular iron uptake of erythroid cells via IRP-2 and TfR-1. Blood. 2008;112:3878–88. doi: 10.1182/blood-2008-02-138339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Ci, Outram SV, Ignacio Saldana J, Furmanski AL, Dessens JT, Crompton T. Regulation of murine normal and stress-induced erythropoiesis by Desert Hedgehog. Blood. 2012;119:4741–51. doi: 10.1182/blood-2011-10-387266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105:2741–8. doi: 10.1182/blood-2004-02-0703. [DOI] [PubMed] [Google Scholar]
- Li H, Rybicki AC, Suzuka SM, von Bonsdorff L, Breuer W, Hall CB, et al. Transferrin therapy ameliorates disease in β-thalassemic mice. Nature Medicine. 2010;16:177–82. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- Libani IV, Guy EC, Melchiori L, Schiro R, Ramos P, Breda L, et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in β-thalassemia. Blood. 2008;112:875–85. doi: 10.1182/blood-2007-12-126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular Events and Intensity of Treatment in Polycythemia Vera. New England Journal of Medicine. 2013;368:22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- Oh P, Lobry C, Gao J, Tikhonova A, Loizou E, Manent J, et al. In Vivo Mapping of Notch Pathway Activity in Normal and Stress Hematopoiesis. Cell Stem Cell. 2013;13:190–204. doi: 10.1016/j.stem.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JM, Harandi OF, Paulson RF. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood. 2007;109:4494–502. doi: 10.1182/blood-2006-04-016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JM, Harandi OF, Porayette P, Hegde S, Kannan AK, Paulson RF. Maintenance of the BMP4-dependent stress erythropoiesis pathway in the murine spleen requires hedgehog signaling. Blood. 2009;113:911–8. doi: 10.1182/blood-2008-03-147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peslak SA, Wenger J, Bemis JC, Kingsley PD, Koniski AD, McGrath KE, et al. EPO-mediated expansion of late-stage erythroid progenitors in the bone marrow initiates recovery from sublethal radiation stress. Blood. 2012;120:2501–11. doi: 10.1182/blood-2011-11-394304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos E, Ruchala P, Goodnough JB, Kautz L, Preza GC, Nemeth E, et al. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood. 2012;120:3829–36. doi: 10.1182/blood-2012-07-440743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Guy E, et al. Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nature Medicine. 2013;19:437–45. doi: 10.1038/nm.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Toudjarska I, Sendamarai AK, Racie T, Milstein S, Bettencourt BR, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe−/− mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood. 2012;121:1200–8. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolovsky M. Ineffective erythropoiesis in Stat5a−/−5b−/− mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–73. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874–81. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulyanova T, Padilla SM, Papayannopoulou T. Stage specific functional roles of integrins in erythropoiesis. Experimental Hematology. 2014 doi: 10.1016/j.exphem.2014.01.007. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio L, Tirelli V, Masselli E, Ghinassi B, Saha N, Besmer P, et al. The Expression of the Glucocorticoid Receptor in Human Erythroblasts is Uniquely Regulated by KIT Ligand: Implications for Stress Erythropoiesis. Stem Cells and Development. 2012;21:2852–65. doi: 10.1089/scd.2011.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula S, Ramdas B, Hanneman P, Martin J, Beggs HE, Kapur R. Essential role for focal adhesion kinase in regulating stress hematopoiesis. Blood. 2010;116:4103–15. doi: 10.1182/blood-2010-01-262790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Vogel O, Kuhn G, Gassmann M, Vogel J. Decreased stability of erythroblastic islands in integrin β3-deficient mice. Physiological Reports. 2013;1:n/a–n/a. doi: 10.1002/phy2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Prak L, Rayon-Estrada V, Thiru P, Flygare J, Lim B, et al. ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature. 2013;499:92–6. doi: 10.1038/nature12215. [DOI] [PMC free article] [PubMed] [Google Scholar]