Abstract
Acute coronary syndromes (ACS) constitute a spectrum of clinical presentations ranging from unstable angina and non-ST-segment elevation myocardial infarction to ST-segment myocardial infarction. Myocardial ischemia in this context occurs as a result of an abrupt decrease in coronary blood flow and resultant imbalance in the myocardial oxygen supply-demand relationship. Coronary blood flow is further compromised by other mechanisms that increase coronary vascular resistance or reduce coronary driving pressure. The goals of treatment are to decrease myocardial oxygen demand, increase coronary blood flow and oxygen supply, and limit myocardial injury. Treatments are generally divided into “disease-modifying” agents or interventions that improve hard clinical outcomes and other strategies that can reduce ischemia. In addition to traditional drugs such as beta-blockers and inhibitors of the reninangiotensin-aldosterone system, newer agents have expanded the number of molecular pathways targeted for treatment of ACS. Ranolazine, trimetazidine, nicorandil, and ivabradine are medications that have been shown to reduce myocardial ischemia through diverse mechanisms and have been tested in limited fashion in patients with ACS. Attenuating the no-reflow phenomenon and reducing the injury compounded by acute reperfusion after a period of coronary occlusion are active areas of research. Additionally, interventions aimed at ischemic pre- and post-conditioning may be useful means by which to limit myocardial infarct size. Trials are also underway to examine altered metabolic and oxygen-related pathways in ACS. This review will discuss traditional and newer anti-ischemic therapies for patients with ACS, exclusive of revascularization, anti-thrombotic agents, and the use of high-intensity statins.
Keywords: Acute coronary syndrome, reperfusion injury, pre-conditioning
Introduction
Pathophysiology of Myocardial Ischemia
Myocardial ischemia derives from an imbalance between myocardial oxygen supply and demand. As myocardial oxygen extraction is near maximal in the resting state, any increase in tissue oxygen delivery in response to increased demand occurs mainly through an increase in coronary blood flow (CBF).1,2 In the setting of an acute coronary syndrome (ACS), ischemia is driven in large measure by an abrupt decrease in CBF consequent to the formation of a fully or partially obstructing platelet-fibrin thrombus at the site of rupture or erosion of a vulnerable plaque.3 CBF can be further compromised by reactive constriction of vascular smooth muscle cells, leading to deleterious increases in coronary vascular resistance (CVR) at the arteriolar level and further narrowing of the larger, epicardial conduit artery. CVR is mediated by a host of factors, including paracrine factors released from activated platelets, adenosine, local availability of nitric oxide (NO), prostacyclin, endothelin, hypoxia, acidosis, adenosine tri-phosphate (ATP)-sensitive K+ channels, neurohumoral agonists, neural tone, and vascular shear stress.4,5 An intact and functional endothelium serves as the gatekeeper. Atherosclerosis leads to a reduction in NO availability and paradoxical increases in CVR on exposure to substances such as acetylcholine, which would otherwise cause vasodilation and a fall in CVR in normal arterial segments.6
Myocardial compressive forces also contribute to CVR. Nutrient supply to the more vulnerable subendocardium can be compromised by increases in left ventricular (LV) diastolic pressure with a resultant decrease in the coronary driving pressure, which is represented by the difference between the aortic and LV diastolic pressures. Isolated reductions in aortic diastolic pressure, as seen in patients with significant aortic regurgitation or in some elderly patients with aggressively treated systolic hypertension, may also decrease coronary driving pressure. Higher LV diastolic pressures in the context of ischemia can further reduce coronary driving pressure. Because the majority of left coronary artery blood flow occurs during diastole, decreases in diastolic filling times at rapid heart rates will also reduce CBF. Myocardial oxygen supply is additionally influenced by the oxygen-carrying capacity of blood, and severe anemia (hemoglobin < 7g/dL) may precipitate ischemia in vulnerable patients.7 In some patients with chronic coronary artery disease (CAD), the extent and severity of an acute ischemic insult can be attenuated by flow through collateral channels.
The three major drivers of myocardial oxygen demand (consumption) are heart rate, contractility, and wall stress.1 Increases in heart rate both reduce diastolic filling time and increase oxygen demand. Compensatory increases in contractility of non-ischemic/non-infarcted myocardial segments can also increase demand. Wall stress is approximated by the Laplace relationship and varies directly as a function of chamber pressure and radius and inversely as a function of wall thickness [stress=(pressure × radius)/(2 × wall thickness)]. Thus, wall stress increases as the LV dilates and chamber pressures rise. Most anti-ischemic pharmacological strategies are targeted to modulating one or more components of the oxygen supply-demand relationship.
Myocardial Ischemia and Reperfusion
The time course of irreversible, ischemic myocardial injury (infarction) depends on the interplay between the forces dictating myocardial oxygen supply and demand. Infarction (myocyte necrosis) typically develops after 20 minutes of complete coronary artery obstruction in the absence of collateral flow. The injury begins in the subendocardial layer and spreads centrifugally as a “wave front” to the subepicardium with completion of a nearly complete transmural infarction by 6 hours. Reperfusion injury with release of oxygen-free radicals and other toxic substances can further accelerate myocardial cell death; migration of leukocytes into the area of injury amplifies the process.8,9 Later in the time course of evolution, programmed cell death (apoptosis) occurs, adding to the amount of injury but doing so independently of the inflammatory cascade. Autophagy is an additional mechanism leading to cell death in the chronic phase of healing.10 Attenuation of these several processes may limit the extent of injury and adverse post-infarction remodeling.
Following acute reperfusion in the setting of coronary occlusion or relief of a severe ischemic insult, regional myocardial function remains depressed for variable periods of time, a process referred to as stunning and indicative of a downward shift in the tightly coupled relationship between CBF and function.11 Functional recovery is anticipated in the absence of repeated ischemic insults. Importantly, restoration of epicardial flow following a period of occlusion does not always correlate with tissue perfusion, which may be further compromised by endothelial cell swelling, micro-embolization, and vasoconstriction.8 The angiographic demonstration of “no-reflow” following culprit artery recanalization equates with larger infarcts and higher risks of complications. Mechanical and pharmacological efforts to improve tissue perfusion in this context have thus far met with neutral or at best mixed results.
Attempts to reduce infarct size and shorten the period of regional myocardial dysfunction via pre- and/or post-conditioning mechanisms may also be of value. Ischemic pre-conditioning attenuates myocyte necrosis by the production of brief, reversible periods of ischemia (induced either locally or remotely) prior to coronary artery occlusion.12,13 Patients with an antecedent history of angina tend to have smaller infarcts than those who do not have angina leading up to the acute event. Similarly, the magnitude of ST-segment elevation seen during coronary artery occlusion at time of PCI decreases with each successive balloon inflation. Delayed pre-conditioning can persist for several days after coronary artery occlusion and may protect the heart from ischemia-reperfusion mediated stunning. Ischemic post-conditioning may reduce the amount of myocardial necrosis by the production of intermittent ischemia or delivery of pharmacological agonists at the time of reperfusion, a strategy that may hold relatively greater promise for clinical application.
Non-Atherosclerotic ACS
There are several non-atherosclerotic causes of ACS, including, among others, primary vasospasm (Prinzmetal or variant angina), stress-cardiomyopathy (Takotsubo), cocaine ingestion, and spontaneous coronary dissection. The several mechanisms by which these entities result in myocardial ischemia include intense vasoconstriction, platelet-activation, endothelial dysfunction, catecholamine toxicity, and/or luminal obstruction from an intimal flap.14-16 In the case of spontaneous or cocaine-induced spasm, vasodilator therapy with nitrates and/or calcium channel blockers constitutes the initial management strategy. There is no effective therapy for stress-cardiomyopathy, a transient phenomenon presumably due to catecholamine excess and regionalized left ventricular stunning. 16,17
Overview of Therapy for ACS
The term, ACS, constitutes a spectrum of clinical disorders, ranging from unstable angina (UA) to non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation MI (STEMI). Differentiation of these entities is based on ECG interpretation and measurement of elevated cardiac biomarker levels. The clinical manifestations of the syndrome, in turn, relate chiefly to the extent and duration of coronary artery obstruction. Therapies in addition to urgent mechanical reperfusion/revascularization and anti-thrombotic agents are important adjuncts to any management strategy that seeks to limit the ischemic insult and its downstream consequences. Whereas some interventions may only attenuate the symptoms and signs of acute ischemia, others may indeed be “disease-modifying,” by virtue of their proven ability to reduce the risk of hard clinical outcomes, including recurrent MI and death. Late events after treatment of ACS can develop as a consequence of the initial injury (arrhythmia, LV remodeling, heart failure, aneurysm formation, functional mitral regurgitation) or because of repeated ischemic insults in the same or remote arterial distributions. Aggressive efforts at secondary prevention, therefore, are critical. In the following sections, we will focus on traditional (Tables 1-2) and newer “anti-ischemic” (Table 3) methods of treatment for ACS, exclusive of revascularization, anti-thrombotic strategies, and lipid lowering treatments. Information about the use of such traditional medical therapies is especially relevant in countries with developing economies where 80% of cardiovascular mortality now occurs and percutaneous coronary intervention (PCI) is either too costly or not available.
Table 1.
Traditional Pharmacological Approaches for Acute Coronary Syndrome (ACS)
| Class of Medications | Mechanism of Action | Efficacy for Hard Clinical Outcomes in ACS |
|---|---|---|
| β-Blockers | Decrease heart rate, blood pressure, and contractility through antagonism of β1 receptors | Decrease mortality in ACS,18–20 Intravenous use increases risk of cardiogenic shock,22 Long-term secondary prevention benefit is less certain in patients without heart failure or reduced left ventricularsystolic function21 |
| Nitrates | Decrease preload through venodilation; vasodilate coronary arteries | No benefit on mortality31,32 |
| Calcium channel blockers | May vasodilate, reduce heart rate, or decrease contractility depending on specific drug | No clear benefit on mortality or reinfarction in ACS,35 Increased reinfarction rate when nifedipine is used alone in ACS34 |
| Angiotensin-converling enzyme inhibitors | Block conversion of angiotensin 1 to angiotensin II and decrease systemic vascular resistance | Decrease mortality and heart failure when used in acute myocardial infarction.31,32,37,38 Long-term secondary prevention benefit in patients with normal ejection fraction is less certain41–44 |
| Angiotensin II receptor blockers | Displace angiotensin II from the angiotensin II type 1 receptor and decrease systemic vascular resistance | Noninferiorto ACE-I in reducing mortality and cardiovascular events after ST-segment elevation myocardial infarction47 |
| Aldosterone antagonists | Competitively inhibit binding of aldosterone to receptors and prevent detrimental effects of renin–angiotensin–aldosterone system cascade | Reduce mortality and sudden cardiac death in patients with acute Ml with reduced ejection fraction and symptoms of heart failure or diabetes mellitus48 |
Table 2.
Summary of Major Society Guideline Recommendations for ACS Therapies
| Class of Medications | ACC/AHA Recommendations | ESC Recommendations |
|---|---|---|
| β-Blockers | •(Vblockers should be initiated <24 h of any ACS if no contraindications (1B} • β-blockers should be continued for <3 y after ACS presentation in the absence of LV dysfunction (1 A); indefinite therapy can be considered (lla B) |
•β-blockers should be considered in all patients witii STEMI without contraindications (lla B) •β-blockers are indicated in all patients with LV dysfunction(STEMI: 1 A; NSTEMI: 1 B) |
| N trates | •Nitrates should be administered for ongoing ischemia in UA/NSTEMI (IC) •No recommendation for use in STEM 1 |
•Nitrates are indicated to relieve angina in NSTEMI (1 C) •Mitrates are recommended in STEMI for Killip class III heart failure (1 C) and for hypertension (lla C) |
| CCBs | •Nonelihydropyridine CCBs should be given for ongoing schem ain NSTEMI if β-blockers are contraindicated (1B) •Nondihydropyridine CCBs should bp given for recurrentischemia in NSTEMI after β-blockers and nitrates have beenfully used (lla C) •Immediate-release dihydropyridine CCBs should not beadministered in the absence of βblockers (III A} •No recornmendat on for use in &TEMI |
•CCBs are recommended for symptom relief in NSTEMI inpatients already receiving βblockers and nitrates or with contraindications to β-blockers (1B) •CCBs are recommended in vasospastic angina (1C) •Dihydropyridine CCBs should not be administered in the absence of (β-blockers (III B) •Mo recommendation for use in STEMI |
| ACE-ls | •ACE-ls should be administered <24 h of STEMI in patients withanterior Ml, HR. or LVEF ≤0.40 (1 A}; reasonable to use in all other patients (lla A) •ACE-ls should be administered <24 h of UA/NSTEMI in patients with pulmonary congestion or LVEF ≤0.40 (1 A); reasonable to use in all other patients (lla B) |
•ACE-ls should be administered <24 h of STEMI to patientswith anterior Ml, HP diabetes mellitus, or LVEF ≤0.40 (1 A); reasonable to use in all other patients (lla A} •ACE-ls should be administered within <24 h of NSTEMI to patients with HF, diabetes mellitus. hypertension, renal diseas or LVEF ≤0.40 (1 A); recommended for all other patients (1 B) |
| ARBs | •ARBs should be given to patients with STEMI who have indications for but are intolerant of ACE-ls (1 B) •ARBs should be given to patients with UA/NSTEMI who are intolerant of ACE-ls with signs of HF or LVEF ≤0.40 (1 A) |
•ARBs (especially valsartan) are alternatives to ACE-ls in patien with STEMI or HF or LVEF≤0.40, especially if intolerant to ACE-ls (1B) •ARBs are recommended in patients with NSTEMI intolerant of ACE-ls (1B) |
| Aldosterone antagonists | • Aldosterone antagonists should be given to patients with ACS without contraindications who are already on an ACE-ls and β-blockers and who have an LVEF ≤0.40, symptomatic HF, or diabetes mellitus (STEMI: 1 B; UA/NSTEMI: 1 A) | • Aldosterone antagonists are indicated in patients with STEMI patients with LVE≤0.40, HF, or diabetes mellitus if no renal failure or hyperkalemia (1 B) • Aldosterone antagonists (epl ere none) are indicated in patients with NSTEM with an LVEF ≤0.35 and HF or diabetes me itus no renal failure or hyperkalemia (1 A) |
Table 3.
Newer and Experimental Agents in ACS
| Therapeutic Agent | Mechanism of Action | Clinical Effects in ACS |
|---|---|---|
| Ranolazine | Inhibits late inward sodium current | Decreases recurrent ischemia and arrhythmias52,53 |
| Trimetazidine | Shifts myocardial metabolism from fatty acid to glucose use | Decreases mortality in subset of patients with ACS not receiving thrombolysis63 |
| Nicorandil | Activates ATP-sensitive K+ channels and dilates arterioles; may have ischemic preconditioning-like effect | Decreases arrhythmias and transient ischemia.72 No effect on infarct size or EF73 |
| Ivabradine | Inhibits inward funny current and decreases heart rate | Not studied yet for efficacy in ACS |
| FXOG | Binds to vascular endothelial-cadherin and prevents leukocyte infiltration and plasma leakage | Reduces necrotic core size but not infarct size39 |
| Pexelizumab | Monoclonal antibody to C5 complement | No effect on mortality or secondary composite cardiovascular end point32 |
| Eniporide | Inhibits Na+/H+ exchange system involved in reperfusion injury | No effect on infarct size or clinical outcomes94 |
| Exenatide | Glucagon-like peptide analog that may activate receptor-mediated survival pathway | Reduces infarct size in relation to area at risk97 |
| Adenosine | Multifactorial effects on endothelium including vasodilation, neutrophil inhibition, decreased free radical formation | No effect on clinical outcomes; reduces infarct size at higher doses98 |
| Cyclosporine | Inhibitor of the mitochondrial permeability transition pore involved in reperfusion injury | Reduces infarct size in small studies; larger clinical trial in progress |
| TR040303 | Reduces opening of the mitochondrial permeability transition pore | Clinical trial in progress |
| Atrial natriuretic peptide | Activates reperfusion injury salvage kinase pathway | Reduces infarct size and reperfusion injury73 |
| Magnesium | Electrolyte repletion | No effect on mortality117 |
| Glucose/insulin/potassiurri | Electrolyte and glucose repletion | No effect on mortality or clinical outcomes119 |
| Metformin | Possibly phosphorylates adenosine monophosphate–activated kinase | Clinical trial in progress |
| Oxvaen | Reduces hvrjoxia | Clinical trial in Droaress |
Traditional Pharmacologic Approaches
Traditional pharmacologic therapies used in the management of ACS include those that reduce oxygen demand and/or increase myocardial oxygen supply chiefly by augmentation of CBF. Reduction of myocardial oxygen demand may be accomplished by decreasing heart rate, lowering wall stress, or reducing myocardial contractility. Wall stress is lowered by decreases in preload (the radius term in the Laplace relationship) and/or afterload (the pressure term in the Laplace relationship). Increases in CBF and augmented myocardial oxygen delivery are generally achieved through vasodilation of coronary resistance and conduit vessels.
The outcomes associated with the agents discussed in this section were studied mostly during an era prior to the use of contemporary methods for coronary reperfusion and revascularization, the routine administration of potent anti-thrombotic agents, and the use of high-intensity statin therapy. It is generally held, however, that they are beneficial for ACS patients managed with either an invasive or conservative strategy. Randomized controlled trials (RCTs) remain the gold standard for the development of evidence-based clinical practice guidelines and landmark trials will be cited here as appropriate.
Beta-adrenergic Receptor Blockers
Beta-adrenergic receptor blockers decrease heart rate, blood pressure, and contractility, resulting in a reduction in myocardial oxygen demand. These effects are primarily the result of competitive antagonism of the beta-1 cardiomyocyte receptors. The evidence supporting their clinical benefit is largely derived from earlier studies in patients with acute MI prior to the current era of reperfusion therapy for STEMI. The findings have been extrapolated to include patients with UA and NSTEMI.
The results of clinical trials studying the use of beta-blockers in ACS indicate improved outcomes from both early and chronic use. A cumulative meta-analysis of 28 trials indicated a mortality decrease of 28% at 1 week after MI with most of the benefit occurring in the first 48 hours.18 There was also an overall 18% reduction in the cumulative rate of reinfarction and a 15% reduction in the cumulative rate of cardiac arrest. A meta-analysis of 82 randomized trials of beta-blockers in patients with acute or previous MI demonstrated a significant reduction in long term mortality.19 The CRUSADE Registry (Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA guidelines) showed that patients with UA/NSTEMI treated with beta-blocker therapy had a 34% risk-adjusted decrease in hospital mortality (p<0.001).20 Conversely, a propensity score-matched analysis of 21860 patients with either CAD risk factors, known CAD, or prior MI in the REACH (Reduction of Atherothrombosis for Continued Health) registry did not show any reduction in a composite of cardiovascular death, nonfatal MI, or nonfatal stroke with beta-blocker use over a median follow-up of 44 months.21
The use of intravenous therapy with beta-blockers has been evaluated in COMMIT/CCS-2 (Clopidogrel and Metoprolol in Myocardial Infarction Trial/Second Chinese Cardiac Study) where early intravenous (IV) metoprolol followed by oral therapy was compared to placebo alone in 45852 patients presenting with ACS, predominantly STEMI.22 There was no difference in the co-primary outcomes of death from any cause or the composite of death/reinfarction/cardiac arrest by either hospital discharge or day 28 (whichever came first). Although rates of ventricular fibrillation (p=0.001) and recurrent myocardial infarction (p=0.001) were lower in the treated group, a significantly higher rate of cardiogenic shock (p<0.00001) was seen after IV metoprolol, especially in certain high-risk subgroups that included patients >70 years old, those with systolic BP < 120 mm Hg, and those presenting with heart rate > 110 beats per minute (bpm).
The ACC/AHA guidelines23 state that oral beta-blockers should be initiated (Class of Recommendation [COR] I, Level of Evidence [LOE] B) in the first 24 hours for patients with STEMI who do not have: signs of heart failure (HF), low output state, increased risk for cardiogenic shock, or contraindications to beta-blocker therapy. Beta-blockers should be continued (COR I, LOE B) during and after hospitalization. Patients with initial contraindications to the use of beta-blockers should be reevaluated (COR I, LOE C) to determine their subsequent eligibility. It is reasonable to administer (COR IIa, LOE B) IV beta-blockers to patients with STEMI and no contraindications who are hypertensive or have ongoing ischemia. Similar recommendations are made for patients with UA/NSTEMI.24,25 In addition, the UA/NSTEMI guidelines indicate that it may be harmful (COR III, LOE A) to give IV beta-blockers to patients with signs of HF, low-output state, or other risk factors for cardiogenic shock. Notably, the current ACC/AHA guidelines on secondary prevention26 state that beta-blocker therapy after MI should be continued for at least 3 years in the absence of LV dysfunction (COR I, LOE A). It is reasonable to continue beta-blocker therapy beyond 3 years but this is now a weaker recommendation than in the past guidelines (COR IIa, LOE B), as corroborated by the findings in the REACH registry. The ESC guideline recommendations for beta-blockers in patients with STEMI and NSTEMI27,28 are generally similar to the ACC/AHA guidelines but include a specific COR I (LOE: A for STEMI, B for NSTEMI) recommendation for the use of oral beta-blockers in all patients with LV dysfunction without contraindications.
Nitrates
Therapy with nitrates exerts beneficial effects by decreasing preload and left ventricular end-diastolic volume, thereby reducing myocardial oxygen demand. Nitrates also dilate both normal and diseased coronary arteries, an action that potentially increases both antegrade and collateral coronary blood flow. When given in the presence of phosphodiesterase inhibitors, the vasodilation resulting from nitroglycerin is substantially increased and prolonged, and results in profound hypotension.29 Thus, its use must be avoided when treating patients with UA/NSTEMI or STEMI who have taken sildenafil or tadalafil within the previous 24 to 48 hours, respectively. Nitrates should also be avoided in patients with right ventricular dysfunction who are exquisitely preload dependent.
Typically, nitrates are used in patients with ACS who have hypertension or heart failure and in those with continued angina. Although a review of small studies in the era before reperfusion suggested a reduction in mortality for MI patients receiving nitrates30, a major benefit was not confirmed in two large RCTs. The ISIS-4 (Fourth International Study of Infarct Survival) trial randomized 58050 patients with acute MI in a 2x2 fashion to various therapies including oral isosorbide mononitrate versus placebo.31 There was no difference in 5-week mortality between the nitrate and placebo groups, but there was an increase in hypotension with nitrate therapy. The GISSI-3 (Gruppo Italiano per lo Studio Della Sopravvivenza nellinfarto Miocardico) trial studied the effects of lisinopril, transdermal glyceryl trinitrate, and their combination in 19394 patients presenting with acute MI.32 The transdermal nitrate did not result in a reduction in 6-week mortality or the combined endpoint of mortality and severe LV dysfunction.
The ACC/AHA guidelines make no formal recommendations for the use of nitrates in patients with STEMI. Nitrate therapy is a recommended for patients with UA/NSTEMI with ongoing ischemic discomfort (COR I, LOE C), for patients with ECG changes indicative of ischemia, and for chest discomfort after cocaine use (COR I, LOE C). The ESC guidelines for STEMI recommend IV nitrate use in patients with elevated blood pressure (COR IIa, LOE C), in patients with HF who are intolerant of both angiotensin converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) (COR IIa, LOE C), and in patients with Killip Class III heart failure without hypotension (COR I, LOE C). Thus, the evidence base for nitrate use in ACS from RCTs is limited, and existing guideline recommendations derive exclusively from expert consensus opinion only (i.e., LOE C).
Calcium Channel Blockers
Calcium channel blockers (CCBs) may cause vasodilation, reduce heart rate, or decrease myocardial contractility. Because these effects occur to a variable extent with the various CCBs, there is not a class effect. Dihydropyridine CCBs such as nifedipine and amlodipine predominately produce vasodilation, resulting in a fall in blood pressure and reflex tachycardia. The non-dihydropyridine CCBs, diltiazem and verapamil, decrease heart rate, the rate of atrio-ventricular (AV) conduction, blood pressure, and myocardial contractility. Short-acting dihydropyridines such as the non-sustained release form of nifedipine must not be given to patients with ACS in the absence of beta-blocker therapy because of the associated deleterious risk of reflex tachycardia. Similarly, verapamil and diltiazem, because of their negative inotropic effects, should be avoided in patients with pulmonary edema or severe heart failure. The DAVIT-II (Danish Verapamil Infarction Trial) study examined 1775 patients with acute MI who were treated with verapamil or placebo.33 There was no difference in 18-month mortality with verapamil therapy compared to placebo. However, when analyzing trial patients without heart failure, verapamil was associated with a reduction in mortality (p=0.02). The HINT (Holland Interuniversity Nifedipine/metoprolol Trial) trial studied nifedipine and metoprolol in patients with UA/NSTEMI and was stopped early because of an increased re-infarction rate in the nifedipine group compared with the metoprolol group.34 In contrast, patients treated with metoprolol benefited from the addition of nifedipine in terms of a reduction in rates of recurrent ischemia or MI within 48 hours. A review of 28 RCTs involving 19000 patients with ACS showed no benefit from CCB therapy on infarct size, rate of reinfarction, or death.35 Thus the major benefit from CCB use in ACS is symptom or augmented hemodynamic control, and they are not recommended as routine, initial therapeutic agents.
The ACC/AHA UA/NSTEMI guidelines recommend that for patients with continuing or frequent ischemia in whom a beta-blocker cannot be given a non-dihydropyridine CCB should be administered (COR I, LOE B) in the absence of clinically significant LV dysfunction or other contraindications. It is reasonable to give a non-dihydropyridine CCB (COR IIa, LOE C) to patients with recurrent ischemia after beta-blockers and nitrates have been fully used. The guidelines emphasize that immediate-release dihydropyridine CCBs should not be administered to patients with UA/NSTEMI in the absence of a beta-blocker (COR III, LOE A). The ACC/AHA STEMI guidelines make no formal recommendations about CCB use. The ESC NSTEMI ACS guidelines have a COR I, LOE B recommendation for CCBs (dihydropyridine type) for symptom relief in patients already receiving nitrates and beta-blockers, and for patients with contraindications to beta-blockade (benzothiazepine or phenylethylamine [nondihydropyridine] type). There is a COR I, LOE C recommendation that CCBs be given to patients with vasospastic angina. The ESC guidelines also state that nifedipine, or other dihydropyridines, are not recommended unless combined with beta-blockers (COR III, LOE B).
Inhibitors of the Renin-Angiotensin-Aldosterone System
Activation of the renin-angiotensin-aldosterone system (RAAS) by a number of factors is known to occur in the setting of ACS, resulting in increased production of angiotensin II with subsequent increases in vascular resistance, myocardial workload, and myocardial oxygen demand. Accordingly, a variety of therapies known to inhibit the activated renin-angiotensin-aldosterone system have been studied in clinical trials to determine their potential efficacy to improve outcomes among patients with ACS. Use of these agents to disrupt renin-angiotensin-aldosterone signaling also inhibits the downstream detrimental pathways of long-term fibrosis and both myocardial and vascular remodeling.36
Angiotensin-Converting Enzyme Inhibitors (ACE-Is)
ACE-Is block the conversion of angiotensin I to angiotensin II thereby reducing the vasoconstrictive and aldosterone stimulating effects of angiotensin II. ACE-Is have been studied extensively in patients with MI both with and without heart failure. The SAVE (Survival And Ventricular Enlargement) trial randomized 2231 patients with LV systolic dysfunction (LV ejection fraction (EF)<0.40) but without overt heart failure after MI to captopril or placebo.37 There was a 19% risk reduction in all-cause mortality over an average follow-up of 42 months (p=0.019), as well as significant reductions in severe heart failure, heart failure hospitalizations, and recurrent MI. The AIRE (Acute Infarction Ramipril Efficacy) study examined the effects of ramipril versus placebo in 2006 patients with clinical evidence of heart failure after an acute MI.38 There was a 27% risk reduction in all-cause mortality (p=0.002). Lastly, the TRACE (TRAndolapril Cardiac Evaluation) study compared treatment with trandolapril versus placebo in 1749 patients with LV dysfunction (EF < 0.35) followed for 24-50 months after MI.39 The relative risk (RR) of all-cause death in the trandolapril group was 0.78 (95% CI 0.67-0.91; p=0.001). Trandolapril also reduced the risk of death from cardiovascular causes (RR 0.75; 95% CI 0.63 to 0.89; p=0.001) and sudden death (RR 0.76; 95% CI 0.59 to 0.98; p=0.03). Progression to severe heart failure was less frequent in the trandolapril group (RR 0.71; 95 % CI 0.56 to 0.89; p=0.003). In addition to these selective trials in post-MI patients with LV dysfunction, two large trials looked at the nonselective use of ACE-s in survivors of MI. The ISIS-4 trial randomized 58050 patients with acute MI in a 2x2 fashion to various therapies including captopril versus placebo.31 There was a 7% reduction in 5-week mortality with captopril (p=0.01), and this survival advantage persisted at 12 months. There was more severe hypotension in the captopril group. The GISSI-3 trial studied the effects of lisinopril, transdermal glyceryl trinitrate, and their combination in 19394 unselected patients with acute MI.32 Lisinopril use was associated with a reduction in 6-week mortality (OR 0.88; 95% CI 0.79-0.99), as well as a composite endpoint of mortality and severe ventricular dysfunction (OR 0.90; 95% CI 0.84-0.98). These findings persisted when lisinopril and nitrates were used in combination. Oral ACE-Is have been shown to improve outcomes among patients with STEMI in a manner that is independent of other therapies, including aspirin, fibrinolytics, and beta-blockers. Their renal protective effects make their use in patients with diabetes and/or chronic kidney disease especially beneficial. The magnitude of their effect on mortality in RCTs has been relatively higher in patients with LV dysfunction after MI. Early administration of ACE-Is to high-risk patients such as those with anterior MI, LVEF <0.40, and heart failure has consistently been shown to improve acute and long-term outcomes.37,40 The long-term secondary prevention benefits of ACE-Is in lower risk patients with atherosclerotic CAD and normal LVEF who have been revascularized and optimally treated with other secondary prevention therapies such as statins and aspirin is less certain.41-44
The ACC/AHA STEMI guidelines state that ACE-Is should be administered within the first 24 hours to all patients with STEMI with anterior location, HF, or LVEF ≤0.40, unless contraindicated (COR I, LOE A). ACE-Is are reasonable for other patients with STEMI (COR IIa, LOE A). The ACC/AHA NSTEMI guidelines are similar except that diabetes is a COR I indication for ACE-I therapy. The ESC recommendations for STEMI similarly state that ACE-Is are indicated starting within the first 24 hours of STEMI in patients with evidence of HF, LV systolic dysfunction, diabetes, or anterior infarct (COR I, LOE A), and ACE-Is should be considered in all patients in the absence of contraindications (COR IIa, LOE A). The ESC NSTEMI guidelines recommend ACE-Is within 24 hours in patients with LVEF ≤0.40 and in patients with HF, diabetes, hypertension, or chronic kidney disease (COR I, LOE A). Importantly, ACE-Is are recommended for all other patients to prevent recurrence of ischemic events, with preference given to agents and doses of proven efficacy (COR I, LOE B).
Despite the extensive study of ACE-Is, there remains some difference of opinion about their need and efficacy among lower risk patients with normal LVEF who have been revascularized and are on optimal medical therapy. There are also questions about potential differences among the ACE-Is utilized in the major trials suggesting that there may not be a class effect. Future trials should be designed to resolve these issues.
Angiotensin-II Receptor Blockers (ARBs)
An alternative method to therapy with ACE-Is is presented by ARBs, which work by displacing angiotensin II from its angiotensin II type 1 (AT-1) receptor, causing a decrease in systemic vascular resistance without an accompanying change in heart rate or cardiac output. The resulting increase in angiotensin II levels has not been associated with deleterious effects. A major difference between ACE-Is and ARBs is the lack of increase in kinin levels with ARB use. Elevated kinins (e.g. bradykinin) may contribute to some of the benefits of ACE-I therapy but also produce the cough that renders ACE-I therapy unacceptable to a sizeable proportion of patients.45 Similar to ACE-Is, ARBs have renal protection benefits for patients with diabetes and/or chronic kidney disease. Treatment of STEMI patients with ARBs has been compared to ACE-I therapy in two major RCTs. The OPTIMAAL (Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan) study of 5477 patients with STEMI failed to show superiority or non-inferiority when losartan was compared to captopril with respect to all-cause mortality during a mean follow-up of 2.7 years.46 However, significantly fewer patients discontinued losartan in comparison to captopril (p<0.0001). The VALIANT (VALsartan In Acute myocardial 10entricula Trial) study compared valsartan with captopril or combined therapy in 14703 patients with recent STEMI complicated by clinical heart failure and/or LV systolic dysfunction.47 Valsartan was found to be non-inferior to captopril in terms of the primary endpoint of all-cause mortality, as well as fatal and nonfatal cardiovascular events during a median follow-up of 24.7 months. Discontinuation of therapy was more frequent in the captopril-treated patients due to cough. Combination therapy had the most drug-related adverse events.
The ACC/AHA STEMI guidelines state that ARBs should be given to patients with STEMI who have indications for but are intolerant of ACE-Is (COR I, LOE B). The ACC/AHA UA/NSTEMI guidelines similarly recommend that an ARB should be given to patients intolerant of ACE-Is who have clinical or radiographic signs of HF or an LVEF <0.40. The ESC STEMI guidelines state that an ARB, preferably valsartan, is an alternative to ACE-I particularly in patients with HF or systolic dysfunction if they are unable to tolerate ACE-I therapy (COR I, LOE B). The ESC NSTEMI guidelines recommend ARB therapy when patients are intolerant of ACE-Is (COR IIa, LOE B).
Aldosterone Antagonists
These agents competitively inhibit the binding of aldosterone to mineralocorticoid receptors. The EPHESUS (Eplerenone Post-acute myocardial infarction Heart failure Efficacy and Survival Study) trial evaluated the effect of eplerenone, a selective aldosterone blocker, on morbidity and mortality in 6642 acute MI patients with LVEF < 0.40 and symptoms of heart failure or diabetes, already treated with a beta-blocker and either an ACE-I or ARB.48 There was a significant reduction in the primary end-point of all-cause death in the eplerenone-treated group over a mean follow-up of 16 months (RR 0.85, 95% CI 0.75-0.96, p=0.008). The rate of secondary cardiovascular endpoints, including sudden death from cardiac causes was also reduced by eplerenone (RR 0.79, 95% CI 0.86-0.98, p=0.02). There was, however, an increased rate of severe hyperkalemia in the eplerenone group (p=0.002).
The ACC/AHA guidelines state that an aldosterone antagonist should be given to patients with STEMI (COR I, LOE B) or UA/NSTEMI (COR I, LOE A) and no contraindications who are already receiving a beta-blocker and ACE-I and have an EF of <0.40 and either symptoms of HF or diabetes. The ESC STEMI guidelines recommend that aldosterone antagonists are indicated in patients with an EF <0.40 and HF or diabetes, provided there is no renal failure or hyperkalemia (COR I, LOE B). The ESC NSTEMI guidelines have a similar recommendation using an EF of <0.35, stating that the findings in STEMI patients may be extrapolated to those with NSTEMI.
Newer Pharmacologic Agents
Ranolazine
Cardiac ischemia is associated with alterations in intracellular sodium and calcium concentrations, leading to arrhythmias, decreased contractility, and myocyte death.49 Ranolazine is piperazine derivative approved for use in the US in 2006 for patients with chronic stable angina. Its anti-ischemic effects are mediated independently of alterations in heart rate and blood pressure. Initially felt to shift myocardial substrate utilization from fatty acid oxidation to glucose utilization, ranolazine also appears to reduce calcium overload in ischemic myocytes via inhibition of the late inward sodium current (INa).49,50 The subsequent reduction in intracellular sodium alters the influx of calcium into the cell via the sodium-calcium exchanger. Ranolazine preserves intracellular ATP levels, improves myocardial function, and limits the extent of ischemic myocardial necrosis. Its role in ACS patients is less well-defined than its demonstrated usefulness in some patients with chronic, stable angina.51 The MERLIN (Metabolic Efficiency with Ranolazine for Less Ischemia in Non–ST-Elevation Acute Coronary Syndromes)-TIMI 36 trial randomized 6560 patients with UA/NSTEMI managed with guideline-directed medical and interventional therapy to ranolazine or placebo and examined a composite primary outcome of CV death, MI, or recurrent ischemia over a median follow-up of 348 days after presentation.52 There was no significant difference in the composite primary endpoint, although there was a reduction in the isolated secondary outcome of recurrent ischemia with ranolazine therapy (HR 0.87, 95% CI 0.76-0.99, p=0.03), particularly among patients with a history of chronic angina. Ranolazine did not affect the incidence of symptomatic arrhythmias during the trial, though 7-day Holter monitoring showed decreased cumulative rates of ventricular tachycardia, supraventricular tachycardia, and ventricular pauses.53 Salutary effects on the development of atrial fibrillation have also been reported.54 The potential role of ranolazine to reduce ACS-related arrhythmias may warrant further investigation. Non-electrophysiologic effects of ranolazine include a reduction in hemoglobin A1c levels.55 The ACC/AHA and ESC guidelines both discuss the results of MERLIN-TIMI 36 but neither makes a formal recommendations for its use.
Trimetazidine
Trimetazidine is also a piperazine derivative that shifts myocardial metabolism from fatty acid oxidation to more oxygen-efficient glucose oxidation pathways, thus reducing ischemia without measurable changes in the heart rate-blood pressure product.56,57 It has been shown to reduce the extent of reperfusion injury in a rat model.58 In small clinical trials, trimetazidine has been shown to reduce ischemic injury when given before PCI59,60 or CABG.61,62 The EMIP-FR (European Myocardial Infarction Project – Free Radicals) trial was a prospective, randomized, double-blind, multicenter trial in which 19.725 patients presenting with symptoms of acute STEMI within the previous 24 hours were randomized to a 48-hour infusion of IV trimetazidine or placebo.63 Stratification was according to thrombolytic therapy (56%) or no thrombolytic therapy (44%). By intention-to-treat analysis, there was no difference between treatment groups in the primary end-point of 35-day all-cause mortality. There was a small but not statistically significant increase in deaths in the trimetazidine group that received thrombolytic therapy. In a per protocol analysis, however, trimetazidine was associated with a reduction in 35-day all-cause mortality among patients who did not receive thrombolysis (13.3% vs 15.1% for placebo; p=0.027). Trimetazidine is approved as a third-line agent for stable angina64 in Europe and other parts of the world.
Nicorandil
Nicorandil is a drug that activates ATP-sensitive K+ channels and dilates peripheral and coronary arterioles.65 This K+ channel function is also postulated to induce cardiac protection via an ischemic preconditioning-like effect.66 In a small study, nicorandil infusion appeared to be as effective as coronary balloon inflation at inducing ischemic pre-conditioning and reducing ST segment changes.67 Nicorandil also has a secondary nitrate-like mechanism of action that results in dilatation of the venous system and coronary arteries.65,66 The drug appears to improve multiple cardiac endpoints in patients with chronic stable angina68,69 and may also be beneficial in variant angina.70,71 In the CESAR 2 (Clinical European Studies in Angina and Revascularization) trial, 188 patients admitted with unstable angina were randomized in a double blind fashion to nicorandil or placebo for a minimum of 48 hours.72 Compared with placebo, nicorandil significantly reduced the incidence of any tachycarrhythmia (p=0.04) and the cumulative number of episodes of transient myocardial ischemia (mostly silent; p=0.003). The anti-ischemic (and secondary anti-arrhythmic) effect was postulated to be due to a pre-conditioning-like effect. The larger J-WIND (Japan-Working groups of acute myocardial Infarction for the reduction of Necrotic Damage) trial enrolled 1216 patients undergoing reperfusion for acute MI to either an IV nicorandil infusion or placebo.73 The primary endpoints of total infarct size (estimated by creatine kinase) and EF at 12 months were not different among the groups. There was, however, a small improvement in EF in a subset of patients who had been placed on chronic oral nicorandil therapy. Currently, nicorandil is used in Japan and Europe for the treatment of stable angina.
Ivabradine
Ivabradine is an agent that specifically inhibits the inward funny (If) current of the sinoatrial node, which controls heart rate.74 Through inhibition of this current, the slope of the rate of diastolic depolarization decreases and heart rate is reduced without effects on blood pressure or contractility. It has shown efficacy similar to atenolol at improving exercise tolerance in patients with stable angina.75 In the large multicenter BEAUTIFUL (morbidity-mortality EvAlUaTion of the If inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction) trial of 10917 patients with CAD and reduced EF, ivabradine did not reduce the rates of death and admission for heart failure, but did decrease the rate of admissions for CAD-related events in select patients.76 In the SHIFT (Systolic Heart failure treatment with If inhibitor ivabradine Trial) study of 6558 heart failure patients with a resting heart rate greater than 70 bpm while on usual therapy, ivabradine reduced the composite outcome of cardiovascular death and heart failure admission (HR 0.82, CI 0.75-0.90, p<0.0001).77 It has yet to be studied, however, in ACS patients with the exception of early studies designed to evaluate clinical safety.78 Its use in Europe is limited to stable angina or heart failure patients with elevated heart rate when beta-blockers are not adequate or cannot be tolerated.
Areas for Investigation
No-Reflow and Reperfusion Injury
No-reflow is a phenomenon characterized by poor tissue perfusion in an ischemic region despite relief of a complete coronary artery occlusion.79 It results from the loss of microvascular integrity consequent to ischemic injury of vascular endothelial cells.8,80,81 Such damage causes microluminal occlusion and prevents restoration of normal tissue blood flow even after infarct artery reperfusion. Continued ischemia, in combination with the inflammation and vasoconstriction seen with reperfusion injury, results in subendocardial myocyte death.82 The lack of adequate blood flow to the vulnerable region prevents the tissue delivery of intravenous or intracoronary medications meant to protect and heal the myocardium. Prompt revascularization is one method to attenuate no-reflow, since a longer period of complete occlusion leads to more microvascular damage. The paradox of reperfusion injury has been described previously.
Prevention of no-reflow with reperfusion injury before they occur is a desirable strategy.80 Mechanical thrombus aspiration during PCI appears to reduce distal embolization, no-reflow, and reperfusion injury; there may be some role for distal embolic protection devices, such as filters, in PCI of saphenous vein grafts but not in native coronary arteries.83,84 Drugs and biologics that target vasoconstriction, free radical formation, and inflammation are some of the agents that have been tested in limited fashion before and during PCI. Intracoronary adenosine and verapamil have shown some promise in preventing no-reflow in small clinical trials.80 In one small study, nitroprusside (a NO donor) did not improve coronary blood flow and reperfusion despite its association with improved clinical outcomes.85 Glycoprotein Iib/IIIa inhibitors may have a beneficial effect by preventing distal platelet-fibrin embolization to the already damaged microvasculature.80,86 In a trial of 368 patients with STEMI, a single administration of IV nicorandil before PCI compared to placebo improved Thrombolysis in Myocardial Infarction (TIMI) flow grade and the long-term clinical endpoints of heart failure hospitalizations or cardiovascular death at a mean follow-up of 2.4 years.87
The investigational agent FX06 is a fibrin-derived peptide that attempts to limit microvascular injury by binding to vascular endothelial (VE)-cadherin and preventing leukocyte infiltration and plasma leakage.88 In the FIRE (Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury) trial, 234 patients with STEMI were randomized to an IV bolus of FX06 or placebo during reperfusion.89 There was no difference in the primary outcome of infarct size at 5 days as measured by cardiac MRI or in the secondary outcomes of infarct size at 40 days and troponin I levels. There was a significant reduction in the secondary outcome of necrotic core zone size at 5 days with FX06 (p<0.025).
Pexelizumab, a humanized monoclonal antibody to C5 complement, was shown in animal models to inhibit apoptosis and inflammatory damage with a resultant decrease in infarct size.90 An initial clinical trial (COMMA: COMplement inhibition in Myocardial infarction treated with Angioplasty) in 960 patients undergoing PCI for STEMI with pexelizumab infusion compared to placebo revealed no change in infarct size between the groups.91 However, there was a statistically significant decrease in 90-day mortality with the agent (1.8% with pexelizumab vs 5.9% with placebo, p=0.014). This observation led to the design and execution of the APEX-AMI (Assessment of PEXelizumab in Acute Myocardial Infarction) trial that randomized 5745 patients undergoing PCI for STEMI to IV pexelizumab or placebo.92 There was no difference between groups in the primary endpoint of 30-day mortality or in the secondary composite endpoint of death, cardiogenic shock, and heart failure at 30-90 days.
Eniporide is a specific inhibitor of the Na+/H+ exchange system that is thought to be activated during reperfusion with consequent calcium overload and myocyte injury.93 In the ESCAMI (Evaluation of the Safety and Cardioprotective effects of eniporide in Acute Myocardial Infarction) trial, patients presenting with STEMI received eniporide or placebo prior to reperfusion. There was no difference between the groups in either enzymatic infarct size or clinical outcomes (death, cardiogenic shock, heart failure, life-threatening arrhythmias).94
Exenatide is a glucagon-like peptide (GLP)-1 analog used in the treatment of type 2 diabetes that has been shown to be cardioprotective during reperfusion in animal models of ischemia-reperfusion.95 Its mechanism of action has not been fully elucidated but is postulated to be partially due to the activation of a receptor-mediated survival pathway.96 In a trial of 172 patients with STEMI undergoing PCI, compared with placebo, IV exenatide increased the salvage index (p=0.003) and reduced infarct size in relation to myocardial area at risk at 90 days (p=0.003).97
Administration of adenosine has been a mainstay of treatment for no-reflow, although verapamil and nicorandil have also been tested.80,81,86 The AMISTAD-II (Acute Myocardial Infarction Study of Adenosine) trial studied 50 and 70 mcg/kg/min infusions of adenosine compared to placebo in 2118 patients with anterior STEMI undergoing either thrombolysis or angioplasty.98 Compared with placebo, adenosine did not result in any change in the primary endpoints of new HF, first re-hospitalization for HF, or death at 6 months. There was no change in infarct size in the pooled adenosine groups compared to placebo, although there was a smaller final median infarct size with the high dose (70 mcg/kg/min) adenosine infusion compared to placebo (p=0.023). Given the lack of effective alternatives, however, adenosine is the primary treatment used to treat no-reflow when it occurs after PCI.81,86 Continued research is clearly needed to discover novel agents that can prevent the no-reflow phenomenon and limit reperfusion injury.
Ischemic Pre- and Post-Conditioning
Further work is also warranted in the field of ischemic pre- and post-conditioning. It was shown first in animal studies that exposure of the heart to brief episodes of ischemia (pre-conditioning) results in improved cardiomyocyte survival during prolonged ischemia.12,13 Subsequent studies in humans have utilized the induction of remote, transient upper limb ischemia through hypersystolic blood pressure cuff inflation to mimic pre-conditioning. Cardiac surgeons have utilized a similar technique, as well as aortic cross-clamping, to induce pre-conditioning.99 Trials in acute MI have shown some benefit with preconditioning in reducing infarct size and clinical endpoints.100,101 A recent 4-year follow-up of 333 patients with STEMI randomized to remote ischemic pre-conditioning before PCI showed a reduction in all-cause mortality compared to standard care (HR 0.32, CI 0.12-0.88, p=0.027).102 Nicorandil, as mentioned previously, has also been shown to induce ischemic pre-conditioning. Diazoxide is a drug for the treatment of hypoglycemia and hypertensive emergencies that in animal studies seems to induce metabolic changes similar to a pre-conditioning state.8,103 This effect is likely mediated through multiple pathways, including mitochondrial K+ channels. It appears currently that the mechanism of ischemic preconditioning may also involve adenosine production, ATP-sensitive K+ channels, inducible nitric oxide synthase (iNOS), reactive oxygen species (ROS), and apoptotic pathways.8,104 Better understanding of the precise molecular mechanisms may enable development of selective drugs and biologics targeting these pathways without the risk of inducing ischemia.
Ischemic post-conditioning is the process of inducing brief cycles of coronary artery reperfusion and occlusion prior to final complete reperfusion in order to attempt to limit myocardial injury. While technically more feasible than pre-conditioning in a clinical context because it can be performed at the same time as, rather than before PCI, results with this intervention in both animal and human models have been inconsistent.8,88,105 The POST (Effects of POSTconditioning on myocardial reperfusion in patients with STEMI) trial was the largest study to examine the efficacy of post-conditioning and randomized 700 patients who had just undergone primary PCI for STEMI to four 1-minute intracoronary angioplasty balloon inflations after initial infarct artery opening or to standard therapy.106 There was no difference between groups in the primary endpoint of complete ST-segment resolution at 30 minutes after PCI. There was also no change in the rate of major adverse cardiac events at 30 days with post-conditioning. Despite this result, there are several ongoing clinical trials to further evaluate potential benefits of ischemic post-conditioning on clinical outcomes and infarct size during primary PCI for STEMI (NCT00922675, NCT01324453, NCT01435408). There is also an active clinical trial to see if remote post-conditioning, induced by occluding blood flow to the thigh, can reduce myocardial infarct size during STEMI (NCT02021760).
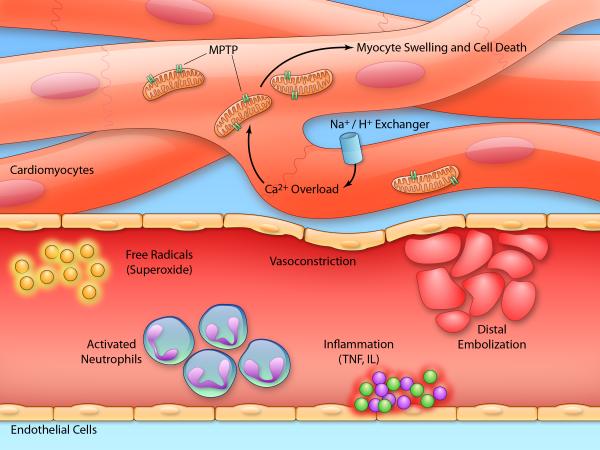
The mechanism of post-conditioning is not fully elucidated but is believed to rely on limiting the magnitude of reperfusion injury and no-reflow during revascularization.84,107 Thus, similar pathologic processes are at work, including distal embolization, myocardial cell swelling and death, localized inflammation, vasoconstriction, and free radical formation. Post-conditioning in part allows brief opportunities for aerobic metabolism to repair the sarcolemma without causing rupture from calcium overload.8 The actions of cyclosporine have shed some light on this mechanism by inducing a postconditioning effect at least partially due to inhibition of the mitochondrial permeability transition pore (MPTP), a key player in reperfusion injury.88 By preventing pore opening during reperfusion, myocytes are thought to be given time to undergo repair processes and survive. Small studies of cyclosporine in acute MI show a benefit in reducing infarct size.108,109 The larger CIRCUS (Cyclosporine and Prognosis in Acute MI Patients; NCT01502774) trial is ongoing in Europe to further examine the benefit of cyclosporine in acute MI. TRO40303 is a novel small molecule that also reduces opening of the MPTP, and its clinical efficacy will be examined in the European MitoCare study.110,111 The Reperfusion Injury Salvage Kinase (RISK) pathway is believed to play a related role in cardioprotection, partly through downstream inhibition of MPTP opening.112 Atrial natriuretic peptide (ANP) administration has been shown to activate the RISK pathway and reduce infarct size.73,113 As is the case with ischemic preconditioning, however, improved understanding of the molecular pathways at play in ischemic postconditioning is needed to enable more effective therapeutic strategies. Figure 1 summarizes the complex, overlapping pathways involved in the pathogenesis of reperfusion injury and no-reflow that may be targeted with therapies including pre/post-conditioning.
Figure 1.
Mechanisms of reperfusion injury and no-reflow in the coronary vasculature (Illustration Credit: Ben Smith).
Other Areas of Investigation
There have been other intriguing areas of investigation for non-antithrombotic therapies for management of ACS. The role of inflammation in the lifecycle of the vulnerable plaque is well-described, although trials of various anti-inflammatory agents in ACS from corticosteroids to monoclonal antibodies have been mostly disappointing.114 However, efforts continue to define more precisely the exact inflammatory mechanisms at play in CAD and ACS and thus identify new agents targeting relevant pathways. For example, an inhibitor of p38 mitogen-activated protein kinase has been shown to decrease inflammation and infarct size in animal models and human studies are underway.115 Previously, it was thought that deficiencies of electrolytes such as magnesium during MI could result in worse clinical outcomes due to effects on multiple ion gradient and energy production processes.116 The MAGIC (MAGnesium In Coronaries) trial randomized 6213 patients with acute STEMI to a magnesium infusion or placebo.117 There was no difference between groups in the primary endpoint of 30-day mortality. Similarly, a glucose/insulin/potassium (GIK) mixture was postulated to be beneficial in MI by suppressing uptake of free fatty acids, providing glucose as fuel for the heart, and reducing ventricular arrhythmias induced by potassium depletion.118 However, the CREATE-ECLA (Clinical Trial of Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation—Estudios Cardiologicos Latinoamerica) trial composed of 20201 patients with acute STEMI randomized to GIK infusion or placebo revealed no difference between groups in the primary outcome of 30-day mortality.119 There was also no effect on rates of development of cardiac arrest, cardiogenic shock, and reinfarction at 30 days. On a related metabolic front, metformin has been proposed to limit myocardial injury and adverse remodeling after MI possibly through phosphorylation of adenosine monophosphate-activated (AMP) kinase.120 The GIPS-III (Glycometabolic Intervention as adjunct to Primary percutaneous intervention in STEMI) trial is currently underway to examine the effects of metformin therapy on EF after STEMI in non-diabetic patients.121 Others have proposed focusing on pathways induced by hypoxia during ischemia and the role of adenosine receptors in this process.122,123 Research on this front remains in an early investigational stage in animal models, although the Detox-AMI trial is underway to determine whether there is any benefit to the standard practice of oxygen supplementation in patients with ACS.
Conclusions
The immediate goals of treatment in patients with ACS are to decrease myocardial oxygen demand, increase coronary blood flow and oxygen supply, and limit myocardial injury, thereby reducing the short and longer-term risks of heart failure, arrhythmia, and death. Traditional pharmacological therapies center around beta-blockers and renin-angiotensin system inhibitors, which have the most robust clinical trials evidence base for efficacy. There is some role for nitrates and calcium channel blockers in specific clinical circumstances. Other agents, such as ranolazine and nicorandil, are primarily used in patients with stable angina though their mechanisms of action suggest possible benefits in patients with ACS, and additional trials are needed. More research is also warranted to further understand the molecular mechanisms of the no-reflow phenomenon, reperfusion injury, and ischemic pre/post-conditioning. Knowledge of these pathways is critical to discovering novel therapeutic agents targeted to key components of these processes. Finally, although this review specifically focuses on non-antithrombotic medical therapies, appropriate secondary prevention should be utilized in all ACS patients to decrease the risk of late cardiac events. Such a program includes lifestyle modification, intensive risk factor intervention, and guideline-directed medical management with anti-thrombotic agents and high-intensity statins. Clearly, much work remains to be done to optimize the use of available therapies and discover new means to reduce the morbidity and mortality associated with ACS.
Supplementary Material
Acknowledgments
Sources of Funding: NIH Training Grant in Cardiovascular Research T32HL007604 (VS)
Non-Standard Abbreviations
- CBF
Coronary blood flow
- COR
Class of recommendation
- CVR
Coronary vascular resistance
- LOE
Level of evidence
- MPTP
Mitochondrial permeability transition pore
- RCT
Randomized controlled trial
Footnotes
Disclosures: No disclosures for any authors
References
- 1.Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald's Heart Disease. Elsevier Health Sciences. 2011 [Google Scholar]
- 2.Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 4.Tune JD. Control of coronary blood flow during hypoxemia. Adv Exp Med Biol. 2007;618:25–39. doi: 10.1007/978-0-387-75434-5_3. [DOI] [PubMed] [Google Scholar]
- 5.Duncker DJ, Bache RJ. Regulation of coronary vasomotor tone under normal conditions and during acute myocardial hypoperfusion. Pharmacol Ther. 2000;86:87–110. doi: 10.1016/s0163-7258(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 6.Quyyumi AA, Dakak N, Mulcahy D, Andrews NP, Husain S, Panza JA, Cannon RO. Nitric oxide activity in the atherosclerotic human coronary circulation. J Am Coll Cardiol. 1997;29:308–317. doi: 10.1016/s0735-1097(96)00472-x. [DOI] [PubMed] [Google Scholar]
- 7.Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med. 2013;159:746–757. doi: 10.7326/0003-4819-159-11-201312030-00007. [DOI] [PubMed] [Google Scholar]
- 8.Jennings RB. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res. 2013;113:428–438. doi: 10.1161/CIRCRESAHA.113.300987. [DOI] [PubMed] [Google Scholar]
- 9.Verma S, Fedak PWM, Weisel RD, Butany J, Rao V, Maitland A, Li R-K, Dhillon B, Yau TM. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332–2336. doi: 10.1161/01.cir.0000016602.96363.36. [DOI] [PubMed] [Google Scholar]
- 10.Przyklenk K, Dong Y, Undyala VV, Whittaker P. Autophagy as a therapeutic target for ischaemia /reperfusion injury? Concepts, controversies, and challenges. Cardiovasc Res. 2012;94:197–205. doi: 10.1093/cvr/cvr358. [DOI] [PubMed] [Google Scholar]
- 11.Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103–114. doi: 10.1161/CIRCULATIONAHA.107.702993. [DOI] [PubMed] [Google Scholar]
- 12.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 13.Reimer KA, Murry CE, Yamasawa I, Hill ML, Jennings RB. Four brief periods of myocardial ischemia cause no cumulative ATP loss or necrosis. Am J Physiol. 1986;251:H1306–15. doi: 10.1152/ajpheart.1986.251.6.H1306. [DOI] [PubMed] [Google Scholar]
- 14.Stern S, Bayes de Luna A. Coronary artery spasm: a 2009 update. Circulation. 2009;119:2531–2534. doi: 10.1161/CIRCULATIONAHA.108.843474. [DOI] [PubMed] [Google Scholar]
- 15.McCord J, Jneid H, Hollander JE, de Lemos JA, Cercek B, Hsue P, Gibler WB, Ohman EM, Drew B, Philippides G, Newby LK. American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117:1897–1907. doi: 10.1161/CIRCULATIONAHA.107.188950. [DOI] [PubMed] [Google Scholar]
- 16.Tranter MH, Wright PT, Sikkel MB, Lyon AR. Takotsubo cardiomyopathy: the pathophysiology. Heart Fail Clin. 2013;9:187–96–viii–ix. doi: 10.1016/j.hfc.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Bossone E, Savarese G, Ferrara F, Citro R, Mosca S, Musella F, Limongelli G, Manfredini R, Cittadini A, Perrone Filardi P. Takotsubo cardiomyopathy: overview. Heart Fail Clin. 2013;9:249–66–x. doi: 10.1016/j.hfc.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med. 1992;327:248–254. doi: 10.1056/NEJM199207233270406. [DOI] [PubMed] [Google Scholar]
- 19.Freemantle N, Cleland J, Young P, Mason J, Harrison J. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller CD, Roe MT, Mulgund J, Hoekstra JW, Santos R, Pollack CV, Ohman EM, Gibler WB, Peterson ED. Impact of acute beta-blocker therapy for patients with non-ST-segment elevation myocardial infarction. Am J Med. 2007;120:685–692. doi: 10.1016/j.amjmed.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, Ohman EM, Cannon CP, Smith SC, Zeymer U, Hoffman EB, Messerli FH, Bhatt DL. REACH Registry Investigators. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–1349. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- 22.Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, Xie JX, Liu LS. COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1622–1632. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 23.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC. 2011 WRITING GROUP MEMBERS, ACCF/AHA TASK FORCE MEMBERS. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 25.2012 Writing Committee Members. Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL. American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 26.Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 27.Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 28.Hamm CW, Bassand J-P, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88–95. doi: 10.1161/CIRCULATIONAHA.110.944603. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Collins R, MacMahon S, Peto R. Effect of intravenous nitrates on mortality in acute myocardial infarction: an overview of the randomised trials. Lancet. 1988;1:1088–1092. doi: 10.1016/s0140-6736(88)91906-x. [DOI] [PubMed] [Google Scholar]
- 31.ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 32.GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto Miocardico. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- 33.Effect of verapamil on mortality and major events after acute myocardial infarction (the Danish Verapamil Infarction Trial II--DAVIT II). Am J Cardiol. 1990;66:779–785. doi: 10.1016/0002-9149(90)90351-z. [DOI] [PubMed] [Google Scholar]
- 34.Early treatment of unstable angina in the coronary care unit: a randomised, double blind, placebo controlled comparison of recurrent ischaemia in patients treated with nifedipine or metoprolol or both. Report of The Holland Interuniversity Nifedipine/Metoprolol Trial (HINT) Research Group. Br Heart J. 1986;56:400–413. doi: 10.1136/hrt.56.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Held PH, Yusuf S, Furberg CD. Calcium channel blockers in acute myocardial infarction and unstable angina: an overview. BMJ. 1989;299:1187–1192. doi: 10.1136/bmj.299.6709.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorn GW. Novel pharmacotherapies to abrogate postinfarction ventricular remodeling. Nat Rev Cardiol. 2009;6:283–291. doi: 10.1038/nrcardio.2009.12. [DOI] [PubMed] [Google Scholar]
- 37.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 38.Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993;342:821–828. [PubMed] [Google Scholar]
- 39.Køber L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 40.Flather MD, Yusuf S, Køber L, Pfeffer M, Hall A, Murray G, Torp-Pedersen C, Ball S, Pogue J, Moyé L, Braunwald E. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355:1575–1581. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- 41.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 42.Fox KM. EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 43.Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, Pfeffer MA, Rice MM, Rosenberg YD, Rouleau JL. PEACE Trial Investigators. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitt B, O'Neill B, Feldman R, Ferrari R, Schwartz L, Mudra H, Bass T, Pepine C, Texter M, Haber H, Uprichard A, Cashin-Hemphill L, Lees RS, QUIET Study Group The QUinapril Ischemic Event Trial (QUIET): evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol. 2001;87:1058–1063. doi: 10.1016/s0002-9149(01)01461-8. [DOI] [PubMed] [Google Scholar]
- 45.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 46.Dickstein K, Kjekshus J. OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet. 2002;360:752–760. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 47.Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau J-L, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 48.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 49.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–2472. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 51.Nash DT, Nash SD. Ranolazine for chronic stable angina. Lancet. 2008;372:1335–1341. doi: 10.1016/S0140-6736(08)61554-8. [DOI] [PubMed] [Google Scholar]
- 52.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, Wolff AA, Skene A, McCabe CH, Braunwald E. MERLIN-TIMI 36 Trial Investigators. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 53.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FWA, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 54.Verrier RL, Kumar K, Nieminen T, Belardinelli L. Mechanisms of ranolazine's dual protection against atrial and ventricular fibrillation. Europace. 2013;15:317–324. doi: 10.1093/europace/eus380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrow DA, Scirica BM, Chaitman BR, McGuire DK, Murphy SA, Karwatowska-Prokopczuk E, McCabe CH, Braunwald E. MERLIN-TIMI 36 Investigators. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119:2032–2039. doi: 10.1161/CIRCULATIONAHA.107.763912. [DOI] [PubMed] [Google Scholar]
- 56.Bucci M, Borra R, Någren K, Pärkkä JP, Del Ry S, Maggio R, Tuunanen H, Viljanen T, Cabiati M, Rigazio S, Taittonen M, Pagotto U, Parkkola R, Opie LH, Nuutila P, Knuuti J, Iozzo P. Trimetazidine reduces endogenous free fatty acid oxidation and improves myocardial efficiency in obese humans. Cardiovasc Ther. 2012;30:333–341. doi: 10.1111/j.1755-5922.2011.00275.x. [DOI] [PubMed] [Google Scholar]
- 57.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 58.Guarnieri C, Muscari C. Effect of trimetazidine on mitochondrial function and oxidative damage during reperfusion of ischemic hypertrophied rat myocardium. Pharmacology. 1993;46:324–331. doi: 10.1159/000139070. [DOI] [PubMed] [Google Scholar]
- 59.Labrou A, Giannoglou G, Zioutas D, Fragakis N, Katsaris G, Louridas G. Trimetazidine administration minimizes myocardial damage and improves left ventricular function after percutaneous coronary intervention. Am J Cardiovasc Drugs. 2007;7:143–150. doi: 10.2165/00129784-200707020-00006. [DOI] [PubMed] [Google Scholar]
- 60.Bonello L, Sbragia P, Amabile N, Com O, Pierre SV, Levy S, Paganelli F. Protective effect of an acute oral loading dose of trimetazidine on myocardial injury following percutaneous coronary intervention. Heart. 2007;93:703–707. doi: 10.1136/hrt.2006.107524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iskesen I, Saribulbul O, Cerrahoglu M, Var A, Nazli Y, Sirin H. Trimetazidine reduces oxidative stress in cardiac surgery. Circ J. 2006;70:1169–1173. doi: 10.1253/circj.70.1169. [DOI] [PubMed] [Google Scholar]
- 62.Martins GF, Siqueira Filho AG de, Santos JB de F, Assunção CRC, Bottino F, Carvalho KG de, Valência A. Trimetazidine on ischemic injury and reperfusion in coronary artery bypass grafting. Arq Bras Cardiol. 2011;97:209–216. doi: 10.1590/s0066-782x2011005000079. [DOI] [PubMed] [Google Scholar]
- 63.Effect of 48-h intravenous trimetazidine on short- and long-term outcomes of patients with acute myocardial infarction, with and without thrombolytic therapy; A double-blind, placebo-controlled, randomized trial. The EMIP-FR Group. European Myocardial Infarction Project--Free Radicals. Eur Heart J. 2000;21:1537–1546. doi: 10.1053/euhj.1999.2439. [DOI] [PubMed] [Google Scholar]
- 64.Ciapponi A, Pizarro R, Harrison J. Trimetazidine for stable angina. Cochrane Database Syst Rev. 2005:CD003614. doi: 10.1002/14651858.CD003614.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Markham A, Plosker GL, Goa KL. Nicorandil. An updated review of its use in ischaemic heart disease with emphasis on its cardioprotective effects. Drugs. 2000;60:955–974. doi: 10.2165/00003495-200060040-00007. [DOI] [PubMed] [Google Scholar]
- 66.Horinaka S. Use of nicorandil in cardiovascular disease and its optimization. Drugs. 2011;71:1105–1119. doi: 10.2165/11592300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Matsubara T, Minatoguchi S, Matsuo H, Hayakawa K, Segawa T, Matsuno Y, Watanabe S, Arai M, Uno Y, Kawasaki M, Noda T, Takemura G, Nishigaki K, Fujiwara H. Three minute, but not one minute, ischemia and nicorandil have a preconditioning effect in patients with coronary artery disease. J Am Coll Cardiol. 2000;35:345–351. doi: 10.1016/s0735-1097(99)00539-2. [DOI] [PubMed] [Google Scholar]
- 68.IONA Study Group Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet. 2002;359:1269–1275. doi: 10.1016/S0140-6736(02)08265-X. [DOI] [PubMed] [Google Scholar]
- 69.Horinaka S, Yabe A, Yagi H, Ishimitsu T, Yamazaki T, Suzuki S, Kohro T, Nagai R. JCAD Study Investigators. Effects of nicorandil on cardiovascular events in patients with coronary artery disease in the Japanese Coronary Artery Disease (JCAD) study. Circ J. 2010;74:503–509. doi: 10.1253/circj.cj-09-0649. [DOI] [PubMed] [Google Scholar]
- 70.Kishida H, Murao S. Effect of a new coronary vasodilator, nicorandil, on variant angina pectoris. Clin Pharmacol Ther. 1987;42:166–174. doi: 10.1038/clpt.1987.127. [DOI] [PubMed] [Google Scholar]
- 71.Lee K-H, Kim SH, Lim HE, Kim EJ, Kim M-K, Park W-J, Cho G-Y. Effectiveness of intravenous administration of nicorandil in a patient with variant angina refractory to continuous intravenous nitroglycerin. Int J Cardiol. 2007;120:e9–12. doi: 10.1016/j.ijcard.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 72.Patel DJ, Purcell HJ, Fox KM. Cardioprotection by opening of the K(ATP) channel in unstable angina. Is this a clinical manifestation of myocardial preconditioning? Results of a randomized study with nicorandil. CESAR 2 investigation. Clinical European studies in angina and revascularization. Eur Heart J. 1999;20:51–57. doi: 10.1053/euhj.1998.1354. [DOI] [PubMed] [Google Scholar]
- 73.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S, J-WIND investigators Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 74.DiFrancesco D, Camm JA. Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs. 2004;64:1757–1765. doi: 10.2165/00003495-200464160-00003. [DOI] [PubMed] [Google Scholar]
- 75.Tardif J-C, Ford I, Tendera M, Bourassa MG, Fox K. INITIATIVE Investigators. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–2536. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- 76.Fox K, Ford I, Steg PG, Tendera M, Ferrari R. BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 77.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, SHIFT Investigators Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 78.Steg PG, Lopez-de-Sà E, Schiele F, Hamon M, Meinertz T, Goicolea J, Werdan K, Lopez-Sendon JL. VIVIFY (eValuation of the IntraVenous If inhibitor ivabradine after STsegment elevation mYocardial infarction) investigators. Safety of intravenous ivabradine in acute ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention: a randomized, placebo-controlled, double-blind, pilot study. Eur Heart J Acute Cardiovasc Care. 2013;2:270–279. doi: 10.1177/2048872613489305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niccoli G, Kharbanda RK, Crea F, Banning AP. No-reflow: again prevention is better than treatment. Eur Heart J. 2010;31:2449–2455. doi: 10.1093/eurheartj/ehq299. [DOI] [PubMed] [Google Scholar]
- 81.Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002;105:656–662. doi: 10.1161/hc0502.102867. [DOI] [PubMed] [Google Scholar]
- 82.Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circ Res. 2012;110:126–144. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- 83.Guo AQ, Sheng L, Lei X, Shu W. Pharmacological and physical prevention and treatment of no-reflow after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. J Int Med Res. 2013;41:537–547. doi: 10.1177/0300060513479859. [DOI] [PubMed] [Google Scholar]
- 84.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 85.Amit G, Cafri C, Yaroslavtsev S, Fuchs S, Paltiel O, Abu-Ful A, Weinstein JM, Wolak A, Ilia R, Zahger D. Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blind, placebo-controlled clinical trial. Am Heart J. 2006;152:887, e9–14. doi: 10.1016/j.ahj.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Ramjane K, Han L, Jin C. The diagnosis and treatment of the no-reflow phenomenon in patients with myocardial infarction undergoing percutaneous coronary intervention. Exp Clin Cardiol. 2008;13:121–128. [PMC free article] [PubMed] [Google Scholar]
- 87.Ishii H, Ichimiya S, Kanashiro M, Amano T, Imai K, Murohara T, Matsubara T. Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation. 2005;112:1284–1288. doi: 10.1161/CIRCULATIONAHA.104.530329. [DOI] [PubMed] [Google Scholar]
- 88.Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res. 2013;113:451–463. doi: 10.1161/CIRCRESAHA.112.300627. [DOI] [PubMed] [Google Scholar]
- 89.Atar D, Petzelbauer P, Schwitter J, Huber K, Rensing B, Kasprzak JD, Butter C, Grip L, Hansen PR, Süselbeck T, Clemmensen PM, Marin-Galiano M, Geudelin B, Buser PT, F.I.R.E. Investigators Effect of intravenous FX06 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction results of the F.I.R.E. (Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury) trial. J Am Coll Cardiol. 2009;53:720–729. doi: 10.1016/j.jacc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 90.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 91.Granger CB, Mahaffey KW, Weaver WD, Theroux P, Hochman JS, Filloon TG, Rollins S, Todaro TG, Nicolau JC, Ruzyllo W, Armstrong PW. COMMA Investigators. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–1190. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 92.APEX AMI Investigators. Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, O'Neill WW, Todaro TG, Vahanian A, Van de Werf F. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 93.Avkiran M. Rational basis for use of sodium-hydrogen exchange inhibitors in myocardial ischemia. Am J Cardiol. 1999;83 doi: 10.1016/s0002-9149(99)00215-5. 10G–17G–discussion 17G–18G. [DOI] [PubMed] [Google Scholar]
- 94.Zeymer U, Suryapranata H, Monassier JP, Opolski G, Davies J, Rasmanis G, Linssen G, Tebbe U, Schröder R, Tiemann R, Machnig T, Neuhaus KL, ESCAMI Investigators The Na(+)/H(+) exchange inhibitor eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction. Results of the evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction (ESCAMI) trial. J Am Coll Cardiol. 2001;38:1644–1650. doi: 10.1016/s0735-1097(01)01608-4. [DOI] [PubMed] [Google Scholar]
- 95.Timmers L, Henriques JPS, de Kleijn DPV, Devries JH, Kemperman H, Steendijk P, Verlaan CWJ, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 96.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 97.Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Køber L, Treiman M, Holst JJ, Engstrøm T. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 98.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW, AMISTAD-II Investigators A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 99.Kloner RA, Rezkalla SH. Preconditioning, postconditioning and their application to clinical cardiology. Cardiovasc Res. 2006;70:297–307. doi: 10.1016/j.cardiores.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 100.Davies WR, Brown AJ, Watson W, McCormick LM, West NEJ, Dutka DP, Hoole SP. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 101.Munk K, Andersen NH, Schmidt MR, Nielsen SS, Terkelsen CJ, Sloth E, Bøtker HE, Nielsen TT, Poulsen SH. Remote Ischemic Conditioning in Patients With Myocardial Infarction Treated With Primary Angioplasty: Impact on Left Ventricular Function Assessed by Comprehensive Echocardiography and Gated Single-Photon Emission CT. Circ Cardiovasc Imaging. 2010;3:656–662. doi: 10.1161/CIRCIMAGING.110.957340. [DOI] [PubMed] [Google Scholar]
- 102.Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE, CONDI Investigators Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 103.Coetzee WA. Multiplicity of effectors of the cardioprotective agent, diazoxide. Pharmacol Ther. 2013;140:167–175. doi: 10.1016/j.pharmthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iliodromitis EK, Lazou A, Kremastinos DT. Ischemic preconditioning: protection against myocardial necrosis and apoptosis. Vasc Health Risk Manag. 2007;3:629–637. [PMC free article] [PubMed] [Google Scholar]
- 105.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 106.Hahn J-Y, Song YB, Kim EK, Yu CW, Bae J-W, Chung W-Y, Choi S-H, Choi J-H, Bae J-H, An KJ, Park J-S, Oh JH, Kim S-W, Hwang J-Y, Ryu JK, Park HS, Lim D-S, Gwon H-C. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128:1889–1896. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- 107.Ovize M, Thibault H, Przyklenk K. Myocardial conditioning: opportunities for clinical translation. Circ Res. 2013;113:439–450. doi: 10.1161/CIRCRESAHA.113.300764. [DOI] [PubMed] [Google Scholar]
- 108.Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G, André-Fouët X, Derumeaux G, Piot C, Vernhet H, Revel D, Ovize M. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–1205. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 109.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André-Fouët X, Revel D, Kirkorian G, Monassier J-P, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 110.Schaller S, Paradis S, Ngoh GA, Assaly R, Buisson B, Drouot C, Ostuni MA, Lacapere J-J, Bassissi F, Bordet T, Berdeaux A, Jones SP, Morin D, Pruss RM. TRO40303, a new cardioprotective compound, inhibits mitochondrial permeability transition. J Pharmacol Exp Ther. 2010;333:696–706. doi: 10.1124/jpet.110.167486. [DOI] [PubMed] [Google Scholar]
- 111.MITOCARE Study Group Rationale and design of the “MITOCARE” Study: a phase II, multicenter, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of TRO40303 for the reduction of reperfusion injury in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Cardiology. 2012;123:201–207. doi: 10.1159/000342981. [DOI] [PubMed] [Google Scholar]
- 112.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 113.Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the “dark side” of reperfusion. Circulation. 2009;120:2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- 114.Matusik P, Guzik B, Weber C, Guzik TJ. Do we know enough about the immune pathogenesis of acute coronary syndromes to improve clinical practice? Thromb Haemost. 2012;108:443–456. doi: 10.1160/TH12-05-0341. [DOI] [PubMed] [Google Scholar]
- 115.Melloni C, Sprecher DL, Sarov-Blat L, Patel MR, Heitner JF, Hamm CW, Aylward P, Tanguay J-F, DeWinter RJ, Marber MS, Lerman A, Hasselblad V, Granger CB, Newby LK. The study of LoSmapimod treatment on inflammation and InfarCtSizE (SOLSTICE): design and rationale. Am Heart J. 2012;164:646–653. e3. doi: 10.1016/j.ahj.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 116.Antman EM. Magnesium in acute MI. Timing is critical. Circulation. 1995;92:2367–2372. doi: 10.1161/01.cir.92.9.2367. [DOI] [PubMed] [Google Scholar]
- 117.Magnesium in Coronaries (MAGIC) Trial Investigators Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) Trial: a randomised controlled trial. Lancet. 2002;360:1189–1196. doi: 10.1016/s0140-6736(02)11278-5. [DOI] [PubMed] [Google Scholar]
- 118.Yusuf S, Mehta SR, Díaz R, Paolasso E, Pais P, Xavier D, Xie C, Ahmed RJ, Khazmi K, Zhu J, Liu L, CREATE-ECLA investigators and steering committee Challenges in the conduct of large simple trials of important generic questions in resource-poor settings: the CREATE and ECLA trial program evaluating GIK (glucose, insulin and potassium) and low-molecular-weight heparin in acute myocardial infarction. Am Heart J. 2004;148:1068–1078. doi: 10.1016/j.ahj.2004.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mehta SR, Yusuf S, Díaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K, Tai J, Orlandini A, Pogue J, Liu L, CREATE-ECLA Trial Group Investigators Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 120.Yin M, van der Horst ICC, van Melle JP, Qian C, van Gilst WH, Silljé HHW, de Boer RA. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H459–68. doi: 10.1152/ajpheart.00054.2011. [DOI] [PubMed] [Google Scholar]
- 121.Lexis CPH, van der Horst ICC, Lipsic E, van der Harst P, van der Horst-Schrivers ANA, Wolffenbuttel BHR, de Boer RA, van Rossum AC, van Veldhuisen DJ, de Smet BJGL, GIPS-III Investigators Metformin in non-diabetic patients presenting with ST elevation myocardial infarction: rationale and design of the glycometabolic intervention as adjunct to primary percutaneous intervention in ST elevation myocardial infarction (GIPS)-III trial. Cardiovasc Drugs Ther. 2012;26:417–426. doi: 10.1007/s10557-012-6413-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eltzschig HK, Bonney SK, Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med. 2013;19:345–354. doi: 10.1016/j.molmed.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koupenova M, Johnston-Cox H, Ravid K. Regulation of atherosclerosis and associated risk factors by adenosine and adenosine receptors. Curr Atheroscler Rep. 2012;14:460–468. doi: 10.1007/s11883-012-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.