Abstract
Metabolic syndrome (MetS) is a clinical condition that includes multiple cardiovascular disease risk factors including obesity, high blood pressure or hypertension, dyslipidemia, and abnormal glucose metabolism. The core metabolic abnormality in MetS is insulin resistance, or impaired insulin mediated glucose regulation that results in elevated plasma insulin concentration. MetS, or multiple cardiovascular risk factors, markedly increase risk for diabetes, atherosclerosis, and adverse metabolic and cardiovascular outcomes. Although commonly associated with adult diseases and aging, MetS is also identifiable in childhood. Because obesity is a key component of the syndrome, the prevalence of MetS in U.S. adults is over 25%, with higher rates among race/ethnic minority groups. The population prevalence of MetS is much less in childhood at approximately 4 to 5%. However, due to the childhood obesity epidemic the prevalence of MetS among obese children and adolescents is approximately 30% with similar race/ethnic disparity among race/minority groups.
Keywords: Insulin resistance, metabolic syndrome, blood pressure, lipids, diabetes, obesity, children, adolescents, adults, race
Introduction
An overlap in occurrence of obesity, hypertension, type 2 diabetes, and atherosclerosis among adults has been commonly observed in clinical practice. This cluster of conditions and risk factors, within individuals, was first described as syndrome X by Reaven1 in the 1988 Banting lecture. Subsequently, other names have been applied to this condition including insulin resistance syndrome, mixed metabolic syndrome, dysmetabolic syndrome, and finally metabolic syndrome (MetS), the term currently used. As proposed by Reaven and others,2,3 insulin resistance, or impaired insulin mediated glucose uptake, is the core abnormality that link the cosegregation of metabolic and hemodynamic abnormalities in MetS. As a consequence of tissue resistance to insulin action, greater amounts of insulin are secreted to achieve glucose control, resulting in relative hyperinsulinemia. Insulin resistance, or impaired insulin sensitivity is difficult to quantify clinically, and the concept of MetS has developed as a strategy to identify individuals with multiple cardiovascular disease risk factors that are linked with insulin resistance. Individuals with MetS experience a high incidence of both diabetes and cardiovascular disease,4, 5 and MetS is strongly associated with higher cardiovascular and total mortality.6
Following the original description of the syndrome, there has been expansion of knowledge on the pathogenesis of metabolic and vascular alterations in MetS. However several issues remain unanswered. For example, while obesity is a very consistent component of MetS, it remains unclear if obesity is a cause or consequence of MetS. Despite several theories and considerable study, the mechanism that links high blood pressure, or hypertension, with insulin resistance remains elusive. There are race and ethnic differences in the prevalence of MetS. Moreover, MetS is not limited to adults, and has been described in children and adolescents as well. This review will focus on race and ethnic variations in MetS in adults and in children, with an emphasis on recent reports that link insulin resistance with high blood pressure in children and adolescents.
Definition of MetS
MetS is a cluster of cardiovascular disease risk factors associated with insulin resistance within individuals that increase the risk for diabetes, cardiovascular disease, and adverse cardiovascular outcomes.7, 8 There is variation in the precise definition of MetS, including which risk factors are key components of the syndrome and threshold values considered to be abnormal. Definitions of MetS have been established by the World Health Organization (WHO),9 the European Group for the Study of Insulin Resistance,10 the National Cholesterol Education Program Adult Treatment Panel III (ATPIII),11 the American Association of Clinical Endocrinologists,12 and the International Diabetes Federation.13 Of these varying definitions, the WHO and ATPIII criteria appear to be more commonly used in clinical reports. Table 1 provides the WHO and ATPIII parameters used for the definition of MetS. According to the ATPIII definition, three of the five criteria are required for diagnosis of MetS. Definition of MetS according to WHO criteria, requires hyperinsulinemia, diabetes, or abnormal fasting glucose plus two other parameters. With these differing definitions of MetS in adults, it has been difficult to compare prevalence of MetS in different populations, especially in comparing ethnic and racial difference in prevalence. Moreover, no consistent definition has been established for definition of MetS in childhood.14 Studies to identify MetS in childhood have generally been based on modifications of either WHO or ATPIII adult criteria. Goodman et al,15 compared the prevalence of MetS in a school based sample of adolescents based on the WHO definition versus the ATPIII definition. Using ATPIII criteria, overall prevalence among adolescents was 4.2% compared to a prevalence of 8.4% when WHO criteria were applied. Among obese adolescents the prevalence of MetS was 19.5% based on ATPIII criteria compared to 38.9% based on WHO criteria. No race or sex differences were identified using the ATPIII definition; in contrast the WHO definition did detect race and sex differences in MetS prevalence. A uniform parameter in all definitions of MetS is obesity, although that criteria also varies according to which measurement of adiposity is the best risk parameter; body mass index (BMI), waist circumference, or some other measurement of visceral adiposity. Despite lack of uniformity on definition, MetS is identifiable in children and adolescence.16, 17,18, 19 The global obesity epidemic, especially the childhood obesity epidemic, has raised both awareness and concern on early expression of obesity associated risk factors represented in MetS.
Table 1.
Criteria for Diagnosis of Metabolic Syndrome: WHO and ATPIII
| Parameter | WHO | ATPIII |
|---|---|---|
| 1. Obesity | BMI ≥ 30 Kg/m2 or WC ≥ 100 cm in males |
WC ≥ 100 cm in males WC ≥ 88 cm in females |
| WC ≥ 88 cm in females | ||
| 2. BP | Hypertension or Systolic BP ≥ 130 mm Hg |
Hypertension or Systolic BP ≥ 130 mm Hg |
| Diastolic BP ≥ 85 mm Hg | Diastolic BP ≥ 85 mm Hg | |
| 3. Lipids | HDL ≤ 35 mg/dL in males | HDL ≤ 40 mg/dL in males |
| HDL ≤ 39 mg/dL in females | HDL ≤ 50 mg/dL in females | |
| 4. Lipids | Triglyceride ≥ 150 mg/dL | Triglyceride ≥ 150 mg/dL |
| 5. Glucose | Diabetes or Fasting glucose ≥ 110 mg/dL | Diabetes or Fasting glucose ≥ 100 mg/dL |
| Insulin* | Hyperinsulinemia |
BMI = body mass index, BP = blood pressure, WC = waist circumference, HDL = high density lipoprotein
WHO requires hyperinsulinemia plus two or more other parameters.
ATPII requires three or more parameters
Prevalence of MetS by Race/Ethnicity
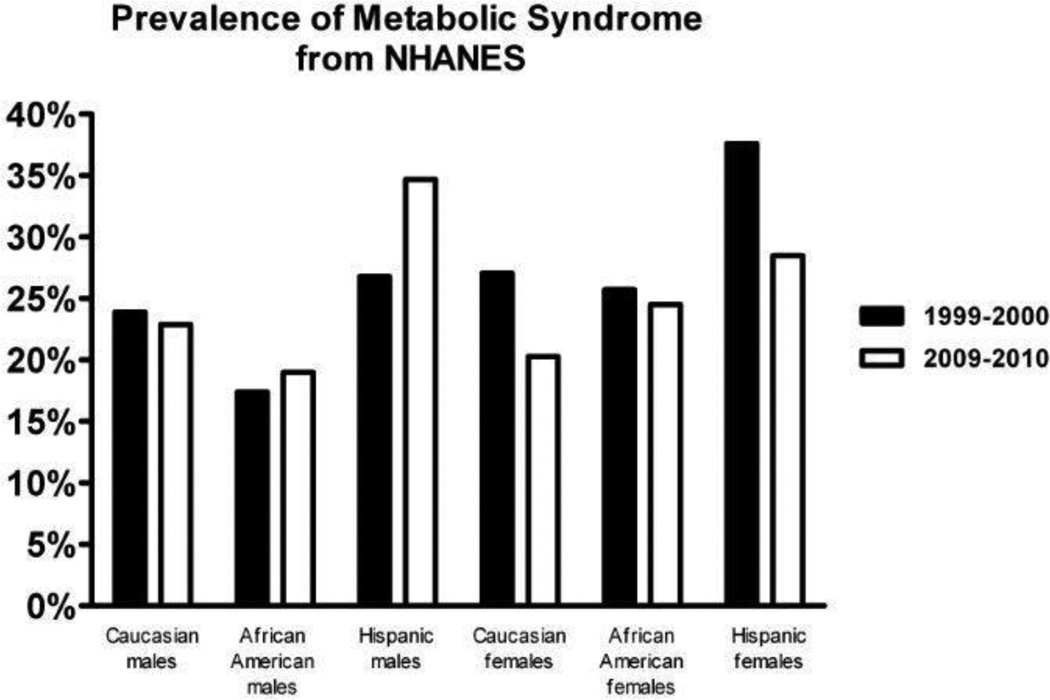
The prevalence of MetS in US adults has been described in several reports based on analysis of National Health and Nutrition Examination Survey (NHANES) data.20,21, 22. The first of these reports based on NHANES data from 1988–1994 estimated the total age–adjusted prevalence of MetS under the ATPIII criteria was 23.97%.21 The most recent of these by Beltran-Sanchez et al,22 examined the trends in prevalence of MetS, based on serial NHANES data from 1999–2000 to 2009–2010. These investigators reported a modest decrease in the age-adjusted prevalence of MetS among US adults, between 1999–2000 when it was 25.5% compared to the 2009–2010 period when the total age-adjusted prevalence was 22.9%. Based upon these results, currently approximately one in five US adults meet criteria for MetS. The prevalence of MetS increased with age in all race/ethnic groups. However, when examining the prevalence of MetS by race/ethnicity, the decrease was only statistically significant for the Caucasian population (25.6% to 21.8%, p for trend = 0.009), whereas there was little change in African Americans and Hispanics, 22,0% to 22.71% and 32.6% to 31.9%, respectively. These race/ethnic differences are summarized in Figure 1. Notably, nearly one third of Hispanics met criteria for MetS in 2009–2010.
Figure 1.
The race/ethnic prevalence of metabolic syndrome in adult males and females, based on two NHANES data periods is depicted: 1999–2000 in black bars and 2009–2010 in gray bars. The prevalence data is based on an analysis of serial NHANES data by Beltrán-Sánchez et al.22
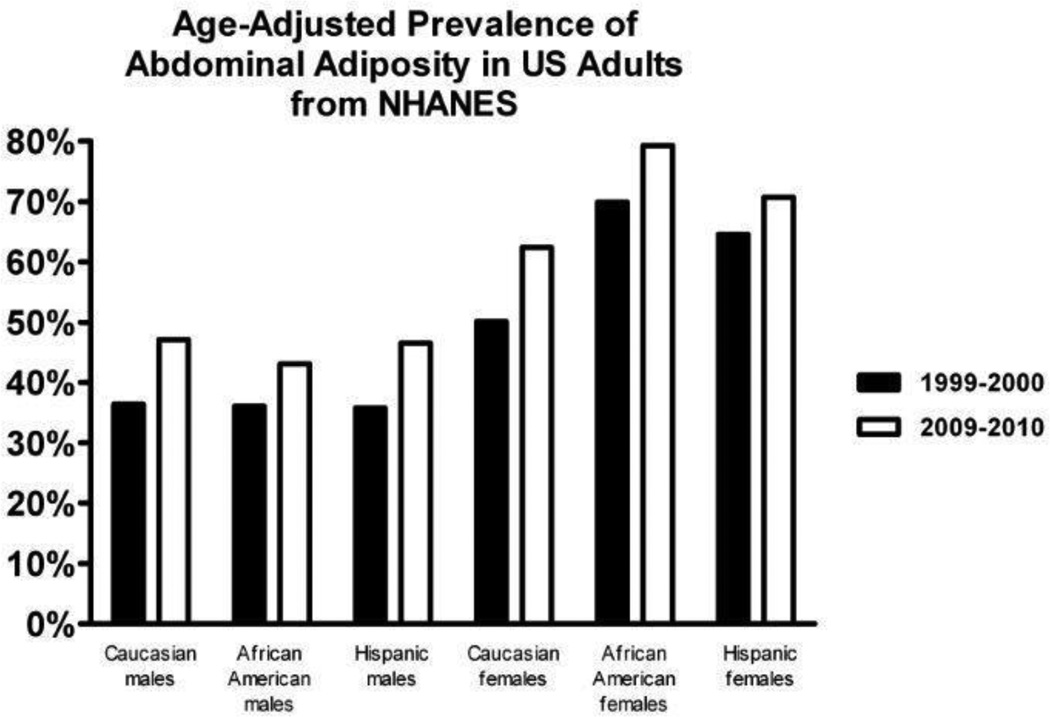
Analysis of NHANES data between 1999–2000 and 2009–2010 demonstrate significant trend differences in individual components of MetS, and also substantial race/ethnic differences in several individual components. Notable differences in race and sex changes were detected for abdominal obesity (Figure 2) and in abnormal glucose (Figure 3). The prevalence of visceral obesity in males, based on waist circumference ≥100 cm, increased for all adult males but was statistically significant only in Caucasian males (p=0.024). Comparing the same two NHANES examination periods, the prevalence of visceral obesity, based on waist circumference ≥88 cm, also increased for all females. African American and Hispanic females have the highest prevalence of visceral obesity. The increase in visceral obesity prevalence was not statistically significant for Hispanic females. 22 Overall, based on this analysis of NHANES data, the prevalence of obesity, based on waist circumference, is extremely high, nearly 50% for males and higher than 60% in all race/ethnic groups.
Figure 2.
The age adjusted prevalence of abdominal adiposity in adult males and females is depicted for race/ethnic populations. The race/ethnic prevalence of abdominal adiposity is compared in two NHANES data periods: 1999–2000 in black bars and 2009–2010 in gray bars. The prevalence data is based on an analysis of serial NHANES data by Beltrán-Sánchez et al.22
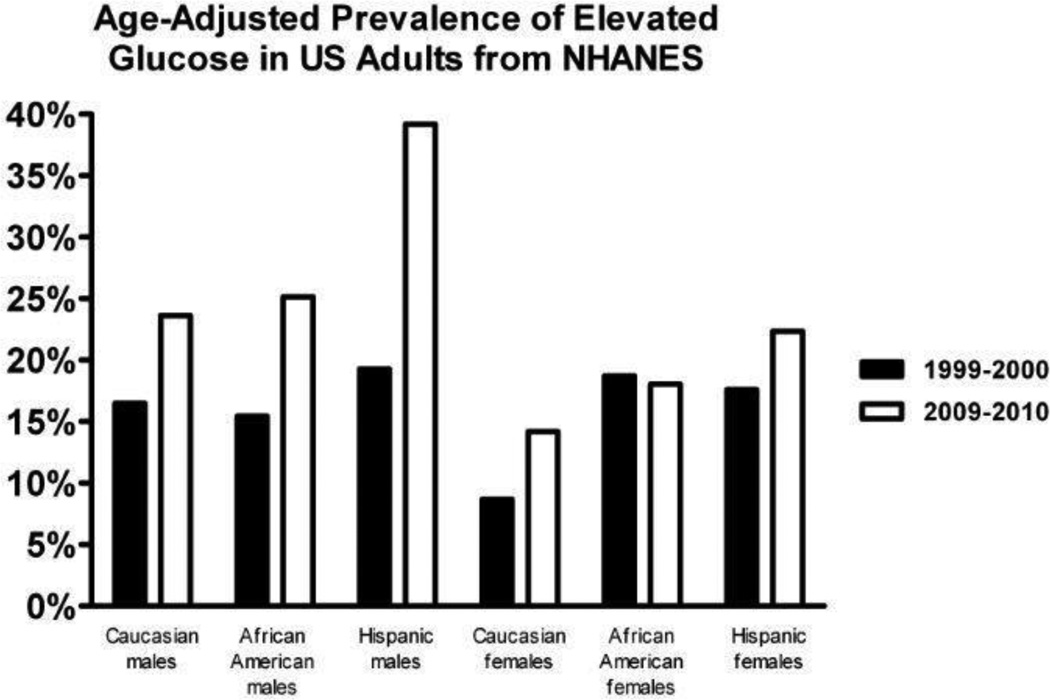
Figure 3.
The age adjusted prevalence of elevated plasma glucose levels in adult males and females is depicted for race/ethnic populations. The race/ethnic prevalence of elevated glucose is compared in two NHANES data periods: 1999–2000 in black bars and 2009–2010 in gray bars. The prevalence data is based on an analysis of serial NHANES data by Beltrán-Sánchez et al.22
Somewhat different levels of obesity are reported when a different measure of obesity is applied to more recent NHANES data. Ogden et al,23 analyzed the population prevalence of obesity using NHANES 2011 and 2012 data, but defined obesity as BMI ≥30 kg/m2. Overall, and for all race groups, except, Asian Americans, the prevalence of obesity exceeded 30%. The age-adjusted prevalence of obesity was highest among Hispanic women (44.4%) and African American women (56.6%). This report also included an analysis of obesity in children. The results indicate that while there are race differences in the prevalence of obesity in pediatric groups according to race, (19.6% for Caucasian children, 22.1% for African American children, and 22.6% for Hispanic children), these race/ethnic differences are more divergent in adults. Overall, the prevalence of obesity is extremely high in adults, whether defined by BMI or by waist circumference, and similar race/ethnic differences are detectable.
Abnormal Glucose Metabolism
In comparing NHANES data from the1999–2000 period to the 2009–2010 period, as reported by Beltran-Sanchez et al,22 the prevalence of abnormal fasting glucose or diabetes increased in all race/ethnic groups; from 12.3% to 18.8% in Caucasians (p<0.001); from 17.0% to 21.8% in African Americans (p=0.006), and from 18.8% to 29.8% in Hispanics (p<0.001). The increase was statistically significant for all males and Caucasian females, although the change was not significant for African American and Hispanic females.
Blood Pressure
The comparison of serial NIHANES population data on blood pressure indicate that there was a statistically significant decrease in the prevalence of high blood pressure, according to ATPIII criteria, in 2009–2010 compared to the 1999–2000 data period. The decrease was apparent in Caucasian males and females and in Mexican-American females, but did not achieve significance in the other race-sex groups. There was also a substantial increase in the use of anti-hypertensive medicines among the NHANES 2009–2010 sample in this time period.22
Dyslipidemia
Similar to blood pressure, there appeared to be a reduction in the prevalence of abnormal HDL-cholesterol in the 2009–2010 NHANES data among Caucasians, compared to the earlier 1999–2000 data period. During the same time interval there was no significant change in the prevalence of abnormal HDL among African Americans and among Hispanics. The prevalence of abnormal HDL was 29.7 for Caucasions, lowest at 27.1% for African Americans, and highest among Hispanics at 34.5%. Nevertheless, more than a quarter of Caucasians and African Americans and more than a third of Mexican-Americans meet the criteria for low HDL-C.
Over this same time interval, there was a decline in prevalence of elevated triglyceride levels in Caucasians but no significant trend in Hispanics or African Americans. In 2009–2010, more than a third of Hispanics (35.7%) have elevated triglycerides and African Americans have the lowest prevalence of elevated triglycerides (14.0%). Overall, from 1999–2000 to 2009–2010, the prevalence of dyslipidemia declined in the US adult population. Although the definitions for high triglycerides and low HDL-C include drug treatment, from 1999–2000 to 2009–2010 there is an inverse association of declining prevalence and increasing pharmacological treatment for dyslipidemia.22
The leading theory to explain the mechanism underlying the detrimental effect of insulin resistance on cardiovascular injury is the association of insulin resistance with atherosclerotic dyslipidemia.24 Several reports describe greater insulin resistance in African Americans compared to Caucasians.25–27 Despite having greater insulin resistance, African Americans have more favorable lipid profiles compared to Caucasians, with lower triglyceride (TG) and higher high-density cholesterol (HDL) concentrations compared to Caucasians.28, 29 In a study on young adult African Americans, age 30–45 years, significant correlations were found for TG, HDL-C, and TG/HDL-C ratio with insulin resistance, quantified by the insulin clamp procedure. Despite obesity in 50% of the sample, only 10% of participants had plasma TG levels ≥150 mg/dL, MetS criterion for both ATPIII and WHO. Participants with TG levels from 110 to 149 mg/dL had measures of insulin resistance comparable to those with TG >150 mg/dL.30 Although African Americans appear to have more favorable lipid profiles, it is possible that they have a different threshold for adverse effects of relative dyslipidemia. Oxidative stress could be a plausible pathway for lipid mediated vascular injury. The effect of acute hyperlipidemia on oxidative stress was studied in African Americans and Caucasians by Lopes et al.31 In both African American and Caucasian groups, a comparable increase in plasma TG concentration occurred following an infusion of Intralipid and heparin. However, F2-isoprostanes, a biomarker of oxidative stress in humans, increased significantly more in African Americans compared to Caucasians. Although this report is based on a short-term rise in TG, the results suggest that African Americans could have greater sensitivity to increases in TG. Some reports on MetS prevalence, based on studies that include various race groups, question the validity of applying the same criteria for MetS to all race and ethnic groups.32, 33
As noted above, the population prevalence of MetS varies considerably, depending on the definition applied. This is especially true for estimated prevalence of MetS in children and adolescents. An early report on the childhood population prevalence of MetS was reported by Cook et al.34 These investigators used ATPIII criteria to estimate prevalence of MetS in adolescents from NHANES 1988–1994 data and reported an overall prevalence of 4.2% with higher prevalence in boys than girls. Among obese adolescents only, the prevalence was 28.7%. A study by Weiss et al,16 included predominately obese children, age 4 to 20 years of age, used a combination of ATPIII and WHO criteria to define MetS. These investigators reported a progressive increase in the prevalence of MetS with increasing degree of obesity and insulin resistance, with MetS identified in 50% of severely obese youth. Subsequently Cook et al,35 used four different definitions to compute the prevalence of MetS in adolescents using the NHANES 1999–2000 data. Under different MetS criteria the overall prevalence ranged from 2.0% to 9.4%. Among obese adolescents the prevalence ranged from 12% to 44%, depending on criteria used. In another report, Ford et al,36 used the International Diabetes Federation criteria to estimate the prevalence of MetS in adolescents on three NHANES data periods from 1999–2000 to 2003–2004 and reported an overall prevalence of 4.5%. There were prevalence variations according to age, sex, and race, with the highest prevalence (7.1%) among Hispanics. As in adults, variation in reported childhood prevalence of MetS depends upon the criteria used to define MetS. In addition, the prevalence estimated on NHANES 1988–1994 data precedes the childhood obesity epidemic, whereas the childhood obesity epidemic was well underway by the 1999–2000 NHANES data period. The childhood population data consistently demonstrates higher prevalence of MetS among obese children relative to non-obese counterparts.
Obesity Associated Hypertension
Obesity is commonly associated with hypertension in adults, and this strong association is consistent in all race/ethnic groups. Campbell et al.37 reported on a study designed to determine if obesity plus high BP could be a clinical phenotype for MetS in African Americans. The investigators stratified a sample of 337 young adult African Americans (mean age 32 years) into four BP-BMI categories: non-obese and normal BP, non-obese and high BP, obese and normal BP, obese and high BP. All participants had fasting lipid levels, an oral glucose tolerance test, and an insulin clamp procedure to quantify insulin resistance. ATPIII criteria were used to identify MetS within each group. No cases of MetS were present among nonobese participants with normal BP. The presence of high BP without obesity was associated with 25% prevalence of MetS, and obesity with normal BP was associated with 44.9% prevalence of MetS. Since the obese with high BP group already had two MetS criteria, 87% of had at least one lipid or glucose abnormality and met criteria for MetS. Among the obese participants, the degree of insulin resistance was equivalent in those with and without high BP. Interestingly, previously unknown type 2 diabetes was identified in 19.2% of the obese with high BP compared to 0% to 2.6% of all other groups. Although this study was limited to African Americans, the results suggest that the combination of obesity and high BP increases the risk for additional metabolic abnormalities.
The prevalence of primary hypertension is much lower in childhood than in adults. However, as in adults, obesity is strongly associated with both hypertension and prehypertension in children and adolescents.38, 39 The prevalence of hypertension in childhood is approximately 3.5%. For prehypertension the prevalence is approximately 3.5% with higher rates of high BP in adolescents.40, 41 However, among obese children and adolescents, the prevalence of both hypertension and prehypertension is much higher compared to normal weight children. This relationship is detectable even in very young children.42 In a study by Boyd et al,43 high BP (prehypertension and hypertension combined) was found in 34.7% of obese children attending a weight management clinic. Although the prevalence of high BP by race was not reported, this rate of high BP among obese children is very similar to the prevalence of high BP detected in school based screening of generally healthy high school students reported by Sorof et al,44 in which 34% of adolescents with BMI ≥95th percentile had high BP. In the study by Boyd et al,43 investigators examined clinical records and computed average BP from up to 5 separate visits and also stratified the patients to moderate and severe obesity according to BMI z score, which adjusts for age and sex. Rates of high BP were greater in obese boys than obese girls both in moderate and severely obese groups. Abnormal plasma lipid levels for HDL and triglycerides were common among all obese children. HDL <40 mg/dL was present in 49.4% of boys with high BP compared to 27.6% of boys with normal BP. In girls, HDL <40 mg/dL was present in 32.9% with high BP and in 27.6% with normal BP. Triglyceride levels were >150 mg/dL in 34.1% of boys with high BP compared to 25.8% in boys with normal BP. Triglyceride levels were similar in girls with high BP (17.1%) and normal BP (18.4%). Although this study was conducted on a sample of obese children referred to a weight management clinic, indicating some selection for severity, the findings do indicate that, especially in boys, dyslipidemia is common among obese children with high BP. Others report that this combination of risk factors, which meet criteria for MetS, are related to atherosclerotic lesions in youth45 and are predictive of subsequent increased carotid intimal-media thickness.46
It is well established that cardiovascular risk factors are present in childhood and it is also known that these risk factors set the stage for cardiovascular pathology. There are also race and ethnic differences in prevalence of individual risk factors that may explain to some extent racial disparities in cardiovascular disease outcomes. Based on recent national survey data in children and adolescents, both BP level and prevalence of high BP are increasing,47 and are consistently higher among minority children.48 The increase in BP level is largely attributed to the increase in BMI and waist circumference. Overall, African American children have the highest rates of high BP and obesity; Hispanic children have highest rates of abnormal glucose levels as well as high rates of obesity; Caucasian children have higher rates of adverse lipid levels, despite less obesity and high BP.
Birth Weight
Following original observations in adults, Barker et al,49 proposed that low birth weight, in response to nutritional deprivation in the intrauterine environment, contributes significantly to susceptibility to cardiovascular and metabolic diseases in later life. Original reports on the birth weight hypothesis linked low birth weight to premature coronary artery disease in men, with high BP thought to be the mediating factor in evolution of cardiovascular disease. While experimental studies tend to support the theory of fetal programing in response to the intrauterine environment, this theory has been more difficult to confirm in human studies. Subsequent investigations on a potential relationship of birth weight with development of chronic disease in later life focused on metabolic diseases. Whincup et al,50 conducted a systematic review on published evidence from human studies on the association of birth weight and type 2 diabetes in adults. These investigators concluded that, based on the body of available data, in most populations low birth weight was inversely related to subsequent type 2 diabetes. While insulin resistance is the core metabolic alteration underlying type 2 diabetes and MetS, clinical investigations conducted on children and adolescents, resulted in inconsistent findings, largely related to variations in adiposity, the rate of weight gain, and nutritional patterns, as well as modest sample sizes.19 For example, Li et al,51 investigated the effects of birth weight on insulin resistance in 139 Caucasian and African American children. They reported a significant association of low birth weight with higher fasting insulin, but no relationship of birth weight with insulin resistance or other metabolic or BP variables. They also detected a much stronger association of low birth weight with higher fasting insulin concentration in African American children compared to Caucasian children.
Prospective studies on fetal programing that begin in the neonatal or perinatal period are limited. A recent report on a prospective study by Lurbe et al52 provides new insights on the fetal programing theory. These investigators enrolled a sample of healthy newborn infants, all products of normal uncomplicated pregnancy. Birth weight and BP were measured at 2 days of age. The infants were then stratified into birth weight groups; small for gestational age (SGA), average for gestational age (AGA), and large for gestational age (LGA). In subsequent exams at 6 months, 2 years, and 5 years BP and growth parameters were measured. At age 5 years a blood sample was obtained for measurement of metabolic parameters. Although the sample size of this study was modest (N=139), novel findings were detected. Weight gain was similar in each birth weight group at each exam interval; the SGA infants remained the smallest and LGA infants remained the largest. Both birth weight and weight gain were positively associated with birth weight; and birth weight was not significantly associated with BP. The metabolic data demonstrated higher fasting insulin levels in the SGA infants who became heavy at age 5 years compared to AGA and LGA infants who became heavy at age 5 years. Most interesting were the measures of insulin resistance, estimated by the homeostatatic model equation (HOMA index). SGA infants were insulin resistant compared to AGA and LGA infants, regardless of their weight status at age 5 years. Even those SGA infants who remained small and thin were relatively insulin resistant compared to those with higher birth weights. This study was limited to Caucasian children and does not provide any information on racial differences. However the data obtained on a sample of healthy infants indicate that some perinatal factor, or combination of exposures, related to lower birth weight could program an individual for relative insulin resistance that is maintained independent of excess weight gain. Similar prospective studies that include other race/ethnic groups of newborn infants are needed to determine if these findings are generalizable and if so, what mechanisms initiate the perinatal programing.
Summary
Insulin resistance is the central metabolic abnormality associated with abnormal glucose metabolism, dyslipidemia, hypertension and obesity. MetS is a clinical construct that is used to link these metabolic and cardiovascular abnormalities. Because MetS is composed of multiple risk factors for type 2 diabetes and cardiovascular disease, the presence of MetS markedly increases the risk for cardiovascular morbidity and mortality. The leading clinical phenotype for MetS is obesity; and MetS is commonly detected among individuals with obesity and high BP. As with obesity, there are race/ethnic differences in prevalence of MetS, with higher rates among African Americans and Hispanic populations. Risk factors within MetS are potentially modifiable by lifestyle changes in diet, physical activity, and weight reduction. In adults, additional primary prevention for adverse cardiovascular outcomes can also be achieved with pharmacologic management of hypertension, dyslipidemia, and diabetes; and some of these drugs may have insulin sensitizing benefits. For children, enduring lifestyle changes are difficult to achieve and results of non-pharmacologic management strategies have been very limited. Use of pharmacologic treatments in MetS risk factors in children is even more complicated due to limited data on intermediate markers of cardiovascular injury and clinical trial data to support long term benefit in arresting or reversing cardiovascular injury. Additional clinical research is needed to develop strategies on type and timing of interventions on MetS, a condition that appears to accelerate the cardiovascular disease process.
Acknowledgments
Bonita Falkner has receive a NIH-NHLBI grant and paid travel expenses from the NIH for grant review meetings (grant application reviewer for NIH and American Association for Advancement of Science).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Nicole D.F.H. Cossrow declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Bonita Falkner, Thomas Jefferson University, 833 Chestnut St, Philadelphia, PA 19107, Bonita.Falkner@jefferson.edu, Phone: 215-5-3-2501, FAX: 215-503-2506.
Nicole D.F.H. Cossrow, 1238 Lenox Rd, Jenkintown, PA 19046, nicolecossrow@msn.com.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 3.Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981;240:E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 5.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. The Journal of clinical endocrinology and metabolism. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 6.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 7.Meigs JB, D'Agostino RB, Sr, Wilson PW, et al. Risk variable clustering in the insulin resistance syndrome. The Framingham Offspring Study. Diabetes. 1997;46:1594–1600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- 8.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. The Journal of clinical endocrinology and metabolism. 1998;83:2773–2776. doi: 10.1210/jcem.83.8.5005. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic medicine : a journal of the British Diabetic Association. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabetic medicine : a journal of the British Diabetic Association. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 11.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Bloomgarden ZT. Definitions of the insulin resistance syndrome: the 1st World Congress on the Insulin Resistance Syndrome. Diabetes care. 2004;27:824–830. doi: 10.2337/diacare.27.3.824. [DOI] [PubMed] [Google Scholar]
- 13.Federation ID. The IDF consensus world wide definition of the metabolic syndrome. 2005 Http://wwwidforg/webdata/docs/Metabolic_syndrome_definitionpdf.
- 14.Ford ES, Li C. Defining the metabolic syndrome in children and adolescents: will the real definition please stand up? The Journal of pediatrics. 2008;152:160–164. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 15.Goodman E, Daniels SR, Morrison JA, et al. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. The Journal of pediatrics. 2004;145:445–451. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 16.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. The New England journal of medicine. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom E, Hernell O, Persson LA, et al. Insulin resistance syndrome in adolescents. Metabolism: clinical and experimental. 1996;45:908–914. doi: 10.1016/s0026-0495(96)90168-7. [DOI] [PubMed] [Google Scholar]
- 18.Young-Hyman D, Schlundt DG, Herman L, et al. Evaluation of the insulin resistance syndrome in 5- to 10-year-old overweight/obese African-American children. Diabetes care. 2001;24:1359–1364. doi: 10.2337/diacare.24.8.1359. [DOI] [PubMed] [Google Scholar]
- 19.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. The Journal of clinical endocrinology and metabolism. 2003;88:1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 20.RB E. Prevalence of metabolic syndrome among adults 20 years of age and over by sex age, race and ethnicity, and body mass index: United States: 2003–2006. National Health Statistics Reports. 2009;13 [PubMed] [Google Scholar]
- 21.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 22. Beltrán-Sánchez HHM, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult US population 1999–2010. Journal of the American College of Cardiology. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. This study estimated the prevalence of metabolic syndrome in US adults and metabolic syndrome components according to sex and race/ethnic populations. The estimates are based on analysis of serial NHANES data periods from 1999–0032000 to 2009–2010. The results of this study depict difference in prevalence by race/ethnic groups
- 23.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States: 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GM R. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: the price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol Metab Clin North Am. 2005;34:49–62. doi: 10.1016/j.ecl.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Ryan AS, Nicklas BJ, Berman DM. Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obesity research. 2002;10:336–344. doi: 10.1038/oby.2002.47. [DOI] [PubMed] [Google Scholar]
- 26.Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. The Journal of clinical endocrinology and metabolism. 1997;82:1923–1927. doi: 10.1210/jcem.82.6.4002. [DOI] [PubMed] [Google Scholar]
- 27.Haffner SM, D'Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 28.Sumner AE, Vega GL, Genovese DJ, et al. Normal triglyceride levels despite insulin resistance in African Americans: role of lipoprotein lipase. Metabolism: clinical and experimental. 2005;54:902–909. doi: 10.1016/j.metabol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Howard BV, Mayer-Davis EJ, Goff D, et al. Relationships between insulin resistance and lipoproteins in nondiabetic African Americans, Hispanics, and non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Metabolism: clinical and experimental. 1998;47:1174–1179. doi: 10.1016/s0026-0495(98)90319-5. [DOI] [PubMed] [Google Scholar]
- 30.Stein E, Kushner H, Gidding S, et al. Plasma lipid concentrations in nondiabetic African American adults: associations with insulin resistance and the metabolic syndrome. Metabolism: clinical and experimental. 2007;56:954–960. doi: 10.1016/j.metabol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes HF, Morrow JD, Stojiljkovic MP, et al. Acute hyperlipidemia increases oxidative stress more in African Americans than in white Americans. American journal of hypertension. 2003;16:331–336. doi: 10.1016/s0895-7061(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 32.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey: 1988–1994. Archives of internal medicine. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112:32–38. doi: 10.1161/CIRCULATIONAHA.104.520130. [DOI] [PubMed] [Google Scholar]
- 34.Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey: 1988–1994. Archives of pediatrics & adolescent medicine. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 35.Cook S, Auinger P, Li C, et al. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey: 1999–2002. The Journal of pediatrics. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Ford ES, Li C, Zhao G, et al. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes care. 2008;31:587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 37.Campbell KLKH, Balkner B. Obesity and high blood pressure: A clinical phenotype for the insulin resistance syndrome in African Americans. Journal of Clinical Hypertension (Greenwich) 2004;6:364–372. doi: 10.1111/j.1524-6175.2004.03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanevold C, Waller J, Daniels S, et al. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–333. doi: 10.1542/peds.113.2.328. [DOI] [PubMed] [Google Scholar]
- 39.Brady TM, Fivush B, Flynn JT, et al. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. The Journal of pediatrics. 2008;152:73–78. doi: 10.1016/j.jpeds.2007.05.053. 78 e71. [DOI] [PubMed] [Google Scholar]
- 40.McNiece KL, Poffenbarger TS, Turner JL, et al. Prevalence of hypertension and prehypertension among adolescents. The Journal of pediatrics. 2007;150:640–644. doi: 10.1016/j.jpeds.2007.01.052. 644 e641. [DOI] [PubMed] [Google Scholar]
- 41.Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. Jama. 2007;298:874–879. doi: 10.1001/jama.298.8.874. [DOI] [PubMed] [Google Scholar]
- 42.Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. The Journal of pediatrics. 2006;148:195–200. doi: 10.1016/j.jpeds.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 43.Boyd GS, Koenigsberg J, Falkner B, et al. Effect of obesity and high blood pressure on plasma lipid levels in children and adolescents. Pediatrics. 2005;116:442–446. doi: 10.1542/peds.2004-1877. [DOI] [PubMed] [Google Scholar]
- 44.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–447. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 45.Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. The New England journal of medicine. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. Jama. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 47. Rosner B, Cook NR, Daniels S, et al. Childhood blood pressure trends and risk factors for high blood pressure: the NHANES experience 1988–2008. Hypertension. 2013;62:247–254. doi: 10.1161/HYPERTENSIONAHA.111.00831. This study examined blood pressure trends in children from serial NHANES data. Analysis of serial NHANES data periods demonstrate a progressive increase in childhood blood pressure, and the increase is attributable to BMI, waist circumference, and dietary sodium intake.
- 48.Rosner B, Cook N, Portman R, et al. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502–508. doi: 10.1161/HYPERTENSIONAHA.109.134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barker DJ, Osmond C, Golding J, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ (Clinical research ed) 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 51.Li C, Johnson MS, Goran MI. Effects of low birth weight on insulin resistance syndrome in caucasian and African-American children. Diabetes care. 2001;24:2035–2042. doi: 10.2337/diacare.24.12.2035. [DOI] [PubMed] [Google Scholar]
- 52. Lurbe EG-VC, Torro MI, Aguilar F, Redon J. Associations of birth weight and postnatal weight gain with cardiometabolic risk parameters at five years of age. Hypertension. 2014;63 doi: 10.1161/HYPERTENSIONAHA.114.03137. in press. This study investigated perinatal programming in a prospective study that enrolled healthy newborn infants, all products of a normal pregnancy, and followed the infants with serial growth and blood pressure measurements. Metabolic assessments at age 5 years. Blood pressure correlated positively with weight at all measurement periods. At age 5 years the low birth weight infants were insulin resistant, regardless of weight at age 5 years, compared to average birth weight and large birth weight infants.