Abstract
Evidence suggests a positive association between administration of psychoactive drugs and rates of cigarette smoking. Prevalence of smoking among opioid-dependent individuals, for example, is four times greater than the general population. We recently completed a randomized double-blind trial evaluating outpatient buprenorphine taper for prescription opioid (PO) abusers, which provided an unique opportunity to examine naturalistic changes in smoking among participants who detoxified without resumption of illicit opioid use. Participants received no smoking-cessation services and were not encouraged to alter their smoking in any way. A subset of 10 opioid-dependent smokers, who were randomized to receive the same 4-week buprenorphine taper and successfully completed detoxification, were included in the present study. They provided staff-observed urine specimens thrice-weekly throughout the 12-week trial. Specimens were analyzed onsite via enzyme-multiplied immunoassay for urinary cotinine, a metabolite of nicotine that provides a sensitive biochemical measure of smoking status. Mean cotinine levels were significantly different across study phases, with significantly lower cotinine levels during taper (1317.5 ng/ml) and post-taper (1015.8 ng/ml) vs. intake (1648.5 ng/ml) phases (p’s<.05). Overall, mean cotinine levels decreased by 38% between intake and end-of-study, reflecting a reduction of approximately eight cigarettes per day. These data provide additional evidence that opioids influence smoking and extend prior findings to include primary PO abusers, rigorous double-blind opioid dosing conditions and urinary cotinine. These results also suggest that, while likely insufficient for complete cessation, patients who successfully taper from opioids may also experience concurrent reductions in smoking and thus may be ideal candidates for smoking cessation services.
Keywords: smoking, cigarettes, buprenorphine, opioid, detoxification
1. Introduction
In the United States, tobacco use is responsible for an estimated 443,000 premature deaths and $96.8 billion in lost productivity annually (CDC, 2008). While rates of smoking in the general U.S. population have declined in recent years (CDC, 2012), smoking remains entrenched among individuals with concurrent substance abuse. Among patients receiving methadone or buprenorphine (Suboxone®) for the treatment of opioid dependence, for example, prevalence of smoking is 4-fold greater than the general population (Guydish et al., 2011).
One possible mechanism underlying the elevated smoking in opioid-dependent patients is a pharmacological interaction whereby opioids directly increase smoking (Chait & Griffiths, 1984; Mello, Lukas, & Mendelson, 1985; Mello, Mendelson, Sellers, & Kuehnle, 1980; Mutschler, Stephen, Teoh, Mendelson, & Mello, 2002; Pickworth, Lee, Abreu, Umbricht, & Preston, 2004; Schmitz, Grabowski, & Rhoades, 1994). It is also worth noting though, that similar associations have been observed between smoking and alcohol (e.g., Griffiths, Bigelow, & Liebson, 1976; Henningfield, Chait, & Griffiths, 1983) and psychomotor stimulants (e.g., Rush et al., 2005; Sigmon, Tidey, Badger, & Higgins, 2003). While the precise mechanism underlying these associations remains unknown, a growing literature suggests that shared genetic or neurobiological risk factors may underlie risk of poly-substance use (See Blum & Braverman, 2003; Blum, Cull, Braverman, & Comings, 1996). Nonetheless, this association holds significant clinical relevance considering approximately 5% of Americans report recent opioid abuse (SAMHSA, 2013) and over 272,000 patients receive opioid maintenance treatment for opioid dependence annually (SAMHSA, 2011). Further, approximately five million adults are receiving long-term opioid treatment for acute or chronic pain, with over 256 million opioid prescriptions filled each year (Boudreau et al., 2009; Governale, 2010; Parsells-Kelly et al., 2008).
Several controlled studies have examined the effects of opioids on smoking (Chait & Griffiths, 1984; Lofwall et al., 2007; Mello et al, 1980; 1985; Mutschler et al., 2002; Pickworth et al., 2004; Schmitz et al., 1994). Of those investigating the effect of opioids on the number of cigarettes smoked per day, all demonstrated a significant positive association between opioids and smoking (Chait & Griffiths, 1984; Mello et al., 1985; Mello et al., 1980; Mutschler et al., 2002; Pickworth et al., 2004; Schmitz et al., 1994). In the four studies that included breath carbon monoxide (CO) as a biochemical measure of smoking status, two demonstrated significant increases in breath CO during opioid administration (Chait & Griffiths, 1984; Lofwall et al., 2007), while two showed no effect (Pickworth et al., 2004; Schmitz et al., 1994).
Taken together, while opioids represent a widely-used approach for managing opioid dependence and pain, their possible effects on smoking warrant an improved understanding of this relationship. We present a secondary analysis of data from a double-blind trial evaluating duration of buprenorphine detoxification for treating PO dependence (Sigmon et al., 2013). Participants did not receive any smoking-cessation services and were not encouraged to alter their smoking in any way. Participants’ urine specimens were analyzed for urinary cotinine as an objective measure of smoking, permitting us to examine whether naturalistic changes in smoking occurred among participants who successfully tapered. We hypothesized that successful opioid detoxification would be associated with a reduction in smoking, as evidenced by reductions in urinary cotinine.
2. Methods
2.1 Participants
Participants were PO-dependent adults enrolled in a NIDA-funded clinical trial investigating the efficacy of buprenorphine detoxification and subsequent oral naltrexone therapy (Sigmon et al., 2013). Eligible participants met DSM-IV criteria for opioid dependence, provided an opioid-positive urine at intake, endorsed an illicit PO as their primary drug of abuse (e.g., oxycodone) and were interested in opioid detoxification. Participants were excluded if they were pregnant or nursing, required opioid therapy for pain, or had a significant psychiatric or medical illness. The local institutional review board approved the study, and participants provided written informed consent prior to participating.
2.2 Study Design
Complete methods of the clinical trial have been described previously (Sigmon et al., 2013). Briefly, participants received an initial buprenorphine stabilization (approximately 2 weeks) wherein they were inducted onto a buprenorphine dose sufficient to achieve withdrawal suppression (Johnson, Strain, & Amass, 2003). Once stabilized, participants were randomized to receive a 1-, 2- or 4-week buprenorphine taper. Following randomization, the study was 12 weeks in duration. During each taper, buprenorphine doses were gradually reduced until placebo (0 mg) was reached. Participants who successfully tapered without resuming illicit opioid use were transitioned to oral naltrexone for the remainder of the study. All medications were administered in a double-blind, double- dummy manner to ensure that participants and staff remained blind to dose, taper duration, and the point at which naltrexone began. Thus, participants received 5.5 sublingual (active buprenorphine and/or color-matched placebo buprenorphine) and 3 capsules (active naltrexone and/or placebo naltrexone) at each study visit.
For the present analyses, our aim was to characterize naturally occurring changes in smoking during successful opioid detoxification. Three criteria were used to identify appropriate participants. First, smokers were defined as those who self-reported smoking ≥ 10 cigarettes per day at intake, which is a commonly-used criterion for identifying regular smokers (Schmitz et al., 1994; Tidey, O’Neill, & Higgins, 2000). Second, to minimize confounding related to illicit opioid use, we included only those participants who successfully tapered off of buprenorphine without resumption of illicit opioid use. Finally, to minimize confounding related to varying taper durations and because results from the parent trial determined that the 4-week taper provided the most complete data for analysis (Sigmon et al., 2013), we focused on participants who were randomly assigned to the 4-week taper duration. While these criteria translated to a limited sample size, they also provided a rigorous evaluation of change in smoking throughout detoxification.
2.3 Biochemical Monitoring
Urine specimens were collected under same-sex staff observation thrice weekly (MWF) and analyzed immediately onsite for cotinine using enzyme multiplied immunoassay (EMIT) (MGC240; Microgenics; Fremont, CA).
2.4 Data Analysis
Mean urinary cotinine levels were compared across intake, stabilization, taper and posttaper study phases. The stabilization phase was defined as the last seven days of the buprenorphine stabilization. The buprenorphine taper phase was defined as Weeks 1–5 (Note: participants received active buprenorphine taper during Weeks 1–4, yet Week 5 was included in this phase to permit buprenorphine to clear the system prior to naltrexone induction in Week 6). The post-taper phase was defined as Weeks 6-12 during which all participants received placebo buprenorphine and active naltrexone. Repeated measures analysis of variance was used to compare mean cotinine values collapsed across study phase. Due to skewed cotinine distributions, values were log-transformed prior to analysis, therefore all means presented represent geometric means and their associated standard errors, which were computed based on the Delta method. Temporal changes in mean cotinine values across study week were analyzed using repeated-measures analysis of variance (PROC MIXED). Paired t-tests were used to compare self-reported number of cigarettes smoked at intake with those smoked at the end of the 12-week study. Pairwise comparisons were performed using Fisher’s LSD. All analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC). Statistical significance was determined using α=.05.
3. Results
3.1 Participants
Participants (n=10) were a mean (SD) of 24.8 ± 2.0 years old, 60% male, and 100% Caucasian. At intake participants smoked 21.0 ± 7.7 cigarettes per day and had a mean score of 4.4 ± 1.8 on the Fagerstrom Test for Nicotine Dependence (Fagerström & Schneider, 1989). In terms of opioid use, 60% of participants endorsed illicit oxycodone as their primary opioid, while 30% endorsed illicit buprenorphine. Participants had used opioids regularly for an average of 3.14 (2.0–5.7) years and were stabilized on a mean dose of 11.4 ± 5.7mg buprenorphine prior to detoxification.
3.2 Cotinine as a Function of Study Phase
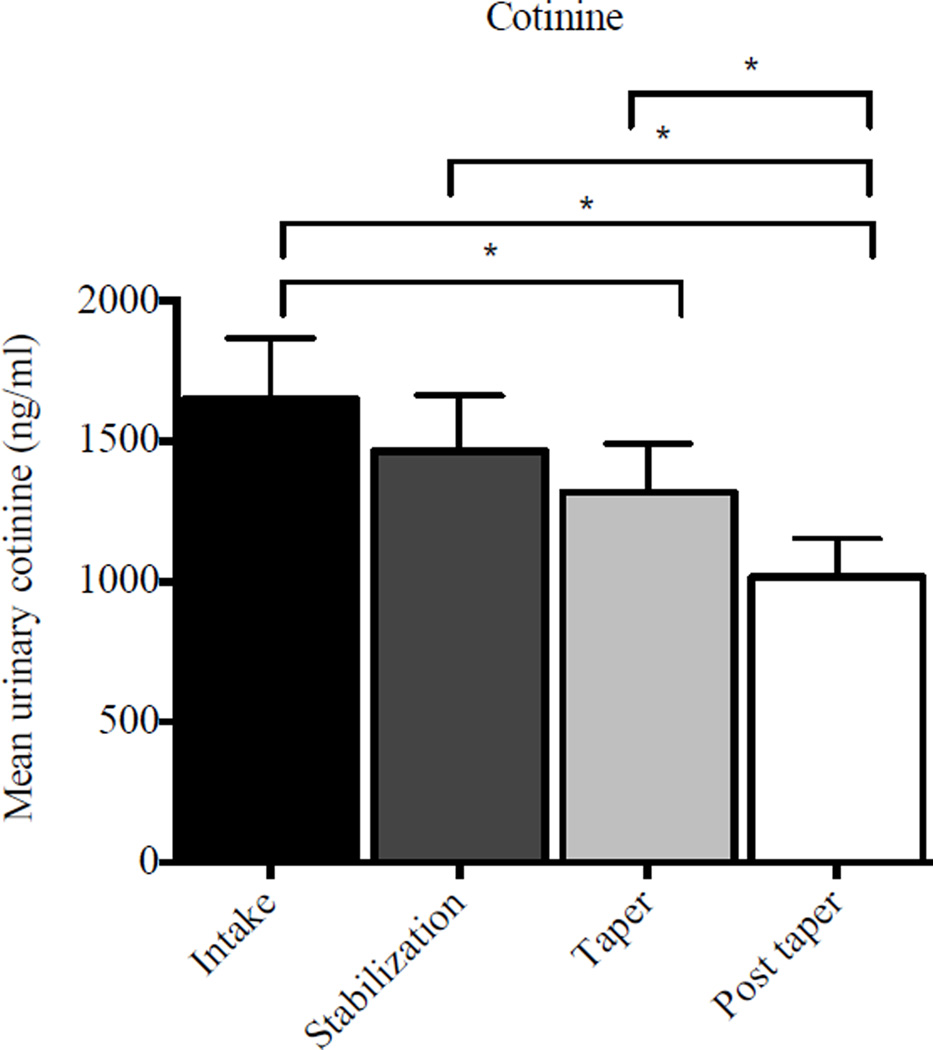
When urinary cotinine levels were compared between intake, stabilization, taper and post-taper phases, there was a significant effect of study phase (F(3, 26) = 6.83, p < .01). Mean cotinine levels were significantly lower during taper (1317.5 ± 173.9 ng/ml) (t(26) = −2.02; p = .05) and post-taper (1015.8 ± 137.1 ng/ml) (t(26) = −4.35; p < .01) phases compared to intake (1648.5 ± 217.6 ng/ml) (Figure 1). Cotinine levels were also significantly lower during the post-taper phase (1015.8 ± 137.1 ng/ml) than stabilization (1464.1 ± 198.1 ng/ml) (t(26) = 3.18, p < .01) and taper phases (1317.5 ± 173.9 ng/ml) (t(26) = 0.92, p = .03). Taken together, mean cotinine levels decreased by approximately 38% between study intake and the post-taper phase (1648.5 vs. 1015.8 ng/ml, respectively), translating to a decrease of approximately eight cigarettes per day (c.f., Benowitz, 1999). A similar reduction was seen in self-reported smoking. Participants reported smoking 21.0 ± 7.7 cigarettes at intake vs. 12.1 ± 5.4 at the end of study (t(9) = 3.47, p = .01.), reflecting a 9-cigarette reduction in number of cigarettes per day that is consistent with the cotinine data.
Figure 1.
Mean urinary cotinine levels as a function of study phase. Data bars represent mean cotinine values (ng/ml) for all specimens collected in each study phase; error bars represent SEM.
3.3 Cotinine as a Function of Study Week
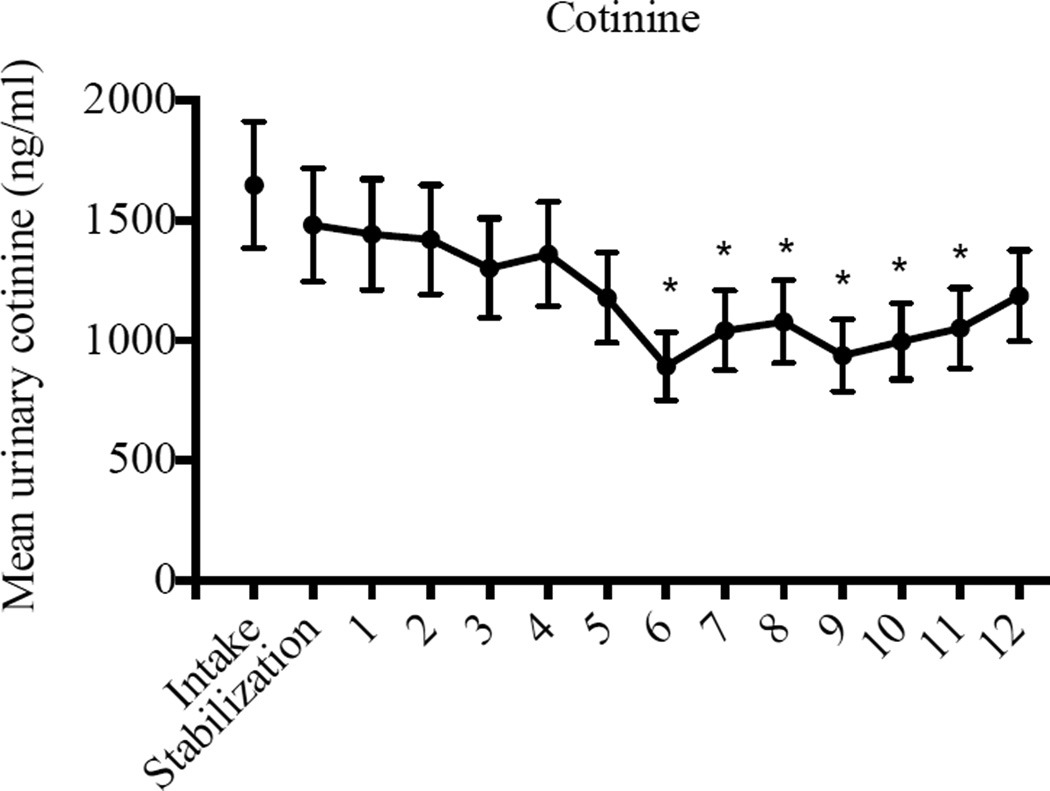
When cotinine levels were examined by study week, there was a significant effect (F(12, 106) = 3.27, p < .01), with significantly lower mean cotinine levels during Study Weeks six (t(106) = 3.74, p < .01), seven (t(106) = 2.61, p < .05), eight (t(106) = 2.35, p < .05), nine (t(106) = 3.38, p < .01), ten (t(106) = 2.93, p < .01), and eleven (t(106) = 2.53, p < .05) compared to the stabilization week (Figure 2).
Figure 2.
Mean urinary cotinine levels as a function of study week. Data points represent mean cotinine values (ng/ml) for all specimens collected during each study week; error bars represent SEM.
4. Conclusions
Our aim was to examine naturally occurring changes in cigarette smoking during outpatient, double-blind buprenorphine detoxification. We observed a gradual decrease in cotinine during opioid detoxification. While no participant quit smoking entirely, mean cotinine levels decreased by 38% over the course of detoxification. Somewhat remarkably, these reductions occurred spontaneously- that is, without any explicit intervention or encouragement for patients to quit or reduce their smoking.
These data are consistent with prior studies suggesting that changes in smoking occur as a function of opioid administration (Bigelow et al., 1981; Chait & Griffiths, 1984; Mello et al., 1980, 1985; Lofwall et al., 2007; Mutschler et al., 2002; Schmitz et al., 1994). However, this study is the first to examine during-treatment changes in smoking among PO-dependent adults. In addition, our examination of smoking during outpatient treatment likely offers generality to the naturalistic smoking behavior seen in typical clinical outpatient settings.
These findings have implications for patients in addiction and pain treatment settings. First, when considered with the existing literature (Chait & Griffiths, 1984; Mutschler et al., 2002; Schmitz et al., 1994), these data suggest that providers should aim to maintain individuals on the lowest effective opioid dose possible without compromising drug or pain management treatment. Second, the interaction between opioids and nicotine may help to explain the poor smoking cessation outcomes among opioid-dependent patients (Richter et al., 2001; but see Dunn, Saulsgiver, & Sigmon, 2011). However, also worth noting is that, if patients who successfully taper from opioids do also experience concurrent reductions levels of nicotine dependence, they may be good candidates for receiving services to support further cessation.
Several limitations should be noted. First, this was a secondary analysis with a limited sample size. We also were unable to examine changes in smoking as a function of taper, given the limited numbers of successful participants in the 1- and 2-week experimental groups (Sigmon et al., 2013). However, limiting our analyses to a uniform taper minimized the influence of potential confounds between groups (e.g., differences in intensity of opioid withdrawal, ancillary medication usage). Second, it is not clear whether naltrexone administration contributed to the decreases in smoking. Although some studies have suggested that naltrexone may reduce smoking (Covey et al., 1999; King et al., 2012), a recent meta-analysis reported no consistent effect and the smoking reductions observed here began well before naltrexone initiation (David et al., 2013).
In summary, this study provides additional evidence that opioid agonist medications influence cigarette smoking, with spontaneous yet meaningful decreases in smoking occurring during outpatient opioid detoxification. These findings may inform and improve efforts to balance effective opioid agonist treatment with smoking-related risks among the five million U.S. patients currently receiving long-term opioid therapy for addiction or pain.
Highlights.
We examined naturalistic changes in smoking throughout a 12-week double-blind, double-dummy buprenorphine detoxification study.
Urinary cotinine levels were analyzed among participants who completed a 4-week buprenorphine taper without resumption to illicit opioid use.
Urinary cotinine levels significantly decreased throughout opioid detoxification.
Overall, mean cotinine levels decreased by 38% between intake and end-of-study, translating to an approximate 8 cigarettes per day reduction.
These data provide additional evidence that opioids influence smoking.
Acknowledgements
We thank Betsy Bahrenburg, RN, Bruce Brown, BS, LICSW, LADC, and Kathryn Saulsgiver, Ph.D. for their assistance conducting this study.
Role of Funding Sources
This study was supported by research (NIDA R01 DA019989; Sigmon), training (NIDA T32 DA007242; Higgins) and center (NIGMS P20 GM103644; Higgins) grants from the National Institutes of Health. Buprenorphine/naloxone and color-matched placebo sublingual tablets were provided by Reckitt Benckiser Pharmaceuticals Inc through the National Institute on Drug Abuse (NIDA). NIDA and Reckitt Benckiser Pharmaceuticals Inc had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Dr Sigmon has received consulting payments from Alkermes and, through her university, has received research support from Titan Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Authors Sigmon, Heil and Higgins designed the study and obtained funding. Author Patrick conducted literature searches and provided summaries of previous research studies. Authors Badger, Patrick, Dunn and Sigmon conducted the statistical analyses and interpretation of results. Author Patrick wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
Conflict of Interest
All other authors declare no conflicts of interest.
References
- Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environmental Health Perspectives. 1999;107:349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow GE, Stitzer ML, Griffiths RR, Liebson IA. Human methadone detoxification: Opioid self-administration behavior and withdrawal signs and symptoms as a function of progressive dose reductions. Federation Proceedings. 1981;40:296. [Google Scholar]
- Blum K, Braverman ER. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2003;32:S1–S112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Blum K, Cull JG, Braverman ER, Comings DE. Reward deficiency syndrome. American Scientist. 1996;84:132–145. [Google Scholar]
- Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, Weisner C. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiology and Drug Safety. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug and Alcohol Dependence. 2004;73:199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Smoking-attributable mortality, years of potential life lost, and productivity losses - United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults—United States, 2011. Morbidity and Mortality Weekly Report. 2011;61:889–894. [PubMed] [Google Scholar]
- Chait LD, Griffiths RR. Effects of methadone on human cigarette smoking and subjective ratings. The Journal of Pharmacology and Experimental Therapeutics. 1984;229:636–640. [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. Journal of Addictive Diseases. 1999;18:31–40. doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- David SP, Lancaster T, Stead LF, Evins AE, Prochaska JJ. Opioid antagonists for smoking cessation. Cochrane Database of Systematic Reviews. 2013;6 doi: 10.1002/14651858.CD003086.pub3. CD003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Saulsgiver KA, Sigmon SC. Contingency management for behavior change: Applications to promote brief smoking cessation among opioid-maintained patients. Experimental and Clinical Psychopharmacology. 2011;19:20–30. doi: 10.1037/a0022039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerström Tolerance Questionnaire. Journal of Behavioral Medicine. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Governale L. Outpatient prescription opioid utilization in the U.S., Years 2000–2009. Presented at the Joint Meeting of the anesthetic and Life Support Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee; Adelphi, MD. 2010. Jul, [Google Scholar]
- Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A. Smoking prevalence in addiction treatment: A review. Nicotine and Tobacco Research. 2011;13:401–411. doi: 10.1093/ntr/ntr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: How to use it right. Drug and Alcohol Dependence. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- King AC, Cao D, O'Malley SS, Kranzler HR, Cai X, deWit H, Stachoviak RJ. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. Journal of Clinical Psychopharmacology. 2012;32:630–636. doi: 10.1097/JCP.0b013e3182676956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Bigelow GE, Strain EC. Experimentally-induced spontaneous opiate withdrawal: Relationship to cigarette craving and expired air carbon monoxide. American Journal on Addictions. 2007;16:310–315. doi: 10.1080/10550490701389799. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology. 1985;86:417–425. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67:45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Stephen BJ, Teoh SK, Mendelson JH, Mello NK. An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependent on cocaine and opioids. Nicotine and Tobacco Research. 2002;4:223–228. doi: 10.1080/14622200210124012. [DOI] [PubMed] [Google Scholar]
- Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. PAIN. 2008;138:507–513. doi: 10.1016/j.pain.2008.01.027. http://dx.doi.org/10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Lee EM, Abreu ME, Umbricht A, Preston KL. A laboratory study of hydromorphone and cyclazocine on smoking behavior in residential polydrug users. Pharmacology, Biochemistry, and Behavior. 2004;77:711–715. doi: 10.1016/j.pbb.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91:296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PE. Methylphenidate increases cigarette smoking. Psychopharmacology. 2005;181:781–789. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Grabowski J, Rhoades H. The effects of high and low doses of methadone on cigarette smoking. Drug and Alcohol Dependence. 1994;34:237–242. doi: 10.1016/0376-8716(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Saulsgiver K, Patrick ME, Badger GJ, Heil SH, Higgins ST. A Randomized, double-blind evaluation of buprenorphine taper for the treatment of prescription opioid dependence. JAMA Psychiatry. 2013;70:1347–1354. doi: 10.1001/jamapsychiatry.2013.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST. Acute effects of amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology. 2003;167:393–402. doi: 10.1007/s00213-003-1416-z. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Medication-Assisted Treatment for Opioid Addiction: 2010 State Profiles. SAMSHA; 2011. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. [Google Scholar]
- Tidey JW, O'Neill SC, Higgins ST. d-amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology. 2000;153:85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- Torrington M, Domier CP, Hillhouse M, Ling W. Buprenorphine 101: Treating opioid dependence with buprenorphine in an office-based setting. Journal of Addictive Disorders. 2007;26:93–99. doi: 10.1300/J069v26n03_10. [DOI] [PubMed] [Google Scholar]
- Wu LT, Woody GE, Yang C, Blazer DG. How do prescription opioid users differ from users of heroin or other drugs in psychopathology: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Addictive Medicine. 2011;5:28–35. doi: 10.1097/ADM.0b013e3181e0364e. [DOI] [PMC free article] [PubMed] [Google Scholar]