Abstract
Clinically useful predictors of treatment outcome in major depressive disorder (MDD) remain elusive. We examined associations between functional magnetic resonance imaging (fMRI) blood oxygen level dependent (BOLD) signal during active negative word processing and subsequent selective serotonin reuptake inhibitor (SSRI) treatment outcome in MDD. Unmedicated MDD subjects (n=17) performed an emotional word processing fMRI task, and then received eight weeks of standardized antidepressant treatment with escitalopram. Lower pre-treatment BOLD responses to negative words in midbrain, dorsolateral prefrontal cortex, paracingulate, anterior cingulate, thalamus and caudate nuclei correlated significantly with greater improvement following escitalopram treatment. Activation of these regions in response to negative words correlated significantly with reaction time for rating word relevance. Maximally predictive clusters of voxels identified using a cross-validation approach predicted 48% of the variance in response to treatment. This study provides preliminary evidence that SSRIs may be most beneficial in patients who are less able to engage cognitive control networks while processing negative stimuli. Differences between these findings and previous fMRI studies of SSRI treatment outcome may relate to differences in task design. Regional BOLD responses to negative words predictive of SSRI outcome in this study were both overlapping and distinct from those predictive of outcome with cognitive behavioral therapy (CBT) in previous studies using the same task. Future studies may examine prediction of differential outcome across treatments in the context of a randomized controlled trial.
Keywords: fMRI, major depressive disorder, biomarker, SSRI, prediction, treatment outcome
1. Introduction
Biomarkers to predict treatment outcome with pharmacotherapy for major depressive disorder (MDD) are needed. Correlates of neural activity in the anterior cingulate cortex (ACC) and its subregions have been implicated in treatment outcome in MDD with some consistency (Pizzagalli, 2011), although no brain imaging measures are used to date in routine clinical practice. Functional MRI (fMRI) studies have found neural response to negative emotional stimuli in anterior cingulate cortex (ACC) (Davidson et al., 2003; Chen et al., 2007), subgenual ACC (sgACC) (Fu et al., 2004; Keedwell et al., 2010), and pregenual/rostral ACC (rACC) (Langenecker et al., 2007; Ruhe et al., 2012) to be associated with better response to antidepressant medication treatment (with selective serotonin reuptake inhibitors (SSRIs), serotonin/norepinephrine reuptake inhibitors (SNRIs), or open treatment). In partial contrast, one of these studies found higher sgACC responses to be associated with worse treatment response (Ruhe et al., 2012). Along the same lines, several (Mayberg et al., 1997; Mayberg et al., 2000) but not all (Konarski et al., 2009) studies of resting glucose metabolism have found that higher pre-treatment activity in sgACC and rACC as well as rACC electroencephalography (EEG) (Pizzagalli et al., 2001) predict better treatment response. While these largely convergent studies implicate ACC in treatment response to antidepressant medications, responses in other brain regions, including amygdala, have also been associated with SSRI outcome in MDD (Victor et al., 2010).
Task design may significantly impact the pattern of neural responses associated with treatment outcome. Many studies described above involved either resting-state acquisitions (Mayberg et al., 1997; Mayberg et al., 2000; Pizzagalli et al., 2001) or task designs emphasizing emotional reactivity (Fu et al., 2004; Langenecker et al., 2007; Keedwell et al., 2010; Ruhe et al., 2012) over cognitive processing of emotion. Others used a paradigm that was purely cognitive (Langenecker et al., 2007). The ACC is involved in connecting frontal cognitive and limbic emotional areas and is therefore critical for the integration of cognition and emotion (Mayberg, 2003; Kemp et al., 2008). Furthermore, there is evidence that SSRIs improve cognitive control of negative emotional material (Chan et al., 2009; Harmer et al., 2009a; Harmer et al., 2009b), which may be mediated by effects on ACC. As a result, ACC activity during a task that involves the cognitive processing of emotional stimuli may produce a different pattern of prediction than noted in studies that focus primarily on emotional reactivity.
One such fMRI task that explicitly engages cognitive processing of emotional stimuli asks subjects to evaluate the personal relevance of emotional words. This task has been used in fMRI studies of cognitive behavioral therapy (CBT) for MDD (Siegle et al., 2006; Siegle et al., 2012), and produced a different pattern of prediction than many of the medication studies described above. Low pre-treatment activity in sgACC in response to emotionally negative words has been found to be predictive of better subsequent treatment outcome with CBT in three independent samples (Siegle et al., 2006; Siegle et al., 2012). This task differs meaningfully from those used in previous studies of antidepressant medication outcome in the degree to which subjects are asked to engage in active processing of the emotional stimuli presented (see discussion for more details).
In the current study, we utilized the same emotional words fMRI paradigm that has been replicated as a predictor of CBT outcome (Siegle et al., 2006; Siegle et al., 2012) to explore its relationship to treatment outcome with standardized SSRI treatment for MDD. We expected that, similar to prediction of outcome with CBT, lower activity in the sgACC in response to negative words would be associated with better treatment outcome. We conducted a whole-brain voxelwise analysis to examine prediction of treatment outcome in an unbiased manner. In addition to identifying regions whose BOLD response was associated with treatment outcome above an a priori statistical threshold, we sought to identify a more limited set of regions that generated the strongest prediction of treatment outcome using a cross-validation approach. Finally, due to the focus in prior research highlighting the relevance of sgACC responses to negative valence and treatment outcome, we conducted an analysis in which we investigated the relationship between sgACC responses to increasing word negativity and SSRI treatment outcome.
2. Methods
2.1 Sample
Subjects gave written informed consent as required by the Institutional Review Board of the New York State Psychiatric Institute. Participants were recruited using online and print advertisements as well as through referrals from surrounding clinics. Inclusion criteria included: 1) DSM-IV criteria for MDD in a current major depressive episode (MDE) assessed using the Structured Clinical Interview for DSM-IV (SCID, (First et al., 1995)); 2) age 18–65; 3) minimum score of 16 on 17-item Hamilton Depression Rating Scale (HDRS, (Hamilton, 1960)); 4) ability to provide informed consent; Exclusion criteria included: 1) significant medical conditions; 2) lifetime history of alcohol abuse or dependence; 3) substance abuse or dependence unless in complete remission for >6 months; 4) ecstasy or intravenous drug use more than two times; 5) presence of major psychiatric disorders including schizophrenia (comorbid anxiety disorders allowed); 6) comorbid anorexia or bulimia nervosa within the past year; 7) first-degree family history of schizophrenia if subject was <33 years old; 8) inability or unwillingness to discontinue any and all antidepressant medications for at least 3 weeks (6 weeks for fluoxetine); 9) pregnancy, current lactation, plans to conceive during study participation or abortion within 2 months of enrollment; 10) medical contraindication to antidepressants; 11) dementia; 12) neurological disease or previous head injury accompanied by loss of consciousness or motor deficits; 13) failure of >2 SSRI or other antidepressant monotherapy trials of adequate dose and duration; 14) metal implants;15) active suicidality or ideation requiring admission or medication intervention; 16) history of significant clinical decompensation in response to prior medication wash-out. One subject had past cannabis dependence in sustained remission and one subject had past bulimia in sustained remission. Subject demographics are presented in Table 1.
Table 1.
Demographic and clinical variables of the sample (n=17)
| Relationship to post-treatment HDRS covarying for baseline HDRS | |||
|---|---|---|---|
|
| |||
| Variable | Mean±S.D. | Ba | p |
| Baseline 24-item HDRS | 25.52±6.86 | 0.37 | 0.12 |
| Post-treatment 24-item HDRS | 12.24±7.12 | N/A | N/A |
| Baseline BDI | 27.4±10.6 | −0.16 | 0.53 |
| Post-Treatment BDI | 8.65±8.13 | −0.78 | 0.41 |
| Baseline Anxiety (HDRS Anxiety Factor)b | 5.3±1.6 | 0.48c | 0.08 |
| Age | 35.65±13.31 | 0.66 | 0.52 |
| Education | 15.59±2.71 | −.116 | 0.86 |
| Frequency (%) | |||
| Female | 10 (58.8) | −0.11 | 0.41 |
| Non-Hispanic | 12 (70.6) | −0.92 | 0.81 |
| Race | |||
| White | 11 (64.7) | −5.88 | 0.12 |
| American Indian | 1 (5.8) | ||
| Asian | 2 (11.8) | ||
| Black | 2 (11.8) | ||
| Multi-racial | 1 (5.8) | ||
| Medication Naïve | 10 (58.9) | −0.2 | 0.96 |
|
| |||
| Non-normally distributed variables | Median (range) | rsd | p |
|
| |||
| Length of Current Episode (weeks) | 104(2–466) | −0.18 | 0.60 |
| Number of Previous Episodes | 1.5 (0–70) | −0.17 | 0.54 |
| Comorbid anxiety disorder | N=7 | 0.02 | 0.96 |
| # of weeks off antidepressant medications (among medication-exposed) | 64 (9–719) | −0.4 | 0.38 |
| Right Handed | N=16 | −0.019 | 0.94 |
| Smoking | N=2 | −0.207 | 0.42 |
Multiple linear regression (DF=2,16);
Sum of items 9, 10, 11, and 15 from HDRS; see (Grunebaum et al, 2005) for details on HDRS factor analysis. Range of scores = 0–12.
Prediction of final HDRS from baseline HDRS anxiety factor while controlling for baseline HDRS excluding anxiety factor items;
Spearman’s rank correlation (DF=17)
2.2. Clinical Procedures and Treatment
24-item HDRS was used as a measure of pre- and post-treatment depression severity. Following baseline MRI scanning, standardized treatment was initiated with escitalopram 10mg. After four weeks, escitalopram dose was increased to 20mg for non-responders (those with <50% decrease in HDRS), and was maintained at 10mg for responders. At six weeks, those subjects still taking escitalopram 10mg who were non-remitters (HDRS ≥10 or <50% decrease in HDRS) had their escitalopram dose increased to 20mg. Post-treatment depression severity (HDRS) was assessed at week eight.
Twenty-five subjects were enrolled in this study. Two subjects dropped out of the study before completing four weeks of treatment and were excluded from the current analysis. Six subjects were excluded due to technical problems with fMRI acquisition. 15 subjects with usable fMRI data completed the eight-week medication trial. Two additional subjects discontinued escitalopram after completing 6 weeks of treatment. We employed last observation carried forward for these two subjects, using their 6-week HDRS rating as the post-treatment HDRS value, in our final analyzed sample of 17 subjects. Among this sample of 17 subjects, 10 were antidepressant naïve, six had a history of prior antidepressant use but were not taking an antidepressant at the time of study enrollment, and one subject was on ineffective antidepressant medication at the time of enrollment and underwent 3-week washout prior to scanning.
2.3. Image Acquisition
MRI scans were acquired on a 3T SignaHDx scanner (General Electric Medical Systems, Milwaukee, WI). T1-weighted MRI scans were acquired for co-registration with functional images. For functional scanning during the emotional words task, an Echo Planar Imaging (EPI) acquisition was obtained for each of three runs using the following parameters: TR = 1500msec, TE = 28 msec, flip angle = 90, FOV = 24cm × 24cm, slice thickness = 4mm, spacing = 0.5mm, number of slices = 24, matrix size = 64×64 pixels, number of volumes = 164.
2.4. Experimental Design
This task was identical to that reported in Siegle et al. (Siegle et al., 2006). Stimuli were presented using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, Pennsylvania) on a rear projection screen viewed using a suspended mirror. Briefly, the task involved visual presentation of a series of 60 words split into 20 emotionally negative, 20 neutral and 20 positive words. Of these, half were standardized words that were identical across subjects, and half were words generated by the subject prior to scanning (personalized words). Subjects were asked to identify “10 personally relevant negative words that best represent what you think about when you are upset, down or depressed; 10 personally relevant positive words that best represent what you think about when you are happy or in a good mood; and 10 personally relevant neutral (i.e., not positive or negative) words that best represent what you think about when you are neither happy nor upset/down or depressed.” Each word trial consisted of a fixation cue (1 second) followed by brief word presentation (200 msec), and then a mask of 4 X’s (10.8 seconds) during which subjects were instructed to rate the personal relevance of the word as quickly and accurately as possible on a three-point scale from not relevant to very relevant. Mapping of buttons to responses was counterbalanced across participants. Relevance ratings and response times were recorded. Following scanning, subjects rated the valence of the words they had viewed on an eight-point scale (very negative to very positive). Three subjects did not complete the post-scan valence ratings, and were excluded from secondary analyses incorporating these ratings. Idiosyncratic and standardized words were used to maximize the likelihood of eliciting emotional responses to words across the sample. There were no hypotheses regarding differences in processing of these words; primary analyses were therefore conducted considering idiosyncratic and standardized words together. Behavioral results showed no significant differences in valence ratings of these words, indicating that they were generally perceived as being negative.
2.5. Image Processing
2.5.1. Pre-Processing
Skull-stripping of T1-weighted structural scans was performed using Atropos (Avants et al., 2011). fMRI data was processed using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). FMRIB’s Linear Image Registration Tool MCFLIRT (Jenkinson et al., 2002) was used for motion correction. Fourier-space time-series phase-shifting was used for slice-timing correction; Brain Extraction Tool (BET) (Smith, 2002) was used to remove non-brain voxels in the EPI image. Spatial smoothing was performed using a Gaussian kernel with full-width half-maximum 6.0mm. Temporal filtering was performed with a highpass cutoff of 50 seconds (Gaussian-weighted least-squares straight line fitting, with sigma=25.0s); Gaussian low-pass filtering was performed with sigma=2.8s. The first four volumes of each run were discarded to allow for intensity equilibration.
Normalization to standard space was conducted via a three- step process using FLIRT (Jenkinson et al., 2002). 4D functional data were (1) registered to the first volume of functional acquisition (using a transformation with 6 degrees of freedom (DF)); (2) registered to the high resolution structural image (7 DF); and (3) normalized to standard space (Montreal Neurological Institute (MNI) space) using 12 DF.
2.5.2 fMRI Statistical Analysis
For all word types, we used a general linear model (GLM) to identify brain activity associated with the time from mask onset to time of response (the period in which subjects rate word relevance), convolved with a double gamma hemodynamic response function, for all word types. The 1-second fixation period served as an implicit baseline.
To identify brain regions whose response to negative words was associated with SSRI treatment outcome, negative word processing activity was regressed onto post-treatment HDRS while covarying for baseline HDRS (FLAME 1+2; FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Clusters were identified by a minimum z-score=2.3, and adjustment for multiple comparisons was effected using a random Markov Field corrected cluster threshold of p<0.05 (Worsley, 2001). As the field of view was cut off ventrally for two subjects, primary analyses were repeated excluding them. We also repeated this analysis covarying for age and gender. To determine whether this finding was specific to negative emotional processing, we repeated this analysis with parameter estimates from both neutral and positive word processing.
2.5.3. Optimizing prediction using cross-validation
We sought to identify the set of voxels that best predicted treatment outcome in an objective manner (Vul, 2009), using a cross-validation approach. We selected voxels showing strong evidence of activation to negative words at the group level, i.e., those with Z-scores exceeding a threshold, and determined whether they were related to treatment outcome. To choose this threshold objectively, we performed a cross-validation procedure for a grid of 100 equally spaced possible Z-score threshold values from −2.24 to 6.12. For each candidate threshold value, we computed a mask of voxels whose Z-score for the group mean response to negative words exceeded the threshold. We set a minimum cluster extent threshold of at least 10 voxels within a sphere of radius 5mm. For each threshold-specific mask, we randomly divided the sample of 17 MDD subjects into 10 subsets. For each subset, we designated it as “validation data” and all other subsets as “training data”. We fit a regression model to the training data with baseline HDRS and the mean response to negative words within the mask as predictors and final HDRS as outcome. This fitted model was applied to the validation data to predict final HDRS, and the out-of-sample predictive ability of the fitted model was measured by the residual sum of squares (RSS). The final mask was formed according to the threshold that minimized the total RSS criterion.
2.5.4. Additional fMRI Analyses
We sought to examine the possible cognitive function of BOLD responses within clusters associated with treatment outcome. We examined the correlation between mean BOLD responses to negative words in regions predictive of treatment outcome and mean reaction time to negative words. This analysis was not confounded by greater hemodynamic fit to shorter responses because the regressors were formed using variable epochs determined by reaction time (Grinband et al., 2008).
We conducted an additional analysis to more closely approximate previous SSRI research that focused on BOLD responses to stimuli with varying levels of negative emotional valence (Fu et al., 2004; Keedwell et al., 2010). For all words, a task regressor modeling the time period from mask onset to rating of word relevance was created. A valence regressor was created by parametrically modulating each word trial by subjects’ post-scan valence rating of the word (coded −3=most negative, 3=most positive). We controlled for task-related activity by including the unmodulated task regressor as a covariate. To control for emotional arousal processed separately from valence (Gerber et al., 2008), we modeled a quadratic valence regressor as a measure of affective intensity irrespective of valence direction. Because of the focus on sgACC in previous studies (Mayberg et al., 1997; Mayberg et al., 1999; Mayberg et al., 2000; Pizzagalli et al., 2001; Davidson et al., 2003; Langenecker et al., 2007; Fu et al., 2008; Keedwell et al., 2010), we limited this analysis to voxels within a standard space mask based on the Talairach atlas (BA 25).
2.6. Behavioral and Demographic Data
We calculated means for all behavioral variables (response time, relevance ratings and valence ratings) for each word condition (3 valences × 2 word types (personalized/standardized)), producing six values per subject. A one way between-subjects ANOVA determined the effects of valence type on response time to words. Associations between behavioral variables as well as normally distributed demographic variables and post-treatment HDRS were calculated with multiple linear regression while controlling for baseline HDRS. Spearman’s rank correlation was used for correlations with non-normally distributed variables. ANOVA, linear regression, and correlation testing were performed in SPSS (IBM SPSS Statistics, v. 18).
3. Results
3.1. Sample
Table 1 provides demographic data for the final sample (N=17). The average age of the sample was 35.7±13.3, (range of 19–56 in men and 18–58 in women). Mean pre-treatment 24-item HDRS was 25.3±6.9, indicating moderate baseline severity of depression. Mean post-treatment HDRS was 12.2±7.1, representing a mean improvement of 51.8%. Baseline HDRS was not correlated with post-treatment HDRS (r=0.352, p=0.166). Seven subjects had a comorbid anxiety disorder; this comorbidity was not associated with treatment outcome (p=0.96). While all fMRI analyses used a continuous measure of treatment outcome and not a categorical measure, response rate (≥50% reduction in HDRS) in the sample was 52%, and remission rate (≥50% reduction in HDRS and final HDRS <10) in the sample was also 52%. Mean final dose of escitalopram across all MDD subjects in the sample was 15.5mg±5mg and was not correlated with treatment outcome (final HDRS while co-varying for baseline HDRS; B=0.23, p=0.81). No demographic variables were associated with post-treatment depression severity or degree of improvement, including some that have previously been identified as predictors of treatment outcome: number of prior major depressive episodes (rs=−0.17, p=0.54), duration of current major depressive episode (rs=−0.18, p=0.60), presence of a current comorbid anxiety disorder (rs=−0.02, p=0.96), or prior medication status (antidepressant-exposed or naïve, B=−0.2, p=0.96). Baseline anxiety severity, measured using an anxiety factor derived from the HDRS (Grunebaum et al., 2005), was associated with worse treatment outcome at a trend level (B=2, p=0.08).
3.2. Behavioral Data from Emotional Words Task
Analyses of behavioral data are summarized in supplemental information (Table S1). Response times to rate word relevance did not differ as a function of emotional valence types (ANOVA, df=2,1017, F=0.026, p=0.97). Subjects responded more quickly to standardized words than to personalized words (ANOVA, df=1,1018, F=4.46, p=0.035). Subjects rated personalized words as more relevant than standardized words. Post-scan valence ratings differed between the three valence categories (positive/negative/neutral), in the expected direction, with negative words being rated as most emotionally negative (see Table S1 for statistical values). Post-treatment HDRS was not correlated with response times (negative p=0.21, positive p=0.98, neutral p=0.43) or valence ratings (negative p=0.85, positive p=0.89, neutral p=0.84).
3.3. Imaging Data
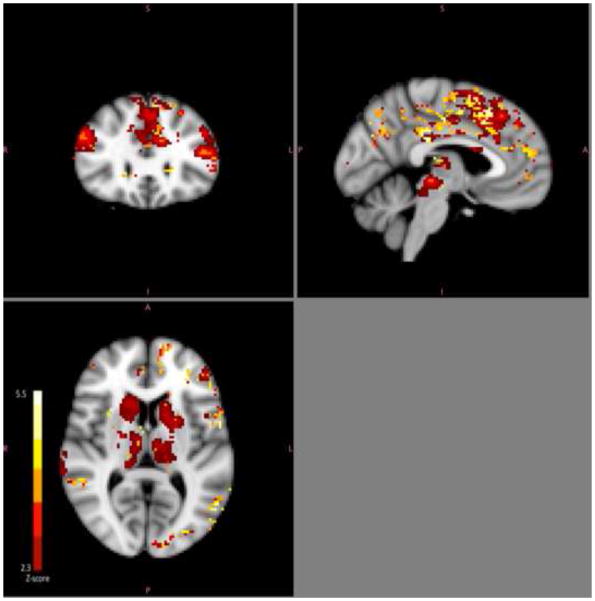
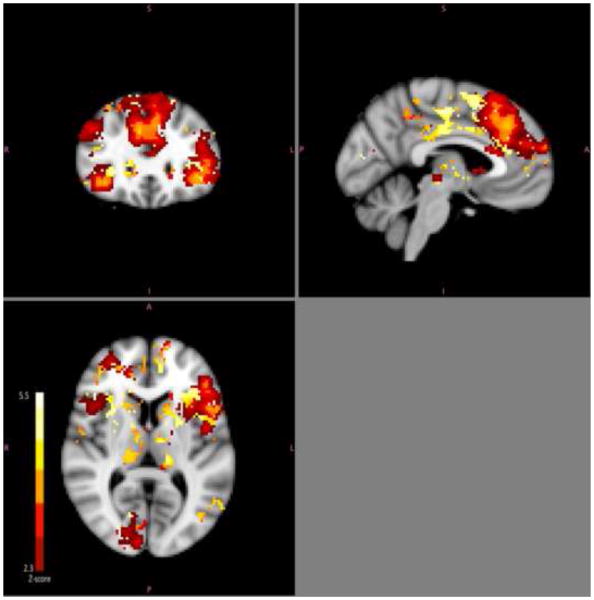
All findings reported below were determined using a minimum Z threshold of 2.3 with a cluster threshold of p<0.05. Lower activation across multiple brain regions during negative word processing was associated with better treatment outcome (Figure 1). Specifically, post-treatment HDRS scores were positively correlated with pre-treatment BOLD signal during processing of negative words in frontal pole, dorsolateral prefrontal cortex, anterior cingulate, paracingulate, middle and superior frontal gyrii, precentral gyrus, insula, posterior cingulate, supramarginal gyrus, lateral occipital cortex, midbrain, caudate and thalamus, while controlling for pre-treatment HDRS. Covarying for age and gender did not significantly affect this contrast, preserving significant clusters related to post-treatment HDRS in all regions mentioned above. Regional maxima, cluster size and average cluster z-score are summarized in Table 2. Activity in these regions was negatively correlated with reaction times to negative idiosyncratic words specifically (r=−0.581 p=.014; scatterplot in supplemental material Figure S1). There were no voxels in which negative word processing activity was inversely correlated with post-treatment HDRS. Sensitivity analyses excluding two subjects whose fMRI scans had a limited field of view yielded similar results and did not reveal activation in additional ventral regions such as amygdala or subgenual cingulate cortex. Regions identified as predictive of treatment outcome overlapped with mean responses to task during processing of negative words (Figure 2).
Figure 1.
Negative word processing regions associated with treatment outcome. Greater pre-treatment BOLD responses to negative words in multiple brain regions were associated with worse subsequent treatment outcome with escitalopram for major depression.
Table 2.
Regional maxima and strength of correlation between negative word processing activity and post-treatment HDRS.
| Cluster Maxima | |||||
|---|---|---|---|---|---|
| X | Y | Z | # of voxels in cluster | r2* | |
| Superior Frontal Gyrus | 46 | 73 | 61 | 1248 | 0.51 |
| Paracingulate | 50 | 78 | 51 | 1216 | 0.43 |
| Middle Frontal Gyrus | 18 | 75 | 54 | 1105 | 0.43 |
| Anterior Cingulate | 42 | 73 | 53 | 55 | 0.39 |
| Precentral Cortex | 66 | 62 | 57 | 1178 | 0.39 |
| Midbrain | 46 | 50 | 32 | 352 | 0.37 |
| Thalamus | 50 | 56 | 83 | 977 | 0.33 |
| Caudate | 36 | 74 | 37 | 445 | 0.31 |
| Dorsolateral Prefrontal Cortex | 18 | 75 | 54 | 775 | 0.31 |
| Post-Central Gyrus | 76 | 46 | 57 | 1317 | 0.29 |
| Frontal Pole | 20 | 83 | 45 | 682 | 0.27 |
| Posterior Cingulate | 46 | 47 | 53 | 188 | 0.21 |
| Lateral Occipital Cortex | 57 | 18 | 39 | 324 | 0.13 |
Effect sizes were calculated using linear regression predicting post treatment HDRS from mean z-score of regional BOLD response to negative words while controlling for baseline HDRS in voxels that were associated with final HDRS. Due to the selection of voxels by association with final HDRS, these effect sizes may be inflated and should only be used to compare relative involvement of each region in predicting final HDRS.
Figure 2.
Group mean of voxels associated with negative word processing.
Analysis of the relationship between BOLD activity during neutral word processing and treatment response revealed a similar but more limited set of clusters in which lower activity was associated with better treatment outcome (Figure 3). Similar results emerged from the analysis of positive words, with a more narrow spatial extent of clusters whose activity during positive words was associated with treatment outcome as compared to the negative words analysis (Figure 3).
Figure 3. Prediction of treatment outcome as a function of word valence.
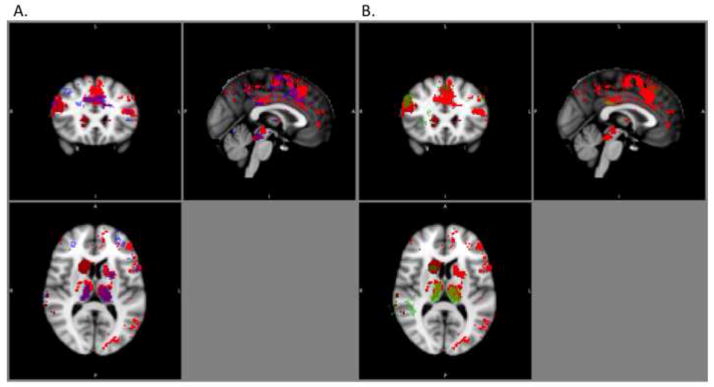
A. Red: Clusters whose activity during negative word processing is associated with treatment outcome. Blue: Clusters whose activity during neutral word processing is associated with treatment outcome (both at cluster corrected p<.05). Neutral word clusters are presented at transparency of 0.5 and overlaid on negative word clusters. B. Red: Clusters whose activity during negative word processing is associated with treatment outcome. Green: Clusters whose activity during positive word processing is associated with treatment outcome (both at cluster corrected p<.05). Positive word clusters are presented at transparency of 0.5 and overlaid on negative word clusters.
A cross-validation method to identify clusters whose responses to negative words were maximally predictive of treatment outcome identified three clusters, in left paracingulate gyrus (center: Xmm=49.4, Ymm=76, Zmm=53.2, #voxels= 23), globus pallidus (center: Xmm=36.5, Ymm=63.9, Zmm=36.8, #voxels=10), and post-central gyrus (center: Xmm=66, Ymm=48.4, Zmm=60.6, #voxels=11) (Figure 4). Post-hoc analysis on the selected clusters using standard linear regression identified a strong association between negative word response in these clusters and final HDRS while adjusting for baseline HDRS, with an r2 of 0.48. On an individual level, the cluster of voxels in the paracingulate gyrus provided the strongest prediction (r2=0.40), followed by globus pallidus (r2=0.26) and post-central gyrus (r2=0.20).
Figure 4.
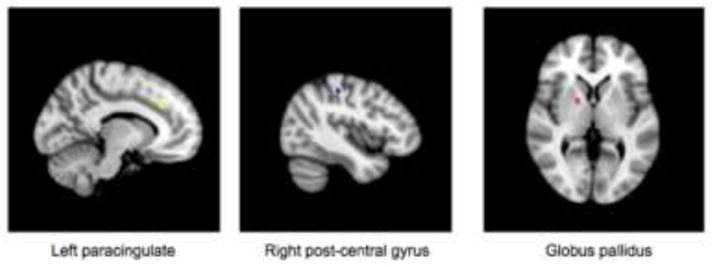
Clusters in which BOLD response to negative words was maximally predictive of treatment outcome as identified using a cross-validation approach. Greater pre-treatment BOLD responses to negative words in these regions were predictive of worse subsequent treatment outcome with escitalopram for major depression.
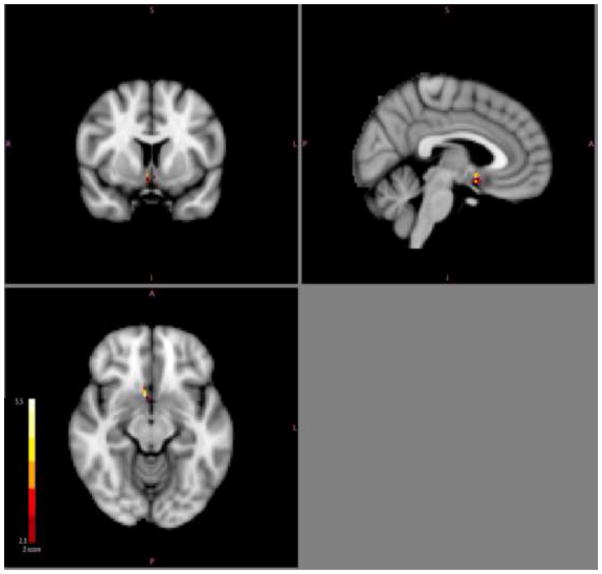
The analysis of responses to emotional valence within sgACC, showed that subjects whose activity in sgACC scaled positively with emotional valence (from emotionally negative to positive) had better treatment outcomes with SSRI treatment (Figure 5, cluster maxima: x=42, y=68, z=30, # of voxels=14). Sensitivity analyses excluding two subjects whose fMRI scans had a limited field of view yielded similar results.
Figure 5.
Cluster in right subgenual cingulate in which the correlation between BOLD response and word valence (negative to positive) was related to better treatment outcome.
4. Discussion
4.1. Treatment Prediction
Pre-treatment, lower fMRI BOLD responses of medication-free MDD subjects during explicit processing of negative emotional words were associated with greater improvement in depression with the SSRI escitalopram. Specifically, lower pre-treatment BOLD responses to negative words in midbrain, dorsolateral prefrontal cortex, insula, middle frontal cortex, premotor cortex, anterior cingulate, thalamus, and caudate were associated with greater improvement (Figure 1). We also identified a highly overlapping set of clusters that were generally involved in negative word processing (Figure 2).
While activity in a large network of regions was associated with treatment outcome, we identified a more limited set of clusters in the paracingulate, globus pallidus and post-central gyrus that were maximally predictive of treatment outcome using a data-driven cross-validation method. Negative word activity across these clusters predicted 48% of the variance in treatment outcome. These data suggest that pre-treatment neural activity during this task may be a robust predictor of treatment outcome and that this methodology may have use in identifying patients who are likely SSRI responders if replicated in subsequent studies.
4.2. Cognitive Processes
The relationship between word processing and treatment outcome was not limited to negative words. Lower activity during neutral and positive words in similar, but more limited, clusters was also associated with better treatment outcome. These findings suggest that it is not reactivity to negative emotional stimuli that predicts treatment response. Rather, it may be the recruitment of cognitive control networks necessary to evaluate and rate the relevance of the word that is predictive. This latter mental process is relevant to all experimental conditions tested here, but is likely to be recruited more heavily when responding to negative words. Therefore, the widest extent of activity and strongest prediction of treatment outcome was generated by the negative word condition, most likely because this condition demanded the greatest degree of cognitive control.
Cognitive control subsumes a wide range of specific cognitive activities involving attention, memory and decision-making. The design of this study precludes definitive conclusions regarding the specific processes represented by these neural activations. A number of brain regions identified here (superior and middle frontal gyrus, paracingulate cortex, dorsolateral prefrontal cortex, thalamus, premotor cortex and insula) are involved in these processes (Beauregard et al., 2001; Ochsner and Gross, 2005; Witt and Stevens, 2012). Lower BOLD responses in these regions may indicate diminished engagement of cognitive control over emotional material that is characteristic of depression (Joormann and Gotlib, 2010; Olafsson et al., 2011). The fact that activation of these regions was also associated with diminished response time to negative idiosyncratic words supports this formulation. This is consistent with findings that demonstrate that SSRIs impact negative cognitive processing biases in MDD (Harmer et al., 2006; Harmer et al., 2009a; Harmer et al., 2009b; Murphy et al., 2009).
The association between midbrain activity and treatment outcome is also of interest. The dorsal midbrain cluster of activity appears to overlap with the dorsal raphe nuclei (DRN), which give rise to the majority of serotonergic projections in the brain (Jasinska et al., 2012). Lower midbrain responses to emotionally negative stimuli may represent a failure of serotonin-mediated responses to affective stimuli (Anderson et al., 2011; Elliott et al., 2011). Individuals with such deficient responses may stand to gain the most from the enhancement of serotonergic neurotransmission that is achieved by SSRI (Blier and De Montigny, 1983).
Among the regions identified as maximally predictive of treatment outcome in our cross-validation analysis, paracingulate activity has been implicated as a biomarker for depression (Zhang et al., 2011) that appears to play a role in cognitive control (Dichter et al., 2010; Kikuchi et al., 2012). The globus pallidus has been implicated in the apathy and anhedonia of major depression in both fMRI (Alexopoulos et al., 2012) and lesion studies (Vataja et al., 2004; Miller et al., 2006).
4.3. Comparison with Previous Literature regarding SSRI Outcome
Our findings suggest that this cognitive and emotional word processing task evoked a different range of activities compared with the entirely emotional processing tasks used in previous studies (Davidson et al., 2003; Fu et al., 2004; Chen et al., 2007; Keedwell et al., 2010; Victor et al., 2010), and thereby identified treatment predictive activity in a different set of regions. Specifically, the network of regions identified here is largely comprised of higher-level cognitive processing areas such as DLPFC, superior frontal gyrus, middle frontal gyrus, and frontal pole as opposed to the limbic and ventral anterior cingulate regions found in the above-mentioned studies.
Our analysis of the sgACC contrasted with findings from previous studies in that lower rather than higher responses to negative valence in this region predicted better treatment response (Fu et al., 2004; Keedwell et al., 2010). Subgenual ACC plays a key role in connecting dorsal cortical and ventral limbic areas (Mayberg, 2003; Kemp et al., 2008). The function of this connectivity during emotional processing (as in (Fu et al., 2004; Keedwell et al., 2010; Ruhe et al., 2012)) is likely to differ from its function during explicit semantic processing of emotional words. We tentatively hypothesize that initially this connectivity brings attention to negative stimuli, whereas later it may facilitate shifting attention away from negative stimuli. Consistent with our findings and this formulation, the cognitive bias hypothesis proposes that people who give greater focus to negative stimuli and less focus to positive stimuli are better candidates for SSRIs (Harmer et al., 2009a).
4.4. Differentiation of CBT and SSRI
In comparison with previous studies using the same fMRI task as a predictor of treatment outcome with CBT (Siegle et al., 2006; Siegle et al., 2012) we identified both overlapping and distinct predictors of outcome with escitalopram treatment. The majority of regions identified in the current study subserve higher-level cognitive activities, such as planning, attention and memory. Activity in many of these regions was not identified as predictive of CBT outcome (Siegle et al., 2006; Siegle et al., 2012). However, hypoactivity of DLPFC in response to negative words was predictive of better outcomes with both CBT in a previous study (Siegle et al., 2012) and with SSRI in the current study, suggesting that this may represent a general predictor of treatment outcome across antidepressant modalities.
Differences between the current findings and studies of CBT may also be partially due to differences in the samples enrolled. There may be differences in the groups that self-select for CBT compared with SSRI treatment. One CBT study using this task primarily enrolled individuals with recurrent MDD (Siegle et al., 2012), whereas 65% of our sample had recurrent MDD. We required a longer minimal antidepressant-free interval prior to fMRI scanning than other studies. Studies also differed in type of scanner and pulse sequences employed (spiral vs. echo planar acquisitions). Another difference between the studies is the time period used to identify activity associated with negative words. We examined activity during the time period in which subjects rated word relevance, whereas the other studies examined a longer predefined period of activity that continued for several seconds following word rating. However, in a secondary analysis, we examined the time period following negative word ratings, and found no associations with treatment outcome, suggesting that the time period examined in our analyses did not explain the differences in findings.
4.5. Predictors of Outcome and Change With Treatment
We identified several brain regions in which low responses to negative stimuli are associated with better SSRI outcome. Consistent with this finding, successful treatment with an SSRI has been associated with increases in resting glucose metabolism or task-based fMRI responses in the majority of these brain regions (caudate and thalamus (Walsh et al., 2007), dorsolateral prefrontal cortex (Fales et al., 2009), premotor cortex, posterior cingulate, anterior cingulate, and prefrontal cortices (Mayberg et al., 2000). This suggests that SSRIs may exert their beneficial effects by enhancing adaptive processing of negative emotional stimuli by this cognitive network. However, some brain imaging studies have found limited correspondence between regions whose pre-treatment activity is predictive of outcome and brain regions where responses change as a function of successful treatment (Davidson et al., 2003; Kennedy et al., 2007; Fu et al., 2008; Siegle et al., 2012).
4.6. Limitations
Replication in a larger sample is needed. Our study used an algorithmic dosing schedule of escitalopram that was standardized across all subjects, but which nonetheless led to differential dosing of escitalopram across subjects in the sample. It remains be determined whether these findings would be similarly predictive of outcome to other SSRIs, non-SSRI antidepressants, placebo, or standardized psychotherapy when compared in a randomized fashion. In addition, fMRI scanning was not repeated post-treatment, which could have determined whether responses in regions identified as predictive of outcome in this study mediate treatment effects.
4.7. Conclusions
Negative word-related activity in a broad range of regions involved in cognitive control and emotional reactivity predicts response to SSRI treatment. Future research should examine this task as a predictor of differential treatment outcome across other treatment modalities.
Supplementary Material
Acknowledgments
Funding for this study was provided by NIMH R01 MH074813 (Dr. Parsey, PI) and a NARSAD Young Investigator Award (Dr. Miller, PI). Some study medication provided by Forest Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, JKS, Lim KO, Gunning FM. Functional connectivity in apathy of late-life depression: A preliminary study. Journal of Affective Disorders. 2012 doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Juhasz G, Thomas E, Downey D, McKie S, Deakin JFW, Elliott R. The effect of acute citalopram on face emotion processing in remitted depression: A pharmacoMRI study. European Neuropsychopharmacology. 2011;21:140–148. doi: 10.1016/j.euroneuro.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. The Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, De Montigny C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. The Journal of Neuroscience. 1983;3:1270–1278. doi: 10.1523/JNEUROSCI.03-06-01270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SWY, Norbury R, Goodwin GM, Harmer CJ. Risk for depression and neural responses to fearful facial expressions of emotion. The British Journal of Psychiatry. 2009;194:139–145. doi: 10.1192/bjp.bp.107.047993. [DOI] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo-Pich E, Bullmore E. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biological Psychiatry. 2007;62:407–414. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. The American Journal of Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. The effects of Brief Behavioral Activation Therapy for Depression on cognitive control in affective contexts: An fMRI investigation. Journal of Affective Disorders. 2010;126:236–244. doi: 10.1016/j.jad.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Zahn R, Deakin JFW, Anderson IM. Affective Cognition and its Disruption in Mood Disorders. Neuropsychopharmacology. 2011;36:153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. Journal of Affective Disorders. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) Biometrics Research Dept., New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biological Psychiatry. 2008;64:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Gerber AJ, Posner J, Gorman D, Colibazzi T, Yu S, Wang Z, Kangarlu A, Zhu H, Russell J, Peterson BS. An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces. Neuropsychologia. 2008;46:2129–2139. doi: 10.1016/j.neuropsychologia.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Wager TD, Lindquist M, Ferrera VP, Hirsch J. Detection of time-varying signals in event-related fMRI designs. Neuroimage. 2008;43:509–520. doi: 10.1016/j.neuroimage.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Keilp J, Li S, Ellis SP, Burke AK, Oquendo MA, Mann JJ. Symptom components of standard depression scales and past suicidal behavior. Journal of Affective Disorders. 2005;87:73–82. doi: 10.1016/j.jad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. The British Journal of Psychiatry. 2009a;195:102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. The American Journal of Psychiatry. 2009b;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Lowry CA, Burmeister M. Serotonin transporter gene, stress and raphe raphe interactions: a molecular mechanism of depression. Trends in Neurosciences. 2012;35:395–402. doi: 10.1016/j.tins.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cognition and Emotion. 2010;24:281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression☆. Journal of Affective Disorders. 2010;120:120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Gordon E, Rush AJ, Williams LM. Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures. CNS Spectrums. 2008;13:1066–1086. doi: 10.1017/s1092852900017120. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. The American Journal of Psychiatry. 2007;164:778–788. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Miller JM, Schneck N, Oquendo MA, Mann JJ, Parsey RV, Keilp JG. Neural responses to incongruency in a blocked-trial Stroop fMRI task in major depressive disorder. Journal of Affective Disorders. 2012;143:241–247. doi: 10.1016/j.jad.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. Journal of Psychiatry and Neuroscience. 2009;34:175–180. [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Young EA, Akil H, Noll DC, Zubieta JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biological Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. The American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Miller JM, Vorel SR, Tranguch AJ, Kenny ET, Mazzoni P, van Gorp WG, Kleber HD. Anhedonia after a selective bilateral lesion of the globus pallidus. The American Journal of Psychiatry. 2006;163:786–788. doi: 10.1176/ajp.2006.163.5.786. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Yiend J, Lester KJ, Cowen PJ, Harmer CJ. Short-term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. The International Journal of Neuropsychopharmacology. 2009;12:169–179. doi: 10.1017/S1461145708009164. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olafsson RP, Smari J, Guethmundsdottir F, Olafsdottir G, Harethardottir HL, Einarsson SM. Self reported attentional control with the Attentional Control Scale: factor structure and relationship with symptoms of anxiety and depression. Journal of Anxiety Disorders. 2011;25:777–782. doi: 10.1016/j.janxdis.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. The American Journal of Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe HG, Booij J, Veltman DJ, Michel MC, Schene AH. Successful pharmacologic treatment of major depressive disorder attenuates amygdala activation to negative facial expressions: a functional magnetic resonance imaging study. Journal of Clinical Psychiatry. 2012;73:451–459. doi: 10.4088/JCP.10m06584. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. The American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Archives of General Psychiatry. 2012;69:913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vataja R, Leppavuori A, Pohjasvaara T, Mantyla R, Aronen HJ, Salonen O, Kaste M, Erkinjuntti T. Poststroke depression and lesion location revisited. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:156–162. doi: 10.1176/jnp.16.2.156. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspectives on Psychological Science. 2009;4:17. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Walsh ND, Williams SCR, Brammer MJ, Bullmore ET, Kim J, Suckling J, Mitterschiffthaler MT, Cleare AJ, Pich EM, Mehta MA, Fu CHY. A Longitudinal Functional Magnetic Resonance Imaging Study of Verbal Working Memory in Depression After Antidepressant Therapy. Biological Psychiatry. 2007;62:1236–1243. doi: 10.1016/j.biopsych.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Witt ST, Stevens MC. Overcoming residual interference in mental set switching: Neural correlates and developmental trajectory. Neuroimage. 2012;62:2055–2064. doi: 10.1016/j.neuroimage.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, PMMaSMS, editors. Functional MRI: An Introduction to Methods. OUP; 2001. [Google Scholar]
- Zhang X, Yaseen ZS, Galynker II, Hirsch J, Winston A. Can depression be diagnosed by response to mother’s face? A personalized attachment based paradigm for diagnostic fMRI. PLoS One. 2011;6:e27253. doi: 10.1371/journal.pone.0027253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.