Abstract
Self-renewal and differentiation by spermatogonial stem cells (SSCs) is the foundation for continual spermatogenesis. SSC self-renewal is dependent on glial cell line-derived neurotrophic factor (GDNF); however, intracellular mechanisms stimulated by GDNF in SSCs are unknown. To investigate these mechanisms we utilized a culture system that maintains a mouse undifferentiated germ cell population enriched for self-renewing SSCs. In these cultures mRNA for the transcription factors Bcl6b, Erm, and Lhx1 are up-regulated by GDNF and decreased in its absence. The expression of all three molecules was further identified in undifferentiated spermatogonia in vivo. Using small interfering RNA to reduce expression and transplantation to quantify stem cell numbers, Bcl6b, Erm, and Lhx1 were shown to be important for SSC maintenance in vitro. Next, GDNF was shown to activate both Akt and Src family kinase (SFK) signaling in SSCs, and culture of SSCs with inhibitors to Akt or SFKs followed by transplantation analysis showed significant impairment of SSC maintenance in vitro. Apoptosis analysis revealed a significant increase in the percentage of apoptotic cells when Akt, but not SFK, signaling was impaired, indicating that multiple signaling pathways are responsible for SSC self-renewal and survival. Biochemical and gene expression experiments revealed that GDNF up-regulated expression of Bcl6b, Erm, and Lhx1 transcripts is dependent on SFK signaling. Overall, these data demonstrate that GDNF up-regulation of Bcl6b, Erm, and Lhx1 expression through SFK signaling is a key component of the intracellular mechanism for SSC self-renewal.
In mammals several tissue systems rely on the function of a small undifferentiated adult stem cell population. The biological function of these cells is to preserve tissue homeostasis by providing differentiated cells while at the same time undergoing self-renewal to ensure that a constant supply of new stem cells is available. These two capabilities, self-renewal and differentiation, are often referred to as fate decisions and are the basis for how adult stem cells are defined. Some adult stem cell-dependent tissues include the epidermis, intestinal epithelium, bone marrow, and testis. The testis is one of the most productive systems, generating millions of sperm each day.
Spermatogenesis, the process of sperm production, is a classic stem cell-dependent system in which external niche stimuli and internal gene expression regulate self-renewal and differentiation of spermatogonial stem cells (SSCs).3 Currently, very little is known about mechanisms regulating these SSC fate decisions, yet deciphering them is important for understanding male fertility and general adult stem cell biology. Study of SSC biology has been hampered because of their rarity in the testis and lack of specific markers for their isolation.
The mammalian testis contains several different germ cell populations. SSCs are present in the undifferentiated spermatogonia population and are often referred to as Asingle (As) spermatogonia (1). Also in this population are Apaired (Apr) and Aaligned (Aal) spermatogonia, which are daughter progeny produced from differentiation of SSCs (1). All three types of undifferentiated spermatogonia (As, Apr, and Aal) are morphologically similar and cannot currently be distinguished based on phenotype. SSCs are rare, estimated to be ~0.03% of the cell population in an adult mouse testis (2). Their differentiating daughters, Apr and Aal, are estimated to be ~10-fold more abundant, constituting 0.3% of the testis cell population (2). Thus, studying the undifferentiated spermatogonia population emphasizes the differentiating daughter progeny of SSCs rather than the SSC itself. The only unequivocal means for identifying SSCs is by functional transplantation (3, 4). Only SSCs are capable of reestablishing spermatogenesis when transferred into a recipient testis; thus, the functional transplantation system is the only current means to distinguish SSCs from Apr and Aal spermatogonia.
Investigating mechanisms regulating SSC fate decisions in vivo is challenging due to their rarity and current lack of means to isolate them. Thus, an in vitro system that supports an enriched self-renewing SSC germ cell population for extended periods of time is essential. A serum-free chemically defined culture system for mouse SSCs has been developed that is useful for cellular, biochemical, and molecular experimentation (5). In this system SSC self-renewal is critically dependent on glial cell line-derived neurotrophic factor (GDNF), a transforming growth factor β family member originally shown to be important for kidney morphogenesis and neuronal progenitor development (6). GDNF has also been shown to influence undifferentiated spermatogonia function in vivo (7). Based on both in vivo and in vitro studies, it has become widely accepted that GDNF is the critical growth factor for mouse SSC self-renewal. Also, GDNF is critical for rat SSC self-renewal in vitro (8), suggesting that the importance of this growth factor for SSC function may be conserved across mammalian species.
Currently the mechanisms by which GDNF exerts its effects on SSCs are largely unknown. However, we have recently identified GDNF-regulated genes in cultured self-renewing germ cell populations proven to be enriched for SSCs by functional transplantation (9) which provided a data base of candidate molecules that may be essential for regulating SSC fate decisions. These gene expression studies revealed that GDNF dramatically regulates expression of the transcription factors B cell CLL/lymphoma 6 member B (Bcl6b), Ets variant 5 (Erm), and LIM homeobox 1 (Lhx1) in cultured SSCs. Using siRNA and functional transplantation analysis, Bcl6b was subsequently shown to be important for maintenance of self-renewing SSCs in vitro, and disrupted spermatogenesis was observed in Bcl6b null testes in vivo (9). These observations established a biological significance for the identified GDNF-regulated genes.
The intracellular signaling pathways stimulated by GDNF that elicit gene expression changes are unknown in SSCs. Studies in kidney morphogenesis and neuronal progenitor function have demonstrated that the GDNF receptor complex on the cell surface consists of Gfrα1 and c-Ret proto-oncogene (6). Gfrα1 functions as a glycosylphosphatidylinositol-anchored docking acceptor that is needed for GDNF to bind with c-Ret, which is a transmembrane tyrosine kinase receptor. In general, the downstream signaling cascades induced by GDNF binding to c-Ret in neuronal cells include the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt (10)), protein kinase C (11), mitogen-activated protein kinase (12), Janus kinase/signal transducer and activator of transcription (13), and Src family kinase (SFK (14)) pathways. PI3K/Akt is a prominent GDNF-induced signaling cascade in neuronal cells, and SFK signaling is essential for embryonic stem cell (ESC) self-renewal (15), which is closely related to SSCs. Thus, the objective of the current study was to determine whether these two signaling pathways, PI3K/Akt and SFK, are critical intracellular mechanisms stimulated by GDNF to regulate SSC self-renewal and survival.
EXPERIMENTAL PROCEDURES
Chemical Signaling Inhibitors and Antibodies
See the supplemental experimental procedures.
Self-renewing SSC Cultures
Cultures of self-renewing SSCs were established from Thy1 MACS selected 6–8 days postpartum (dpp) donor B6.129S7-Gtrosa26 (designated Rosa; The Jackson Laboratory) male mice as previously described (16). Cultures were maintained in serum-free media with the addition of GDNF (20 ng/ml), Gfrα1 (150 ng/ml), and basic fibroblast growth factor (1 ng/ml). All experiments were performed on 1–3-month-old cultures.
SSC Transplantation Analysis
For all functional transplantation experiments cultured donor Rosa cells were transferred into the seminiferous tubules of adult busulfan-treated (60 mg/kg) 129 SvCP (129) × C57BL/6 recipient male mice at a concentration of 106 cells/ml. Two months after transplantation recipient testes were analyzed for donor-derived colonies of spermatogenesis by X-gal (5-bromo-4-chloro-3-indolyly β-d-galactosidase) staining. The number of donor colonies, which is a direct reflection of SSC number in the injected cell suspension (17), was manually quantified for each recipient testis. The Animal Care and Use Committee of the University of Pennsylvania approved all experimental procedures in accordance with The Guide for Care and Use of Laboratory Animals (National Academy of Sciences, Assurance no. A3079-0).
siRNA Transfections
All gene-specific (Silencer® Pre-designed) and negative control (Silencer® Negative Control #1) siRNAs were purchased from a commercial vendor (Ambion Inc., Austin, TX). Approximately 0.7–1.2 × 105 clump-forming germ cells were transfected with 75 pmol of siRNA using Lipofectamine 2000 (Invitrogen) as previously described (9). To validate knock-down of transcript levels, RNA was isolated from cells 24 h after transfection using the Trizol method (Invitrogen), and gene expression levels were determined by qRT-PCR. For transplantation experiments the cells were maintained on STO feeders for 7 days followed by transplantation into busul-fan-treated recipient testes.
Quantitative Real-time PCR
In all experiments RNA was isolated using the Trizol method (Invitrogen). The quality of each RNA sample was determined based on spectrophotometry and denaturing agarose gel electrophoresis. Only intact samples with a 260/280 ratio of 1.8 or higher were used. All RNA samples were subsequently DNase-treated (DNA-free kit, Ambion Inc.) to remove possible contaminating genomic DNA. One microgram of RNA was reverse-transcribed using oligo(d)T priming and SuperScript II reverse transcriptase (Invitrogen). The expression levels of specific genes were measured using SYBR Green assays and ABI 7300 sequence detection system (Applied Biosystems Inc.; Foster City, CA). Melt curve analyses were conducted to ensure specificity of the amplicon. Primer sequences for all genes analyzed were designed using Primer Express 3.0 software (Applied Biosystems Inc.) and are available as supplemental data (Table 1). Quantitative comparisons were made by normalizing gene-of-interest expression values to that of ribosomal protein S2. Relative transcript abundance was determined using the formula: Relative transcript abundance = 1/2(CT of gene-of-interest − CT S2) (18, 19).
Bcl6b, Erm, and Lhx1 Immunohistochemistry and Immunofluorescence
To examine Bcl6b, Erm, and Lhx1 in normal spermatogenesis, testis cross-sections created from formalin- fixed 8 dpp and adult (3 mo) mouse testes were utilized. To examine Bcl6b, Erm, and Lhx1 expression in vitro, paraformaldehyde-fixed cultures of clump-forming germ cells were used. See the supplemental experimental procedures for more detailed procedures.
Immunoblotting
For immunoblotting experiments, clump-forming germ cells were lysed in SDS sample buffer, and 50 µg of total protein was resolved by SDS-PAGE (10% Ready Gel, Bio-Rad) and transferred to nitrocellulose membranes. Blots were blocked and probed with primary antibodies against specific antigens. Digital images were captured with phosphorimaging (VersaDoc, Bio-Rad), and density measurements were made using commercial software (Quantity One, Bio-Rad). See the supplemental experimental procedures for detailed immunoblot procedures.
Statistics
All quantification data are presented as the mean ± S.E. All statistical tests were performed using SPSS 13.0 software (SPSS Inc., Chicago, Ill.). Significant differences between means for single comparisons were determined using Student’s t-tests. Multiple comparison analyses were performed using a univariate analysis of variance with Tukey honestly significant difference or least significance difference post hoc tests.
RESULTS
Expression of Bcl6b, Erm, and Lhx1 in Cultured SSCs and during Postnatal Spermatogenesis
In our previous studies GDNF was shown to up-regulate Bcl6b, Erm, and Lhx1 transcript levels in cultured self-renewing SSC populations established from 6–8-dpp C57BL/6 mouse pup Thy1+ germ cells (9). In the current study these results were confirmed using 129× C57 Rosa donor pups, because a reporter gene was required for quantitative transplantation studies to investigate intracellular signaling. These germ cells in culture form tight clumps of cells loosely attached to an underlying STO feeder (Fig. 1A). Growth and maintenance of self-renewing SSCs is supported in a chemically defined serum-free media with the addition of only three factors, GDNF, Gfrα1, and basic fibroblast growth factor (5). The stem cell potential and enrichment of these cultures was confirmed by functional transplantation in which the cells generated colonies of donor-derived spermatogenesis in recipient testes (Fig. 1B). GDNF up-regulation of Bcl6b, Erm, and Lhx1 transcript expression, identified in our previous studies, was re-confirmed in these cultures. Also, the specificity of gene expression responses to GDNF was evaluated in these cultures by measuring expression of Plzf and Pou5f1 (Oct4), which are SSC-expressed but not GDNF-regulated genes. Expression levels for Bcl6b, Erm, and Lhx1 were significantly reduced 6-fold or greater after 18 h withdrawal of GDNF and subsequently up-regulated 2-fold or greater after 4 hGDNF replacement, whereas Plzf and Pou5f1 expression was unaffected by GDNF treatments (see supplemental Fig. 1). The expression of Bcl6b, Erm, and Lhx1 proteins were also identified by both immunofluoresence and Western blotting in these self-renewing SSC cultures (Fig. 1, C and D).
FIGURE 1. Expression of the GDNF-regulated transcription factors Bcl6b, Erm, and Lhx1 in self-renewing SSCs in vitro and undifferentiated spermatogonia in vivo.
A, cultured germ cell clumps (arrows) established from 6–8-dpp mouse pup Thy1+ germ cells and maintained in a serum-free environment with GDNF, Gfrα1, and basic fibroblast growth factor. Self-renewing SSCs grow within these clumps and are loosely attached to an underlying STO feeder (arrowhead). B, recipient mouse testis transplanted with cultured clump-forming Rosa donor germ cells, demonstrating the SSC content of these cultures. Each blue colony of spermatogenesis is derived from a single donor SSC that was present in the injected cell population (17). C, immunofluorescent detection of Bcl6b, Erm, and Lhx1 protein expression in SSC-enriched cultured germ cell clumps. Negative control was omission of primary antibody. D, immunoblot detection of Bcl6b, Erm, and Lhx1 expression in two independent SSC enriched germ cell clump cultures. Glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) was used as a loading control. E, examination of Bcl6b, Erm, and Lhx1 expression in cross-sections of 8-dpp mouse pup testes using immunohistochemistry. Expression of all three molecules was detected in undifferentiated spermatogonia. Both Bcl6b and Lhx1 expression was observed in individual spermatogonia (arrows), whereas Erm expression was detected in most spermatogonia (arrows) as well as Sertoli cell nuclei (arrowhead). Negative control was omission of primary antibody. Scale bars, 100 µm (A), 2 mm (B), 50 µm (C and E).
To further characterize Bcl6b, Erm, and Lhx1 in SSCs, we examined their expression in postnatal spermatogenesis. Transcript levels for all three molecules were measured using qRTPCR at specific ages of development (see supplemental Fig. 2A). Expression for all three molecules was elevated at 0 and 6–8 dpp, developmental stages at which undifferentiated spermatogonia and SSCs are most abundant. A dramatic reduction in expression then occurred as spermatogenesis progressed and more differentiating germ cells appeared at 10–12 dpp. In adult (3 mo) testis, expression levels of all three transcripts then increased. Both Bcl6b and Lhx1 levels were only elevated compared with the 10–12 dpp stage, but Erm expression was dramatically higher in adult testes even compared with 6–8 dpp, which likely reflects its expression in differentiated Sertoli cells (20). As expected, transcript levels were significantly higher in freshly isolated Thy1+ germ cells from 6–8-dpp testes compared with whole tissue for all three molecules. These data demonstrate a developmental profile suggestive of Bcl6b, Erm, and Lhx1 expression in SSCs and undifferentiated spermatogonia.
Cross-sectional immunohistochemistry was then used to localize Bcl6b, Erm, and Lhx1 protein expression to specific cell types within pup and adult testes. Mouse pup testes at 8 dpp were primarily examined because they contain a full complement of undifferentiated and differentiating spermatogonia populations (21). In the 8-dpp testes, expression of all three molecules was detected in undifferentiated spermatogonia and possibly differentiating spermatogonia since these two types of spermatogonia are not easily distinguished (Fig. 1E). Bcl6b and Lhx1 expression was detected in individual spermatogonia, and Erm expression was localized to multiple types of spermatogonia lining the basement membrane of pup testes. Nuclear expression of Erm was also detected in Sertoli cells of pup testes. Expression of all three molecules was also examined in cross-sections of adult testes. Similar to pup testes, both Bcl6b and Lhx1 were localized to individual spermatogonia, and only a few tubules could be found that contained positive germ cells (see supplemental Fig. 2B). Surprisingly, Bcl6b and Lhx1 also appeared to be expressed in stage 7 round spermatids in adult testes. Erm expression was observed in all spermatogonia lining the basement membrane of adult seminiferous tubules, similar to its expression in pup testes. These data reveal expression of Bcl6b and Lhx1 at two stages of spermatogenesis and demonstrate the expression of all three molecules in undifferentiated spermatogonia as would be expected if they are expressed in the SSC population in vivo.
Bcl6b, Erm, and Lhx1 Are Essential for SSC Maintenance in Vitro
The regulation of Bcl6b, Erm, and Lhx1 transcript expression by GDNF suggests that these molecules are important for SSC self-renewal and survival. However, loss-of-function combined with functional transplantation analysis is the only unequivocal means to examine their actual biological importance. We have previously shown that transient reduction of Bcl6b expression with siRNA impairs SSC maintenance in vitro (9). Using a similar approach we examined the effects of reducing Erm and Lhx1 expression with siRNA treatment on SSC maintenance in vitro. Also, Bcl6b was examined as a positive control. Transient transfection of cultured Rosa donor SSCs with synthetic siRNA targeted against Bcl6b, Erm, or Lhx1 significantly (p ≤ 0.01) reduced transcript levels by 87.2 ± 0.1% (mean ± S.E., n = 3), 62.5% ± 0.6% (n = 3), and 52.5 ± 0.4% (n = 3), respectively, compared with cells transfected with scrambled negative control siRNA (Fig. 2A). Off-target effects of each specific siRNA were assessed by BLAST analysis and determination of transcript levels for the other non-targeted GDNF-regulated genes (i.e. Bcl6b, Erm, Lhx1) and the SSC expressed non- GDNF-regulated genes Plzf and Pou5f1. BLAST analysis for each siRNA revealed no significant homology to any known mammalian gene other than the targeted gene-of-interest. Gene expression analysis using qRT-PCR revealed specific knock-down of each targeted gene without reducing the expression level of the other GDNF-regulated genes or non-regulated Plzf and Pou5f1 (see supplemental Fig. 3). For example, Bcl6b siRNA treatment specifically reduced Bcl6b transcript levels without reducing the levels of Erm, Lhx1, Plzf, or Pou5f1. These data provide confidence that the siRNA treatments specifically reduced targeted genes without significant off-target effects that could contribute to observed biological responses.
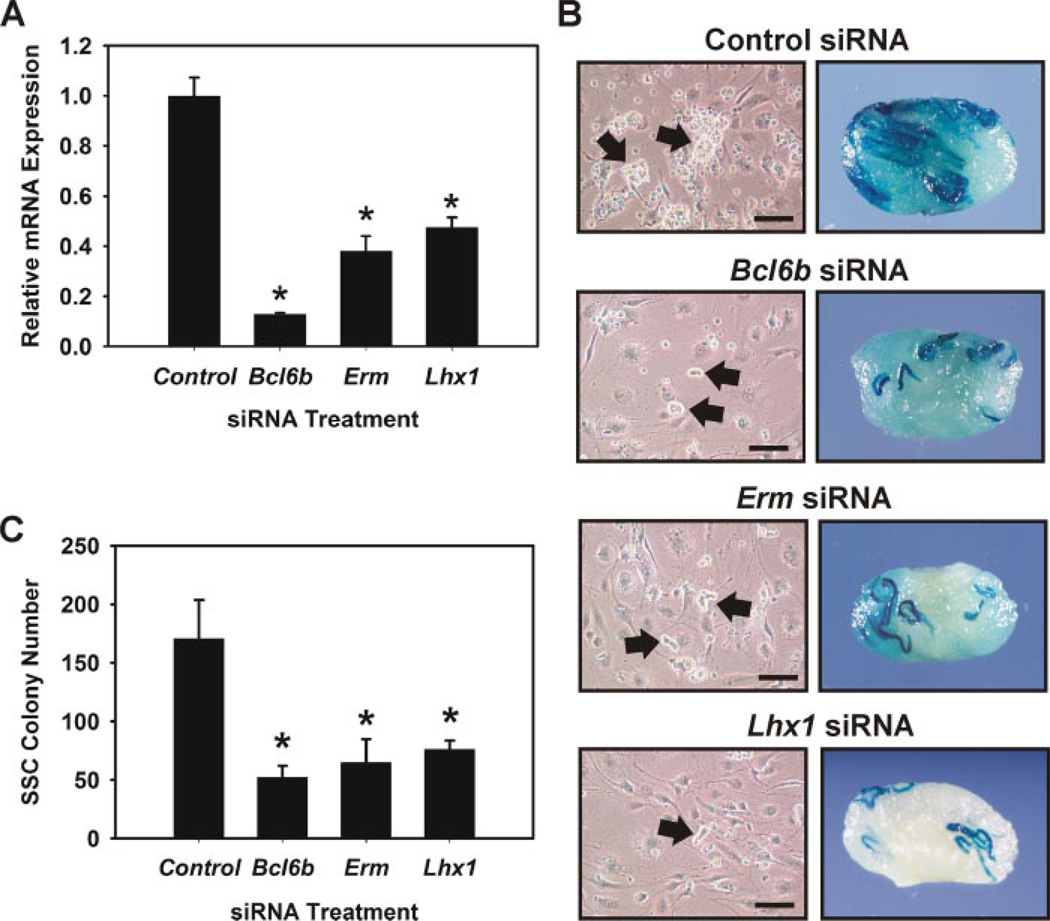
FIGURE 2. Effects of reducing Bcl6b, Erm, and Lhx1 transcript expression by siRNA treatment on SSC maintenance in vitro.
A, qRT-PCR analysis of siRNA treatment on reduction of Bcl6b, Erm, and Lhx1 transcript levels. Data are presented as expression levels (n = 3, mean ± S.E.) relative to cells transfected with scrambled negative control siRNA. B, effects of Bcl6b, Erm, and Lhx1 siRNA treatment on formation of germ cell clumps in vitro. In control siRNA cultures large robust germ cell clumps (arrows) formed, whereas small clumps of cells formed in Bcl6b, Erm, and Lhx1 siRNA cultures after 7 days (arrows). Scale bars, 100 µm. C, functional transplantation analysis of Bcl6b, Erm, and Lhx1 siRNA treatment on SSC maintenance in vitro. Clump-forming germ cells were transiently transfected with gene specific siRNA or negative control siRNA and maintained for 7 days, one self-renewal cycle, under conditions that support SSC self-renewal. The cells were then transplanted into recipient testes to determine the number of SSCs present within each culture. SSC colony number is the number of colonies within recipient testes/105 cells cultured. Data are presented as mean ± S.E. for three independent experiments. Asterisks denote significantly (p ≤ 0.05) different from control.
The functional transplantation assay was then used to unequivocally determine the effects of reducing Bcl6b, Erm, and Lhx1 transcripts on SSC maintenance in vitro. Clump-forming germ cells established from Rosa donors, which express the LacZ marker transgene, were transfected with Bcl6b, Erm, Lhx1, or scrambled negative control siRNA and maintained in vitro for 7 days before transplantation into recipient testes. The doubling time for SSCs in vitro is ~6 days (5); therefore, we were able to assay treatment effects after an entire self-renewal period. Germ cell clump formation after 7 days was clearly affected in Bcl6b-, Erm-, and Lhx1-treated cultures, which formed small cell clumps, compared with the large robust clumps that formed in control siRNA cultures (Fig. 2B). To determine direct effects on SSC maintenance in vitro, the functional transplantation assay was then utilized (Fig. 2C). Evaluation of recipient testes for colonies of donor-derived spermatogenesis, which is a direct measure of SSC number in the injected cell population (17), revealed significantly (p ≤ 0.05) fewer SSCs within cultures treated with Bcl6b (52.3 ± 9.8 colonies/105 cells cultured, mean ± S.E., n = 3 replications, 24 recipient testes), Erm (64.8 ± 20.1 colonies/105 cells cultured, n = 3 replications, 24 recipient testes), and Lhx1 (76.0 ± 7.6 colonies/105 cells cultured, n = 3 replications, 24 recipient testes) siRNAs compared with control siRNA cultures (170.8 ± 32.8 colonies/105 cells cultured, n = 3 replications, 24 recipient testes) (Fig. 2C). Thus, there were ~3.3 (170.8/52.3)-, 2.6 (170.8/64.8)-, and 2.3 (170.8/76.0)-fold fewer SSCs present after one self-renewal cycle when Bcl6b, Erm, and Lhx1 expression was reduced, respectively. These data demonstrate that GDNF regulation of Bcl6b, Erm, and Lhx1 expression is an important mechanism for SSC maintenance in vitro.
GDNF Activates Akt Signaling in Cultured Self-renewing SSCs
The intracellular signaling mechanisms utilized by GDNF to drive self-renewal in SSCs are unknown. The cell surface receptor signaling complex for GDNF consists of c-Ret tyrosine kinase receptor and the co-receptor Gfrα1 (6). Previous studies have shown that c-Ret is important for establishment of postnatal spermatogenesis (22), and both the long and short c-Ret isoforms are expressed by long-term cultured self-renewing SSCs (see supplemental data Fig. 4).
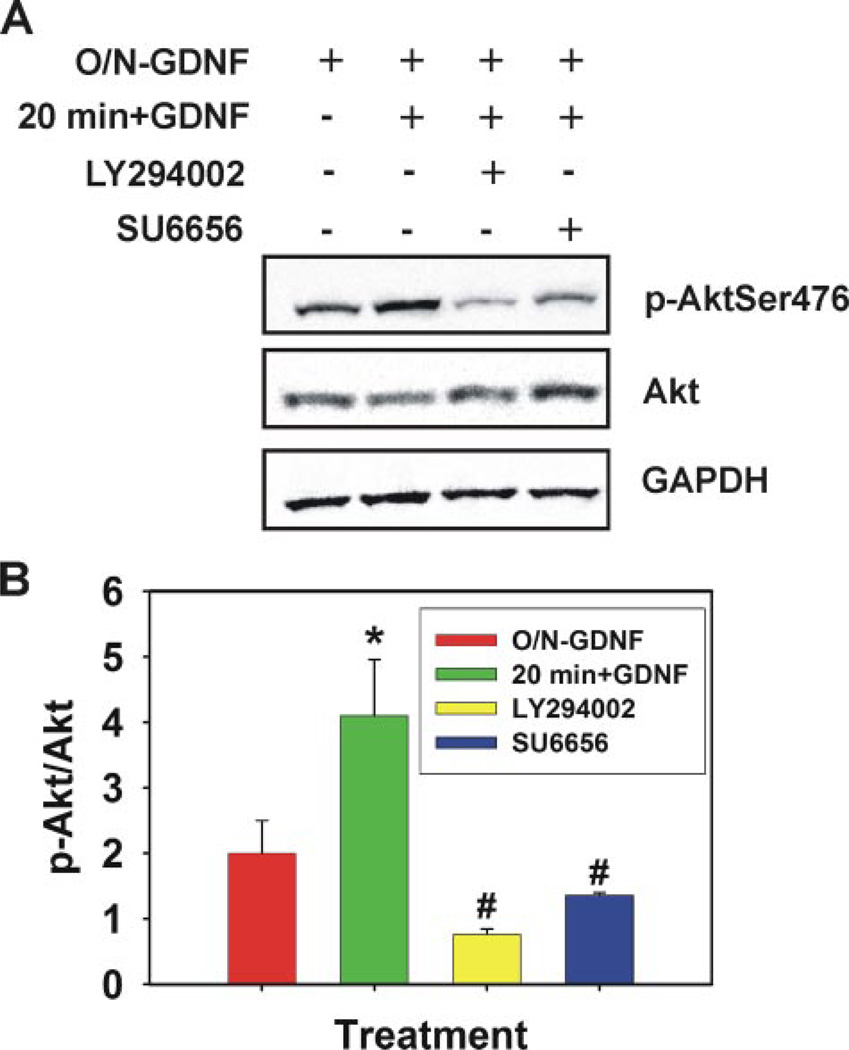
The intracellular signaling mechanisms, stimulated by GDNF downstream of c-Ret, which regulate expression of specific genes (e.g. Bcl6b, Erm, and Lhx1) and activation of proliferation and survival molecules, which ultimately lead to self-renewal, are unknown in SSCs. In other cell types the Akt signaling cascade is often stimulated by GDNF (6). Akt itself does not interact with transmembrane receptors and is often stimulated via PI3K. Therefore, we examined whether GDNF would also stimulate this signaling mechanism in self-renewing SSC populations. Overnight (18 h) GDNF removal from culture media followed by a 20-min replacement resulted in a significant (p = 0.005) increase in Ser-476-phosphorylated Akt levels within cultured self-renewing SSCs, and this activation could be blocked by preincubation of the cells with 10 µm LY294002, a PI3K specific chemical inhibitor (Fig. 3). These data clearly show that stimulation of the Akt signaling cascade is one of the intracellular mechanisms utilized by GDNF in self-renewing SSCs, and this response is mediated through PI3K.
FIGURE 3. GDNF activation of Akt in cultured self-renewing SSCs.
A, representative image of immunoblot analysis for phosphorylated Ser476 Akt levels in cultured self-renewing SSCs. GDNF was removed from culture media overnight for 18 h (O/N-GDNF) followed by a 30-min preincubation with Me2SO (control), 10 µm LY294002 (PI3K specific inhibitor), or 1 µm SU6656 (selective SFK inhibitor). GDNF was then replaced in the media for 20 min before cell lysis. Fifty µg of total cell lysate was resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with a phosphorylated Ser-476 Akt-specific antibody. Blots were stripped and re-probed for total Akt and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). B, graphic representation of the average density ratio of phosphorylated (p) Ser-476 Akt to total Akt from three independent immunoblot experiments. Data are presented as the mean ± S.E. The asterisk denotes significant difference (p = 0.024) between O/N-GDNF and 20 min + GDNF. The number symbol (#) denotes significant difference between 20 min + GDNF and LY294002 (p = 0.002) and SU6656 (p = 0.003).
Akt Function Is Essential for SSC Survival in Vitro
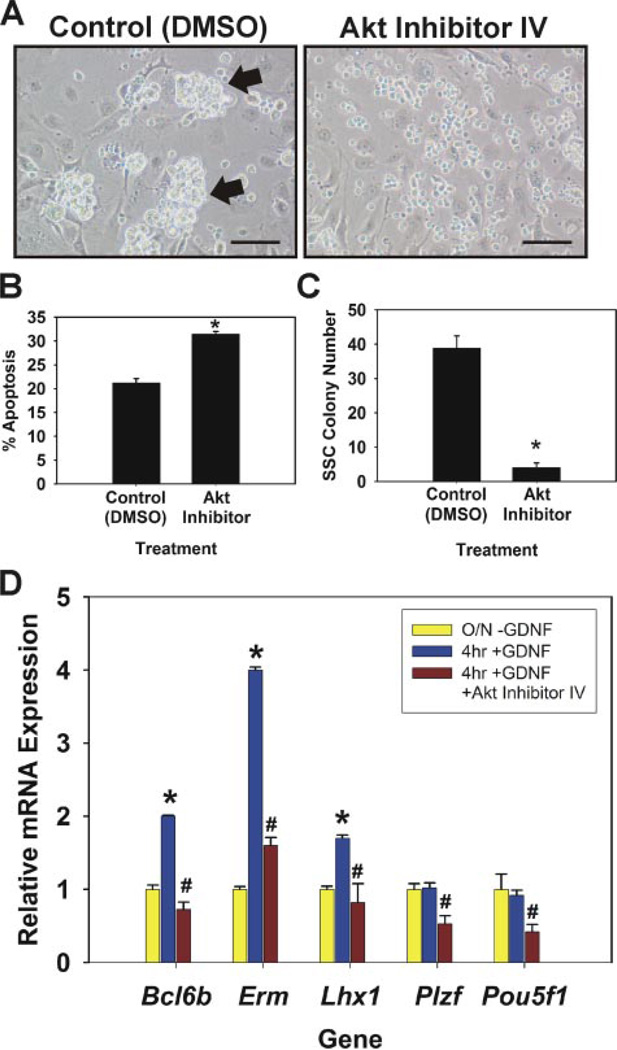
The above experiments demonstrating GDNF activation of Akt signaling strongly suggested that this intracellular pathway would be involved in SSC self-renewal and survival. To examine this hypothesis we used the functional transplantation assay to determine the effects of inhibiting Akt function on SSC maintenance in vitro. Cultures of self-renewing SSCs were visibly affected when Akt function was impaired with a chemical inhibitor (Akt Inhibitor IV, Calbiochem) (Fig. 4A). Germ cell clumps did not form in cultures maintained with a relatively low concentration of 20 nm Akt inhibitor IV, and only single cells were present after 7 days. In contrast, control cultures maintained with the addition of Me2SO to the media formed large robust clumps of germ cells. These observations indicated that SSC survival was dramatically impaired when Akt signaling was inhibited. Further analysis revealed a significant increase (p = 0.001) in the percentage of apoptotic cells within Akt inhibitor cultures (31.5 ± 0.5%, n = 3) compared with controls (21.2 ± 0.9%, n = 3) 24 h after the addition of 20 nm Akt inhibitor or Me2SO solvent (Fig. 4B), respectively. To directly examine the effects of Akt inhibition on SSC survival in vitro we next utilized the functional transplantation assay. Germ cell cultures were maintained for 7 days, 1 SSC self-renewal cycle, in the presence of 20 nm Akt inhibitor or Me2SO (control) and subsequently transplanted into recipient testes to determine SSC number (Fig. 4C). The average number of colonies was significantly reduced (p = 0.001) in recipient testes transplanted with Akt inhibitor cultures (4.1 ± 1.3 colonies/105 cells cultured, n = 2 replications, 16 recipient testes) compared with those transplanted with control cultures (38.9 ± 3.5 colonies/105 cells cultured, n = 2 replications, 16 recipient testes). Nearly all (~90%, 4.1/38.9) of the SSCs were lost over a 7-day period when a relatively low concentration of Akt inhibitor was added, strongly suggesting that SSC survival is sensitive to Akt signaling. Based on morphological observations, STO feeders did not appear to be affected by the relatively low level of Akt inhibitor added to the media.
FIGURE 4. Evaluation of Akt inhibition on self-renewing SSCs in vitro.
A, morphological observation of germ cell clump formation 7 days after inclusion of Me2SO (DMSO) or 20 nm Akt inhibitor IV in culture media. In control Me2SO cultures large robust germ cell clumps formed (arrows), whereas only single cells were present in Akt inhibitor cultures. Scale bars, 100 µm. B, percentage of apoptotic cells in clump-forming germ cell cultures 24 h after addition of Me2SO (control) or 20 nm Akt inhibitor IV to culture media. C, functional transplantation analysis of Akt inhibitor treatment on SSC maintenance in vitro. Clump-forming germ cells were cultured for 7 days in conditions that support SSC self-renewal with the addition of Me2SO or 20 nm Akt inhibitor IV. Cells were subsequently transplanted into recipient testes to determine the number of SSCs present in each culture. SSC colony number is the number of colonies within recipient testes/105 cells cultured. Data are presented as the mean ± S.E. for two independent experiments. Asterisks denote significant difference from control. D, qRT-PCR analysis of Akt inhibition on GDNF-regulated gene expression. Cultured SSCs were deprived of GDNF for 18 h (O/N-GDNF, yellow bars) followed by 4 h GDNF replacement (4 h + GDNF, blue bars) with the addition of Me2SO (control) or 20 nm Akt inhibitor IV (red bars). Relative mRNA expression levels for each gene were normalized to that of ribosomal protein S2 and are presented as -fold change compared with O/N-GDNF (yellow bars). Data are the mean ± S.E. for three independent experiments. The asterisk denotes significant difference (p = 0.05) between O/N-GDNF and 4 h + GDNF. The number symbol (#) denotes significant difference (p ≤ 0.01) between 4 h + GDNF and 4 h + GDNF + Akt Inhibitor IV.
Next, we examined whether Akt signaling is directly involved in GDNF-regulated gene transcription within self-renewing SSCs. In previous studies we have demonstrated that a 4-h GDNF stimulation after overnight withdrawal elicits an increase in Bcl6b, Erm, and Lhx1 transcript levels within cultured self-renewing SSCs (9). The addition of 20 nm Akt inhibitor along with GDNF was able to completely block the GDNF up-regulation of all three transcripts (Fig. 4D), suggesting that this signaling pathway is involved in GDNF-regulated gene transcription and, thus, specific for SSC self-renewal. However, the expression of both Plzf and Pou5f1, two genes expressed by SSCs but not GDNF-regulated, were also significantly (p = 0.05) reduced when Akt was inhibited (Fig. 4D). Together these data demonstrate that GDNF induction of Akt signaling plays a general, yet critical role to promote SSC survival but not specifically self-renewal.
SFK Signaling Is Essential for GDNF Regulation of SSC Self-renewal in Vitro
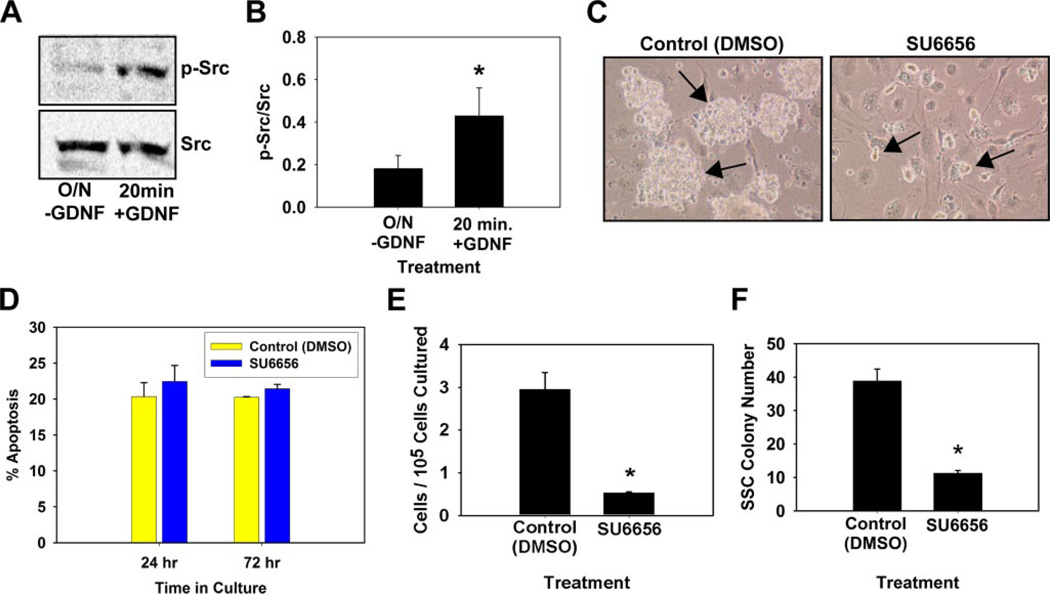
In neurons c-Ret has been shown to interact with SFKs at the plasma membrane (23) and influence Akt activation (24); thus, we reasoned that the same mechanisms could also function in SSCs to promote self-renewal and survival. Similar to PI3K inhibition, preincubation of cells with the selective SFK chemical inhibitor SU6656 (100 nm) blocked the GDNF-stimulated increase in phosphorylated Ser-476 Akt levels (Fig. 3). First, we examined whether GDNF directly activates SFK signaling in cultured SSCs. The addition of GDNF for 20 min after an overnight withdrawal significantly (p = 0.01) increased phosphorylated SFK levels within cultured SSCs (Fig. 5, A and B), further strengthening the hypothesis that SFK signaling is involved in GDNF regulation of SSC self-renewal. Next we examined whether inhibiting SFK function would affect SSC maintenance in vitro. Germ cell clump formation was dramatically impaired when cultures were maintained with the addition of 100 nm SU6656 (Fig. 5C). In contrast to Akt inhibition, which resulted in cultures of single cells after 7 days, SFK inhibition resulted in the formation of small germ cell clumps, whereas large robust clumps of cells were generated in control cultures. These morphological observations closely resembled the appearance of the small clump formation when Bcl6b, Erm, or Lhx1 expression was reduced by siRNA treatment (Fig. 2B), indicating that SFK signaling may be directly involved in GDNF regulation of SSC self-renewal. Furthermore, the percentage of apoptotic cells in SSC cultures was not significantly (p = 0.1) increased 24 or 72 h after the addition of 100 nm SU6656 to culture media (Fig. 5D), in contrast to the effects seen 24 h after Akt inhibitor addition in previous experiments (Fig. 4B). As expected from observed impaired clump formation, total cell numbers were significantly (p = 0.012) reduced 7 days after SU6656 compared with controls (Fig. 5E). Transplantation analyses were subsequently conducted to directly determine the effects of SFK inhibition on SSC maintenance after one self-renewal cycle of 7 days (Fig. 5F) and revealed a significant (p = 0.001) decrease in colony numbers within recipient testes transplanted with SU6656 (100 nm)-treated cultures (7.5 ± 3.8 colonies/105 cells cultured, n = 2 replications, 16 recipient testes) compared with control Me2SO-treated cultures (38.9 ± 3.5 colonies/105 cells cultured, n = 2 replications, 16 recipient testes). There were ~81% (7.5/38.9) fewer SSCs when SFK function was impaired compared with normal control cultures after a single self-renewal cycle, strongly suggesting that SFK signaling is essential for SSC self-renewal in vitro. Based on morphological examination, the addition of 100 nm SU6656 to culture media did not appear to affect STO feeders.
FIGURE 5. Evaluation of SFK signaling in SSC self-renewal in vitro.
A, representative image of immunoblot analysis for GDNF activation of SFK signaling in cultured self-renewing SSCs. Cultures were subjected to an 18-h withdrawal of GDNF (O/N-GDNF) followed by replacement for 20 min before cell lysis. Fifty µg of total cell lysate was resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with a phosphorylated (p−) SFK antibody. Blots were then stripped and re-probed with an antibody for total Src levels. B, graphic representation of the ratio of phosphorylated SFK to total Src from three independent immunoblot experiments. Data are the mean ± S.E. Asterisks denote significant difference (p = 0.016). C, morphological observation of germ cell clump formation in vitro 7 days after addition of Me2SO (DMSO, Control) or 100 nm concentrations of the selective SFK inhibitor SU6656. Large robust germ cell clumps (arrows) formed in control cultures, whereas SU6656 cultures contained only small clumps of germ cells (arrows). Scale bars, 100 µm. D, percentage of apoptotic cells in clump-forming germ cell cultures 24 or 72 h after the addition of Me2SO (control) or 100 nm SU6656. Data are the mean ± S.E. of three independent experiments. E, total number of remaining cells 7 days after SU6656 (100 nm) treatment per 105 cells originally cultured. F, functional transplantation analysis of the effect of SU6656 treatment on SSC maintenance in vitro. Clump-forming germ cells were cultured for 7 days in conditions that support SSC self-renewal with the addition of Me2SO or 100 nm SU6656. Cells were subsequently transplanted into recipient testes to determine the number of SSCs present in each culture. SSC colony number is the number of colonies within recipient testes/105 cells cultured. Data are presented as mean ± S.E. for two independent experiments. Asterisks denote significantly different from control (p = 0.008).
To assess further whether SFK signaling is involved directly in GDNF influence on SSC self-renewal, we examined whether chemical inhibition could specifically block GDNF-regulated gene expression. Similar to Akt inhibition, SFK inhibition also completely blocked an increase in Bcl6b, Erm, and Lhx1 gene expression generated by 4 h GDNF stimulation after overnight withdrawal (Fig. 6). However, SFK inhibition did not effect expression of the SSC expressed non-GDNF-regulated genes Plzf and Pou5f1, whereas Akt inhibition did have a significant impact on expression of these two genes. These data strongly suggest that SFK signaling is one intracellular mechanism directly involved in GDNF-regulated SSC self-renewal. Collectively, the data demonstrate a dynamic role for SFK signaling in GDNF influence on SSC self-renewal, first by activating Akt to prevent apoptosis and, second, by regulating the expression of specific genes essential for SSC self-renewal.
FIGURE 6. Evaluation of SFK signaling importance for GDNF-regulated expression of self-renewal genes in cultured SSCs.
qRT-PCR analyses was used to determine whether SFK inhibition effects GDNF-regulated expression of Bcl6b, Erm, and Lhx1. Cultured SSCs were deprived of GDNF for 18 h (O/N-GDNF, yellow bars) followed by 4 h of GDNF replacement (4 h + GDNF, blue bars) with the addition of Me2SO (control), or 100 nm SU6656 (red bars). Relative mRNA expression levels for each gene were normalized to that of ribosomal protein S2 and are presented as -fold change compared with the O/N-GDNF treatment (yellow bars). Data are the mean ± S.E. for three independent experiments. The asterisk denotes significant difference (p ≤ 0.05) between O/N-GDNF and 4 h + GDNF. The number symbol (#) denotes significant difference (p ≤ 0.01) between 4 h + GDNF and 4 h + GDNF + SU6656.
There are eight reported SFK members in mammals, c-Src, Yes, Fyn, Lyn, Hck, Lck, Fgr, and Blk (25). All of these share very similar roles and have the capability of compensating for one another in cell signaling processes. However, not all members are expressed in the same cell types; for example, Blk expression is restricted to the hematopoietic lineage (25). We examined which of the different SFKs are expressed in self-renewing SSCs using RT-PCR (see supplemental Fig. 5). In cultured clump-forming SSCs the expression of c-Src, Yes, Fyn, Lyn, and Hck was clearly detectable, whereas expression of Lck, Fgr, and Blk could not be detected. Expression of 7 of 8 isoforms was detected in cultured embryonic stem cells (AB1 cells, gift from Alan Bradley), serving as a positive control, and only Blk could not be clearly detected. Thus, at least five SFKs are present in GDNF-stimulated self-renewing SSCs in vitro. The functional importance of each specific isoform is unclear at this time and must be determined by future experimentation.
DISCUSSION
The ability to study SSC biology has dramatically increased since development of the functional transplantation system (3, 4). However, the intrinsic molecular mechanisms that control SSC self-renewal and differentiation fate decisions are largely unknown. We recently developed a culture system that maintains a self-renewing SSC population for extended periods of time, making it possible to examine these molecular mechanisms. This system has considerable strength because the medium is chemically defined and serum-free, allowing precise molecular and biochemical experimentation of growth factor effects on SSCs. Importantly, results of experimental modifications in culture conditions regarding SSC function can be evaluated using the functional transplantation assay. It is critically important to validate treatment effects on cultured cells by transplantation to recipient testes to unequivocally demonstrate effects on SSCs.
Through both in vitro and in vivo experimentation it has been determined that GDNF is the essential growth factor controlling SSC self-renewal. Our approach to understand the intrinsic molecular mechanisms regulating this process has been to examine the intracellular pathways activated or suppressed by GDNF. Recently, we identified GDNF-regulated genes in functionally proven self-renewing SSC populations maintained in a serum-free environment (9). From these studies we were able to identify up-regulation of the transcription factors Bcl6b, Erm, and Lhx1, and Bcl6b was further shown to have a biological importance in SSC maintenance in vitro and in vivo. In the current study we examined the biological importance of Erm and Lhx1 gene expression in SSC self-renewal in vitro and further characterized the expression of all three transcription factors. As expected, the protein for Bcl6b, Erm, and Lhx1 was detected in cultured self-renewing SSCs using two different methods. In vivo, the expression of all three molecules was seen in undifferentiated and perhaps differentiating spermatogonia in the case of Erm. In 8-dpp mouse pup testes, both Bcl6b and Lhx1 were detected in individual spermatogonia along the basement membrane, strongly suggesting expression in undifferentiated spermatogonia. Erm expression was observed in most spermatogonia, suggesting expression in undifferentiated and differentiating spermatogonia as well as Sertoli cells. In adult testes, Bcl6b and Lhx1 expression was again localized to individual spermatogonia, and Erm expression was observed in most spermatogonia lining the basement membrane. The detection of Erm expression in cultured SSCs and spermatogonia in vivo contrasts to reports of exclusive Erm expression in Sertoli cells within adult mouse testes (20). The data in this study demonstrating expression of Erm by cultured SSCs and spermatogonia in vivo and the importance of Erm gene expression in SSC maintenance in vitro strongly suggests that the dramatic infertility experienced by Erm null male mice is at least in part due to impaired function of spermatogonia and not solely Sertoli cell malfunction. Bcl6b null male mice also exhibit impaired spermatogenesis (9). Thus, two of the three GDNF-regulated genes in cultured self-renewing SSCs that we have focused on have a demonstrated biological significance in vivo. Unfortunately, Lhx1 null mice die before or shortly after birth (26); thus, effects of Lhx1 loss-of-function on male fertility are not easily assessed. However, based on the in vitro data from this study demonstrating an importance for Lhx1 in SSC maintenance and the spermatogenic defects seen from Bcl6b and Erm loss-of-function, it is reasonable to assume that Lhx1 also has an important role in normal mouse spermatogenesis in vivo. Surprisingly, both Bcl6b and Lhx1 expression appeared to be present in step 7 round spermatids within adult mouse testes. The functional importance of this is unclear at the present time. Overall, the data derived in this study from combining functional transplantation experiments with gene expression analysis demonstrates that regulation of Bcl6b, Erm, and Lhx1 expression is an important mechanism stimulated by GDNF to regulate SSC self-renewal.
To understand more completely the regulation of SSC self-renewal by GDNF, the intracellular signaling pathways that elicit intrinsic responses, such as changes in gene expression, must be determined. The Akt signaling pathway is one of the most prominent intracellular mechanisms stimulated by GDNF to prevent apoptosis and induce proliferation of neuronal progenitor cells (6). In this study we determined that GDNF also activates Akt signaling in cultured self-renewing SSCs. Using GDNF-regulation of Bcl6b, Lhx1, and Erm gene expression as an end point, we were able to determine that Akt is not specifically involved in SSC self-renewal. Although GDNF upregulation of these genes was blocked, expression of the non GDNF-regulated genes Plzf and Pou5f1 was also significantly reduced when Akt function was impaired. Thus, Akt signaling is not specific for SSC self-renewal and has a general, but essential role promoting their survival in vitro.
In neuronal cells, complete GDNF biological activity requires SFK signaling (23). Also, SFK signaling has been shown to be essential for ESC self-renewal (15). Therefore, we hypothesized that SFK signaling may also be essential for GDNF-regulation of SSC self-renewal and survival. The data from this study demonstrate that SFK signaling utilizes at least two important pathways to serve as a major intracellular mechanism for SSC self-renewal and survival (Fig. 7). First, SFK signaling is involved in activating Akt to promote SSC survival. However, a significant increase in apoptosis was not observed when SFK signaling was impaired with a chemical inhibitor. Thus, this mechanism is likely secondary to the main SSC survival pathway, which is PI3K activation of Akt. Second, SFK signaling is directly involved in SSC self-renewal by specifically regulating expression of self-renewal genes (e.g. Bcl6b, Erm, and Lhx1) upon GDNF stimulation. The downstream effectors of SFK signaling, which ultimately influence specific changes in gene expression, must be determined by future experiments to continue to develop our understanding of intracellular signaling mechanism induced by GDNF in SSCs.
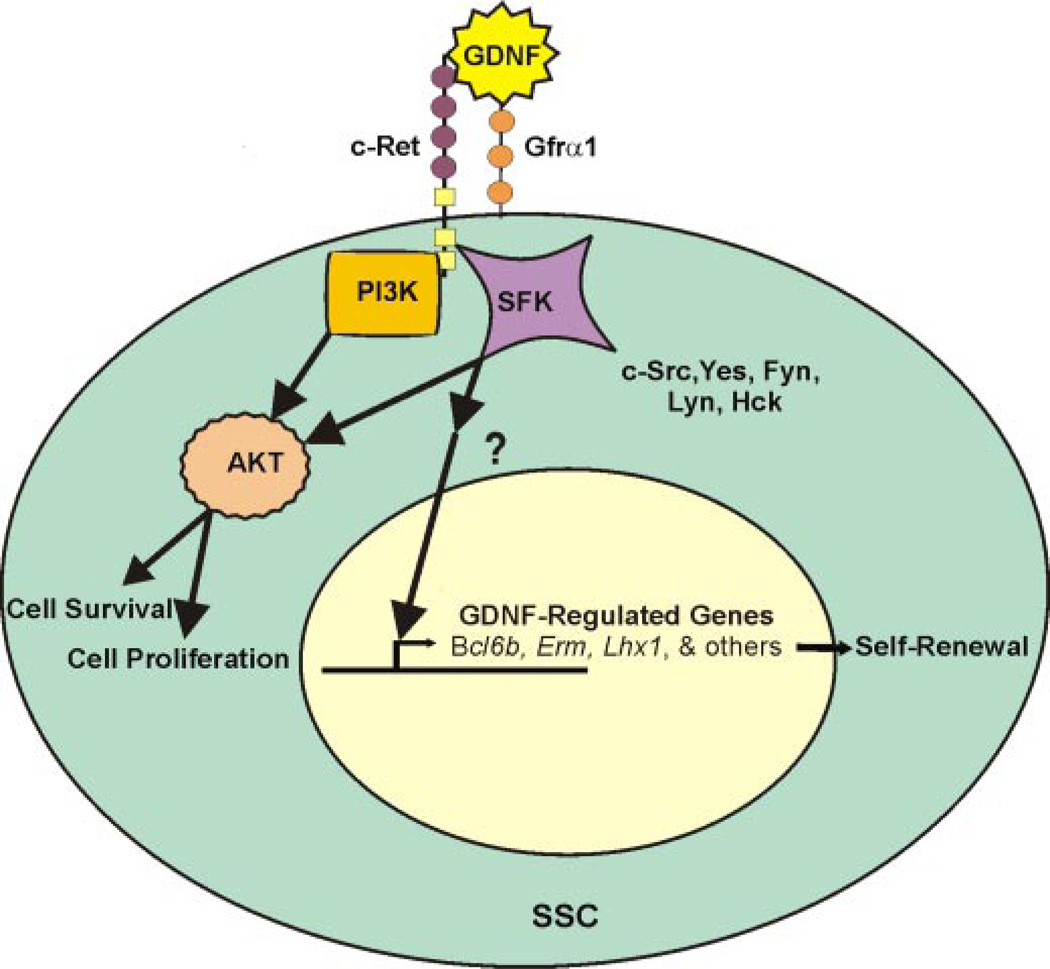
FIGURE 7. Proposed model for GDNF regulation of SSC self-renewal and survival.
In this model GDNF signals through c-Ret tyrosine kinase to activate Akt and SFK intracellular signaling. SSC survival is promoted via Akt activation from both PI3K and SFK. The expression of specific genes necessary for self-renewal, such as Bcl6b, Erm, and Lhx1, is regulated via activation of SFK signaling cascades. The downstream effectors of SFK activation are currently unknown in SSCs.
Recently, GDNF has been connected to SFK signaling in mouse pup germ cells (27). In that study SFKs were shown to be important for maintenance of Gfrα1+ testis cells in vitro via activation of Akt signaling. These results are difficult to interpret in terms of SSCs, since no functional studies were performed (i.e. transplantation analysis). Because Gfrα1 is expressed by all undifferentiated spermatogonia, examination of testis cells based on Gfrα1 expression does not distinguish between SSCs and their differentiating daughter cells, which are much more abundant in both the neonatal and adult testis. Without functional transplantation experiments it is impossible to separate treatment effects on SSCs and those on the more abundant non SSC undifferentiated spermatogonia. Both the Braydich-Stolle et al. (27) study and our results agree that GDNF activates Akt signaling via both PI3K and SFKs in undifferentiated spermatogonia. However, our functional transplantation experiments demonstrate a distinct role of GDNF-induced SFK signaling in SSCs. One effect is general in nature and supports SSC survival via Akt activation. The second effect is more specific and is by an undefined pathway involved in regulating the expression of specific genes (e.g. Bcl6b, Erm, and Lhx1) important for SSC self-renewal in vitro (Fig. 7).
Another recent study suggests that overexpression of an activated form of Akt results in GDNF-independent SSC self-renewal in vitro (28). However, without comparing the stem cell potential in a modified culture-adapted cell population to that of wild-type cells by functional transplantation, it is difficult to make a distinction between non-GDNF supported SSC self-renewal and general proliferation or survival, since wild-type SSCs can survive in vitro for extended periods without the addition of GDNF as well (29). Also, the increase in expression of differentiation markers in Akt overexpressing cells (28) suggests that Akt alone is not sufficient for maintaining SSC self-renewal, and other signaling pathways must be involved.
Although the above two studies suggest that SFK or Akt signaling are involved in SSC self-renewal, important differences in the function of the two pathways cannot be distinguished due to absence of quantitative transplantation data. In the current study the data clearly indicate the function of the Akt pathway is primarily in SSC survival, whereas SFK signaling has a specific involvement in regulating the genes involved in and necessary for SSC self-renewal.
The self-renewal, survival, and differentiation of SSCs are the foundation for continual sperm production in males. Defining the intracellular mechanisms that control these abilities is essential for understanding spermatogenesis as well as treating infertility due to defects in SSC function. Also, understanding mechanisms that govern SSC self-renewal increases our knowledge of general adult stem cell biology, which may provide critical insights into the self-renewal mechanisms of other stem cell populations. Results from this study establish an important intracellular signaling pathway for SSC self-renewal and survival.
Supplementary Material
Acknowledgments
We thank Dr. J. A. Schmidt for manuscript review, C. Freeman and R. Naroznowski for assistance with animal maintenance, J. Hayden for photography, and the University of Pennsylvania Cell Morphology Core histological preparations. Also, thanks to Drs. D. Fearon for Bcl6b antibody and R. Behringer for Lhx1 antibody.
Footnotes
The research was supported by the NICHD, National Institutes of Health Grants HD044445 and HD052728 and by the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5, Table 1, and experimental procedures.
The abbreviations used are: SSC, spermatogonial stem cell; siRNA, small interfering RNA; qRT, quantitative real-time; GDNF, glial cell line-derived neurotrophic factor; PI3K, phosphatidylinositol 3-kinase; SFK, Src family kinase; dpp, days post-partum.
REFERENCES
- 1.de Rooij DG, Russell LD. J. Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 2.Tegelenbosch RAJ, de Rooij DG. Mutat. Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 3.Brinster RL, Avarcbock MR. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinster RL, Zimmermann JW. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubota H, Avarbock MR, Brinster RL. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sariola H, Saarma M. J. Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 7.Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 8.Ryu B-Y, Kubota H, Avarbock MR, Brinster RL. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oatley JM, Avarbock MR, Teleranta AI, Fearon DT, Brinster RL. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besset V, Scott RP, Ibanez CF. J. Biol. Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- 11.Iwahashi N, Murakami H, Nimura Y, Takahashi M. Biochem. Biophys. Res. Commun. 2002;294:642–649. doi: 10.1016/S0006-291X(02)00528-4. [DOI] [PubMed] [Google Scholar]
- 12.Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- 13.Plaza-Menacho I, van der Sluis T, Hollema H, Gimm O, Buys CH, Magee AI, Isacke CM, Hofstra RM, Eggen BJ. J. Biol. Chem. 2007;282:6415–6424. doi: 10.1074/jbc.M608952200. [DOI] [PubMed] [Google Scholar]
- 14.Popsueva A, Poteryaev D, Arighi E, Meng X, Angers-Loustau A, Kaplan D, Saarma M, Sariola H. J. Cell Biol. 2003;161:119–129. doi: 10.1083/jcb.200212174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anneren C, Cowan CA, Melton DA. J. Biol. Chem. 2004;279:31590–31598. doi: 10.1074/jbc.M403547200. [DOI] [PubMed] [Google Scholar]
- 16.Oatley JM, Brinster RL. Methods Enzymol. 2006;419:259–282. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- 17.Nagano M, Avarbock MR, Brinster RL. Biol. Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson UE, Heid CA, Williams PM. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 19.Heid CA, Stevens J, Livak KJ, Williams PM. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM, Hassell JA, Chandreshekar V, Hofmann MC, Hess RA, Murphy KM. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell LD, Ettlin RA, Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. pp. 1–40. [Google Scholar]
- 22.Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Biol. Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 23.Melillo RM, Barone MV, Lupoli G, Cirafici AM, Carlomagno F, Visconti R, Matoskova B, Di Fiore PP, Vecchio G, Fusco A, Santoro M. Cancer Res. 1999;59:1120–1126. [PubMed] [Google Scholar]
- 24.Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM., Jr J. Neurosci. 2001;21:1464–1472. doi: 10.1523/JNEUROSCI.21-05-01464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas SM, Brugge JS. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 26.Shawlot W, Behringer RR. Nature. 1995;374:425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- 27.Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Dev. Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 29.Nagano M, Avarbock MR, Leonida EB, Brinster CJ, Brinster RL. Tissue Cell. 1998;30:389–397. doi: 10.1016/s0040-8166(98)80053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.