Abstract
SLP-76 is an adapter protein expressed in T cells and myeloid cells that is a substrate for ZAP-70 and Syk. SLP-76–deficient mice exhibit a profound block in T-cell development. We found that although SLP-76 is expressed in mouse mast cells, SLP-76–/– mice have normal numbers of mast cells in their skin and bronchi. SLP-76–/– mice are resistant to IgE-mediated passive anaphylaxis. SLP-76–/– mice sensitized with IgE anti-dinitrophenyl (DNP) and then challenged with DNP-HSA developed only mild and transient tachycardia, failed to increase their plasma histamine level, and all survived the antigen challenge. Bone marrow–derived mast cells (BMMCs) from SLP76–/– mice failed to release β-hexosaminidase and to secrete IL-6 after FcεRI cross-linking. Tyrosine phosphorylation of phospholipase C-γ1 (but not of Syk) and calcium mobilization in response to IgE cross-linking were reduced in SLP-76–deficient BMMCs. These results suggest that SLP-76 plays an important role in FcεRI-mediated signaling in mast cells.
J. Clin. Invest. 103:1737–1743 (1999).
Introduction
Allergic reactions are mediated by products released from mast cells and basophils after ligation of their high-affinity receptor for IgE (FcεRI) by allergen. FcεRI is a multimolecular complex of the IgE-binding α subunit, 2 signal transducing γ subunits, and a β subunit that promotes assembly of the receptor and amplifies signal transduction (1, 2). Both γ and β chains contain immunoreceptor tyrosine-based activation motifs (ITAMs) within their intracellular domains. Signaling pathways activated upon cross-linking of FcεRI are similar to those activated by ligation of other ITAM-containing members of the immune antigen receptor family. These include the T-cell receptor (TCR), the B-cell receptor (BCR), and several Fc receptors. Upon receptor cross-linking, ITAMs become tyrosine phosphorylated by Src family tyrosine kinases (Lyn, in the case of FcεRI) and recruit Syk family tyrosine kinases (Syk, in the case of FcεRI), resulting in their phosphorylation and activation (3). Both Src and Syk family protein tyrosine kinases (PTKs) then phosphorylate multiple intracellular proteins such as PLC-γ, Vav, and the adapter protein SLP-76 (4).
SLP-76 (Src homology 2 domain–containing [SH2-containing] leukocyte protein of 76 kDa) is an adapter protein predominantly expressed in hematopoietic cells, notably in T cells and myeloid cells (5, 6). SLP-76 contains multiple NH2-terminal tyrosine phosphorylation sites, a central proline-rich region, and a COOH-terminal SH2 domain (5). After ligation of the T-cell antigen receptor, SLP-76 is rapidly tyrosine phosphorylated by the protein tyrosine kinase (PTK) ZAP-70, which is recruited to the ITAM of CD3ζ (7–9). SLP-76 associates via tyrosine-phosphorylated residues in its NH2-terminal domain with Vav (10), a guanine nucleotide exchange factor for GTPases Cdc42 and Rac1, which activate the JNK (Jun amino terminal kinase) pathway (11). SLP-76 associates via its proline-rich domain with the SH3 domains of the adapter protein Grb2 and may be coupled through Grb2 and Sos to the Ras/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway (12). In addition, SLP-76 interacts through its SH2 domain with the Fyn-binding protein (FYB), also known as SLP-76–associated phosphoprotein of 130 kDa (SLAP-130) (13, 14), and with other proteins. These include a Ser/Thr kinase, a 62-kDa phosphoprotein, phospholipase C (PLC-γ), c-Cbl, SHP-1, Shc, and SKAP-55, although many of these interactions may be indirect (12).
A role for SLP-76 in signal transduction has been established in T cells. Overexpression of SLP-76 in the human T-cell line Jurkat results in marked augmentation of TCR-stimulated IL-2 and nuclear factor of activated T cells (NFAT) promoter activity and in increased IL-2 production (15). We and others have recently shown, using a gene-targeting approach, that SLP-76 is necessary for T-cell development. SLP-76–/– mice lack peripheral T cells and double-positive (DP) CD4+CD8+ thymocytes as well as CD25–CD44– and CD4–CD8– double-negative (DN) thymocytes (16, 17). Although DN thymocytes in these mice express a pre-TCR/CD3 complex, administration of antibodies to CD3 fails to drive the differentiation of their DN thymocytes into DP cells in vivo (16). This suggests that SLP-76 links TCR/CD3 to downstream signaling cascades. Support of this notion has come from studies of an SLP-76–deficient variant of the Jurkat T-cell line. Activation of PLC-γ1, calcium mobilization, phosphorylation of the ERK, and IL-2 production in response to TCR/CD3 cross-linking all were severely compromised in SLP-76–deficient Jurkat cells (18).
SLP-76 is a substrate of FcεRI-stimulated PTKs in the rat basophilic cell line RBL-2H3 (19). The role of SLP-76 in mast cell development and function is not known. We show that although SLP-76 is expressed in mouse bone marrow–derived mast cells (BMMCs), SLP-76–/– mice have normal numbers of mast cells in their skin and bronchi. SLP-76–/– mice were resistant to IgE-mediated passive anaphylaxis, and their BMMCs failed to release β-hexosaminidase and secrete IL-6 after FcεRI cross-linking. These results suggest that SLP-76 plays an important role in FcεRI-mediated signaling in mast cells.
Methods
Mice.
The construction of mice deficient for the adapter protein SLP-76 has been described in detail (16). SLP-76+/+ and SLP-76+/– littermates were used as a control. All mice were of mixed 129Sv/C57BL6 background and were housed under pathogen-free conditions.
Derivation and characterization of BMMCs.
Bone marrow cells obtained by flushing the femur and tibia bones with PBS were cultured in WEHI-3–conditioned medium as a source of IL-3 (20). Passages were made every week by replating the cells in fresh medium. After 3–5 weeks of culture, 90% or more of the cells derived from SLP-76+/+ and SLP-76–/– bone marrow are mast cells, as evidenced by FACS analysis for IgE binding. To assess IgE binding, the cells were incubated with 1 μg/mL of mouse IgE anti-dinitrophenyl (DNP) mAb SPE-7 (Sigma Chemical Co., St. Louis, Missouri, USA), followed by washing once and then adding biotinylated rat anti-mouse IgE and streptavidin-CyChrome (both from PharMingen, San Diego, California, USA). The cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). Data on 5 × 106 to 20 × 106 viable, nonerythroid cells (as determined by forward versus side scatter) were collected for each sample.
Histological studies.
Mice were sacrificed by cervical dislocation. Bronchial and cutaneous tissues were fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, and 0.025% CaCl2 in 0.1 M sodium cacodylate buffer (pH 7.3), and were stored overnight at 4°C. They were then washed in 0.1 M sodium cacodylate buffer and stored in the same buffer at 4°C until processing into 3-μm-thick, paraffin-embedded, Giemsa-stained sections. Tissues were examined by light microscopy for determination of mast cell numbers. One complete mainstem bronchial cross-section per mouse was examined. Back skin was examined at ×400 because the mast cells are relatively sparse and easily distinguished from surrounding cells; ear skin, in which mast cells are more frequent but more difficult to distinguish, was assessed at ×1,000. In each tissue, mast cells in 6 randomly chosen fields were counted.
Heart rate and blood histamine measurements.
SLP-76–/– mice and SLP-76+/+ controls were sensitized with 3 μg of mouse IgE anti-DNP mAb SPE-7 by intravenous injection in the tail vein. Twenty-four hours later, the mice were challenged with intravenous injection of DNP-HSA (500 μg/mouse). Blood histamine levels were determined by competitive RIA (Immunotech, Westbrook, Maine, USA) on ∼100 μL of blood 1.5 minutes after antigen challenge.
For heart rate measurements, according to our previously published method (21), mice sensitized as just described were anesthetized with sodium pentobarbital (70–90 mg/kg intraperitoneally). A 19-gauge tubing adapter (Becton Dickinson and Co., Franklin Lakes, New Jersey, USA) was inserted into the trachea, and a silastic catheter was inserted into a jugular vein for injection of antigen (500 μg of DNP-HSA per mouse). Mechanical ventilation was instituted via the tracheostomy tube using a tidal volume of 5–7 mL/kg at a rate of 150 breaths per minute. Transpulmonary pressure was detected with a pressure transducer (Celesco, Canoga Park, California, USA). The signal was amplified, and the cardiac artifact was recorded with the ventilator turned off during 10-second intervals of apnea at different times before and after injection of the antigen. The difference between controls and SLP-76–/– mice was analyzed by two-way ANOVA, with time and mouse group as the variables.
β-hexosaminidase release assay.
BMMCs (1 × 106) were incubated in 50% WEHI-3–conditioned medium containing the indicated concentrations of rat IgE (monoclonal rat anti-DNP hapten, clone LO-DNP-30; Serotec Ltd., Oxford, United Kingdom) for 1 hour on ice. After being washed once by centrifugation at 4°C, pellets were resuspended on ice in their original volume with 50% WEHI-3–conditioned medium with or without 25 μg/mL F(ab′)2 fragments of mouse anti-rat immunoglobulins (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) and incubated for 15 minutes at 37°C. The reaction was stopped by centrifugation. The pellets were resuspended in their original volumes with WEHI-3–conditioned medium and were lysed by 3 cycles of freezing and thawing in an alcohol/dry ice bath. Aliquots (5 μL) of supernatants and cell lysates were incubated for 30 minutes with 80 μL of substrate solution (1.3 mg/mL p-nitrophenyl-b-D-2-acetamido-2-deoxyglucopyranozide in 0.1 M citrate [pH 4.5]). The reaction was stopped by the addition of 200 μL of 0.2 M glycine (pH 10.7). OD was read at 405 nm in an ELISA reader.
The percent release values for each experimental condition were calculated by the formula [S/(S + P)] × 100, where S and P are the β-hexosaminidase contents of the supernatant (S) and pellet (P) from each sample. The net percent release values were obtained by subtracting the percent release of sensitized cells incubated in IgE alone from that of replicate cells sensitized with IgE and challenged with cross-linking antibodies.
Measurement of IL-6 secretion.
BMMCs (1 × 106) preloaded for 1 hour with 10 μg/mL IgE anti-DNP mAb SPE-7 were incubated with different concentrations of DNP-HSA or with ionomycin (10 μM) for 24 hours in culture medium. Supernatants were assayed for their IL-6 content using an ELISA kit (R&D Systems Inc., Minneapolis, Minnesota, USA).
Western blotting and immunoprecipitation.
Lysates were prepared from 1 × 106 BMMCs by boiling in an SDS loading buffer and were separated on a 9% gel by PAGE and transferred to nitrocellulose membrane. The blots were developed using anti-phosphotyrosine mAb 4G10 (Upstate Biotechnology Inc., Lake Placid, New York, USA), followed by protein G linked to horseradish peroxidase (Protein G-HRP; Bio-Rad Laboratories Inc., Hercules, California, USA) or enhanced chemiluminescence (ECL; Amersham Life Sciences Inc., Arlington Heights, Illinois, USA) or Super Signal Ultra system (Pierce Chemical Co., Rockford, Illinois, USA), according to the manufacturers’ instructions.
For immunoprecipitation, cell lysates prepared in RIPA buffer were precleared for 2 hours with normal rabbit serum coupled to protein G-Sepharose beads, and then immunoprecipitated overnight with antibodies to Syk, PLC-γ1, and Vav (Upstate Biotechnology Inc.) on protein G-Sepharose beads. The immunoprecipitates were washed 5 times in lysis buffer and eluted by boiling with SDS-sample buffer. Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Blots were probed with anti-phosphotyrosine mAb 4G10 or RC20 (Signal Transduction Laboratories, San Diego, California, USA) and developed with ECL as already described. To ensure equal loading, blots were stripped and then reprobed with the appropriate antibody.
Measurement of [Ca2+]i.
BMMCs were sensitized with 10 μg/mL IgE anti-DNP mAb SPE-7 for 1 hour on ice and loaded with Fluo-3/AM for 30 minutes at 37°C in PBS supplemented with 10 mM HEPES, 0.1% BSA, 0.5 mM MgCl2, 1 mM CaCl2, and 5 mM glucose. After loading, the cells were washed twice, resuspended in 1 mL of the same buffer at 1 × 106 cells/mL, and analyzed with FACScalibur (before and after antigen challenge) for their content of [Ca2+]i, as described (22).
Results
SLP-76 is expressed in mast cells but is not required for their development.
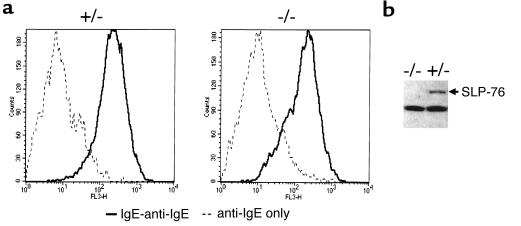
We examined SLP-76 expression in BMMCs derived from bone marrow of SLP-76+/– control mice after 5 weeks of culture in WEHI-3–conditioned medium. Figure 1a shows that ∼90% of the cells in these cultures were mast cells, as evidenced by their capacity to bind IgE. Furthermore, the same proportion of cells contained metachromatic granules when stained with toluidine blue (data not shown). Figure 1b shows that SLP-76 protein was readily detectable by Western blotting in lysates of SLP-76+/– BMMCs.
Figure 1.
Surface IgE binding and SLP-76 expression in BMMCs. BMMCs from SLP-76–/– and SLP-76+/– littermates were examined after 4 weeks of culture of bone marrow cells in WEHI-3–conditioned medium. (a) FACS analysis of IgE binding. Cells were incubated with mouse IgE followed by biotinylated anti-mouse IgE and streptavidin-CyChrome. (b) Western blot analysis of SLP-76 expression. Similar results were obtained in 2 different experiments.
As in SLP-76+/– controls, ∼90% of the cells derived from SLP-76–/– bone marrow cells cultured in WEHI-3–conditioned medium bound IgE (Figure 1a) and contained metachromatic granules (data not shown). The kinetics of cell growth and differentiation of SLP-76–/–, SLP-76+/–, and SLP-76+/+ BMMCs were similar (data not shown). As expected, SLP-76 protein was not detectable in SLP-76–/– BMMCs (Figure 1b).
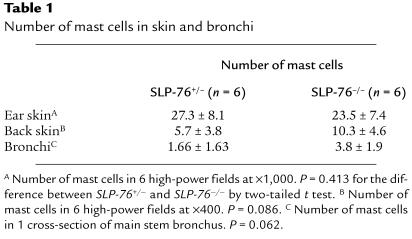
We next asked whether SLP-76 deficiency impairs the capacity of mast cells to populate tissues. We examined the presence of mast cells in 1-μm tissue sections stained with Giemsa. Table 1 shows that there was no significant difference (P > 0.05) in the number of mast cells in 2 different skin sites (ear and back) and in bronchi of SLP-76–/– and SLP-76+/– controls. Taken together, these results indicate that although SLP-76 is expressed in mast cells, it is not essential for their development.
Table 1.
Number of mast cells in skin and bronchi
Passive systemic IgE-dependent anaphylaxis is greatly diminished in SLP-76–/–mice.
Adoptive transfer of IgE antibodies to normal mice primes them to express passive systemic anaphylactic reactions to intravenous challenge with specific antigen. These responses are associated with extensive mast cell degranulation, marked alterations in cardiopulmonary function, and considerable mortality. IgE-mediated anaphylaxis depends on FcεRI signaling, as it is virtually absent in FcεRIα-deficient mice (23). IgE-mediated anaphylaxis also depends on mast cells, as it is diminished in mast cell–deficient W/Wv mice (24, 25) and virtually absent in mast cell–deficient Sl/Sld mice (26).
To evaluate the role of SLP-76 in IgE-dependent systemic anaphylaxis, we passively sensitized 10 SLP-76–/– mice and 10 control littermates (5 SLP-76+/+ and 5 SLP-76+/– mice) with mouse IgE anti-DNP mAb. This was followed 24 hours later by challenge with DNP-HSA. Figure 2a shows that control mice rapidly developed tachycardia. The mean increase in heart rate reached 160% of baseline 1 minute after challenge, peaked at 175% of baseline 5 minutes after challenge, and was sustained for the remaining 25 minutes of the observation period. There was no difference in the heart rates between SLP-76+/–and 5 SLP-76+/+ controls. SLP-76–/– mice exhibited a mild and transient rise in their heart rate after antigen challenge (Figure 2a). Their mean increase in heart rate averaged 105% of baseline at 1 minute after challenge, peaked at 125% of baseline 5 minutes after challenge, and returned to baseline by 15 minutes after challenge. The difference in heart rate response between the SLP-76–/– and control groups was significant (P < 0.0001, ANOVA). More importantly, 5 of the 10 control mice (3 SLP-76+/+ and 2 SLP-76+/–) died — 2 at 5 minutes, 1 at 15 minutes, and 2 at 20 minutes after antigen challenge — whereas all 10 SLP-76–/– mice survived antigen challenge.
Figure 2.
IgE-mediated passive systemic anaphylaxis. (a) Heart rate expressed as percent of baseline in SLP-76–/– mice (n = 10) and control littermates (n = 10) challenged with antigen at time 0. *P < 0.0001 by two-way ANOVA. (b) Plasma histamine level 1.5 minutes after challenge in SLP-76–/– mice (n = 5) and control littermates (n = 6).
A major vasoactive mediator released by activated mast cells and basophils is histamine. Systemic anaphylaxis has been associated with notable increase in plasma histamine levels (27). Plasma histamine content was determined before and 1.5 minutes after challenge with antigen. Plasma histamine was undetectable before antigen administration (data not shown). Figure 2b shows that there was a marked rise in plasma histamine in control mice after antigen challenge (8,726 ± 655 nM; n = 6). The increase in plasma histamine was almost completely abolished in SLP-76–/– mice (588 ± 247 nM; n = 5). These results suggest that SLP-76 is essential for in vivo IgE-mediated mast cell and basophil degranulation.
BMMCs from SLP-76–/– mice fail to degranulate and secrete cytokine after FcεRI cross-linking in vitro.
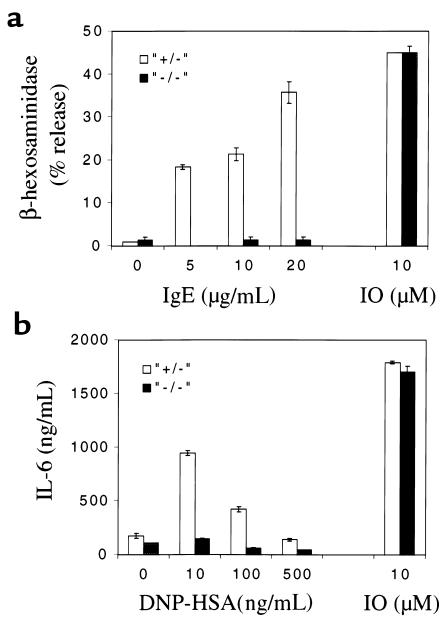
To confirm that the resistance of SLP-76–/– mice to passive anaphylaxis is due to defective FcεRI-mediated degranulation of mast cells, we examined the capacity of SLP-76–/– BMMCs to degranulate upon FcεRI cross-linking in vitro. BMMCs were incubated with rat IgE (5–20 μg/mL), followed 1 hour later by cross-linking with mouse anti-rat Ig (25 μg/mL); then the release of β-hexosaminidase, an enzyme found in preformed mast cell granules (28), was measured. Figure 3a shows that control SLP-76+/– BMMCs released β-hexosaminidase in a dose-dependent manner. β-hexosaminidase release, at the highest concentration of IgE antibody used, reached a level equivalent to that released in response to the calcium ionophore ionomycin. In contrast, SLP-76–/– mast cells showed no detectable release of β-hexosaminidase in response to FcεRI cross-linking. Treatment of SLP-76–/– BMMCs with ionomycin at a concentration found optimal for β-hexosaminidase release by normal BMMCs (10 μM) resulted in normal degranulation, demonstrating that their cellular machinery for degranulation was intact.
Figure 3.
IgE-mediated in vitro degranulation and cytokine release by BMMCs. SLP-76–/– and SLP-76+/– BMMCs were examined for (a) release of β-hexosaminidase by BMMCs sensitized with indicated concentrations of rat IgE and challenged with F(ab′)2 mouse anti-rat immunoglobulin; and (b) release of IL-6 by BMMCs sensitized with mouse IgE anti-DNP and challenged with DNP-HSA. Results represent the mean of 2 experiments, each performed in duplicate. IO, ionomycin (10 μM).
FcεRI cross-linking causes mast cells to secrete cytokines that include IL-4 (29), IL-13 (30), TNF-α (31), and IL-6 (32). To assess the role of SLP-76 in FcεRI-mediated cytokine release, BMMCs were sensitized with IgE anti-DNP and challenged with DNP-HSA, and IL-6 release was examined. Figure 3b shows that BMMCs from SLP-76+/– controls, but not from SLP-76–/– mice, secreted IL-6 within 24 hours of challenge. SLP-76–/– BMMCs secreted normal amounts of this cytokine after stimulation with ionomycin, which bypasses FcεRI signaling.
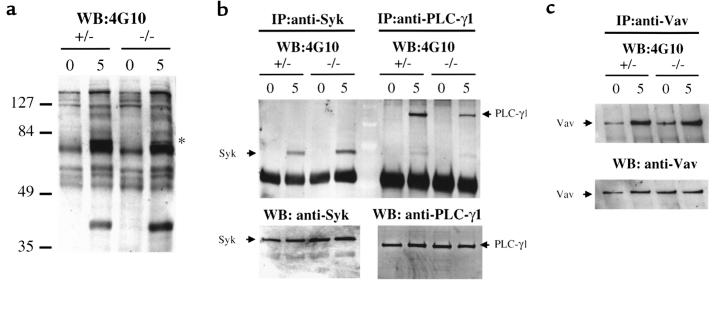
Protein tyrosine phosphorylation and calcium mobilization in response to FcεRI cross-linking are abnormal in BMMCs from SLP-76–/– mice. FcεRI cross-linking activates tyrosine kinases that include Lyn, Blk, Syk, and Btk (3, 33), and results in tyrosine phosphorylation of a number of targets that include the kinases themselves as well as PLC-γ, Vav, and other intracellular proteins (34, 35). Figure 4a shows that after FcεRI cross-linking, protein tyrosine phosphorylation of protein(s) at ∼75 kDa was diminished in SLP-76–/– BMMCs compared with SLP-76+/– controls. Tyrosine phosphorylation of Syk was equivalent in SLP-76–/– BMMCs and controls, and in some experiments was even increased (Figure 4b). In contrast, tyrosine phosphorylation of PLC-γ1 was markedly and consistently diminished in SLP-76–/– BMMCs (Figure 4b) up to 15 minutes after stimulation (data not shown). Tyrosine phosphorylation of Vav was equivalent in SLP-76–/– and SLP-76+/– BMMCs (Figure 4c).
Figure 4.
Protein tyrosine phosphorylation in BMMCs in response to IgE cross-linking. SLP-76–/– and SLP-76+/– BMMCs were sensitized with 20 μg/mL rat IgE, followed by cross-linking with F(ab′)2 mouse anti-rat immunoglobulin. Five minutes later, cell lysates were examined for total protein tyrosine phosphorylation (a) by Western blotting with the anti-phosphotyrosine 4G10 mAb, and for tyrosine phosphorylation of Syk and PLC-γ1 (b) and Vav (c) by immunoprecipitation with the indicated antibodies, followed by Western blotting with mAb 4G10. The star in a indicates phosphoprotein(s) present in controls but diminished in SLP-76–/– BMMCs. Equal loading of Syk, PLC-γ1, and Vav was verified by Western blotting. Results shown are representative of 2 experiments.
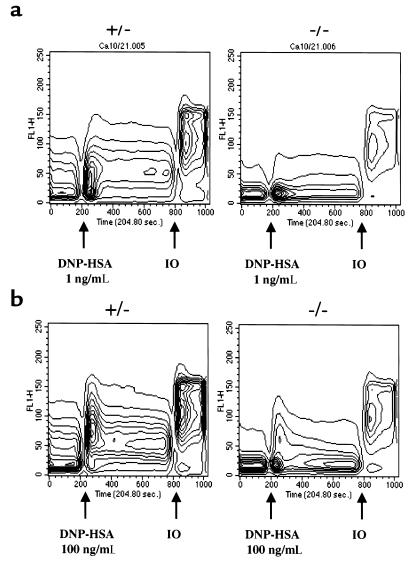
Calcium flux is an early event induced by FcεRI cross-linking in mast cells (3). Calcium mobilization after ligation of ITAM-containing receptors depends on tyrosine phosphorylation and activation of PLC-γ by ZAP-70/Syk. Activated PLC-γ breaks down membrane phosphoinositides to generate the second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which mobilizes calcium from internal stores (4). Given the diminished tyrosine phosphorylation of PLC-γ1 in BMMCs from SLP-76–/– after FcεRI cross-linking, calcium mobilization was examined. BMMCs were sensitized with IgE anti DNP (10 μg/mL), loaded with Fluo-3/AM dye, and then challenged with DNP-HSA (1 ng/mL and 100 ng/mL). Figure 5a shows that calcium mobilization in response to the lower concentration of cross-linking agent was barely detectable in SLP-76–/– BMMCs but readily detectable in SLP-76+/– BMMC controls. At the higher concentration of cross-linker, calcium mobilization became detectable in SLP-76–/– BMMCs but was greatly diminished in both amplitude and duration compared with controls (Figure 5b).
Figure 5.
Ca2+ mobilization in BMMCs in response to IgE cross-linking. [Ca2+]i response of Fluo-3/AM–loaded SLP-76–deficient (–/–) and control (+/–) BMMCs incubated with mouse IgE anti-DNP mAb and then challenged with the cross-linking antigen DNP-HSA at 1 ng/mL (a) and 100 ng/mL (b). Results shown are representative of 2 experiments. IO, ionomycin (10 μM).
Discussion
The results of this study show that SLP-76 plays a critical role in FcεRI-mediated activation of mast cells in vivo and in vitro. SLP-76–/– mice were resistant to passive IgE-mediated anaphylaxis, and their BMMCs failed to degranulate and secrete the cytokine IL-6 upon FcεRI cross-linking.
SLP-76 protein was detected in normal BMMCs. However, the development of BMMCs from bone marrow of SLP-76–/– was normal (Figure 1). Furthermore, we detected normal numbers of connective tissue mast cells in the skin and normal numbers of mucosal mast cells in the bronchi of SLP-76–/– mice (Table 1). Thus, although SLP-76 is tyrosine phosphorylated after FcεRI ligation (19) and is required for FcεRI-induced mediator release (Figure 3), it is not required for mast cell development. This is consistent with the lack of requirement for FcεRI signaling in mast cell development, as evidenced by the presence of normal numbers of mast cells in mice that lack IgE, FcεRIα (23), FcRγ (36), Lyn (37), and Syk (38).
SLP-76–/– mice were resistant to passive IgE-mediated anaphylaxis. SLP-76–/– mice sensitized with IgE antibody and then challenged with antigen developed only mild and transient tachycardia, exhibited a negligible rise in plasma histamine levels, and survived the challenge (Figure 2). The resistance of SLP-76–/– mice to IgE-mediated anaphylaxis is not related to their lack of T cells, as IgE-mediated anaphylaxis in RAG-2–/– and control mice was comparable (data not shown). Because IgE-mediated anaphylaxis depends on FcεRI (23), our results strongly suggest that SLP-76 is a critical component of the FcεRI signaling pathway. The residual response of SLP-76–/– mice in passive IgE-mediated anaphylaxis, manifested by mild and transient tachycardia, is similar to that of mast cell–deficient W/Wv mice, which exhibit cardiac acceleration, but not hypotension or death, after IgE-mediated anaphylaxis (24, 25). The residual response of SLP-76–/– mice could be due to residual signaling via FcεRI. Alternatively, cross-linking of surface IgE receptors other than FcεRI may trigger mediator release. These may include CD23 expressed on B cells, macrophages, and mast cells (39), and the IgE-binding lectin expressed on mast cells and macrophages (40). Finally, IgE immune complexes have been shown to bind to FcγRIII, and it is possible that this receptor may signal independently of SLP-76, e.g., in cells that express the SLP-76 homologue BLNK (41, 42).
FcεRI-mediated mast cell degranulation and cytokine release were deficient in SLP-76–/– BMMCs. This was evidenced by their inability to release β-hexosaminidase (Figure 3a) and secrete IL-6 (Figure 3b) after IgE cross-linking. Degranulation and IL-6 secretion are early and late events, respectively, triggered by FcεRI ligation (43). The signaling pathways leading to the release of these mediators are likely to be distinct. This places SLP-76 in a pivotal position in FcεRI signaling. Our studies of early events in FcεRI signaling in BMMCs of SLP-76–/– mice revealed that tyrosine phosphorylation of Syk was intact, whereas that of PLC-γ1 was markedly diminished (Figure 4b). This places SLP-76 early in the FcεRI signaling pathway, downstream of Syk and upstream of PLC-γ1. It is also consistent with data from SLP-76–deficient Jurkat cells and from platelets of SLP-76–/– mice that suggest that SLP-76 is downstream of ZAP-70 and Syk and upstream of PLC-γ in the TCR/CD3 (18) and the GPVI/FcRγ collagen activation receptor signaling pathways (44). In some experiments, we observed increased tyrosine phosphorylation of Syk after FcεRI cross-linking in SLP-76–/– BMMCs. SLP-76 associates with the tyrosine phosphatase SHP-1 (45) and may recruit it to downregulate protein tyrosine phosphorylation after FcεRI ligation. Tyrosine phosphorylation of Vav was normal in SLP-76–/– BMMCs. This is consistent with findings in SLP-76–deficient Jurkat T cells (18). Further studies are needed to asses Vav activation after FcεRI ligation in SLP-76–/– BMMCs.
Activated PLC-γ1 hydrolyzes membrane phosphoinositides to generate IP3, which mobilizes calcium. Activation of PLC-γ1 was deficient in SLP-76–/– BMMCs after FcεRI cross-linking, as evidenced by their impaired ability to mobilize calcium. This is consistent with the defective FcεRI-mediated degranulation in these cells. The residual PLC-γ1 phosphorylation and the weak and transient rise in intracellular calcium suggest that some signals may be transduced via FcεRI in the absence of SLP-76. However, the residual calcium mobilization in SLP-76–/– BMMCs was associated with minimal or absent mediator release. This is consistent with the notion that the amplitude and duration of the rise in intracellular calcium play a critical role in the activation of calcium-dependent signaling pathways (46).
Both Lyn and Syk are required for mast cell activation after FcεRI ligation (36, 38). Our results show that the same is true for SLP-76. Furthermore, they place SLP-76 between Syk and PLC-γ1. Taken together, these findings suggest that FcεRI ligation activates the following signaling cascade: FcεRI→Lyn→Syk→SLP-76→PLC-γ1→IP3→calcium mobilization, which is critical for mediator release in mast cells. FcεRI-triggered mediator release involves reorganization of the actin cytoskeleton (47, 48). Given that SLP-76 is implicated in actin reorganization in T cells (49), it would be of interest to examine the reorganization of the actin cytoskeleton in SLP-76–deficient mast cells. Furthermore, it will be important to examine the role of SLP-76 in signaling via receptors, other than FcεRI, that cause mast cell mediator release, particularly the ITAM-containing receptors FcγRII/FcγRIII and PIR-A (50). Experiments are under way to address these questions.
Acknowledgments
The authors thank Alison Kisselgof, Joanne Brewer, and Ross Fujita for technical assistance. This work was supported by National Institutes of Health grants AI-35714 and AI-41144; a Pew Scholarship; an Asthma and Allergy Foundation of America Investigator Award (to H.C. Oettgen); a postdoctoral fellowship from the Arthritis Foundation (to J.M. Lu-Kuo); and grants from the Baxter, Olsten, and Centeon Corporations and the Jeffrey Modell Foundation.
References
- 1.Blank U, et al. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature (Lond) 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 2.Lin S, Cicala C, Scharenberg AM, Kinet J. The FcεRIβ subunit functions as an amplifier of FcεRIγ-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 3.Kinet JP, Jouvin MH, Paolini R, Numerof R, Scharenberg A. IgE receptor (Fc epsilon RI) and signal transduction. Eur Respir J Suppl. 1996;22:116s–118s. [PubMed] [Google Scholar]
- 4.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 5.Jackman JK, et al. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 6.Robinson A, et al. Characterization of Grb2-binding proteins in human platelets activated by Fc gamma RIIA cross-linking. Blood. 1996;88:522–530. [PubMed] [Google Scholar]
- 7.Wardenburg JB, et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 8.da Silva AJ, Raab M, Li Z, Rudd CE. TcR zeta/CD3 signal transduction in T-cells: downstream signalling via ZAP-70, SLP-76 and FYB. Biochem Soc Trans. 1997;25:361–366. doi: 10.1042/bst0250361. [DOI] [PubMed] [Google Scholar]
- 9.Raab M, da Silva AJ, Findell PR, Rudd CE. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR zeta/CD3 induction of interleukin-2. Immunity. 1997;6:155–164. doi: 10.1016/s1074-7613(00)80422-7. [DOI] [PubMed] [Google Scholar]
- 10.Tuosto L, Michel F, Acuto O. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J Exp Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 12.Koretzky G. The role of Grb2-associated proteins in T-cell activation. Immunol Today. 1997;18:401–406. doi: 10.1016/s0167-5699(97)01088-8. [DOI] [PubMed] [Google Scholar]
- 13.da Silva AJ, et al. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain–containing leukocyte protein 76 and modulates interleukin 2 production. Proc Natl Acad Sci USA. 1997;94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musci MA, et al. Molecular cloning of SLAP-130, an SLP-76–associated substrate of the T cell antigen receptor–stimulated protein tyrosine kinases. J Biol Chem. 1997;272:11674–11677. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- 15.Motto DG, Ross SE, Wu J, Hendricks-Taylor LR, Koretzky GA. Implication of the GRB2-associated phosphoprotein SLP-76 in T cell receptor–mediated interleukin 2 production. J Exp Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pivniouk V, et al. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 17.Clements JL, et al. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 18.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76- deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 19.Hendricks-Taylor LR, Motto DG, Zhang J, Siraganian RP, Koretzky GA. SLP-76 is a substrate of the high affinity IgE receptor–stimulated protein tyrosine kinases in rat basophilic leukemia cells. J Biol Chem. 1997;272:1363–1367. doi: 10.1074/jbc.272.2.1363. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M, et al. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med. 1997;185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin TR, Galli SJ, Katona IM, Drazen JM. Role of mast cells in anaphylaxis. Evidence for the importance of mast cells in the cardiopulmonary alterations and death induced by anti-IgE in mice. J Clin Invest. 1989;83:1375–1383. doi: 10.1172/JCI114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsitsikov EN, Gutierrez-Ramos JC, Geha RS. Impaired CD19 expression and signaling, enhanced antibody response to type II T independent antigen and reduction of B-1 cells in CD81-deficient mice. Proc Natl Acad Sci USA. 1997;94:10844–10849. doi: 10.1073/pnas.94.20.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet J-P. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor α chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 24.Takeishi T, Martin TR, Katona IM, Finkelman FD, Galli SJ. Differences in the expression of the cardiopulmonary alterations associated with anti-immunoglobulin E-induced or active anaphylaxis in mast cell-deficient and normal mice. Mast cells are not required for the cardiopulmonary changes associated with certain fatal anaphylactic responses. J Clin Invest. 1991;88:598–608. doi: 10.1172/JCI115344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin TR, et al. Mast cells contribute to the changes in heart rate, but not hypotension or death, associated with active anaphylaxis in mice. J Immunol. 1993;151:367–376. [PubMed] [Google Scholar]
- 26.Ando A, Martin TR, Galli SJ. Effects of chronic treatment with the c-kit ligand, stem cell factor, on immunoglobulin E–dependent anaphylaxis in mice. Genetically mast cell–deficient s1/s1d mice acquire anaphylactic responsiveness, but the congenic normal mice do not exhibit augmented responses. J Clin Invest. 1993;92:1639–1649. doi: 10.1172/JCI116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oettgen HC, et al. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 28.Stevens RL, Austen KF. Recent advances in the cellular and molecular biology of mast cells. Immunol Today. 1989;10:381–386. doi: 10.1016/0167-5699(89)90272-7. [DOI] [PubMed] [Google Scholar]
- 29.Bradding P, et al. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992;176:1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toru H, Pawankar R, Ra C, Yata J, Nakahata T. Human mast cells produce IL-13 by high-affinity IgE receptor cross-linking: enhanced IL-13 production by IL-4-primed human mast cells. J Allergy Clin Immunol. 1998;102:491–502. doi: 10.1016/s0091-6749(98)70140-x. [DOI] [PubMed] [Google Scholar]
- 31.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor α (TNF-α)/cachectin by mouse mast cells stimulated via the FcεRI. A mechanism for the sustained action of mast cell-derived TNF-α during IgE-dependent biological responses. J Exp Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plaut M, et al. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 33.Hata D, et al. Involvement of Bruton’s tyrosine kinase in FcepsilonRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JS, Gomez J, Stancato LF, Rivera J. Association of a p95 Vav-containing signaling complex with the FcepsilonRI gamma chain in the RBL-2H3 mast cell line. Evidence for a constitutive in vivo association of Vav with Grb2, Raf-1, and ERK2 in an active complex. J Biol Chem. 1996;271:26962–26970. doi: 10.1074/jbc.271.43.26962. [DOI] [PubMed] [Google Scholar]
- 35.Teramoto H, Salem P, Robbins KC, Bustelo XR, Gutkind JS. Tyrosine phosphorylation of the vav proto-oncogene product links FcepsilonRI to the Rac1-JNK pathway. J Biol Chem. 1997;272:10751–10755. doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- 36.Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in Lyn-deficient bone marrow–derived mast cells. J Immunol. 1997;158:2350–2355. [PubMed] [Google Scholar]
- 37.Miyajima I, et al. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costello PS, et al. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- 39.Takizawa F, Adamczewski M, Kinet J-P. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as FcγRII and FcγRIII. J Exp Med. 1992;176:469–476. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frigeri LG, Liu F-T. Surface expression of functional IgE binding protein, and endogenous lectin, on mast cells and macrophages. J Immunol. 1992;148:861–867. [PubMed] [Google Scholar]
- 41.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 42.Wienands J, et al. SLP-65: A new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki H, et al. Early and late events in Fc epsilon RI signal transduction in human cultured mast cells. J Immunol. 1997;159:5881–5888. [PubMed] [Google Scholar]
- 44.Clements JL, et al. Fetal hemorrhage and platelet dysfunction in SLP-76 deficient mice. J Clin Invest. 1999;103:19–25. doi: 10.1172/JCI5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binstadt BA, et al. SLP-76 is a direct substrate of SHP-1 recruited to killer cell inhibitory receptors. J Biol Chem. 1998;273:27518–27523. doi: 10.1074/jbc.273.42.27518. [DOI] [PubMed] [Google Scholar]
- 46.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer JR, Seagrave JC, Davis BH, Deanin GG, Oliver JM. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 1985;101:2145–2155. doi: 10.1083/jcb.101.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massol P, Montcourrier P, Guillemot JC, Chavrier P. Fc receptor-mediated phagocytosis requires CDC42 and rac1. EMBO J. 1998;17:6219–6229. doi: 10.1093/emboj/17.21.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bubeck Wardenburg J, et al. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 50.Maeda A, Kurosaki M, Kurosaki T. Paired immunoglobulin-like receptor (PIR)-A is involved in activating mast cells through its association with Fc receptor gamma chain. J Exp Med. 1998;188:991–995. doi: 10.1084/jem.188.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]