Abstract
Preeclampsia, the major cause of maternal morbidity and mortality in developed countries, is associated with abnormalities of placenta function due to shallow invasion of the maternal decidua by trophoblasts. Data suggest that TGF-β may play a role in inhibiting trophoblast outgrowth or invasion, or both. We report that placental TGF-β3 expression is high in early pregnancy but falls at around 9 weeks’ gestation. This pattern is inversely correlated with trophoblast outgrowth and fibronectin synthesis, markers of early trophoblast differentiation toward an invasive phenotype. We demonstrate that TGF-β3 is overexpressed in preeclamptic placentae. In contrast to control placentae, explants from preeclamptic pregnancies fail to exhibit spontaneous invasion in vitro. Significantly, antisense-induced inhibition of TGF-β3 expression, and inhibition of TGF-β3 activity with antibodies, induces the formation of columns of trophoblast cells, which migrate out of the explant into the underlying Matrigel. To our knowledge, this is the first demonstration that the hypoinvasive placental phenotype characteristic of preeclampsia can be essentially normalized in vitro by biochemical manipulation. We speculate that a failure to downregulate expression of TGF-β3 at around 9 weeks’ gestation results in shallow trophoblast invasion and predisposes the pregnancy to preeclampsia.
Introduction
Successful human placentation depends on adequate transformation of the uteroplacental circulation by extravillous trophoblast (EVT) proliferation, migration, and invasion into the maternal decidua (1–3). This process rises to a peak by the end of first trimester and declines rapidly thereafter (4).
Preeclampsia occurs in 7–10% of pregnancies and remains the major cause of maternal morbidity and mortality in developed countries. Insufficient trophoblast invasion of maternal spiral arteries contributes to the development of preeclampsia, which, when severe, results in coexistent intrauterine growth restriction (IUGR) (5–8). The placenta plays a central role in the pathogenesis of preeclampsia, as removal of this organ at delivery normally results in prompt resolution of the disease. In addition, molar pregnancies, in which there is placental tissue without a fetus, are often complicated with preeclampsia (9, 10).
Histological examination of placental bed biopsies from preeclamptic women demonstrates trophoblast proliferation but limited migration into superficial decidua (11). Consequently, invasion of the cells into the myometrial portions of the spiral arteries is severely reduced (12, 13), resulting in reduced intervillous blood flow and placing the fetus at risk of oxygen and nutrient deprivation (14). Progress has been made toward understanding the molecular basis of these observations. Villous trophoblasts from preeclamptic placentae have been found to exhibit an immature phenotype, ultrastructurally and biochemically, when compared with normal placentae (15, 16). Specifically, EVT cells in the decidua of women whose pregnancies were complicated by preeclampsia exhibit a less invasive and more proliferative phenotype than normal (11). They continue to express α6β4 as well as abnormally high levels of α5β1 integrins, but they fail to express the α1β1 integrin normally expressed during EVT invasion (12). They also fail to adopt the vascular adhesion phenotype, characteristic of differentiating/invading trophoblasts (17). Various growth factors and cytokines, such as EGF, TGF-α, amphiregulin, IGF-II, and IL-1β, stimulate trophoblast differentiation toward an invasive phenotype (18–21). By contrast, limited data exist regarding possible inhibitory regulators of trophoblast development. Members of the TGF-β superfamily of growth factors, known to inhibit cell invasion (22–24), may be involved in this process. Some studies have reported that TGF-β1 inhibits trophoblast invasion, possibly through an induction of tissue inhibitor of metalloproteinases (TIMP) expression (25), whereas others found no such effect (18). Other studies using isolated first-trimester villous cytotrophoblasts have shown that TGF-β1 inhibits the differentiation of trophoblasts toward an invasive phenotype and suppresses trophoblast endocrine differentiation (26). We have recently reported that activin, a member of the TGF-β superfamily, stimulates trophoblast differentiation toward an invasive phenotype (including trophoblast outgrowth and proliferation from villous tips, integrin switching, fibronectin synthesis, and induction of gelatinase activity) in first-trimester placental explants (27). Surprisingly, antisense-induced downregulation of the TGF-β receptor endoglin also induces trophoblast differentiation in this system (28).
In the present study, we have investigated the role of the 3 mammalian isoforms of TGF-β in normal pregnancies and in pregnancies complicated by preeclampsia. We report that TGF-β3, but not TGF-β1 or TGF-β2, inhibits trophoblast differentiation toward an invasive phenotype in first-trimester human placental explants. Preeclamptic placentae overexpress TGF-β3 and exhibit a hypoinvasive phenotype in vitro. Differentiation toward the invasive phenotype can be restored in these explants by antibody and antisense disruption of endogenous TGF-β3 activity and synthesis, respectively.
Methods
Human chorionic villous explant culture.
Villous explant cultures were established from first-trimester human placentae (5–13 weeks’ gestation) obtained from elective terminations of pregnancies by dilatation and curettage. Villous explant cultures were also prepared from preeclamptic and age-matched control placentae (30 and 32 weeks’ gestation) collected from deliveries at Mount Sinai Hospital. The preeclamptic group was selected to represent classic preeclampsia according to both clinical and pathological criteria (29). The age-matched control groups were primiparous but did not show clinical and pathological signs of preeclampsia or other placental disease. Villous explants cultures were established as described previously (28, 30). Briefly, placental tissue was placed in ice-cold PBS and processed within 2 hours of collection. The tissue was aseptically dissected to remove decidual tissue and fetal membranes. Small fragments of placental villi (15–20 mg wet weight) were teased apart and placed on Millicell-CM culture dish inserts (Millipore Corp., Bedford, Massachusetts, USA) precoated with 0.2 mL of undiluted Matrigel (Collaborative Biomedical Products, Bedford, Massachusetts, USA), and placed in a 24-well culture dish. Explants were cultured in serum-free DMEM/F12 (GIBCO BRL, Grand Island, New York, USA) supplemented with 100 μg/mL streptomycin, 100 U/mL penicillin, and 0.25 μg/mL ascorbic acid (pH 7.4) overnight at 37°C in 5% CO2 in air to allow attachment. In all experiments, a single placenta was used, and for each treatment, explants cultures were set up in triplicate. Morphological integrity and viability of villous explants and their EVT differentiation were monitored daily for up to 6 days as reported previously (30). EVT cell outgrowth from the distal end of the villous tips and their migration into the surrounding Matrigel were consistently monitored and quantitated as described previously (28).
Antisense oligonucleotides and their effects on trophoblast differentiation.
Phosphorothioate oligonucleotides were synthesized on a DNA synthesizer and purified by capillary electrophoresis. Oligonucleotides of 16 bp targeted against sequences adjacent to the AUG initiation codon of different human TGF-β isoforms mRNA were synthesized (31). Previous studies have demonstrated that antisense oligonucleotides, targeted to sequences adjacent to initiation codons, are most efficient in inhibiting translation (32). Furthermore, 16-mer oligonucleotides are short enough to be taken up efficiently and provide sufficient specificity for hybridization to the corresponding target mRNA (32). The sequences of the antisense and sense TGF-β oligonucleotides were TGF-β1: 5′-CCCCGAGGGCGGCATG-3′ and 5′-CATGCCGCCCTCGGGG-3′, respectively; TGF-β2: 5′-CACACAGTAGTGCATG-3′ and 5′-CATGCACTACTGTGTG-3′; TGF-β3: 5′-CCTTTGCAAGTGCATC-3′ and 5′-GATGCACTTGCAAAGG-3′. Oligonucleotides were dissolved in water, and their concentration was estimated by optical density at OD260. Villous explants, prepared from placentae of 5–13 weeks’ gestation and from preeclamptic and age-matched control placentae of 29–32 weeks, were incubated overnight in DMEM/F12 alone. Explant cultures were then incubated in DMEM/F12 alone or medium containing antisense or sense oligonucleotides (10 μM). Culture media in the presence or absence of treatments were routinely changed every 48 hours. Experiments were carried out in triplicate and repeated at least 3 times.
TGF-β antibodies and their effect on trophoblast differentiation.
Villous explants, prepared from placentae of 5–13 weeks’ gestation and from preeclamptic and age-matched control placentae of 29–32 weeks, were incubated overnight in DMEM/F12 alone. Explant cultures were then incubated in DMEM/F12 alone or medium containing antibodies to TGF-β1, TGF-β2, TGF-β3, or control IgG (10 μg/mL). Culture media in the presence or absence of treatments were routinely changed every 48 hours. Experiments were carried out in triplicate and repeated at least 3 times.
RT-PCR and Southern blot analysis.
Total RNA was extracted from the placenta, reverse transcribed, and amplified by 15 cycles of PCR using TGF-β–specific primers. RT-PCR products were analyzed by Southern blotting using 32P-labeled TGF-β cDNAs (33). Primers used for amplification were as follows: (a) TGF-β1 cDNA: (forward primer) 5′-GCCCTGGACACCAACTATTGCT-3′, (reverse primer) 5-AGGCTCCAAATGTAGGGGCAGG-3′ (predicted product size = 161 bp); (b) TGF β2 cDNA: (forward primer) 5′-CATCTGGTCCCGGTGGCGCT-3′, (reverse primer) 5′- GACGATTCTGAAGTAGGG-3′ (predicted product size = 353 bp); (c) TGF-β3 cDNA: (forward primer) 5′-CAAAGGGCTCTGGTGGTCCTG-3′, (reverse primer) 5′-CTTAGAGGTAATTCCCTTGGGG-3′ (predicted product size = 374 bp); and (d) β-actin cDNA: (forward primer) 5-CTTCTACAATGAGCTGGGTG-3′, (reverse primer) 5-TCATGAGGTAGTCAGTCAGG-3 (predicted product size = 307 bp). The identity of the PCR reaction products was also confirmed by sequencing.
In situ hybridization.
Antisense and sense digoxigenin-labeled TGF-β3 riboprobes (33) were generated as described in the RNA labeling and detection kits (nonradioactive) from Boehringer Mannheim (Laval, Quebec, Canada). In situ hybridization to placental tissue from the first trimester (5–13 weeks’ gestation) and from normal pregnancies and pregnancies complicated by preeclampsia at delivery (26–34 weeks’ gestation) was performed according to Braissant and Wahli (34).
Immunohistochemistry.
Placental tissue from the first trimester (5–13 weeks’ gestation) and from normal pregnancies and pregnancies complicated by preeclampsia at delivery (26–34 weeks’ gestation) was fixed for 2–4 hours at 4°C in 4% (vol/vol) paraformaldehyde, cryoprotected by incubation in 10% (vol/vol) glycerol for 30 minutes and 50% (vol/vol) OCT compound (Tissue-Tek; Miles Inc., Elkhart, Indiana, USA) for 18 hours, embedded in 100% OCT, and frozen in liquid nitrogen. Sections of 7-μm width were cut. To verify the quality of the tissue and select the most representative sections, every 10th section was stained with hematoxylin and eosin. Neighboring sections were stained using the avidin-biotin immunoperoxidase method. Endogenous peroxidase enzyme activity was quenched with 3% (vol/vol) hydrogen peroxide in methanol for 30 minutes. Nonspecific binding sites were blocked using 5% (vol/vol) normal goat serum (NGS) and 1% (wt/vol) BSA in Tris-buffer for 40 minutes at room temperature. Purified rabbit polyclonal antibodies directed against TGF-β1, TGF-β2, and TGF-β3 (Santa Cruz Biotechnology, Santa Cruz, California, USA) were used each at 1:50 dilution. The slides were washed 3 times with Tris-buffer and were incubated with a 200-fold dilution of biotinylated goat anti-rabbit IgG for 1 hour at 4°C. After washing 3 times with Tris- buffer, the slides were incubated with an avidin-biotin complex for 1 hour. Slides were washed again in Tris-buffer and developed in 0.075% (wt/vol) 3,3-diaminobenzidine in Tris-buffer (pH 7.6) containing 0.002% (vol/vol) H2O2, giving rise to a brown product. After light counterstaining with toluidine blue, slides were dehydrated in an ascending ethanol series, cleared in xylene, and mounted. In control experiments, primary antibodies were replaced with antiserum preincubated with an excess of TGF-βs (competing peptide) or blocking solution (5% [vol/vol] NGS and 1% [wt/vol] BSA).
Fibronectin synthesis and release.
Villous explants of 5–13 weeks’ gestation were incubated overnight in DMEM/F12. Explants were then washed and incubated in DMEM/F12 alone or in medium containing either 10 μM antisense or sense TGF-β1, TGF-β2, or TGF-β3 oligonucleotides. The medium with or without the various agents was changed on day 3 of culture and was replaced on day 5 by methionine/cysteine–free DMEM supplemented with 25 μCi/mL of [35S]methionine/cysteine with or without the same oligonucleotides. After labeling for 18 hours, conditioned culture media were collected and diluted with an equal amount of 25 mM Tris-HCl buffer (pH 7.4), 0.15 M NaCl, and 0.5% (vol/vol) Triton X-100. Fibronectin was isolated using gelatin-Sepharose as described previously (28, 35). Briefly, 50 μL of the gelatin-Sepharose suspension was added to 500 μL of medium, and the samples were incubated overnight at 4°C. The gelatin-Sepharose beads were centrifuged and then washed 3 times in Tris/Triton X-100 buffer. Fibronectin was eluted by boiling for 5 minutes in 1% (vol/vol) SDS and was electrophoresed on 4–12% (wt/vol) polyacrylamide gradient gels. Radiolabeled fibronectin was revealed by autoradiography and quantitated using a PhosphorImager (410A and ImageQuant software; Molecular Dynamics, Sunnyvale, California, USA).
Detection of metalloproteinases by zymography and Western blot analysis.
Analysis of gelatinolytic activity was performed using 10% (wt/vol) polyacrylamide gel impregnated with 0.1% (wt/vol) gelatin (Novex, San Diego, California, USA). Four microliters of conditioned media harvested from the explant cultures at day 5 of treatment was mixed with 10% (vol/vol) glycerol, 2% (wt/vol) SDS, 0.0025% (wt/vol) bromophenol blue, and 0.5 M Tris (pH 6.8) and subjected to substrate-gel electrophoresis. Gels were then washed twice in 2% (vol/vol) Triton X-100 for 30 minutes at room temperature to remove the SDS. After this time, gels were equilibrated with developing buffer (50 mM Tris-HCl, 0.2 M NaCl, 5 M CaCl2, Brij 35; pH 7.2) for 30 minutes at room temperature and incubated overnight with the same buffer at 37°C. Gels were then stained with 0.1% (wt/vol) Coomassie brilliant blue G-250 to view zones of gelatinase activity.
For Western blot analysis of metalloproteinase expression, 5 μL of conditioned media was subjected to gel electrophoresis using 10% (wt/vol) polyacrylamide gels. Proteins were then blotted to Westran PVDF membrane. Primary antibodies were used at 1:100 dilution and were detected using horseradish peroxidase–conjugated anti-mouse IgG (1:10,000-fold dilution; Amersham, Baie d’Urse, Quebec, Canada) followed by enhanced chemiluminescence (ECL; Amersham).
Statistical analysis.
All data are presented as mean ± SEM of at least 3 separate experiments carried out in triplicate. Statistical significance was determined by Student’s t test for paired groups, and by one-way ANOVA followed by assessment of differences using Student Newman-Keuls test for nonpaired groups. Significance was defined as P < 0.05.
Results
Developmental regulation of TGF-β3 expression in the first trimester.
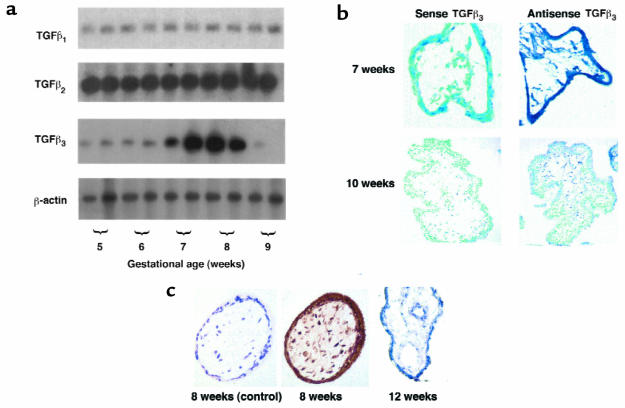
Low-cycle RT-PCR followed by Southern blot analysis and immunohistochemistry was used to define the pattern of expression of TGF-β isoforms in the human placenta during the first trimester of pregnancy. All 3 isoforms of TGF-β were expressed during the first trimester (Figure 1a). However, although transcripts corresponding to TGF-β1 and TGF-β2 were uniformly expressed throughout this period, the expression of TGF-β3 exhibited a striking pattern of developmental regulation. TGF-β3 mRNA levels were relatively low at 5–6 weeks, increased markedly between 7 and 8 weeks, and then fell precipitously at 9 weeks. To determine the spatial pattern of TGF-β3 mRNA expression, we performed in situ hybridization to placental sections from 5–10 weeks’ gestation using a TGF-β3 riboprobe. Figure 1b shows a representative experiment demonstrating that high levels of TGF-β3 mRNA were detected in sections of 7-week placentae in syncytiotrophoblast, cytotrophoblast, and stromal cells. By 9–10 weeks’ gestation, the mRNA expression of TGF-β3 in all cell layers declined. This spatial and temporal expression pattern for TGF-β3 was confirmed at the protein level by immunohistochemistry (Figure 1c). Villous trophoblasts from a placenta of 8 weeks stained positively for TGF-β3. In contrast, TGF-β3 immunoreactivity was noticeably low or absent in villous trophoblast cells of 12-week placental tissue. No reactivity was observed in placental sections stained with antisera preabsorbed with peptide.
Figure 1.
Expression of TGF-β isoforms in human placenta in the first trimester of gestation. (a) Message expression of TGF-β isoforms was assessed by low-cycle RT-PCR followed by Southern blot analysis using specific probes for TGF-β1, TGF-β2, TGF-β3, and the control housekeeping gene β-actin. Note that TGF-β3 expression increases at around 7–8 weeks’ gestation and declines thereafter. (b) Expression of TGF-β3 mRNA was also assessed by in situ hybridization to placental sections at 7 and 10 weeks’ gestation with digoxigenin-labeled sense and antisense TGF-β3 riboprobes. Endogenous alkaline phosphatases were blocked by the addition of levamisole. Sections were counterstained with methyl green. Note that TGF-β3 mRNA expression, viewed by blue staining, is high at 7 weeks in chorionic villi and decreases around 10 weeks. Control experiments were performed using sense TGF-β3 riboprobes. ×100. (c) Immunoperoxidase staining of TGF-β3 was performed in placental sections at 8 and 12 weeks’ gestation. Sections of placental tissue of 8 weeks’ gestation show strong positive immunoreactivity viewed as brown staining in the cytotrophoblast, syncytiotrophoblast, and stromal cells of the chorionic villi. Sections of placenta at 12 weeks’ gestation demonstrate low or absent TGF-β3 immunoreactivity in the villi. There is no immunoreactivity when antiserum was preincubated with an excess of TGF-β3 competing peptide (8 weeks, control). ×200.
Temporal and spatial differentiation of first-trimester villous trophoblasts toward an invasive phenotype in vitro.
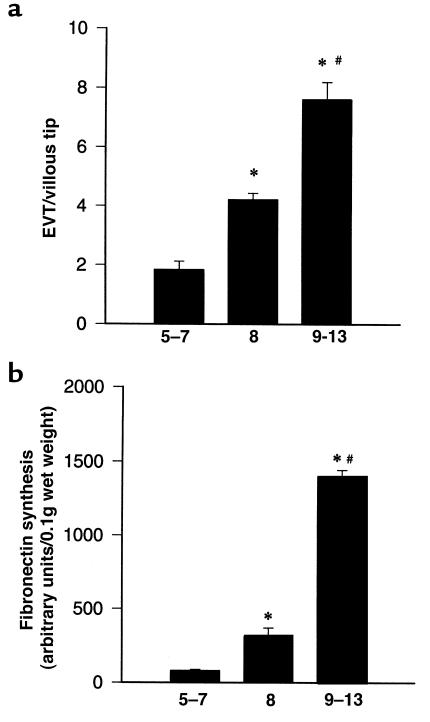
Because TGF-β regulates interactions of cells with the extracellular matrix molecules, as well as interactions between cells, it was particularly relevant to study its effects on trophoblast differentiation toward an invasive phenotype in a system in which the placental villous tissue architecture is maintained. Placental villous explants in culture preserve the topology of intact villi and mimic closely the formation of anchoring villi occurring in vivo during the first trimester of pregnancy (27, 28, 30). We first investigated the temporal differentiation of first-trimester villous trophoblasts toward an invasive phenotype using the villous explant system. Explants from placentae of 5–7, 8, or 9–13 weeks’ gestation were cultured on a Matrigel base for 6 days, and their differentiation was assessed using morphological and biochemical markers of early trophoblast differentiation, namely, outgrowth of EVT cells (EVT outgrowth) from the explant and fibronectin synthesis, respectively. Explants at 9–13 weeks’ gestation showed significantly greater EVT outgrowth and fibronectin synthesis compared with those at 5–8 weeks (Figure 2, a and b).
Figure 2.
Trophoblast differentiative capability of villous explants is gestational age dependent. Villous explants from first-trimester gestation (5–13 weeks) were cultured for 6 days. Differentiation was monitored by measuring morphological and biochemical markers of trophoblast differentiation. (a) EVT cell outgrowth and migration were expressed as the ratio of EVT outgrowths per villous tip, where the nominator, EVT outgrowths, represents the number of EVT columns sprouting from the villous tips plus the number of islands of EVT invading into the Matrigel. The denominator represents the total number of villous tips in a single explant culture. (b) At day 5, explants were metabolically labeled with [35S]methionine for 18 hours. Fibronectin was isolated from conditioned medium using gelatin-Sepharose beads on the protein extract separated by SDS PAGE. Radiolabeled bands were quantified with the use of a PhosphorImager. Data represent the mean ± SEM of 6–10 different experiments performed in triplicate. *P < 0.05, by ANOVA, compared with 5–7 weeks’ gestation. #P < 0.05, by ANOVA, compared with 8 weeks’ gestation.
Inhibition of TGF-β3 expression and activity initiates trophoblast differentiation toward an invasive phenotype.
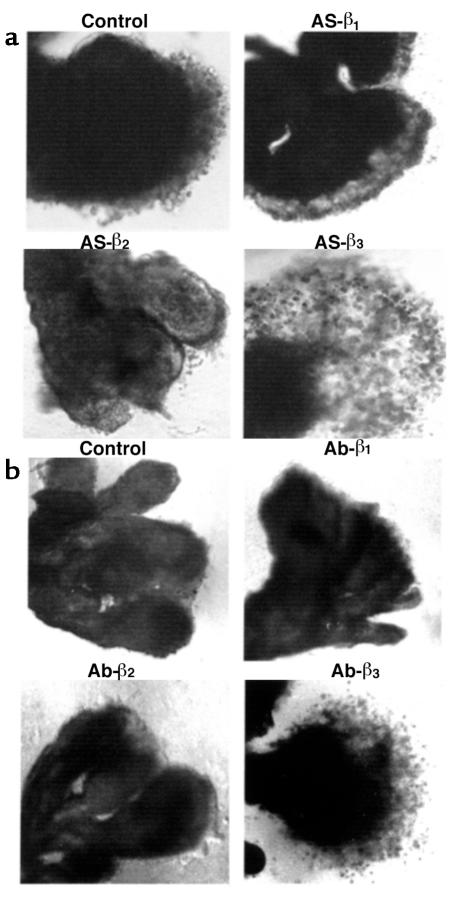
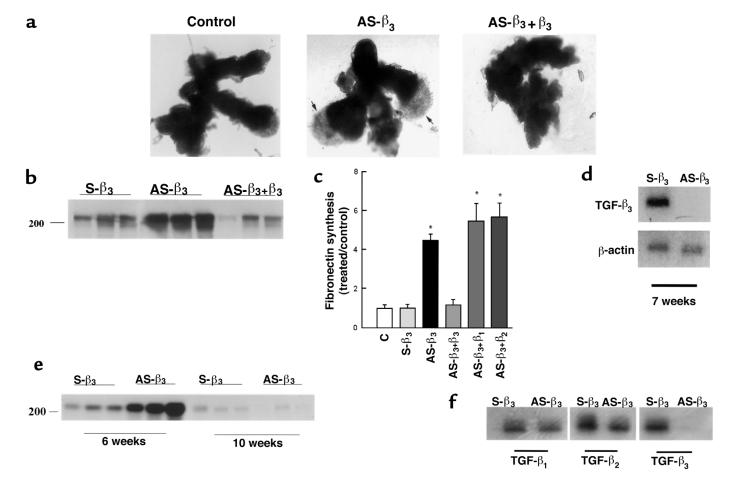
To determine the functional significance of the TGF-β expression patterns, we monitored trophoblast differentiation in response to both TGF-β antibody– (10 μg/mL) and antisense oligonucleotide–induced inhibition of TGF-β expression in explants at 5–8 weeks’ gestation. EVT outgrowth was not significantly increased after exposure to antisense oligonucleotides or antibodies to TGF-β1 or TGF-β2 (Figure 3, a and b). In contrast, explants exposed to either antisense TGF-β3 oligonucleotide or TGF-β3 antibody displayed prominent EVT outgrowth from the distal end of the villous tip and an increased number of cells migrating into the surrounding matrix (Figure 3, a and b; see Figure 5a). Characterization of the EVT in the villous outgrowths of antisense TGF-β3 oligonucleotide–treated explants with various markers was performed as described earlier (27, 28). During the differentiation pathway, EVT start to express fibronectin. We thus investigated the effect of antisense oligonucleotide to the different TGF-β isoforms on fibronectin synthesis by villous explants of 5–8 weeks’ gestation. Explants were metabolically labeled on day 5 with [35S]methionine, and newly synthesized fibronectin released into the medium over a period of 18 hours was measured. Antisense oligonucleotide to TGF-β3, but not to TGF-β1 or TGF-β2, significantly increased the production of fibronectin when compared with that observed in control cultures treated with sense oligonucleotide or medium alone (Figure 4). Thus the morphological response after antisense TGF-β3 treatment is associated with a significant increase in fibronectin synthesis. The specificity of the antisense TGF-β3 response was demonstrated by reversal of both morphological and biochemical indices when antisense-treated explants were cultured in the presence of TGF-β3 but not TGF-β1 or TGF-β2 (Figure 5, a–c). Further experiments demonstrated that treatment of villous explants with antisense oligonucleotide to TGF-β3 abolished TGF-β3 mRNA expression (Figure 5, d and f), but not that of TGF-β1 or TGF-β2 (Figure 5f), corroborating the specificity of the antisense TGF-β3 effect. TGF-β3 is not expressed in villous trophoblasts at ≥9 weeks (Figure 1). Indeed, antisense oligonucleotide to TGF-β3 had no effect on fibronectin synthesis in villous explants of 10 weeks’ gestation (Figure 5e).
Figure 3.
Antisense oligonucleotides and antibody to TGF-β3, but not to TGF-β2 or TGF-β1, induce trophoblast outgrowth and migration in villous explant cultures. (a) Villous explants from 5–8 weeks’ gestation were maintained in culture for 5 days in the presence of 10 μM antisense oligonucleotides to TGF-β1 (AS-β1), TGF-β2 (AS-β2), and TGF-β3 (AS-β3). Control experiments were run in parallel using explants from the same placenta cultured in either medium alone or medium containing sense oligonucleotides. (b) Villous explants from 5–8 weeks’ gestation were maintained in culture for 5 days in the presence of 10 μg/mL of neutralizing antibody to TGF-β1 (Ab-β1), TGF-β2 (Ab-β2), and TGF-β3 (Ab-β3). Control experiments were run in parallel using explants from the same placenta cultured in either medium alone or in medium containing IgG control. Note that both antisense TGF-β3 (AS-β3) and antibody TGF-β3 (Ab-β3) treatments dramatically increase budding and outgrowth of EVT from the distal end of the villous tips when compared with control villous explants or explants exposed to TGF-β1 and TGF-β2 antisense oligonucleotides and antibodies. ×50.
Figure 5.
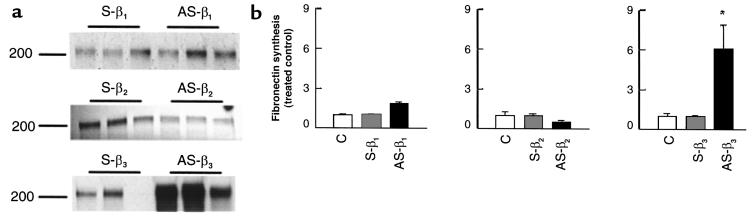
The antisense TGF-β3 stimulatory effect on trophoblast migration and on fibronectin production is specific. Explants of 5–8 weeks’ gestation were treated for 5 days with 10 μM antisense oligonucleotides to TGF-β3 (AS-β3), plus 10 ng/mL recombinant TGF-β3 (AS-β3+β3), TGF-β1 (AS-β3+β1), or TGF-β2 (AS-β3+β2). Control experiments were run in parallel using sense TGF-β3 (S-β3) or medium alone (C). (a) Shown is a representative experiment demonstrating that addition of recombinant TGF-β3 to antisense TGF-β3–treated explants (AS-β3+β3) abolishes the antisense stimulatory effect on trophoblast budding and outgrowth (arrows). ×25. (b) Similar reversal of AS-β3 stimulation of fibronectin synthesis by exogenous TGF-β3. A representative analysis of triplicate samples from a single experiment is shown. The position of the marker with Mr = 200,000 is indicated. (c) Radiolabeled bands were quantified with the use of a PhosphorImager. Shown are the changes in fibronectin estimated after normalization to control cultures. Antisense TGF-β3 treatment (AS-β3) significantly increased the amount of labeled fibronectin compared with both medium alone (C) or sense oligonucleotide (S-β3). Addition of exogenous TGF-β3 (AS-β3+β3), but not TGF-β1(AS-β3+β1) or TGF-β2 (AS-β3+β2), to the antisense-treated explants abolished the antisense stimulatory effect on fibronectin production. (d) mRNA expression of TGF-β3 in explants treated with antisense (AS-β3) or control sense (S-β3) oligonucleotides was measured by low-cycle RT-PCR followed by Southern blot analysis using specific probes for TGF-β3 and the control housekeeping gene β-actin. (e) The antisense TGF-β3 stimulatory effect on fibronectin production is lost by 10 weeks’ gestation. Explants of 6 and 10 weeks’ gestation were treated with 10 μM antisense (AS-β3) or control sense (S-β3) oligonucleotides to TGF-β3. Newly synthesized fibronectin was isolated from the medium as already described here. Representative analysis of triplicate samples from a single experiment is shown. (f) Antisense TGF-β3 treatment of villous explants (AS-β3) inhibits TGF-β3 mRNA but not TGF-β1 or TGF-β2 mRNA.
Figure 4.
Antisense oligonucleotides to TGF-β3, but not to TGF-β2 or TGF-β1, increase fibronectin synthesis in villous explant cultures. Explants were incubated for 4 days in medium alone, or in the presence of antisense oligonucleotides to TGF-β1 (AS-β1), TGF-β2 (AS-β2), TGF-β3 (AS-β3), and sense oligonucleotides (S-β1, S-β2, S-β3) (10 μM), and then metabolically labeled with [35S]methionine for 18 hours in the presence or absence of oligonucleotides. (a) Fibronectin was isolated from conditioned medium using gelatin-Sepharose beads and subjected to SDS-PAGE. The position of the marker with Mr = 200,000 is indicated. (b) Radiolabeled bands were quantified with the use of a PhosphorImager. Shown are the changes in fibronectin estimated after normalization to control cultures. Antisense TGF-β3 treatment (AS-β3; filled bar in third panel) significantly increased the amount of labeled fibronectin compared with both medium alone (C; open bars) or sense (S-β3; cross-hatched bar in third panel), whereas antisense TGF-β1 (AS-β1; filled bar in first panel) and TGF-β2 (AS-β2; filled bar in middle panel) did not. *P < 0.05 by ANOVA. All data are expressed as the mean ± SEM of 5 separate experiments carried out in triplicate.
Preeclampsia is associated with a failure to downregulate trophoblast expression of TGF-β3.
We next characterized the expression of the 3 TGF-β isoforms in villous placental tissue from patients across a range of gestational ages who were diagnosed as severe preeclamptics. TGF-β expression in these samples was compared with that in an age-matched control group of nonpreeclamptic patients by low-cycle RT-PCR followed by Southern blotting and immunohistochemistry. We detected mRNA encoding TGF-β3, but not TGF-β1 or TGF-β2, at higher levels in preeclamptic placenta than in controls (Figure 6a). We also observed elevated levels of α5-integrin and fibronectin mRNA (Figure 6a) and absence of α1-integrin mRNA (data not shown) in preeclamptic placentae, consistent with previous data (12) suggesting that trophoblast differentiation is arrested at an early stage along the invasive pathway in these placentae. The aberrant expression of TGF-β3 in preeclamptic placentae was further confirmed by histochemical analysis. In situ hybridization and immunohistochemistry showed high TGF-β3 expression in the syncytiotrophoblast cells in villous tissues from preeclamptic patients, whereas little or no signal was present in the age-matched controls (Figures 6b and 7). In contrast, immunohistochemical analysis demonstrated no differences for TGF-β1 and TGF-β2 expression between preeclamptic and age-matched control placentae (Figure 7).
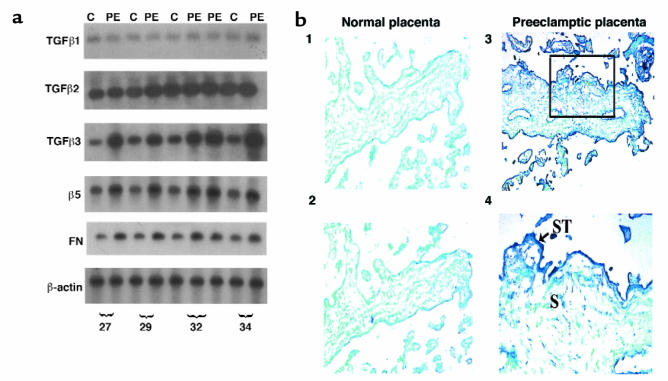
Figure 6.
TGF-β3 is overexpressed in preeclamptic placentae. (a) Message expression of TGF-β isoforms, α5 integrin receptor, and fibronectin in preeclamptic (PE) and age-matched control placentae (C) was assessed by low-cycle RT-PCR followed by Southern blot analysis using appropriate probes. Note that expression of TGF-β3, α5, and fibronectin, but not TGF-β1 or TGF-β2, was higher in preeclamptic placentae than in controls. (b) Expression of TGF-β3 mRNA was also assessed by in situ hybridization to placental sections from normal and preeclamptic pregnancies using digoxigenin-labeled sense and antisense TGF-β3 riboprobes. Sections were counterstained with methyl green. Endogenous alkaline phosphatase were blocked by the addition of levamisole. Panel 2 shows a section of normal placenta at 29 weeks, with little or absent expression of TGF-β3. Panels 3 and 4 show sections of preeclamptic placental tissue of the same gestation, with high TGF-β3 expression viewed by blue staining in the syncytiotrophoblast (ST; arrow) and to a lesser extent in stromal cells (S) of the chorionic villi. Control experiments were performed using sense TGF-β3 riboprobes (panel 1). Panels 1–3: ×100; panel 4: ×200.
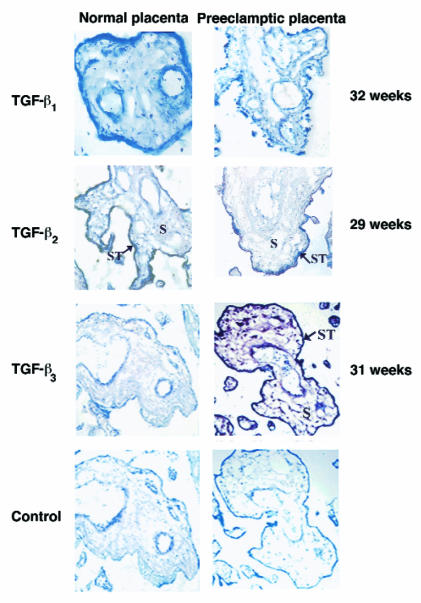
Figure 7.
Immunoperoxidase staining of TGF-β1, TGF-β2, and TGF-β3 was performed in placental sections from normal pregnancies and pregnancies complicated by preeclampsia. Sections of both normal and preeclamptic placental tissue of 29 weeks’ gestation show low/absent TGF-β1 immunoreactivity in cells of the chorionic villi. Sections of both normal and preeclamptic placental tissue of 32 weeks’ gestation show positive TGF-β2 immunoreactivity in the syncytium of the chorionic villi (ST, arrow). Sections of normal placental tissue of 31 weeks’ gestation show low/absent TGF-β3 immunoreactivity in cells of the chorionic villi. Sections of preeclamptic placental tissue of the same gestation show strong TGF-β3 immunoreactivity viewed by brown staining in the syncytiotrophoblast (ST, arrow) and in stromal cells (S) of the chorionic villi. Control experiments were performed using antiserum preabsorbed with an excess of peptide. ×100. Immunostaining for TGF-β1, TGF-β2 , and TGF-β3 was repeated in 5 different preeclamptic and age-matched control placentae ranging from 27 to 34 weeks’ gestation.
Inhibition of TGF-β3 expression and activity in preeclamptic placentae restores invasive capability of trophoblasts.
To determine whether there was functional significance associated with overexpression of TGF-β3 in preeclamptic placentae, we analyzed trophoblast differentiation in explants from control and preeclamptic patients. When cultured on Matrigel, explants from a nonpreeclamptic patient showed formation of EVT columns that spontaneously invaded into the surrounding Matrigel (Figure 8a, panels 1 and 5). In contrast, explants from preeclamptic placentae failed to exhibit EVT outgrowth or invasion (Figure 8a, panels 2 and 6). These data are consistent with the view that preeclampsia is associated with reduced invasive capability of trophoblasts. To determine whether this reduced invasive capability was due to TGF-β3 overexpression, we monitored trophoblast differentiation in villous explants from preeclamptic patients cultured in the presence of antibody and antisense oligonucleotide to TGF-β3. In contrast to untreated or sense-treated controls, treatment of explants from preeclamptic patients with antisense TGF-β3 restored the invasive capability, as demonstrated by the formation of EVT columns migrating through the Matrigel (Figure 8a, panel 5). Similar stimulatory effect on EVT column formation was observed with an antibody to TGF-β3 (Figure 8a, panel 3). The specificity of the antisense TGF-β3 response was demonstrated by reversal of this effect when antisense-treated explants were cultured in the presence of 10 ng/mL TGF-β3 (Figure 8a, panel 4). Further experiments demonstrated that treatment of explants from preeclamptic pregnancies with antisense oligonucleotide or antibodies to TGF-β1 or TGF-β2 did not show any effect on EVT column formation (data not shown), indicating that only the inhibition of the TGF-β3 isoform induces the invasive phenotype. The invasive nature of this antisense-induced phenotype was confirmed by the finding that explants treated with antisense TGF-β3 acquired the expression of gelatinase B/MMP-9, an enzyme that is normally only expressed in trophoblast cells that are highly invasive (Figure 8b). Treatment of explants from age-matched control patients with antibody or antisense TGF-β3 did not show any effect on gelatinase production (data not shown).
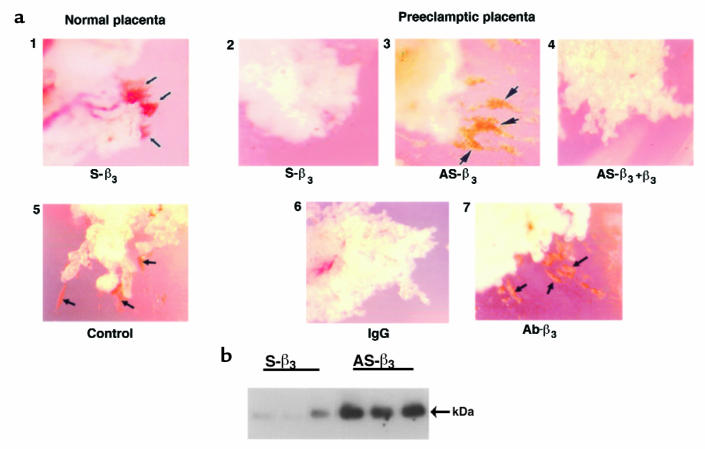
Figure 8.
Antisense oligonucleotides and antibody to TGF-β3 induce the formation of columns of trophoblast cells and gelatinase expression in preeclamptic villous explants. Villous explant cultures were prepared from preeclamptic and age-matched control placentae. (a) Explants were maintained in culture in the presence of either control sense or antisense oligonucleotide to TGF-β3 for 5 days. Shown is a representative experiment (n = 3 experiments carried out in triplicate). Panel 1 shows explants from a normal placenta (32 weeks) exposed to sense oligonucleotide (or medium alone; data not shown) spontaneously form columns of trophoblast cells that migrate and invade into the surrounding Matrigel (arrows), whereas explants from a preeclamptic placenta (32 weeks) do not (panel 2). Panel 3 shows antisense treatment of explants from a preeclamptic placenta (AS-β3) triggers the formation of invading trophoblast columns (arrows), an effect that is reversed by addition of recombinant TGF-β3 (panel 4). Similarly, explants from a normal placenta (29 weeks) exposed to control IgG (or medium alone; data not shown) spontaneously form columns of invasive trophoblast cells (arrows) (panel 5), whereas explants from a preeclamptic placenta (29 weeks) do not (panel 6). Panel 7 shows that antibody treatment (Ab-β3) triggers the formation of invading trophoblast columns (arrows) (n = 1 experiment carried out in triplicate). ×50. (b) Explants from preeclamptic placentae (32 weeks) were treated with antisense (AS-β3) or control sense (S-β3) oligonucleotides to TGF-β3 for 5 days. Samples of conditioned medium were collected at day 5 and subjected to Western blotting with MMP-9 antisera. Arrows indicate positions of MMP-9 (92 kDa).
Discussion
Here we show that TGF-β3 is a regulator of human trophoblast differentiation toward an invasive phenotype. A failure to downregulate expression of this cytokine is associated with, and may contribute to, the pathogenesis of preeclampsia in humans. The conclusion that TGF-β3 is a major factor in the pathogenesis of preeclampsia is based on 3 observations: first, the distinct temporal and spatial patterns of TGF-β3 expression in normal and preeclamptic pregnancies; second, the demonstration that the invasive phenotype of trophoblasts can be prematurely triggered in first-trimester placentae by downregulation of TGF-β3; and third, the observation that the normal invasive phenotype was restored in preeclamptic placentae by both antibody and antisense inhibition of trophoblast TGF-β3 expression.
Expression of TGF-β3 in placental trophoblasts peaks at 6–8 weeks’ gestation and then falls precipitously around 9 weeks, precisely at the time of maximal trophoblast invasion in vivo (4). This downregulation of TGF-β3 expression is temporally associated with earlier reports of major changes in the expression of markers of trophoblast differentiation toward an invasive phenotype in anchoring villi, including switching of integrin isoforms (36–38), synthesis of matrix ligands for these integrins (39, 40), and upregulation of gelatinase A (MMP-2) (21). Previous studies have suggested that the trophoblasts from preeclamptic placentae exhibit a relatively immature phenotype. Thus, preeclamptic placentae fail to complete integrin switching (i.e., the trophoblasts remain positive for α5 and fail to express β1) (12); do not acquire an endovascular adhesion phenotype (41); demonstrate an excess of proliferative immature intermediate trophoblasts (11); and also maintain elevated plasma levels of fibronectin (40, 42) (a marker of the initial phase of trophoblast differentiation). Our observation of increased fibronectin and α5 mRNA levels (with no α1 expression) in preeclamptic versus age-matched control placentae supports these findings. Moreover, we now report that TGF-β3, expressed in first-trimester trophoblasts before their differentiation along the invasive pathway, is also overexpressed in preeclamptic placentae.
Antisense and antibody inhibition of TGF-β3 in human villous explants at 6–8 weeks of pregnancy induced a premature switch to the differentiative/invasive phenotype normally seen at 9–13 weeks (when endogenous expression of TGF-β3 is reduced). The data strongly suggest that TGF-β3 acts as an inhibitor of trophoblast differentiation toward an invasive phenotype. This conclusion is consistent with reports of the anti-invasive actions of other TGF-β isoforms (primarily TGF-β1) on other cell/tissue systems including colon carcinoma and thyroid cells (22–24). There have been conflicting data as to the actions of TGF-β1 on trophoblast invasion. Some investigators have reported inhibition of the invasive capability of isolated first-trimester trophoblasts, possibly through an induction of TIMP expression or reduced urokinase plasminogen activator (uPA) activity (25). In other studies, TGF-β1 was not able to inhibit invasion of isolated first-trimester trophoblasts (18). It is likely that the isolation of different trophoblast subpopulations (due to different preparation methodologies or pooling of late first-trimester placentae) may underlie these contradictory reports. Cell-matrix and cell-cell interactions are known to be critical modulators of TGF-β action, and the maintenance of such interactions in the placental explants may be important in revealing the anti-invasive actions of TGF-β3.
There have been several descriptive reports of various markers associated with preeclampsia, including TNF-α, human leukocyte antigen-G, VEGF, M-CSF, uPA, and IGFs (43–48). The precise role, if any, of these proteins in preeclampsia is unclear. The data presented here demonstrate not only that abnormalities in TGF-β3 expression are associated with preeclampsia but also that inhibition of TGF-β3 with antibody and antisense oligonucleotides restores the invasive capability of preeclamptic trophoblasts. Thus, we found morphological (formation of invading trophoblast columns) and biochemical (induction of MMP-9, a marker of trophoblast invasion; ref. 21) evidence of induction of an invasive phenotype following antisense TGF-β3 treatment. Our data are consistent with a model of normal placentation in which downregulation of TGF-β3 expression in trophoblasts around 9 weeks of pregnancy permits differentiation of trophoblasts to EVT that form the anchoring villi and from which derive the α1-integrin–positive EVT that can invade deep into the maternal uterus. This invasion contributes to the remodeling of the uterine spiral arteries and ultimately enables the establishment of increased vascular perfusion of the placenta. In placentae predisposed to preeclampsia, TGF-β3 expression remains abnormally elevated and trophoblasts remain in a relatively immature state of differentiation. As a direct consequence, trophoblast invasion into the uterus is limited and uteroplacental perfusion is reduced. This conclusion is consistent with the clinical manifestations of preeclampsia, including shallow trophoblast invasion into the uterus and abnormally high uteroplacental vascular resistance. Nevertheless, the aberrant expression of a large number of factors in preeclamptic pregnancies suggests that the pathogenesis of this disease is complex and likely involves many regulatory systems.
The data in this article suggest that the monitoring of TGF-β3 expression may be a useful diagnostic marker of preeclampsia. Equally important, these data suggest a novel target for therapeutic intervention to ameliorate or prevent this life-threatening condition.
Acknowledgments
We thank Lindsay McWhirter for providing the placental samples. We also thank Alan Bernstein, John Kingdom, James Copeman, and Jay Cross for carefully reading the manuscript, and Knox Ritchie for the constant support. This work was supported by the Department of Obstetrics and Gynaecology, Medical Research Council of Canada (MRC) grant MT-14096 (to I. Caniggia), MRC Group Grant in Developmental and Fetal Health (to S.J. Lye), and MRC Group Grant in Lung Development (to M. Post).
References
- 1.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 2.Strickland S, Richards WG. Invasion of trophoblasts. Cell. 1992;71:355–357. doi: 10.1016/0092-8674(92)90503-5. [DOI] [PubMed] [Google Scholar]
- 3.Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway in vivo. J Clin Invest. 1992;89:221–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–692. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- 5.Redman CWG. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 6.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational-age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JM, Taylor RN, Friedman SA, Goldfien A. New development in preeclampsia. Fetal Matern Med Rev. 1990;2:125–141. [Google Scholar]
- 8.Broughton Pipkin F, Rubin PC. Pre-eclampsia the “disease of theories.”. Br Med Bull. 1994;50:381–396. doi: 10.1093/oxfordjournals.bmb.a072898. [DOI] [PubMed] [Google Scholar]
- 9.Pritchard, J.A., and Mc Donald, P.C. 1980. Hypertensive disorders of pregnancy. InWilliams’ obstetrics. 16th edition. J.A. Pritchard and P.C. McDonald, editors. Appleton-Century-Croft. New York, NY. 665–700.
- 10.Slattery MA, Khong TY, Dawkins RR, Pridemore BR, Hague WM. Eclampsia in association with partial molar pregnancy and congenital abnormalities. Am J Obstet Gynecol. 1993;169:1625–1627. doi: 10.1016/0002-9378(93)90452-o. [DOI] [PubMed] [Google Scholar]
- 11.Redline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol. 1995;26:594–600. doi: 10.1016/0046-8177(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeGroot CJ, O’Brien TJ, Taylor RN. Biochemical evidence of impaired trophoblastic invasion of decidual stroma in women destined to have preeclampsia. Am J Obstet Gynecol. 1996;175:24–29. doi: 10.1016/s0002-9378(96)70245-4. [DOI] [PubMed] [Google Scholar]
- 14.Kingdom JCP, Kaufmann P. Current topic: oxygen and placental villous development: origin of fetal hypoxia. Placenta. 1997;18:613–621. doi: 10.1016/s0143-4004(97)90000-x. [DOI] [PubMed] [Google Scholar]
- 15.Arkwright PD, Rademacher TW, Dwek RA, Redman CW. Pre-eclampsia is associated with an increase in trophoblast glycogen content and glycogen synthase activity, similar to that found in hydatidiform moles. J Clin Invest. 1993;91:2744–2753. doi: 10.1172/JCI116515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross JC. Trophoblast function in normal and preeclamptic pregnancy. Fetal Matern Med Rev. 1996;8:57–66. [Google Scholar]
- 17.Zhou Y, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bass KE, et al. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev Biol. 164:550–561. doi: 10.1006/dbio.1994.1223. [DOI] [PubMed] [Google Scholar]
- 19.Lala, P., and Lysiak, J. 1994. Role of locally produced growth factors in human placental growth and invasion with special reference to transforming growth factors. In Immunology of Reproduction. J.S. Hunt, editor. Springer-Verlag. New York, NY. 57–81.
- 20.Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF-β, IGF-II and IGFBP-1. Exp Cell Res. 1995;217:419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- 21.Librach CI, et al. Interleukin-1β regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994;269:17125–17131. [PubMed] [Google Scholar]
- 22.Massague J, Weis-Garcia F. Serine/threonine kinase receptors: mediators of transforming growth factor beta family signals. Cancer Surv. 1996;27:41–64. [PubMed] [Google Scholar]
- 23.Blaydes JP, Wynford-Thomas D. Loss of responsiveness to transforming growth factor beta (TGF beta) is tightly linked to tumorigenicity in a model of thyroid tumor progression. Int J Cancer. 1996;65:525–530. doi: 10.1002/(SICI)1097-0215(19960208)65:4<525::AID-IJC22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, et al. Reduced expression of transforming growth factor beta type I receptor contributes to the malignancy of human colon carcinoma cells. J Biol Chem. 1996;271:17366–17371. doi: 10.1074/jbc.271.29.17366. [DOI] [PubMed] [Google Scholar]
- 25.Graham CH, Lala PK. Mechanism of control of trophoblast invasion in situ. J Cell Physiol. 1991;148:228–234. doi: 10.1002/jcp.1041480207. [DOI] [PubMed] [Google Scholar]
- 26.Morrish DW, Bhardwaj D, Paras MT. Transforming growth factor β1 inhibits placental differentiation and human chorionic gonadotropin and human placental lactogen secretion. Endocrinology. 1991;129:22–26. doi: 10.1210/endo-129-1-22. [DOI] [PubMed] [Google Scholar]
- 27.Caniggia I, Lye SJL, Cross JC. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology. 1997;138:3976–3986. doi: 10.1210/endo.138.9.5403. [DOI] [PubMed] [Google Scholar]
- 28.Caniggia I, Taylor CV, Ritchie JWK, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997;138:4977–4988. doi: 10.1210/endo.138.11.5475. [DOI] [PubMed] [Google Scholar]
- 29.Chesley L. Diagnostic criteria of preeclampsia. Obstet Gynecol. 1985;65:423–425. [PubMed] [Google Scholar]
- 30.Genbacev O, Schubach SA, Miller RK. Villous culture of first trimester human placenta-model to study extravillous trophoblast (EVT) differentiation. Placenta. 1992;13:439–461. doi: 10.1016/0143-4004(92)90051-t. [DOI] [PubMed] [Google Scholar]
- 31.Chai Y, et al. Specific transforming growth factor-β subtypes regulate embryonic mouse Meckel’s cartilage and tooth development. Dev Biol. 1994;162:85–103. doi: 10.1006/dbio.1994.1069. [DOI] [PubMed] [Google Scholar]
- 32.Malcolm ADB. Uses and applications of antisense oligonucleotides: uses of antisense nucleic acids—an introduction. Biochem Soc Trans. 1992;20:745–746. doi: 10.1042/bst0200745. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, et al. Cloning and characterization of glucocorticoid-induced genes in fetal rat lung fibroblasts: transforming growth factor β3. J Biol Chem. 1995;270:2722–2728. doi: 10.1074/jbc.270.6.2722. [DOI] [PubMed] [Google Scholar]
- 34.Braissant O, Wahli W. A simplified in situ hybridization protocol using non-radioactively labeled probes to detect abundant and rare mRNAs on tissue sections. Biochemica. 1998;1:10–16. [Google Scholar]
- 35.Engvall E, Ruoslhati E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 36.Damsky CH, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 37.Bischof P, Redard M, Gindre P, Vassilakos P, Campana A. Localization of alpha2, alpha5 and alpha6 integrin subunits in human endometrium, decidua and trophoblast. Eur J Obstet Gynecol Reprod Biol. 1993;51:217–226. doi: 10.1016/0028-2243(93)90038-e. [DOI] [PubMed] [Google Scholar]
- 38.St.-Jacques S, Forte M, Lye SJ, Letarte M. Localization of endoglin a transforming growth factor-β binding protein, and of CD44 and integrins in placenta during the first trimester of pregnancy. Biol Reprod. 1994;51:405–413. doi: 10.1095/biolreprod51.3.405. [DOI] [PubMed] [Google Scholar]
- 39.Bischof P, Haenggeli L, Campana A. Gelatinaseoncofetal fibronectin secretion is dependent on integrin expression on human cytotrophoblasts. Hum Reprod. 1995;10:734–742. doi: 10.1093/oxfordjournals.humrep.a136024. [DOI] [PubMed] [Google Scholar]
- 40.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol MA. Fetal fibronectin levels are elevated in maternal plasma and amniotic fluid of patients with severe preeclampsia . Am J Obstet Gynecol. 1995;172:649–653. doi: 10.1016/0002-9378(95)90587-1. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblast to mimic a vascular adhesion phenotype. J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brubaker DB, Ross MG, Marinoff D. The function of elevated plasma fibronectin in preeclampsia. Am J Obstet Gynecol. 1992;166:526–531. doi: 10.1016/0002-9378(92)91663-u. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Wals SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol. 1996;32:157–169. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- 44.Hara N, et al. Altered expression of human leukocyte antigen G (HLA-G) on extravillous trophoblast in preeclampsia: immunohistological demonstration with anti-HLA-G specific antibody “87G” and anti-cytokeratin antibody “CAM5.2.”. Am J Reprod Immunol. 1996;36:349–358. doi: 10.1111/j.1600-0897.1996.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 45.Cooper JC, Sharkey AM, Charnock-Jones DS, Palmer CR, Smith SK. VEGF mRNA levels in placentae from pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1996;103:1191–1196. doi: 10.1111/j.1471-0528.1996.tb09627.x. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi M, Numaguchi M, Watabe H, Yaoi Y. High blood levels of macrophage colony-stimulating factor in preeclampsia. Blood. 1996;88:4426–4428. [PubMed] [Google Scholar]
- 47.Graham CH, McCrae KR. Altered expression of gelatinase and surface-associated plasminogen activator activity by trophoblast cells isolated from placentas of preeclamptic patients. Am J Obstet Gynecol. 1996;175:555–562. doi: 10.1053/ob.1996.v175.a74404. [DOI] [PubMed] [Google Scholar]
- 48.Giudice LC, Martina NA, Crystal RA, Tazuke S, Druzin M. Insulin-like growth factor binding protein-1 at the maternal-fetal interface and insulin-like growth factor I, insulin-like growth factor II, and insulin-like growth factor binding protein-1 in the circulation of women with severe preeclampsia. Am J Obstet Gynecol. 1997;176:751–757. doi: 10.1016/s0002-9378(97)70598-2. [DOI] [PubMed] [Google Scholar]